Abstract
The recepteur d’origine nantais (RON) receptor tyrosine kinase, belonging to the mesenchymal-to-epithelial transition proto-oncogene family, has been implicated in the pathogenesis of cancers derived from the colon, lung, breast, and pancreas. These findings lay the foundation for targeting RON for cancer treatment. However, development of RON-targeted therapeutics has not gained sufficient attention for the last decade. Although therapeutic monoclonal antibodies (TMABs) targeting RON have been validated in preclinical studies, results from clinical trials have met with limited success. This outcome diminishes pharmaceutical enthusiasm for further development of RON-targeted therapeutics. Recently, antibody–drug conjugates (ADCs) targeting RON have drawn special attention owing to their increased therapeutic activity. The rationale for developing anti-RON ADCs is based on the observation that cancer cells are not sufficiently addicted to RON signaling for survival. Thus, TMAB-mediated inhibition of RON signaling is ineffective for clinical application. In contrast, anti-RON ADCs combine a target-specific antibody with potent cytotoxins for cancer cell killing. This approach not only overcomes the shortcomings in TMAB-targeted therapies but also holds the promise for advancing anti-RON ADCs into clinical trials. In this review, we discuss the latest advancements in the development of anti-RON ADCs for targeted cancer therapy including drug conjugation profile, pharmacokinetic properties, cytotoxic effect in vitro, efficacy in tumor models, and toxicological activities in primates.
Keywords: antibody–drug conjugates, clinical trials, epithelial cancer, pharmacokinetic profile, receptor tyrosine kinase, therapeutic efficacy, therapeutic target, toxicological activity
Introduction
For the last 25 years, the role of recepteur d’origine nantais (RON) in tumorigenesis has been studied extensively in various cancer model systems.1,2 As a receptor tyrosine kinase belonging to the mesenchymal-to-epithelial transition (MET) receptor proto-oncogene family,3–5 RON is actively involved in various aspects of tumorigenesis including tumor progression, cellular invasiveness, chemoresistance, and cancer stemness.1,2 Clinically, aberrant RON expression, featured by overexpression of the receptor and generation of active splicing variants, exists in various types of cancer.1,2,6–13 Increased RON expression also has the prognostic value for disease progression and patient survival.14–19 These findings not only validate the significance of RON in clinical oncology, but also provide the rationale to develop RON-targeted therapeutics for cancer therapy. Here, we focus our attention on the latest information about aberrant RON expression in tumorigenesis and the progression in development of anti-RON antibody–drug conjugates (ADCs) for potential cancer treatment.
Aberrant RON expression and signaling in cancer pathogenesis
Expression of RON exists at relatively low levels in various types of normal epithelial cells including those from the colon, lung, and breast, but is not present in cells from mesenchymal origin.1,2 Functional studies using cancer cell lines and immunohistochemical (IHC) staining of tumor specimens confirm that aberrant RON expression and signaling are associated with cancer pathogenesis.1,2 In this sense, RON is a tumor-associated antigen. Aberrant RON expression is mainly featured by overexpression of the receptor and generation of active isoforms.1,2 Genetic alterations, such as point mutations and amplifications of the RON gene, are rarely observed. Overexpression of RON in cancerous tissues, but not in normal or benign cells, was first reported in breast cancer.9 Since then, increased RON expression has been documented in various types of cancer including those from colorectal, lung, breast, pancreatic, and others.6–13 A systematic analysis using tumor tissue microarrays demonstrates that RON overexpression at the rate of 30% and above occurs in tumors including colorectal, breast, and pancreatic cancers.6 Recently, increased RON expression has also been documented in bladder and prostate cancers.12–15 These findings help identify tumors for focused analysis of RON pathogenesis. In breast cancer, RON is known to be expressed in more than 80% of samples with overexpression in ~36% of cases.6,9,10 A recent study of primary triple negative breast cancer (TNBC) samples further demonstrates that RON is widely expressed in ~75% of samples with overexpression in 45% of cases.20 These findings mark aberrant RON expression as a pathogenic feature of breast cancer. Increased RON expression also is associated with the production of oncogenic RON isoforms such as RONΔ160, a variant with the deletion of 109 amino acids coded by exons 5 and 6 in the RON β-chain extracellular sequence.1,11,21–24 The majority of RON isoforms are mRNA splicing variants with deletions in certain exons.1,11,21–24 The frequency of RON variants detected in primary cancer samples and cell lines is relatively high with positive samples ranging from 40% to 60% of cases.1,23,24 In pancreatic cancer, the existence of different RON variants including the one with partial 5 and partial 6 exon splicing (designated as P5P6) is a pathogenic feature.23,24 In this sense, a splicing RON transcript profile for pancreatic cancer can be created.23,24 At the transcription level, hypermethylation in the RON gene promoter appears as a mechanism for altered mRNA splicing.24 Heterogeneous nuclear ribonucleoprotein A1 (hnRNP-A1), a nuclear splicing regulator that controls mRNA synthesis, splicing, and translation,25 has been shown to regulate alternative RON mRNA splicing.26 Thus, aberrant RON expression manifested at transcriptional and translational levels serves as a common pathogenic event for various types of cancer.
The roles of RON in regulating cancer cell invasiveness have been established.1,2 Multiple signaling mechanisms upon RON activation appear to be involved in this process.1,2 In the case of pancreatic cancer, aberrant RON expression and signaling play a critical role in regulating cancer cells growth, invasiveness, and chemoresistance.7,23,24,26–29 Studies using a mouse model of K-RAS-driven pancreatic cancer further demonstrate that RON overexpression not only increases acinar-ductal metaplasia formation but also accelerates the progression of pancreatic intraepithelial neoplasia towards adenocarcinomas.27 In breast cancer, RON activates several signaling pathways critical for proliferation, invasiveness, and stemness.26,30–36 In the case of breast cancer, aberrant RON signaling activates the cellular Abelson murine leukemia viral oncogene homolog (c-Abl), which leads to phosphorylation of proliferating cell nuclear antigen,36 a protein responsible for maintaining cellular growth machinery.37 In addition, RON signaling regulates breast cancer cell invasiveness through activation of DEK,34 a non-histone nuclear phosphoprotein that binds to DNA and induces positive supercoils into closed circular DNA.38 This effect appears to be channeled through an autocrine/paracrine canonical β catenin signaling loop,34 which plays a role in breast cancer stemness.31,39 Clinical studies indicate that aberrant RON signaling in collaboration with the DEK activity correlates with breast cancer recurrence and metastasis.34 Moreover, by reprogramming DNA methylation at specific target genes, aberrant RON signaling enhances breast cancer cell invasive growth and metastasis as evident in tumor xenograft models.35 This intriguing effect appears to be a result from RON-mediated phosphatidylinositol 3 kinase (PI3K)-dependent upregulation of the methyl-CpG binding domain protein 4 (MBD4),40 a thymine DNA glycosylase.40 Analysis of clinical data supports this notion, which demonstrates that the RON-MBD4 epigenetic pathway is associated with poor prognosis for breast cancer patients.40 In addition, the high-throughput proteomic analysis finds RON as a regulator for mammalian target of rapamycin complex 1 (mTORC1)/ ribosomal protein S6 kinase β-1 (p70S6k),30 a downstream signaling protein complex of the PI-3 kinase pathway.41 Suppression of mTORC1 by small molecule kinase inhibitors (SMKIs) is able to attenuate RON-dependent breast cancer cell metastasis in animal models.30 Thus, aberrant RON expression and signaling serve as the tumorigenic determinant in these cancer cells.
Progression in development of RON-targeted biotherapeutics
The concept of using anti-RON therapeutic monoclonal antibodies (TMABs) for cancer treatment emerged in the middle 2000s.42 Since then, anti-RON TMABs including IMC-41A10, narnatumab (also known as IMC-RON8), Zt/f2, Zt/g4, and others have been reported for their anti-tumor activities (Table 1).42–47 A recent study reported two anti-RON TMABs, namely 6E6 and 7G8, with variable therapeutic activities in mice models.48 As a single agent, anti-RON TMABs are able to delay tumor growth initiated by multiple cancer cell lines from different tissues.42–48 Different activities such as attenuation of RON signaling and stimulation of immune functions have been suggested as the mechanism of action.43–49 In addition, anti-RON TMABs have been reported in combination with chemotherapeutics to achieve a synergistic effect.45 However, the major issue associated with anti-RON TMABs is the limited anti-tumor activity. As evident from various in vivo studies, anti-RON TMABs only partially inhibit tumor growth.42–48 Complete inhibition by a single anti-RON TMAB has not been reported. In light of these facts, a concern has surfaced regarding the feasibility of anti-RON TMABs for clinical application. Indeed, the clinical phase I trials of narnatumab in patients with advanced solid tumors show limited activity within the dosing regimen,43 leading to the discontinuation of the clinical trials.49 Considering the fact that the majority of cancer cells are not addicted to RON signaling for survival, the outcome from this clinical trial is not surprising. In this sense, strategies aimed to maximize the therapeutic activity of anti-RON TMABs need to be further explored.
Table 1.
Biochemical and biological features of therapeutic and drug-delivering monoclonal antibodies specific to RON.
| Monoclonal antibodies analyzed | Producer/year | Chains or domains recognized | IgG subtype | Binding affinity (Kd) of mAbs | Current research status | Drug conjugated | Effect cellular model in vitro | Effect in xenograft tumors in mouse model | Clinical trial information | References |
|---|---|---|---|---|---|---|---|---|---|---|
| IMC-41A10 (human) | Imclone (USA)/2006 | Sema domain in RON | IgG1 | 0.15 nM | Preclinical | N/A | Prevents MSP binding, blocks RON signaling, and inhibits RON-mediated activity | Single agent at particular doses partially inhibits delay tumor growth | N/A | O’Toole et al.42 |
| Narnatumab /IMC-RON8 (human) | Eli Lilly (USA)/2011 | Sema domain in RON | IgG (subtype N/A) | Unknown | Phase I | N/A | Prevents MSP binding, blocks RON signaling, and inhibits RON-mediated activity | Single agent at particular doses partially inhibits delay tumor growth | Discontinued | LoRusso et al.43 |
| 29B06/07F01 (humanized) | AVEO (USA)/2010 | Sema domain in RON | IgG1 | 0.31 nM & 0.19 nM | Preclinical | N/A | Prevents MSP binding, blocks RON signaling, and inhibits RON-mediated activity | Single agent at particular doses partially inhibits delay tumor growth | N/A | Han et al.47 |
| Ig4/Ig7/Ig10 (human) | IRBM (Italy)/2011 | Sema domain in RON | IgG (subtype N/A) | 0.17, 0.43, and 0.64 nM | Preclinical | N/a | Prevents MSP binding, blocks RON signaling, and inhibits RON-mediated activity | Single agent at particular doses partially inhibits delay tumor growth | N/A | Gunes et al.46 |
| Zt/f2 (mouse) | TTUHSC (USA) /2006 | Sema domain in RON β-chain | IgG1/κ | 0.39 nM | Preclinical | N/A | Partially blocks RON signaling, and induces RON internalization | Single agent at particular doses partially inhibits tumor growth | N/A | Yao et al.44 |
| Zt/g4 (humanized) | TTUHSC (USA) /2006 | Sema domain in RON β-chain | IgG1/κ | 0.54 nM | preclinical | DM1, DCM, MMAE, and PBD | Moderated activate RON signaling and strongly induces RON endocytosis | No effect as naked antibody but completely inhibits tumors used as ADCs | N/A | Guin et al.45 |
| PCM5B14 (humanized) | PCM Targetech (USA)/2019 | PSI domain in RON β-chain | IgG1/κ | 0.27 nM | preclinical | DM1, DCM, and MMAE | Moderately activates RON signaling, and robustly induces RON endocytosis | No effect as naked antibody but completely inhibits tumors used as ADCs | N/A | Tong et al.50 |
| 6E6/7G8 (mouse) | A*STAR (Singapore)/2019 | IPT3 domain in β chain | IgG2a/IgG1 | 0.49 nM /unknown | preclinical | N/A | Specifically binds RON, partially blocks RON signaling, and inhibits RON-mediated activity | Single agent at particular doses partially inhibits or delay tumor growth | N/A | Koh et al.48 |
DCM, duocarmycin; DM1, matensinoid derivative 1; IPT, immunoglobulin-like plexin and transcription; MMAE, monomethyal auristatin E; MSP, macrophage-stimulating protein; PBD, pyrrolobenzodiazepine; PSI, plexin-semaphorin-integrin; Sema, semaphorin.
We are the first to use RON as a target moiety for drug delivery.48 Results from proof-of-concept studies using anti-RON immunoliposomes confirm the effectiveness of this approach.45,51,52 Since then, the advancement in ADC technology makes anti-RON ADCs a practical and applicable reality.53–55 Several features render RON a valuable target for ADC development. First, RON is preferentially expressed in a group of cancers but minimal in their corresponding normal cells.6–11 In addition, RON is not expressed in fibroblasts, endothelial cells, or blood cells that originate from the mesoderm.6–11 Such an expression pattern is critical for achieving the maximal therapeutic delivery with manageable safety profiles. Second, the selected anti-RON MABs rapidly induce cell surface RON internalization upon binding to cancer cells.45,51,52 It appears that MABs that interact with the unique RON extracellular domain and cause transient RON phosphorylation are the key for efficient receptor endocytosis. Third, levels of RON expression correlate with the number of drug molecules delivered to the cell, which helps to predict the antibody-directed cytotoxic activity.45,51,52 Considering these facts, it is believed that development of RON-targeted ADCs is a promising strategy for treatment of cancers overexpressing RON. This approach should also overcome the shortcomings associated with SMKI- and TMAB-targeted cancer therapy that rely on RON signaling addiction by cancer cells for survival. Fourth, cancer cells with different phenotypes such as hypoxia, stemness, and chemoresistance, are susceptible to anti-RON MAB-directed drug delivery.45,51,52
Anti-RON ADCs with superior anti-cancer activity
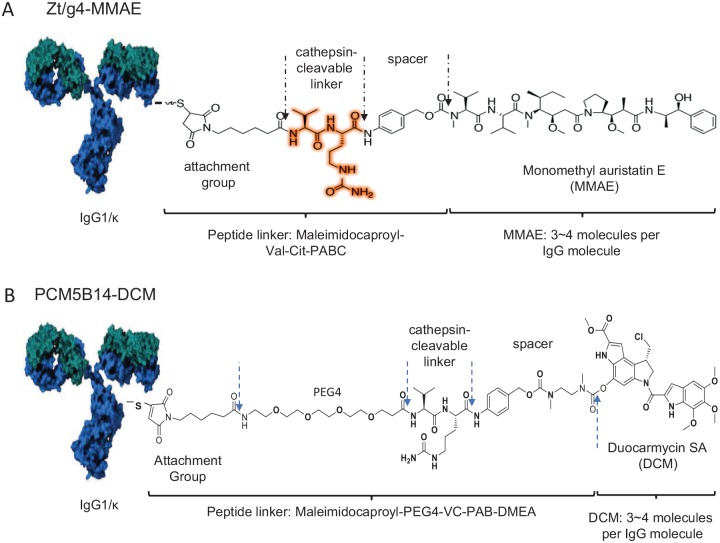
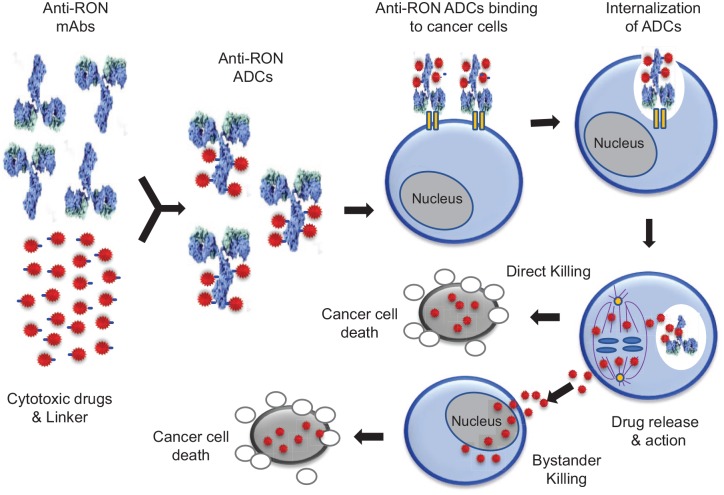
ADCs are a combination of a target-specific antibody, highly potent compound, versatile chemical linker, and controlled drug payload.53–55 Design and generation of lead candidate ADCs require innovative considerations for selection of a target-specific antibody, suitable chemical linker, and cytotoxic payloads. Various articles that review the latest advances and different aspects of ADCs are available.56–58 Currently, two lead candidate MABs, namely Zt/g4 and PCM5B14, have been selected for development of RON-targeted ADCs, resulting in Zt/g4-based ADCs (Zt/g4-matensinoid derivative 1 (DM1) and Zt/g4-monomethyal auristatin E (MMAE) and PCM5B14-based ADCs (PCM5B14-MMAE and PCM5B14-duocarmycin (DCM; Figure 1).20,50,59–63 The mechanisms underlying the action of anti-RON ADCs are shown in Figure 2. The following is a summary of pharmacological and therapeutic features of anti-RON ADCs.
Figure 1.
Schematic representation of anti-RON ADCs conjugated with MMAE and DCM. Anti-RON mAbs Zt/g4 and PCM5B14 are selected as lead candidates for drug conjugation. Zt/g4 14 is conjugated with MMAE linked to the synthetic dipeptide linker Mc-Val-Cit-PABC to generate Zt/g4-MMAE. PCM5B14 is conjugated with DCM linked to the synthetic dipeptide linker MA-PEG4-VC-PAB-DMEA to generate PCM5B14-DCM. The conjugation is aimed to reach the drug to antibody ratio of 4:1.
Figure 2.
Schematic representation of generation of anti-RON ADC and its mechanism of action in killing cancer cells. Anti-RON MABs such as Zt/g4 and PCM5B14 are conjugated with cytotoxic drugs including MMAE and DCM through the thioether linkers and protease-sensitive linker, respectively, to form anti-RON ADCs with a drug to antibody ratio (DAR) of 3~4 to 1. Anti-RON ADCs bind to RON expressed by cancer cells, which leads to internalization of ADCs into the intracellular compartments. Intracellular cleavage of the linker by lysosomal enzymes results in the release of cytotoxic drugs, which either blocks tubulin polymerization or inhibits DNA synthesis leading to cancer cell death (direct killing effect). Dissociated free drugs also diffuse into neighboring cancer cells causing apoptosis (bystander killing effect).
Criteria for selection of anti-RON MABs for ADC development
Selection of Zt/g4 and PCM5B14 is based on the following. (a) Both MABs are specific to RON with the binding affinities in the range of 0.47 µg/ml for Zt/g4 and 0.35 µg/ml for PCM5B14, respectively. Both MABs also are specific to monkey RON with no cross-reactivity to MET, plasminogen, or other proteins with similar domains.59,61 Humanization of both MABs in the IgG1 backbone retains all these features.56,61 (b) Both MABs bind to their distinct epitopes on the RON extracellular domain without overlapping to the ligand macrophage-stimulating protein (MSP)-binding site.50,59 Zt/g4 specifically recognizes the RON semaphorin (SEMA) domain,60 whereas PCM5B14 interacts with the RON β-chain plexin-semaphorin-integrin (PSI) domain.50 The binding of both MABs results in transient RON phosphorylation,50,59 a process critical for induction of cell surface RON internalization by various types of cancer cells.1,2 (c) Both MABs induce a robust cell surface RON internalization.20,50,59–63 The average internalization efficacy (IE50) from more than 20 cancer cell lines is ~15 h for Zt/g4 and ~9 h for PCM5B14, respectively.20,50,59–63 These features ensure the delivery of a sufficient amount of drug molecules for cell cytotoxicity. (d) Both MABs have been conjugated with drugs including DM1, MMAE, MMAF, and DCM with proper conjugation profiles and stability in serum.20,50,59–63 Thus, Zt/g4 and PCM5B14 meet the criteria required for ADC development.
Functional domains in ADC-induced RON internalization for drug delivery
Internalization of the cell surface receptor upon the MAB binding serves as the first step for payload delivery to achieve cancer cell killing. There is evidence indicating that certain domains in the RON extracellular sequence regulate this process.50,59 The RON β-chain extracellular sequence is composed of the SEMA domain followed by the PSI domain and three immunoglobulin-like plexin and transcription (IPTs) motifs.3 The SEMA domain possesses a high-affinity MSP-binding pocket, which, upon ligand interaction, causes RON dimerization for signaling transduction.64,65 The PSI domain acts as a wedge between the SEMA domain and IPT motifs and facilitates the formation of an interface through the SEMA domain by a RON homodimer.64,65 In this sense, the PSI domain is responsible for the correct positioning of the ligand pocket for MSP binding. Evidence from studying cell surface MET internalization by cancer cells supports this notion.66
Various approaches have been applied to accelerate receptor internalization for payload delivery.67–70 The intention appears to believe that an acceleration in the MAB-induced receptor internalization will result in a rapid delivery of payloads leading to an enhancement in ADC-mediated cytotoxicity. To improve RON-mediated drug delivery, a panel of anti-RON MABs specific to SEMA, PSI, and IPTs, respectively, have been studied for their activities in induction of RON internalization.50,59 It appears that PCM5B14 binding to the RON PSI domain is most effective in induction of RON internalization with an IE50 of ~9 h.50 In contrast, the average IE50 for Zt/g4 is ~15 h.63 The effect of Zt/c1, which recognizes the IPT motifs, appears to be weak with an average IE50 at ~20 h.50 These results confirm that MABs binding to different regions in the RON extracellular sequences have a profound impact on RON internalization. PCM5B14 binding to the RON PSI domain is superior over other anti-RON MABs in induction of RON internalization.
By rapid induction of RON internalization, PCM5B14-based ADCs display cellular cytotoxicity in a relatively early stage when compared with that of Zt/g4, which has a moderate internalization efficacy.50,63 Cell cycle changes and reduction of cell viability mediated by PCM5B14-based ADCs occur as early as 3 h after initiation of experiments.50 In contrast, significant changes in cell cycle and in cell viability reduction are observed around 12 h after treatment with Zt/g4-based ADCs.20,50,59–63 To our surprise, an increase in induction of RON internalization does not translate into an enhancement in ADC cytotoxicity.50 The IC50 values in vitro between Zt/g4- and PCM5B14-based ADCs are at comparable level without statistical differences.50,63 Studies from ADC-treated tumor xenografts also suggest that the efficacies of Zt/g4- and PCM5B14-based ADCs, as judged by the rate of tumor inhibition and eradication, are at similar levels.50,63 Thus, the outcomes from using antibodies with different capabilities in induction of RON internalization have no difference. Nevertheless, the effect of PCM5B14-mediated robust RON internalization occurs rapidly at early stages,50 which may have pharmacological advances in controlling cancer cell growth and survival.
Cell surface RON density in correlation with anti-RON ADC efficacy
Various factors such as cellular drug sensitivity, the receptor internalization process, and levels of RON expression affect the anti-cancer efficacy of anti-RON ADCs. IHC staining and flow cytometric analysis indicate that RON is differentially expressed at variable levels in different types of cancer cells.6–9,20,50,59–63 For example, RON is wildly expressed in ~75% of TNBC samples by IHC analysis.20 However, overexpression occurs only in 45% of cases.20 Similarly, RON expression by different cancer cell lines has been shown to vary significantly, ranging from less than 100 to more than 70,000 cell surface RON molecules per cell.20,50,59–63 This expression pattern led to a question of what levels of RON expression is sufficient for anti-RON ADCs to exert a significant activity. To address this issue, a model using various types of cancer cell lines with different levels of RON expression in conjunction with cytotoxic efficacy of anti-RON ADCs was applied.20,50,59–63 The amount of anti-RON ADCs needed to achieve an IC50 value were used as the effective dose to determine the required receptor number to reach the EC95 significance.20,50,59–63 Although sensitivities of individual cell lines to ADCs were different, the patterns of their responsiveness to Zt/g4- and PCM5B14-based ADCs were highly similar.20,50,59–63 A decrease in the levels of RON expression proportionally correlates with the diminished efficacy of anti-RON ADCs. In conclusion, a minimal of 8000 RON receptors per cell is required for anti-RON ADCs to achieve a 95% reduction in cancer cell viability.20,50,59–63 These findings have important clinical implication, which implies that a particular level of RON expression is required for anti-RON ADCs to achieve significant therapeutic activity. In other words, the level of RON expression by cancer cells is a critical criterion for selecting suitable cancers for the use of anti-RON ADCs.
Stability, pharmacokinetic profiles, and toxicological activities of anti-RON ADCs
All anti-RON ADCs described here are produced either through thioether linkers (non-cleavable) to generate DM1-based ADCs or through protease-sensitive dipeptide linkers (cleavable) to form MMAE-based ADCs.20,50,59–63 The formed ADCs, in general, have a DAR of ~ 3.7. Both MMAE- and DM1-based ADCs are stable in phosphate buffered saline for 30 days with less than 7% of changes in the DARs as analyzed by hydrophobic interaction chromatography.50,59–63 They also are stable in human plasma at 37°C for up to 28 days with only ~3.5% MMAE and ~4.0% DM1 dissociated from the antibody.56–61 Studies from cynomolgus monkeys also confirm the stability of anti-RON ADCs.60 In case of Zt/g4-MMAE, the maximal levels of free MMAE detected by the liquid chromatography tandem mass spectrometry method peaked around 3 h after ADC injection. The calculated free MMAE was equivalent to ~0.058% of total MMAE conjugated to Zt/g4.63 This decomposition ratio was steadily maintained during the period of the study, suggesting that anti-RON ADCs are stable in vivo.63
The pharmacokinetic (PK) profiles of Zt/g4-MMAE in both mice and cynomolgus monkeys are similar in many aspects and fit the two-compartment model.63 Zt/g4-MMAE has an average mean plasma clearance of 0.12 ml/day/kg, a t½ of 6.54 days, and a mean residential time of 7.40 days in the cynomolgus monkey.63 These values were similar to those found in the mouse study.60 To study the impact of RON-positive tumors on the PK dynamics, Zt/g4-MMAE was administrated in three doses and evaluated in tumor-bearing and nonbearing mice. Since Zt/g4 does not recognize mouse RON, the goals were to determine: (a) any alterations of the Zt/g4-MMAE PK profile in tumor-bearing mice and (b) RON-independent behavior of Zt/g4-MMAE in tumor-nonbearing mice. The obtained results show that the PK of Zt/g4-MMAE, even at the different doses, is in a two-compartment model from both tumor-bearing and nonbearing mice. Overall, the calculated data from tumor-bearing mice overlapped with those from the tumor-nonbearing mice with 95% prediction intervals.60,63 It is concluded that there is no differences in the PK parameters of Zt/g4-MMAE between tumor-bearing and nonbearing mice. In other words, tumor growth does not affect the dynamics of Zt/g4-MMAE. Moreover, RON expression by tumor cells has no impact on Zt/g4-MMAE disposition in vivo.
Toxicological activities of anti-RON ADCs have been studied in both mice and cynomolgus monkeys.50,59,63 At therapeutic doses up to 20 mg/kg in a Q12 × 2 schedule in mice, anti-RON ADCs have been shown to be safe without visible abnormalities or bodyweight reduction.50,59,63 The maximum tolerated dose for both MMAE- and DCM-based ADCs has been determined as 60 mg/kg as judged by animal daily activity, food consumption, and bodyweight.50,63 In the monkey toxicological study, Zt/g4-MMAE was evaluated in a single injection at 10 or 30 mg/kg.63 No evidence-based abnormalities were documented, judged by various parameters including daily activity, bodyweight, body temperature, food consumption, heart rate, breathing, vision, and urination. In addition, analysis of electrocardiograms, urine samples, and histological changes of multiple tissues at the end-point found no evidence of tissue inflammation, cell death, structural alteration, hemorrhage, and other pathological changes in all animals tested.63 Nevertheless, adverse reactions in blood chemistry tests reflecting the hematopoietic system and liver function were observed as evident by a moderate increase and/or decrease in blood leukocytes, or reticulocytes, and evaluation of a panel of liver enzymatic activities. These effects were observed in a dose-dependent manner, reversible, and manageable. At the end of the study, all changes were restored to the baseline.63 The toxic effect of anti-RON ADCs on reproductive tissues has not been examined. In reviewing literature related to ADC toxicity, we noticed that toxicological profiles of Zt/g4-MMAE are highly similar to those of ADCs approved by the US Food and Drug Administration (FDA) or currently under clinical trials.71 Specifically, toxicities of ADCs conjugated with MMAE all have a similar profile affecting the hematopoietic system, liver, and reproductive organs regardless of their reactivity to target antigens.71 In case of five ADCs conjugated with MMAE tested in cynomolgus monkeys, prominent organ toxicities have been observed mainly in the hematopoietic system, liver, and reproductive organs.71 The toxic effect of DCM-based anti-RON ADCs appears to be severe in cynomolgus monkeys (our unpublished data). Zt/g4-DCM at a single injection of 30 mg/kg leads to death of the animal (our unpublished data). In light of these facts, it is reasoned that Zt/g4-MMAE generated through classical drug-linkage technology is relatively safe when used at the therapeutic dose. Furthermore, these data should help design a phase I clinical trial for Zt/g4-MMAE.
Cytotoxic effect of anti-RON ADCs in vitro
The cytotoxic effect is defined as the action of a drug that leads to disruption of cell cycle, inhibition of cellular proliferation, and cell apoptotic death. Up to now, four highly potent cytotoxic compounds with different mechanisms of action, including DM1, MMAE, MMAF, and DCM have been conjugated to generate Zt/g4- and PCM5B14-based ADCs.20,50,59–63 DM1, MMAE and MMAF belong to a group of antimitotic agents that inhibit cell division by blocking polymerization of tubulins.59–63 In contrast, DCM acts as a DNA minor groove alkylating agent, which damages nucleic architecture leading to cancer cell death.50 Conjugation of Zt/g4 with pyrrolobenzodiazepine (PBD) to generate Zt/g4-PBD has also been achieved (our unpublished data). Judging from a pharmaceutical standard, anti-RON ADCs conjugated with MMAE or DCM as the payload fulfill criteria for further development. Activities of anti-RON ADCs conjugated with DM1 or MMAF are relatively weak.50,56,63 An interesting observation is from the study that evaluated Zt/g4-PBD cytotoxicity in vitro. PBD has been used as a cytotoxic drug payload for ADC development.72,73 We generated Zt/g4-PBD according to a previously described method, which has been used for the development of anti-MET ADC TR1801-ADC.72 TR1801-ADC is currently under clinical trials (ClinicalTrials.gov identifier: NCT03859752). Unexpectedly, Zt/g4-PBD behaves significantly different from other anti-RON ADCs in terms of cytotoxic activity in vitro. Specifically, Zt/g4-PBD kills cancer cells in a RON-independent manner (our unpublished data). Surprisingly, biochemical and biological analysis find no obvious defects in terms of drug conjugation or alterations in antigen-binding specificity after conjugation. The underlying mechanism is currently unknown. Since PBD is extremely toxic in comparison with DM1, MMAE, and DCM72,73 and the MET expression is often at high levels by cancer cells,72 we suspect that cancer cells with relatively low levels of RON expression may not serve as suitable targets for PBD-based ADCs. More studies are needed to determine the underlying mechanism associated cell cytotoxicity mediated by Zt/g4-PBD.
Cytotoxic effects of anti-RON ADCS in vitro have been evaluated using a model of three groups of cancer cells (Table 1). The regular cancer cell group included more than 20 established cancer cell lines, representing colorectal, lung, breast, and pancreatic cancers. The use of these cell lines was primarily based on their tissue origination, malignant status, variable drug sensitivities, and levels of RON expression.50,59–63 Eight primary cancer cell lines from patient-derived pancreatic cancer samples (PDX) were chosen based on their similarities to the original pancreatic cancer cases.74 Several populations of cancer stem cells (CSCs) with CD44+/CD24– phenotypes or with aldehyde dehydrogenase activity from TNBC cell lines, and with CD44+/epithelium-specific antigen+ phenotypes from pancreatic cancer cell lines were also used as the target cells for the ADC study.20,50,75 The objective was to determine whether anti-RON ADCs are effective in killing CSCs.
Both Zt/g4- and PCM5B14-based ADCs had a profound effect on all three groups of cancer cells based on changes in cell cycle, cell viability reduction, and massive cell death.20,50,59–63 Several features are worth mentioning: First, the effectiveness of ADCs is highly dependent on the levels of RON expression by cancer cells.50,63 In other words, anti-RON ADC-mediated killing proportionally correlates with levels of RON expression. Nevertheless, a few exceptions exist. For instance, Zt/g4-MMAE shows an IC50 of ~0.80 µg for HCC2185 cells expressing ~31,000 cell surface RON molecules but only has an IC50 of ~1.50 µ for HCC1937 cells, which express more than 70,000 cell surface RON molecules.20 Second, the effect of PCM5B14-based ADCs appears much earlier than that of Zt/g4-based ADCs.50 PCM5B14-based ADCs at a therapeutic dose causes more than 40% cell viability reduction within a 24 h treatment. In contrast, Zt/g4-based ADCs needs 48 h to achieve the similar levels of reduction.50,63 Third, although MMAE and DCM have different mechanisms of action, the outcomes as judged by IC50 values for cell viability and cell death are at similar levels.50,63 In analysis of ~20 cancer cell lines with RON expression from 8000 to 70,000 molecules per cell, the average IC50 value from the PCM5B14-MMAE treated group is ~3.0 µg/ml, which is similar to that (~2.9 µg/ml) from the PCM5B14-DCM treated group.50,63 Fourth, there are differences in drug sensitivity among cancer cell lines in response to anti-RON ADCs. Notably, breast cancer cells, including TNBC cell lines, appear to be more sensitive than colorectal, lung, and pancreatic cancer cell lines in response to anti-RON ADCs. The average IC50 value from four TNBC cell lines is ~1.2 µg/ml, which is statistically significant in comparison with those from other cell groups.20,50,59–63 The sensitivity among colorectal, lung, and pancreatic cancer cell lines are very similar.50,59–63 Fifth, cells from primary cancer cell lines display a similar ADC sensitivity pattern as those from the regular cancer cell lines.63 Among eight cell lines tested, the average IC50 value is ~3.8 µg/ml, which is comparable with regular cancer cells treated with Zt/g4- and PCM5B14-based ADCs.50 Finally, anti-RON ADCs are highly effective against CSCs derived from TNBC and pancreatic cancer cells.20,50 Seven CSC populations have shown to respond well to anti-RON ADCs with an average IC50 value at ~0.78 µg/ml, which is relatively lower than those from parental TNBC cell lines.20
Inhibition and eradication of tumors by anti-RON ADCs in vivo
Xenograft tumors from multiple sources have been used as a model for validation of anti-RON ADCs. The obtained results are exciting (Table 2).
Table 2.
Efficacy of Zt/g4 and H5B14-based ADCS in inhibition of xenograft tumors derived from various types of human cancer cell lines*.
| Anti-RON ADCs Evaluated | Tumor growth inhibition based on average tumor weights (g) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Colorectal Cancer |
Pancreatic cancer |
Lung and breast cancer |
CSCs |
PDXs |
|||||||||
| LoVo | HCT116 | HT29 | SW620 | Pan-1 | BxPc-3 | FG | L3.6pl | H358 | T-47D | MDA-MB468 | FGCSCs | SNU2491 | |
| M-Zt/g4-DM1 | ND | D16/D16:6.23/156 (96.0%) | D16/D16:5.92/180 (96.7%) | D16/D16:110/624 (82.4%) | ND | D32/D32: 0.06/0.7 (91.8%) | D20/D32: 1.89/2.49 (24.1%) | D16/D28: 1.41/2.54 (44.6%) | D42/52: 1.78/0.31 (82.6%) | D36/D36: 1.34/0.18 (86.6%) | ND | ND | ND |
| H-Zt/g4-DM1 | D36/D36: 1.11/1.0 (9.0%) | D36/D36: 0.32/1.88 (83.0%) | D32/D36: 0.21/1.71 (87.7%) | D36/D36: 0.14/1.95 (92.8%) | D32/D32: 1.25/1/32 (–5.3%) | D32/D32 0.08/0.81 (90.1%) | D32/d32 1.67/2.33 (28.3%) | D32/D32: 1.31/2.44 (46.3%) | ND | ND | ND | ND | ND |
| M-Zt/g4-MMAE | ND | ND | ND | ND | D44/D44: 0.44/0,41 (–7.3) | D44/D52: 0.01/1.19 (99.2%) | D24/D44: 0.03/1.54 (98.1) | D24/D44: 0.02/1.58 (98.7) | ND | ND | ND | ND | ND |
| H-Zt/g4-MMAE | D44/D44: 1.33/1.56 (14.7%) | D36/D52: 0.01/2.12 (99.5%) | D32/D52: 0.01/1.76 (99.4%) | D36/D52: 0.02/1.63 (98.8%) | D44/D44: 1.29/1.3 (3.70%) | D40/D52: 0.01/1.97 (99.9%) | D28/D52: 0.01/1.62 (99.4%) | D28/D52: 0.01/2.0 (99.5%) | ND | ND | D68/D68: 0.42/0.00 (100%) | D24/D40:1.83/0.25 (86.3%) | D36/D40:1.77/0.04 (97.7%) |
| H5B14-MMAE | D32/D32: 1.03/1.08 (–4.9%) | ND | D24/D32: 2.51/0.07 (97.2%) | ND | ND | ND | ND | D32/D32: 2.96/0.06 (98.4%) | D32/D32: 1.42/0.21 (85.2%) | D32/D32: 2.59/0.10 (96.2%) | ND | ND | ND |
| H5B14-DCM | D36/D36: 0.55/0.58 (–5.5%) | ND | D36/D36: 1.15/0.05 (95.7%) | ND | ND | ND | ND | D28/D36: 3.46/0.05 (98.6%) | D36/D36:1.82/0.03 (98.4%) | D36/D36: 2.13/0.01 (99.5%) | ND | ND | ND |
ADC, antibody-drug conjugates; CSC, cancer stem cells; DCM, duocarmycin; DM1, matensinoid derivative 1; MMAE monomethyal auristatin E; PDX, patient-derived pancreatic cancer samples.
The significance (p < 0.05) of differences was indicated as bold values.
Various Anti-RON ADCs were prepared as described in previous reports.50,59–63 Xenograft tumors were initiated by colorectal, pancreatic, lung, and breast cancer cell lines. CSCs derived from FG cells and primary SNU2491 cells from pancreatic PDXs were also used for induction of xenograft tumors. Individual ADCs were used at 20 mg/kg in the Q12 × 2 schedule. At the end of the study dependent on growth rate of individual models, tumors were collected and weighted to reach an average value for each group. The percentages of inhibition for tumor growth were calculated as previously described.50,59–63
(a) Anti-RON ADCs are highly effective against tumors mediated by 15 regular cancer cell lines (representing breast, colorectal, lung, and pancreatic tumors), by 4 primary pancreatic cancer cell lines from PDX, and by 5 CSC populations with defined phenotypes.20,50,59–63 The broad anti-cancer activity of anti-RON ADCs highlights the usefulness of targeting RON-expressing tumors from different sites.
(b) The efficacy of anti-RON ADCs has manifested not only inhibition but also eradication of xenograft tumors regardless of their chemoresistance or metastatic status.20,50,59–63 In xenograft tumors that originated from three pancreatic cancer cell lines, Zt/g4-MMAE mediated tumor growth inhibition was reported in the range of 95–99%.62 Moreover, four out of five tumors were completely eradicated.62 This effect has been further confirmed in other studies using colorectal, lung, and breast cancer cell lines as the model.20,50,56–63
(c) Both MMAE and DCM-based ADCs appear to be equally effective as judged by their efficacies in vivo. An interesting finding is that the efficacy of DM1-based ADCs such as Zt/g4-DM1 is relatively weak in comparison with that of MMAE- and DCM-based ADCs.59–61 Among nine models of xenograft tumors tested, which are mediated by colon, lung, pancreatic, and breast cancer cell lines, Zt/g4-DM1 has been shown to significantly delay tumor growth. Nevertheless, tumor eradication has not been observed in all models tested.59–61 For this reason, Zt/g4-MMAE is favored and selected as the lead candidate.
(d) The effect of anti-RON ADCs is highly RON specific owning to the antigen specificity of MABs. Tumor xenografts lacking RON expression are not affected.20,50,59–63 A partial effect is seen in tumors expressing low to moderate levels of RON expression (500–5000 RON molecules per cells).20,50,59–63 This is consistent with the in vitro findings showing that the minimal RON expression at ~8000 is required for ADCs to achieve maximal activity.20,50,59–63 As validated by Western blot analysis, RON expression in residual tumor xenografts is either not detected or at a trace level, suggesting the elimination of RON-expressing cancer cells after ADC treatment.50,59–63 An interesting observation is that MMAE-based ADCs are effective in killing RON-negative cancer cells through bystander mechanisms.20 This could well explain that MMAE-based ADCs but not DM1-based ADCs are capable of eradicating tumor xenografts.
(e) Analysis of the dose-time relationship reveals that anti-RON ADCs have tumor static concentrations (TSCs) ranging from 1 to 3 mg/kg for all tumor xenografts tested.20,50,59–63 The TSCs are the minimal doses that balance the tumor growth and inhibition, which reflects the effectiveness of an ADC in vivo.71 It is worth mentioning that the TSCs of anti-RON ADCs are in line with the doses of ADCs currently approved by the FDA for clinical application.71
(f) Breast and pancreatic CSCs are highly sensitive to anti-RON ADCs.20 Results from both in vitro and in vivo studies confirm that anti-RON ADCs are effective in vitro in killing CSCs.20,50 In a recent study, tumor xenografts mediated by TNBC-CSCs with CD44+/CD24– phenotypes were completely inhibited by anti-RON ADCs (Suthe et al., manuscript submitted). Significantly, more than 80% of tumor xenografts were eradicated. These findings highlight the usefulness of anti-RON ADCs for eradication of TNBC CSCs for clinical benefits.
(g) The presence of residual tumors with minimal RON expression after anti-RON ADC treatment suggests a necessity in combination with other anti-cancer agents for combating tumor recurrence. In this sense, anti-RON ADCs in combination with chemotherapeutics or immune-checkpoint antibodies should be considered for future application.
Conclusion
Aberrant RON expression is the pathological feature in various types of primary cancers and a critical determinant in regulating the cancer cell invasive phenotype. Preclinical studies have validated anti-RON ADCs with a significantly improved therapeutic index. Considering their superiority in inhibition and eradication of tumors from multiple xenograft models with favorable pharmacological profiles and manageable adverse activities, both Zt/g4- and PCM5B14-based anti-RON ADCs hold the promise as a novel modality for cancer treatment in the future.
Acknowledgments
We thank Ms. Rachel Hudson (TTUHSC School of Pharmacy in Amarillo, TX) for carefully editing and proofreading the manuscript.
Footnotes
Author contributions: HPY, SRS, XMT, and MHW discussed the necessity for writing this manuscript. MHW wrote the original draft. HPY, SRS, and XMT reviewed the draft with detailed comments. MHW made the revision. All authors read and approved the final manuscript for submission.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by funds from Major Project of Zhejiang Provincial Science and Technology Department (grant number 2019C03038 to XMT) and by the National Natural Sciences Foundation of China (grant number 81872883 to HPY).
Contributor Information
Hang-Ping Yao, State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China; National Clinical Research Center for Infectious Diseases, First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China.
Sreedhar Reddy Suthe, Cancer Biology Research Center, Department of Pharmaceutical Sciences, Jerry H. Hodge School of Pharmacy, Texas Tech University Health Sciences Center, Amarillo, TX, USA.
Xiang-Min Tong, Department of Hematology, Zhejiang Provincial People’s Hospital and People’s Hospital of Hangzhou Medical College, Hangzhou, China.
Ming-Hai Wang, State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China; Cancer Biology Research Center, Texas Tech University Health Sciences Jerry H. Hodge School of Pharmacy, 1406 Coulter Street, Amarillo, TX 79106, USA.
References
- 1. Yao HP, Zhou YQ, Zhang R, et al. MSP-RON signalling in cancer: pathogenesis and therapeutic potential. Nat Rev Cancer 2013; 13: 466–481. [DOI] [PubMed] [Google Scholar]
- 2. Faham N, Welm AL. RON signaling is a key mediator of tumor progression in many human cancers. Cold Spring Harb Symp Quant Biol 2016; 81: 177–188. [DOI] [PubMed] [Google Scholar]
- 3. Ronsin C, Muscatelli F, Mattei MG, et al. A novel putative receptor protein tyrosine kinase of the met family. Oncogene 1993; 8: 1195–1202. [PubMed] [Google Scholar]
- 4. Gaudino G, Follenzi A, Naldini L, et al. RON is a heterodimeric tyrosine kinase receptor activated by the HGF homologue MSP. EMBO J 1994; 13: 3524–3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang MH, Ronsin C, Gesnel MC, et al. Identification of the ron gene product as the receptor for the human macrophage stimulating protein. Science 1994; 266:117–119. [DOI] [PubMed] [Google Scholar]
- 6. Wang MH, Lee W, Luo YL, et al. Altered expression of the RON receptor tyrosine kinase in various epithelial cancers and its contribution to tumourigenic phenotypes in thyroid cancer cells. J Pathol 2007; 213: 402–411. [DOI] [PubMed] [Google Scholar]
- 7. Thomas RM, Toney K, Fenoglio-Preiser C, et al. The RON receptor tyrosine kinase mediates oncogenic phenotypes in pancreatic cancer cells and is increasingly expressed during pancreatic cancer progression. Cancer Res 2007; 67: 6075–6082. [DOI] [PubMed] [Google Scholar]
- 8. Kanteti R, Krishnaswamy S, Catenacci D, et al. Differential expression of RON in small and non-small cell lung cancers. Genes Chromosomes Cancer 2012; 51: 841–851. [DOI] [PubMed] [Google Scholar]
- 9. Maggiora P, Marchio S, Stella MC, et al. Overexpression of the RON gene in human breast varcinoma. Oncogene 1998; 16: 2927–2933. [DOI] [PubMed] [Google Scholar]
- 10. Yao HP, Zhuang CM, Zhou YQ, et al. Oncogenic variant RON160 expression in breast cancer and its potential as a therapeutic target by small molecule tyrosine kinase inhibitor. Curr Cancer Drug Targets 2013; 13: 686–697. [DOI] [PubMed] [Google Scholar]
- 11. Zhou YQ, He C, Chen YQ, et al. Altered expression of the RON receptor tyrosine kinase in primary human colorectal adenocarcinomas: generation of different splicing RON variants and their oncogenic potential. Oncogene 2003; 22: 186–197. [DOI] [PubMed] [Google Scholar]
- 12. Bedolla RG, Shah DP, Huang SB, et al. Receptor tyrosine kinase recepteur d’origine nantais as predictive marker for aggressive prostate cancer in African Americans. Mol Carcinog 2019; 58: 854–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brown NE, Paluch AM, Nashu MA, et al. Tumor cell autonomous RON receptor expression promotes prostate cancer growth under conditions of androgen deprivation. Neoplasia 2018; 20: 917–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cheng HL, Liu HS, Lin YJ, et al. Co-expression of RON and MET is a prognostic indicator for patients with transitional-cell carcinoma of the bladder. Br J Cancer 2005; 92: 1906–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Comperat E, Roupret M, Chartier-Kastler E, et al. Prognostic value of MET, RON and histoprognostic factors for urothelial carcinoma in the upper urinary tract. J Urol 2008; 179: 868–872. [DOI] [PubMed] [Google Scholar]
- 16. Lee CT, Chow NH, Su PF, et al. The prognostic significance of RON and MET receptor coexpression in patients with colorectal cancer. Dis Colon Rectum 2008; 51: 1268–1274. [DOI] [PubMed] [Google Scholar]
- 17. Ferrandina G, Martinelli E, Petrillo M, et al. Prognostic role of the recepteur d’origine nantais (RON) expression in ovarian cancer patients. Gynecol Oncol 2008; 111: 237–243. [DOI] [PubMed] [Google Scholar]
- 18. Catenacci DV, Cervantes G, Yala S, et al. RON (MST1R) is a novel prognostic marker and therapeutic target for gastroesophageal adenocarcinoma. Cancer Biol Ther 2011; 12: 9–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee WY, Chen HH, Chow NH, et al. Prognostic significance of co-expression of RON and MET receptors in node-negative breast cancer patients. Clin Cancer Res 2005; 11: 2222–2228. [DOI] [PubMed] [Google Scholar]
- 20. Suthe SR, Yao HP, Weng TH, et al. RON receptor tyrosine kinase as a therapeutic target for eradication of triple-negative breast cancer: efficacy of anti-RON ADC Zt/g4-MMAE. Mol Cancer Ther 2018; 17: 2654–2664. [DOI] [PubMed] [Google Scholar]
- 21. Krishnaswamy S, Bukhari I, Mohammed AK, et al. Identification of the splice variants of Recepteur d’Origine nantais (RON) in lung cancer cell lines. Gene 2018; 679: 335–340. [DOI] [PubMed] [Google Scholar]
- 22. Ling Y, Kuang Y, Chen LL, et al. A novel RON splice variant lacking exon 2 activates the PI3K/AKT pathway via PTEN phosphorylation in colorectal carcinoma cells. Oncotarget 2017; 8: 39101–39116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chakedis J, French R, Babicky M, et al. A novel protein isoform of the RON tyrosine kinase receptor transforms human pancreatic duct epithelial cells. Oncogene 2016; 35: 3249–3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chakedis J, French R, Babicky M, et al. Characterization of RON protein isoforms in pancreatic cancer: implications for biology and therapeutics. Oncotarget 2016; 7: 45959–45975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bonomi S, di Matteo A, Buratti E, et al. HnRNP A1 controls a splicing regulatory circuit promoting mesenchymal-to-epithelial transition. Nucleic Acids Res 2013; 41: 8665–8679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cammas A, Lacroix-Triki M, Pierredon S, et al. hnRNP A1-mediated translational regulation of the G quadruplex-containing RON receptor tyrosine kinase mRNA linked to tumor progression. Oncotarget 2016; 7: 16793–16805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Babicky ML, Harper MM, Chakedis J, et al. MST1R kinase accelerates pancreatic cancer progression via effects on both epithelial cells and macrophages. Oncogene 2019; 38: 5599–5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li C, Morvaridi S, Lam G, et al. MSP-RON signaling is activated in the transition from pancreatic intraepithelial neoplasia (PanIN) to pancreatic ductal adenocarcinoma (PDAC). Front Physiol 2019; 10: 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhao S, Cao L, Freeman JW. Knockdown of RON receptor kinase delays but does not prevent tumor progression while enhancing HGF/MET signaling in pancreatic cancer cell lines. Oncogenesis 2013; 2: e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Faham N, Zhao L, Welm AL. mTORC1 is a key mediator of RON-dependent breast cancer metastasis with therapeutic potential. NPJ Breast Cancer 2018; 4: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ruiz-Torres SJ, Benight NM, Karns RA, et al. HGFL-mediated RON signaling supports breast cancer stem cell phenotypes via activation of non-canonical beta-catenin signaling. Oncotarget 2017; 8: 58918–58933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Andrade K, Fornetti J, Zhao L, et al. RON kinase: a target for treatment of cancer-induced bone destruction and osteoporosis. Sci Transl Med 2017; 9: eaai9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bieniasz M, Radhakrishnan P, Faham N, et al. Preclinical efficacy of ron kinase inhibitors alone and in combination with PI3K inhibitors for treatment of sfRon-expressing breast cancer patient-derived xenografts. Clin Cancer Res 2015; 21: 5588–5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Privette Vinnedge LM, Benight NM, Wagh PK, et al. The DEK oncogene promotes cellular proliferation through paracrine Wnt signaling in Ron receptor-positive breast cancers. Oncogene 2015; 34: 2325–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cunha S, Lin YC, Goossen EA, et al. The RON receptor tyrosine kinase promotes metastasis by triggering MBD4-dependent DNA methylation reprogramming. Cell Rep 2014; 6: 141–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhao H, Chen MS, Lo YH, et al. The Ron receptor tyrosine kinase activates c-Abl to promote cell proliferation through tyrosine phosphorylation of PCNA in breast cancer. Oncogene 2014; 33: 1429–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang J, Pendergast AM. The emerging role of ABL kinases in solid tumors. Trends Cancer 2015; 1: 110–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hacker KE, Bolland DE, Tan L, et al. The DEK oncoprotein functions in ovarian cancer growth and survival. Neoplasia 2018; 20: 1209–12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tharmapalan P, Mahendralingam M, Berman HK, et al. Mammary stem cells and progenitors: targeting the roots of breast cancer for prevention. EMBO J 2019; 38: e100852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bellacosa A. Role of MED1 (MBD4) gene in DNA repair and human cancer. J Cell Physiol 2001; 187: 137–44. [DOI] [PubMed] [Google Scholar]
- 41. Davis NM, Sokolosky M, Stadelman K, et al. Deregulation of the EGFR/PI3K/PTEN/Akt/mTORC1 pathway in breast cancer: possibilities for therapeutic intervention. Oncotarget 2014; 5: 4603–4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. O’Toole JM, Rabenau KE, Burns K, et al. Therapeutic implications of a human neutralizing antibody to the macrophage-stimulating protein receptor tyrosine kinase (RON), a c-MET family member. Cancer Res 2006; 66: 9162–9170. [DOI] [PubMed] [Google Scholar]
- 43. LoRusso PM, Gounder M, Jalal SI, et al. Phase 1 study of narnatumab, an anti-RON receptor monoclonal antibody, in patients with advanced solid tumors. Invest New Drugs 2017; 35: 442–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yao HP, Zhou YQ, Ma Q, et al. The monoclonal antibody Zt/f2 targeting RON receptor tyrosine kinase as potential therapeutics against tumor growth-mediated by colon cancer cells. Mol Cancer 2011; 10: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Guin S, Yao HP, Wang MH. RON receptor tyrosine kinase as a target for delivery of chemodrugs by antibody directed pathway for cancer cell cytotoxicity. Mol Pharm 2010; 7: 386–797. [DOI] [PubMed] [Google Scholar]
- 46. Gunes Z, Zucconi A, Cioce M, et al. Isolation of fully human antagonistic RON antibodies showing efficient block of downstream signaling and cell migration. Transl Oncol 2011; 4: 38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Han M, Whalen K, Gifford J, et al. Antitumor activity of anti-RON antibodies and biomarker of response Presented at EORTC-NCI-AARC International Symposium, November 16–19, 2010. Berlin, Germany. [Google Scholar]
- 48. Koh XY, Koh XH, Hwang LA, et al. Therapeutic anti-cancer activity of antibodies targeting sulfhydryl bond constrained epitopes on unglycosylated RON receptor tyrosine kinase. Oncogene 2019; 39: 7342–7356. [DOI] [PubMed] [Google Scholar]
- 49. Williams R. Discontinued in 2013: oncology drugs. Expert Opin Investig Drugs 2015; 24: 95–110. [DOI] [PubMed] [Google Scholar]
- 50. Tong XM, Feng L, Suthe SR, et al. Therapeutic efficacy of a novel humanized antibody-drug conjugate recognizing plexin-semaphorin-integrin domain in the RON receptor for targeted cancer therapy. J Immunother Cancer 2019; 7: 250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Guin S, Ma Q, Padhye S, et al. Targeting acute hypoxic cancer cells by doxorubicin-immunoliposomes directed by monoclonal antibodies specific to RON receptor tyrosine kinase. Cancer Chemother Pharmacol 2011; 67: 1073–1083. [DOI] [PubMed] [Google Scholar]
- 52. Padhye SS, Guin S, Yao HP, et al. Sustained expression of the RON receptor tyrosine kinase by pancreatic cancer stem cells as a potential targeting moiety for antibody-directed chemotherapeutics. Mol Pharm 2011; 8: 2310–2319. [DOI] [PubMed] [Google Scholar]
- 53. Sievers EL, Senter PD. Antibody-drug conjugates in cancer therapy. Annu Rev Med 2013; 64: 15–29. [DOI] [PubMed] [Google Scholar]
- 54. Birrer MJ, Moore KN, Betella I, et al. Antibody-drug conjugate-based therapeutics: state of the science. J Natl Cancer Inst 2019; 111: 538–549. [DOI] [PubMed] [Google Scholar]
- 55. Abdollahpour-Alitappeh M, Lotfinia M, Gharibi T, et al. Antibody-drug conjugates (ADCs) for cancer therapy: strategies, challenges, and successes. J Cell Physiol 2019; 234: 5628–5642. [DOI] [PubMed] [Google Scholar]
- 56. Yaghoubi S, Karimi MH, Lotfinia M, et al. Potential drugs used in the antibody-drug conjugate (ADC) architecture for cancer therapy. J Cell Physiol 2020; 235: 31–64. [DOI] [PubMed] [Google Scholar]
- 57. Ricciuti B, Lamberti G, Andrini E, et al. Antibody-drug conjugates for lung cancer in the era of personalized oncology. Semin Cancer Biol 2019; S1044-579X(19)30424-9. [DOI] [PubMed] [Google Scholar]
- 58. Goulet DR, Atkins WM. Considerations for the design of antibody-based therapeutics. J Pharm Sci 2020; 109: 74–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Feng L, Yao HP, Wang W, et al. Efficacy of anti-RON antibody Zt/g4-drug maytansinoid conjugation (Anti-RON ADC) as a novel therapeutics for targeted colorectal cancer therapy. Clin Cancer Res 2014; 20: 6045–6058. [DOI] [PubMed] [Google Scholar]
- 60. Yao HP, Feng L, Zhou JW, et al. Therapeutic evaluation of monoclonal antibody-maytansinoid conjugate as a model of RON-targeted drug delivery for pancreatic cancer treatment. Am J Cancer Res 2016; 6: 937–956. [PMC free article] [PubMed] [Google Scholar]
- 61. Feng L, Yao HP, Zhou YQ, et al. Biological evaluation of antibody-maytansinoid conjugates as a strategy of RON targeted drug delivery for treatment of non-small cell lung cancer. J Exp Clin Cancer Res 2016; 35: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yao HP, Feng L, Weng TH, et al. Preclinical efficacy of anti-RON antibody-drug conjugate Zt/g4-MMAE for targeted therapy of pancreatic cancer overexpressing RON receptor tyrosine kinase. Mol Pharm 2018; 15: 3260–3271. [DOI] [PubMed] [Google Scholar]
- 63. Yao HP, Feng L, Suthe SR, et al. Therapeutic efficacy, pharmacokinetic profiles, and toxicological activities of humanized antibody-drug conjugate Zt/g4-MMAE targeting RON receptor tyrosine kinase for cancer therapy. J Immunother Cancer 2019; 7: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chao KL, Tsai IW, Chen C, et al. Crystal structure of the Sema-PSI extracellular domain of human RON receptor tyrosine kinase. PLoS One 2012; 7: e41912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chao KL, Gorlatova NV, Eisenstein E, et al. Structural basis for the binding specificity of human Recepteur d’Origine Nantais (RON) receptor tyrosine kinase to macrophage-stimulating protein. J Biol Chem 2014; 289: 29948–29960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Park H, Kim D, Kim E, et al. Tumor inhibitory effect of IRCR201, a novel cross-reactive c-met antibody targeting the PSI domain. Int J Mol Sci 2017; 18: pii: E1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Pedersen MW, Jacobsen HJ, Koefoed K, et al. Sym004: a novel synergistic anti-epidermal growth factor receptor antibody mixture with superior anticancer efficacy. Cancer Res 2010; 70: 588–597. [DOI] [PubMed] [Google Scholar]
- 68. Durbin KR, Phipps C, Liao X. Mechanistic modeling of antibody-drug conjugate internalization at the cellular level reveals inefficient processing steps. Mol Cancer Ther 2018; 17: 1341–1351. [DOI] [PubMed] [Google Scholar]
- 69. Maass KF, Kulkarni C, Betts AM, et al. Determination of cellular processing rates for a TrastuzuMAB-Maytansinoid antibody-drug conjugate (ADC) highlights key parameters for ADC design. AAPS J 2016; 18: 635–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. de Goeij BE, Vink T, Ten Napel H, et al. Efficient payload delivery by a bispecific antibody-drug conjugate targeting HER2 and CD63. Mol Cancer Ther 2016; 15: 2688–2697. [DOI] [PubMed] [Google Scholar]
- 71. Saber H, Leighton JK. An FDA oncology analysis of antibody-drug conjugates. Regul Toxicol Pharmacol 2015; 71: 444–452. [DOI] [PubMed] [Google Scholar]
- 72. Gymnopoulos G, Betancourt O, Blot V, et al. TR1801-ADC: a highly potent cMet antibody-drug conjugate with high activity in patient-derived xenograft models of solid tumors. Mol Oncol 2020; 14: 54–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Saber H, Simpson N, Ricks TK, et al. An FDA oncology analysis of toxicities associated with PBD-containing antibody-drug conjugates. Regul Toxicol Pharmacol 2019; 107: 104429. [DOI] [PubMed] [Google Scholar]
- 74. Jung J, Lee CH, Seol HS, et al. Generation and molecular characterization of pancreatic cancer patient-derived xenografts reveals their heterologous nature. Oncotarget 2016; 7: 62533–62546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rodriguez-Torres M, Allan AL. Aldehyde dehydrogenase as a marker and functional mediator of metastasis in solid tumors. Clin Exp Metastasis 2016; 33: 97–113. [DOI] [PMC free article] [PubMed] [Google Scholar]