Abstract
Triple negative breast cancer (TNBC) is a heterogenous subtype of breast cancer often associated with an aggressive phenotype and poor prognosis. Antibody–drug conjugate (ADC), comprising of a monoclonal antibody linked to a cytotoxic payload by a linker, is gaining increasing traction as an anti-cancer therapeutic. Emerging ADC drugs such as sacituzumab govitecan (IMMU-132) and trastuzumab deruxtecan (DS-8201a) are in late stages of clinical development for patients with metastatic breast cancer, including TNBC. In this article, we review and discuss the development and clinical application of ADCs in patients with advanced TNBC.
Keywords: antibody–drug conjugates, breast cancer, triple negative breast cancer
Introduction
Triple negative breast cancer (TNBC) is a heterogeneous tumor subtype conventionally defined by the absence of expression of the estrogen receptor (ER), progesterone receptor (PgR), and human epidermal growth factor receptor 2 (HER2) amplification, and accounts for approximately 15–20% of all breast carcinomas. Clinically, TNBC is often an aggressive tumor subtype associated with an earlier age of onset, higher rate of relapse, and a relatively short survival of 10–13 months from the time of metastasis.1 TNBC is often the most challenging subtype of breast cancer to treat for various clinical and biological reasons. Aside from germline BRCA1/2 deficiency, which now has an approved targeted therapy, PARP inhibition, and programmed cell death ligand 1 (PD-L1)-positive tumors, for which immunotherapy with a PD-L1 inhibitor is now approved, attempts to identify biomarkers that can help guide treatment decisions with targeted therapies have yet to improve the outcomes of patients with metastatic TNBC, and cytotoxic chemotherapy remains the mainstay of treatment. Although recent efforts to characterize TNBC tumors based on the profiling of its transcriptome, proteome, genome, epigenome, and immunological microenvironment are helping to increase understanding of the molecular heterogeneity of TNBC,1 the development of novel treatment strategies is an area of unmet clinical need. As a first-line treatment for patients with PD-L1-positive metastatic TNBC, the PD-L1 inhibitor, atezolizumab plus nab-paclitaxel, showed a survival benefit compared with nab-paclitaxel alone [progression-free survival (PFS); 7.5 months versus 5.0 months, hazard ratio (HR), 0.62; p < 0.001; overall survival (OS) from 25.0 months and 15.5 months, HR 0.62; p < 0.001].2 However, not all patients may benefit from immune checkpoint blockade, as only about 40% of triple-negative tumors are PD-L1 positive, and the tumor will ultimately become resistant to first-line therapy, necessitating the development of additional beneficial targeted therapies.
Antibody–drug conjugates (ADC) are immunoconjugate agents engineered to deliver potent small molecules preferentially to cancer cells. This novel approach combines the specificity of a monoclonal antibody (mAb) with the high potency of small molecules and has the potential to improve cancer treatment. This category of drug is one of the fastest growing classes of cancer therapeutics in the past few decades. The clinical application of ADCs utilizes mAbs, which were first described in 1975.3 The “magic bullet” concept, described by Paul Ehrlich, originally referred to a chemical that specifically targeted microorganisms.4 The mAb-based strategy was developed as a biological tool for a cancer therapeutic that specifically targets cancer cells while minimizing toxicity to normal tissues. As the first generation of ADC, gemtuzumab ozogamicin, which is an anti-CD33 mAb conjugated with the potent DNA-targeting antibiotic calicheamicin, was given accelerated approval by the US Food and Drug Administration (FDA) for the treatment of patients with acute myeloid leukemia (AML) in 2000.5,6 However, the drug was voluntarily withdrawn from the US market in 2010 due to its failure to demonstrate improved survival, and with more fatal toxicity in a required post-approval study. Reasons for failure may include off-target toxicity due to its unstable linker and the heterogeneous mixture of drugs comprising 1–8 calicheamicin moieties per IgG molecule and 50% unconjugated antibody,6,7 which competes with the ADC for cancer cell internalization. In September 2017, the low-fractionated dose of gemtuzumab ozogamicin with chemotherapy for CD33-positive refractory AML gained approval by the FDA.8 This unusual story of the first ADC was an insightful example of how successful drug development of ADCs depends critically on the suitable engineering of the ADC components as well as adequate selection of the antigen to generate efficacy while minimizing toxicity of the drug.
Despite the advancement of development and engineering of ADCs, the majority of ADCs utilize payload from only a few categories of cytotoxic agents: antimitotic agents, microtubule inhibitor, antitumor antibiotics, and DNA damaging agents. The largest group of ADCs in clinical trials use antimitotic monomethyl auristatin E (MMAE) and MMAF, based on their high potency, water solubility, and stability under physiological conditions. The second largest class of payload of ADCs in clinical trials is microtubule inhibiting maytansinoids (DM1 and DM4), which have excellent stability and acceptable water solubility. Calicheamicin is a highly potent antibiotic that binds to the minor groove of DNA and creates double-stranded DNA breaks. Camptothecin analogues such as SN-38 and exatecan mesylate are potent DNA-damaging agents that exhibit topoisomerase 1 inhibitory activity.9 So far, the criteria for suitable payload include high level of potency, hydrophilicity, a lack of susceptibility to multidrug resistance protein 1 (MDR1), making it challenging to identify the suitable payload; thus, there is no gold-standard payload for TNBC. With improvements in payload application and conjugation chemistry, second generation ADCs, such as brentuximab vedotin for Hodgkin’s lymphoma and anaplastic large-cell lymphoma,10,11 and trastuzumab emtansine (T-DM1) for HER2-positive breast cancer,12 have been approved. Most recently, in June 2019, polatuzumab vedotin-piiq, a CD79b-directed ADC carrying MMAE by protease-cleavable peptide linker in combination with bendamustine plus rituximab was approved for the indication of relapsed diffuse large B-cell lymphoma. As a result of these efforts to improve the therapeutic index of the drug by maximizing tolerated dose and minimizing effective dose, novel ADCs are emerging in clinical development for patients with TNBC. Here, we will review and discuss the clinical application and development of ADCs for patients with TNBC.
Mechanism of action of ADCs
ADCs are composed of three well-defined components: an antibody directed to a tumor antigen, a highly potent cytotoxic payload, and a linker between the former two components. The most appealing mechanism of ADCs is a selective delivery of the drug to a cancer cell. The drug is administered intravenously to avoid the degradation by gastric acid within the gastrointestinal tract, finds and binds to the target antigens, following which the drug–antigen complex undergoes internalization with the cell via receptor-mediated endocytosis. This process results in the formation of an early endosome; an influx of proton ions into the endosome creates an acidic environment. The late endosome fuses with the cell lysosome, which contains proteases and undergoes lysosomal degradation. The cleavage of the linker due to acidic pH or the presence of protease in the lysosome allows the release of payloads into cytoplasm and the payloads to take effect.
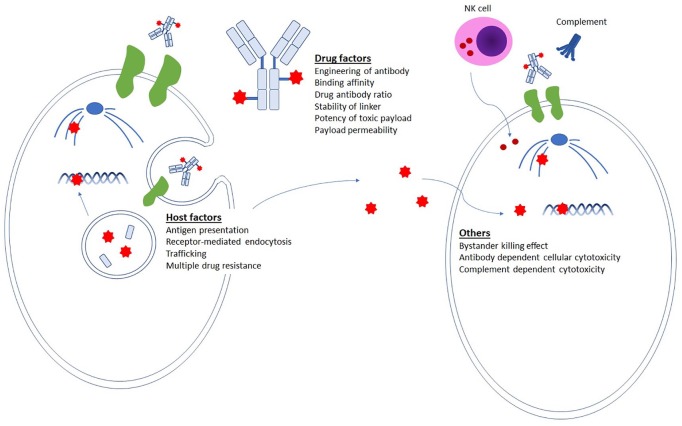
As this is a multi-step mechanism of action involving cancer cells, ADC molecules, and the microenvironment, various factors are implicated in influencing the outcome of ADC treatment (Figure 1). Firstly, optimal antigen selection is critical. Target antigens should ideally have a relatively high expression in tumors, with little or no expression in normal tissues, but be present on the cell surface for the drug to access and be an internalizing antigen so that the ADC is transported into the cancer cell via receptor-mediated endocytosis. The degree of surface antigen expression is not always predictive of response to ADCs since slow internalization kinetics or inefficient trafficking may influence overall drug efficacy. Secondly, the payload is ideally nonsusceptible to multidrug resistance protein, to avoid efflux of the drug. Thirdly, linker optimization is also a key feature; linkers must be stable while the ADC is in circulation to avoid off-target toxicity, but be capable of releasing the drug once inside cancer cells.
Figure 1.
Factors implicated in influencing the impact of ADCs. For efficient selective drug delivery, the antibody must have high binding affinity to the target antigen and the payload conjugation should not alter the pharmacokinetics. As host factors, the expression of target antigen should ideally be abundant on cancer cells but not on normal tissues. Upon receptor-mediated endocytosis, a fraction of the ADCs binds to FcRns in endosomes and are recycled back outside the cell. If the payload has high permeability, it may induce bystander killing effects. Extracellular mechanisms of action, such as antibody-dependent cellular cytotoxicity or complement dependent cytotoxicity, are potential additional mechanisms that could impact efficacy.
ADC, antibody–drug conjugate.
In addition to direct cytotoxicity, there are other mechanisms by which ADCs can exert anti-tumor effects. ADCs can induce antibody-dependent cellular cytotoxicity (ADCC), whereby natural killer (NK) cells recognize and kill antibody-coated cancer cells by activating cascades of apoptosis-inducing cytotoxic granules such as perforin and granzyme.13 Apart from selective drug delivery, bystander killing effects have been reported with some ADCs, in which free drugs, such as those released nonspecifically from conjugates or released by apoptotic cells, cross the plasma membrane and kill neighboring cells regardless of the presence or absence of antigen presentation. 14,15 In addition, cytotoxic mechanisms of action such as ADCC and complement-dependent cytotoxicity (CDC) may be triggered by ADCs. For example, trastuzumab emtansine binds to FcγRIII on immune effector cells and mediates ADCC by activating cytotoxic granules such as perforin and granzyme.16
ADCs for metastatic TNBC
The clinical development of ADCs and the composition of the various drugs in development are summarized in Tables 1 and 2.
Table 1.
Clinical development of antibody–drug conjugates for patients with metastatic TNBC.
| Drug | Target | Antibody | Linker-payload | DAR | Study phase | # of patients | Patient cohort | Treatment arms | ORR | PFS | ClinicalTrials.gov identifier |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sacituzumab govitecan (IMMU-132) | Trop-2 | RS7 | CL2A-SN-38 | 7.6 | I/II | 108 | Metastatic TNBC | IMMU-132 | 33.3 | 5.5 | NCT01631552 |
| III | 488 | Metastatic TNBC | IMMU-132 | NR | NR | NCT02574455 | |||||
| Eribulin/Cape/VNR/GEM | |||||||||||
| Ladiratuzumab vedotin (SGN-LIV1a) | LIV-1 | hLIV22 | vc-MMAE | 4 | I | 366 | Metastatic BC | SGN-LIV1A | 32* | 0.94* | NCT01969643 |
| Ib/II | 310 | Metastatic TNBC | SGN-LIV1A + atezo | NR | NR | NCT03424005 | |||||
| Cape | |||||||||||
| I/II | 97 | Metastatic TNBC | SGN-LIV1A + pembro | NR | NR | NCT03310957 | |||||
| II | NR | BC | SGN-LIV1A followed by AC | NR | NR | NCT01042379 | |||||
| Standard therapy | |||||||||||
| Trastuzumab deruxtecan (DS-8201a) | HER2 | Anti-HER2 IgG | Peptide linker with DXd | 8 | I | 278 | Advanced solid malignant tumors | DS-8201a | 43%** | NR | NCT02564900 |
| III | 540 | HER2-low BC | DS-8201a | NR | NR | NCT03734029 | |||||
| Cape/eribulin/GEM/PTX/nab-PTX | |||||||||||
| AVID100 | EGFR | MAB100 | Cleavable linker with DM1 | Undisclosed | Ia/IIA | 90 | TNBC, HNSCC, NSCLC | AVID100 | NR | NR | NCT03094169 |
| U3-1402 | HER3 | Patritumab | Tetra-peptide based linker with DXd | 8 | I/II | 180 | Metastatic BC | U3-1402 | 42.9*** | NR | NCT02980341 |
| CAB-ROR2-ADC (BA3021) | Ror2 | CAB | Undisclosed | Undisclosed | I/II | 120 | Solid tumor, NSCLC, TNBC, Soft tissue sarcoma | BA3021 | NR | NR | NCT03504488 |
| Anti-CA6-DM4 immunoconjugate (SAR566658) | CA6 | DS6 | SPDB-DM4 | 1 | I | 114 | Solid tumors | SAR566658 | 60****/35***** | NR | NCT01156870 |
| II | 23 | TNBC | SAR566658 | NR | NR | NCT02984683 |
AC, doxorubicin plus cyclophosphamide; AF-HPA, auristatin F-hydroxypropylamide; atezo, atezolizumab; BC, breast cancer; Cape, capecitabine; DAR, drug antibody ratio; DXd, extecan derivative; EGFR, epidermal growth factor receptor; GEM, gemcitabine; HER2, human epidermal growth factor receptor 2; HNSCC, head and neck squamous cell carcinoma; MMAE, monomethyl auristatin E; NSCLC, non-small cell lung cancer; q2w, every 2 weeks; ORR, objective response rate; pembro, pembrolizumab; PFS, progression-free survival; PTX, paclitaxel; TNBC, triple negative breast cancer; vc, valine-citrulline; VNR, vinorelbine.
The ORR is evaluated in TNBC dose escalation and expansion cohorts.
The ORR is evaluated in 23 patients including 6 patients with low HER2-expressing breast or gastric or gastro-esophageal carcinomas.
The ORR is evaluated in 34 patients with HER3-positive breast cancer.
The ORR is evaluated in patients with dosing of 190 and 90 mg/m2 on day 1 and day 8.
The ORR is evaluated in patients with dosing of 150 and 120 mg/m2 q2w.
Table 2.
Discontinued antibody–drug conjugates for patients with metastatic breast cancer.
| Drug | Target | Antibody | Linker-payload | DAR | Patient cohort | Main reason for discontinuation |
|---|---|---|---|---|---|---|
| PF-06664178 | Trop-2 | RN926 | AcLys-VCAur0101 | 2 | BC, NSCLC, ovarian cancer | Toxicity |
| Trastuzumab tesirine conjugate (ADCT-502) | HER2 | Anti-HER2 IgG1 | Cathepsin B-cleavable valine-alanine PBD | 1.7 | BC, NSCLC, bladder cancer, gastroesophageal cancer | Narrow therapeutic index |
| XMT-1522 | HER2 | HT-19 | Dolaflexin, AF-HPA | 12 | BC, NSCLC, gastric cancer | Company’s decision due to the competitive environment for HER2-targeted therapies |
| Glembatumumab vedotin (CDX-011) | gpNMB | CR011 | vc-MMAE | 2.7 | BC | No significant advantage of response and survival for CDX-011 |
AF-HPA, auristatin F-hydroxypropylamide; BC, breast cancer; DAR, drug antibody ratio; HER2, human epidermal growth factor receptor 2; MMAE, monomethyl auristatin E; NSCLC, non-small cell lung cancer; PBD, pyrrolobenzodiazepines; VC, valine-citrulline.
Sacituzumab govitecan
Mechanism of action
Sacituzumab govitecan is an anti-trophoblast cell-surface antigen (Trop-2) antibody conjugated with a potent DNA damaging agent, SN-38, by a pH-sensitive cleavable linker. Trop-2 is a Ca2+ signal transducer that was initially identified as a transmembrane glycoprotein in a trophoblast cell.17,18 Functionally, it is associated with cell migration and anchorage-independent growth; elevated expression of Trop-2 in breast cancer is correlated with poor prognosis.19 It is highly expressed in many epithelial cancers such as breast, colorectal, pancreatic, lung, esophageal, and ovarian cancers, with lower expression in certain normal tissues,20–22 and is expressed in 90% of TNBC tumors.23 SN-38, the payload of the drug, is an active metabolite of irinotecan, which binds reversibly to the topoisomerase 1 cleavage complex on DNA and slows down DNA replication by interfacial inhibiting mechanism, which causes S-phase-specific cell death.24 Although SN-38 is moderately toxic, with an IC50 in the nanomolar range, the average drug-to-antibody ratio (DAR) of 7.6 makes it possible to reach a sufficient drug concentration in cancer cells.25 Sacituzumab govitecan delivers SN-38 in its most active, non-glucuronidated form, which may explain the lower frequency of severe diarrhea than with irinotecan. In a preclinical study, sacituzumab govitecan, as well as SN-38, mediated pro-apoptotic signals such as upregulation of p21WAF1/Cip1 and cleavage of pro-caspase-3 and PARP in breast cancer cell lines, and demonstrated higher antitumor activity in a xenograft mouse model compared with irinotecan.25
Clinical results
In a single-arm phase I/II study, 108 patients with metastatic TNBC who had received at least two prior therapies were enrolled.23,26 Patients received 10 mg/kg of sacituzumab govitecan on days 1 and 8 in a 21-day cycle until disease progression or unacceptable toxicity occurred. The majority of patients had received prior treatment with anthracycline, taxane, and platinum chemotherapy, with a median of three prior therapies. The objective response rate (ORR) was 33.3% (95% CI 24.6–43.1%), the median PFS was 5.5 months (95% CI 4.1–6.3 months), and median OS was 13.0 months (95% CI 11.2–13.7 months). Notably, patients appeared to benefit from the drug regardless of age, number of previous treatments, or receipt of a prior immune checkpoint inhibitor, although the sample size of these subgroups were too small to draw statistically significant conclusions. These results compared quite favorably with prior studies of therapies for pretreated mTNBC, which typically show response rates around 12% and PFS less than 3 months.26
In the patients treated with sacituzumab govitecan, grade 3 or 4 adverse events (AEs) included neutropenia (42%), anemia (11%), hypophosphatemia (9%), diarrhea (8%), and fatigue and asthenia (8%). Based on the clinical activity demonstrated in this phase I/II trial, sacituzumab govitectan received a breakthrough therapy designation for the treatment of metastatic TNBC in February 2016. Final drug approval by the FDA is currently pending due to chemistry and manufacturing issues.
To verify the findings seen in the initial early phase study, the international, open-label, randomized, confirmatory phase III ASCENT trial is currently ongoing. This trial is enrolling 488 patients with refractory or metastatic TNBC who received at least two prior chemotherapies in the metastatic setting including a taxane. Patients are assigned to treatment with either sacituzumab govitecan or to physician’s choice of single-agent chemotherapy (eribulin, capecitabine, gemcitabine, or vinorelbine). The primary outcome measure is PFS and secondary outcome measures are OS, ORR, duration of response, and time to onset of response [ClinicalTrials.gov identifier: NCT02574455].
Ladiratuzumab vedotin (SGN-LIV1a)
Mechanism of action
Ladiratuzumab vedotin is a humanized antibody targeting the zinc transporter LIV-1 conjugated with a potent microtubule-disrupting agent, monomethyl auristatin E (MMAE) by a proteolytically cleavable linker. LIV-1 is a multi-span transmembrane protein with putative zinc transporter and metalloproteinase activity expressed in 68% of metastatic TNBC tumors.27 LIV-1 is expressed frequently in breast, prostate, and melanoma, but less in ovarian, uterine, and lung cancers. Expression of LIV-1 was initially identified in primary estrogen receptor-positive breast cancer, found to be maintained after endocrine therapy in metastatic sites, and is also upregulated in TNBC.28 Expression of LIV-1 is reported to be associated with lymph node metastasis and metastatic progression by promoting epithelial-mesenchymal transition (EMT) through downregulation of E-cadherin via interaction with signal transducer and activator of transcription 3 (STAT3) and Snail.29 The payload, MMAE, is a synthetic analogue of dolastatin 10, which is a natural antimitotic drug extracted from the sea hare Dolabella auricularia and causes G2/M phase cell cycle arrest by interfering with the polymerization of microtubules upon binding to the β-subunit of tubulin dimers. The drug is highly potent (free drug IC50: 10–10M) in cell lines, water soluble, and stable under physiological conditions, and therefore is preferred as an ADC payload. In a preclinical study, ladiratuzumab vedotin was shown to bind to the target antigen, internalize over a 24-h period, traffic to the lysosome, release the payload by proteolysis, and mediate the disruption of microtubules. Also, the antitumor activity of ladiratuzumab vedotin was demonstrated in breast cancer and cervical cancer xenograft models.28
Clinical results
The ongoing phase I study has been evaluating the safety, tolerability, pharmacokinetics, and antitumor activity of ladiratuzumab vedotin in patients with LIV1-positive advanced or metastatic breast cancer [ClinicalTrials.gov identifier: NCT01969643]. At completion of dose escalation, expansion cohorts were opened to further evaluate safety and antitumor activity of monotherapy in TNBC patients at 2.0 mg/kg and 2.5 mg/kg dosing every 3 weeks. Among the 44 patients with TNBC in the combined dose escalation and expansion cohorts, the ORR was 32% and the median PFS was 11.3 weeks (95% CI 6.1–17.1 weeks). In the entire cohort, grade 3 and 4 AEs included neutropenia (25%) and anemia (15%). These interim results of the phase I study showed encouraging antitumor activity and tolerability of ladiratuzumab vedotin. Enrollment is ongoing in the triple negative monotherapy expansion cohort.30 Other clinical trials are evaluating the efficacy and safety of ladiratuzumab vedotin as monotherapy in I-SPY 2 trial [ClinicalTrials.gov identifier: NCT0102379], and in combination with either pembrolizumab31 [ClinicalTrials.gov identifier: NCT03310957] or atezolizumab32 [ClinicalTrials.gov identifier: NCT03424005].
Trastuzumab deruxtecan (DS-8201a)
Mechanism of action
Trastuzumab deruxtecan is a humanized antibody against HER2 conjugated with a topoisomerase I inhibitor, extecan derivative (DX-8951 derivative, DXd) by a cleavable peptide-based linker. HER2 is a member of the epidermal growth factor receptor (EGFR) family of receptor tyrosine kinases and is overexpressed in a broad spectrum of cancers, such as bladder, breast, cervical, cholangiocarcinoma, colorectal, endometrial, esophageal, gastric, and lung. Overall, amplification and/or overexpression of HER2 occurs in 15–20% of breast cancer tumors, and is associated with poor prognosis due to its promotion of cell proliferation, adhesion, migration, and apoptosis.33,34 DXd was demonstrated to be approximately 10 times more potent than SN-38 as evidenced by the DNA relaxation assay.35 Trastuzumab deruxtecan has a relatively higher DAR of 7–8, and releases the payload in the presence of lysosomal enzyme such as cathepsin B.36 In vitro, trastuzumab deruxtecan induced ADCC and inhibited phosphorylated Akt as well as phosphorylation of Chk1 and Histone H2A.X, implying that the drug is capable of inducing HER2-specific cell growth inhibition and DNA damage and apoptosis via delivery of the payload in HER2-positive cancer cell lines. In vivo, growth inhibition of both high and low HER2-expressing tumors in patient-derived xenograft models as opposed to less inhibition by low-DAR ADC control drug were observed, suggesting its efficient delivery of payload with higher DAR were effective even in low HER2-expressing tumors.35 Moreover, trastuzumab deruxtecan demonstrated a bystander killing effect with antitumor activity in both HER2-positive and HER2-negative tumors only when they were neighboring, likely due to the high permeability of the payload.15
Clinical results
In a dose escalation study, patients with breast or gastric or gastro-esophageal carcinoma refractory to standard therapy regardless of HER2 status were enrolled. Among 24 patients in the entire cohort, 8 had low-HER2 expressing tumors, which were defined to be either IHC 1+ and IHC 2+/ISH–. Although the greatest response to the drug was observed in HER2 IHC 3+ patients, two responders had low-HER2 expressing tumors, suggesting that trastuzumab deruxtecan might offer activity even in patients with low-HER2 expression. This ongoing, open-label, multicenter phase III study [ClinicalTrials.gov identifier: NCT03734029] is recruiting patients with HER2-low, unresectable and/or metastatic breast cancer. Approximately 540 patients will be randomized in a 2 to 1 ratio to trastuzumab deruxtecan (5.4 mg/kg every 3 weeks) versus physician’s choice of chemotherapy and PFS, OS, ORR, duration of response, and safety will be evaluated.37
AVID100
Mechanism of action
AVID100 is an anti-EGFR ADC that targets both wild-type and mutant forms of EGFR and is conjugated with DM1 (derivative of maytansine). EGFR is highly expressed in many epithelial cancers such as lung, breast, and head and neck cancer, making it a promising target antigen for cancer therapeutics. Among patients with TNBC, 20% highly overexpress EGFR. However, due to EGFR expression in normal skin cells, on-target and off-target toxicity has been a major concern. The antibody moiety of AVID100, MAB100, exhibits antagonist activity in the EGFR signaling pathway by competing with EGFR for binding. Although AVID100 demonstrated cytotoxicity in breast, head and neck, and lung cancer cell lines, the drug did not demonstrate increased cytotoxicity compared with MAB100 in keratinocytes.38
Clinical results
In a phase I dose-escalation study, 24 patients with advanced or metastatic epithelial malignancies without available standard of care therapy and likely to express EGFR received a 2-h infusion of AVID100 every 3 weeks. The most common AEs experienced were rash, nausea, and fatigue, but the drug was generally well tolerated. Durable responses were observed in three of these patients not selected for EGFR overexpression (colorectal, ovarian, cervical).39 The multicenter, dose-expansion, phase IIa trial is recruiting patients with advanced, EGFR-overexpressing TNBC to evaluate the efficacy, safety, and tolerability of AVID100 (AVID100-01 [ClinicalTrials.gov identifier: NCT03094169]). In this study, patients will receive 220 mg/m2 (~6 mg/kg) of AVID100 every 3 weeks.
U3-1402
Mechanism of action
U3-1402 is an anti-HER3 ADC that is conjugated with a topoisomerase I inhibitor exatecan derivative (DXd) via a peptidyl linker.40 HER3 is one of the HER family molecules and is highly expressed in various tumor types. Unlike other HER family molecules, HER3 has a feature of lacking, or having minimal, intrinsic kinase activity.41 HER3 can be phosphorylated by forming a heterodimer with other receptor tyrosine kinases such as HER2, which results in activating the intracellular signaling pathways, mainly the PI3K/AKT and MAPK/ERK pathways. HER3 is implicated in causing resistance to anti-HER2 agents,42,43 and may be associated with poor prognosis in solid tumors including breast, gastric, ovarian cancer, and melanoma.44 Similar to the naked, fully human anti-HER3 mAb patritumab, which binds to the extracellular domain of HER3 and inhibits HER3-activated PI3K/AKT signaling pathway, U3-1402 showed efficient internalization into the cancer cell and release of the payload. In addition, the cytotoxic activity of the drug was found to be mediated predominantly by its payload, DXd, via DNA damage and apoptosis induction in an in vitro pre-clinical study. In in vivo experiments, U3-1402 showed potent antitumor activity in a dose-dependent and HER3-dependent manner without unacceptable toxicity in rats and monkeys.40
Clinical Results
The ongoing phase I/II trial is evaluating the safety, tolerability, and efficacy of U3-1402 in patients with HER3-overexpressing metastatic breast cancer. According to the most recent report, 21 evaluable patients received U3-1402, and the grade 3/4 toxicity included thrombocytopenia and liver enzyme increase. Other common toxic effects included nausea, vomiting, and decreased appetite. The ORR was 33% and disease-control rate [DCR, complete response (CR) + partial response (PR) + stable disease (SD)] was 95%.45 This preliminary data suggests that U3-1402 has promising antitumor activity with manageable toxicity in patients with HER3-overexpressing metastatic breast cancer.
CAB-ROR2-ADC
Mechanism of action
CAB-ROR2-ADC is an ADC composed of a conditionally active biologic (CAB) antibody directing receptor tyrosine kinase-like orphan receptor 2 (ROR2) conjugated to an undisclosed payload. The ROR2 belongs to the ROR subfamily of cell surface receptors and is an onco-fetal protein that acts as a non-canonical Wnt 5A receptor; ROR2 expression is correlated with clinical outcome.46 Upon administration of the drug, the antibody becomes activated under the unique microphysical conditions present in the tumor microenvironment. The CAB proteins are generated using BioAtla’s proprietary protein technologies and are specifically activated by the glycolytic metabolism of cancer cells, but not in the microenvironment of normal tissues. In vitro and in vivo, CAB-ROR2-ADC was demonstrated to mediate cytotoxicity and tumor growth inhibition in ROR2-expressing cell lines and human xenograft models.47
Clinical results
A multi-center, open-label, phase I/II study is currently recruiting patients with advanced solid tumors such as TNBC, non-small cell lung cancer, and soft tissue sarcoma, to assess the safety and efficacy of CAB-ROR2-ADC.
Anti-CA6-DM4 immunoconjugate (SAR566658)
Mechanism of action
SAR566658 is a humanized DS6 antibody directed against tumor-associated sialoglycotope CA6 conjugated to the maytansinoid DM4. DS6 specifically recognizes a MUC-1 tandem repeat domain formed by tumor-associated glycosylation. The expression of CA6 was observed in breast, ovarian, and bladder cancer. In in vivo models, SAR566658 induced antitumor activity against CA6-positive solid tumor and in breast patient-derived xenograft models.48
Clinical results
A phase I dose escalation and expansion study was conducted to evaluate the safety and the maximum tolerated dose of SAR566658. A total of 114 patients with heavily pretreated solid tumors, including breast cancer with CA6 expression, were enrolled. The most common AEs were reversible keratopathy (grade 2/3; 36%) and fatigue (32.6%), peripheral neuropathy (31.6%), and gastrointestinal disorders. Tumor regression was observed in about 60% of patients at 190 and 90 mg/m2 on day 1 and day 8 dosing and 35% of patients at 150 and 120 mg/m2 every 2 weeks (q2w). Among eight patients with PR, three had breast cancer. The further clinical development of the drug will be based on 90 mg/m2 day 1 and day 8 every 3 weeks (q3w).49
Discontinued ADCs for patients with metastatic breast cancer
Discontinued ADCs for patients with metastatic breast cancer are listed in Table 2.
Glembatumumab vedotin
Mechanism of action
Glembatumumab vedotin is a glycoprotein NMB (gpNMB)-specific monoclonal antibody conjugated to the MMAE by a proteolytic linker. gpNMB is present in 40–60% of TNBC, and its overexpression was demonstrated to promote invasion and metastasis of glioma and breast cancer cells.50
Clinical results
Patients (n = 124) with refractory breast cancer that expressed gpNMB in ⩾5% of epithelial or stromal cells by immunohistochemistry were enrolled and randomized to glembatumumab vedotin or investigator’s choice in a 2:1 ratio, with stratification by gpNMB expression. Glembatumumab vedotin achieved 40% ORR versus 0% with investigator’s choice of therapy in a subgroup of patients with gpNMB-overexpressing TNBC.51 However, the drug failed to demonstrate a PFS, ORR, or OS benefit in a phase II randomized trial compared with capecitabine in patients with gpNMB-overexpressing metastatic TNBC [ClinicalTrials.gov identifier: NCT01997333], which led to discontinuation of the development of this ADC.
Conclusion
The development of ADCs has emerged as an important targeted therapeutic strategy for TNBC. Although the approach to combine the specificity of mAbs with potency of payload is novel, the success of the drug in clinics depends critically on optimal engineering of the drug and matching the right drug to the right patient. The relatively few identified ideal target antigens that are expressed highly in cancer tissues but at a low level in normal tissues have created a highly competitive environment for the development of new ADCs in the drug development industry. In the past few decades, many important lessons have been learned from ADCs that have resulted in discontinuation. For example, PF06664178, an anti-Trop-2 antibody with Aur0101, demonstrated excess toxicities, due primarily to skin/mucosal and neutropenia with minimal antitumor activity. The AEs seen with PF06664178, and their severity, were notably different from those of sacituzumab govitecan, which also targets Trop-2. This is possibly explained by the difference of DAR and potency of the payload between those two drugs; whereas sacituzumab govitecan had higher DAR with the lower toxicity of SN-38, PF06664178 had lower DAR with more toxic Aur0101.52
Since ADCs can provide a broader therapeutic window than conventional chemotherapy, combination therapy with other agents is a potentially effective strategy to enhance synergy as well as target tumor heterogeneity. For example, the combination of sacituzumab govitecan with PARP inhibition in TNBC models in vitro and in vivo demonstrated increased dsDNA breaks and synergistic growth inhibition regardless of BRCA1/2 status in a preclinical study.53 To validate the feasibility of this combination therapy, a phase I/II clinical trial to investigate the dose limiting toxicity of sacituzumab govitecan with talazoparib in patients with metastatic TNBC is ongoing [ClinicalTrials.gov identifier: NCT04039230].
While the off-target toxicity of ADCs is generally caused by non-optimal engineering of the drug, such as premature release of payload due to unstable linker, or non-specific distribution of payload from apoptotic cells, the on-target toxicity profile is usually reflected by that of the payload itself. Thus, the strategy of combining ADCs with immune checkpoint inhibitors with non-overlapping toxicity that target the interaction between microenvironment and cancer cells has emerged as a promising option. As part of an open-label, multicenter, randomized umbrella phase Ib/II trial, MORPHEUS-TNBC is recruiting patients with metastatic or inoperable locally advanced TNBC to evaluate the efficacy and safety of a combination of atezolizumab and sacituzumab compared with nab-paclitaxel plus atezolizumab [ClinicalTrials.gov identifier: NCT03424005]. The rationale of the combination of ADCs with immune checkpoint inhibitors is based on preclinical evidence that ADCs can mediate increased infiltration of cytotoxic T cells and antigen-presenting cells in the microenvironment.
A recent study reported enhanced antitumor activity by incorporating a bispecific antibody that binds to Mesothelin, a glycosyl-phosphatidyl inositol-linked membrane protein and CD16 in the context of TNBC.54 The key advantage of targeting two molecules through a single platform points to unique approaches such as the targeting of two different epitopes on the same antigen or the use of antibody to redirect immune effectors. Although the potency of the payload is the critical component in antitumor activity in ADCs, bispecific ADCs may complement efficacy by improving internalization, trafficking, or extracellular effects of cell killing such as ADCC.55 If these attempts to improve the efficacy of ADCs demonstrate success, ADCs could potentially replace a chemotherapy backbone as the preferred therapeutic partner for treatment of TNBC.
Footnotes
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: A. Nagayama owns stock of Chugai, Inc. A. Nagayama’s immediate family member has a leadership position with Chugai, Inc. and Roche, Inc.
N. Vidula reports consulting/advisory board to AbbVie; and research funding to the institution (Massachusetts General Hospital): Merck, Radius, Daehwa, Pfizer, and Novartis.
A. Bardia reports consultant/advisory board to Genentech/Roche, Immunomedics, Novartis, Pfizer, Merck, Radius Health, Diiaichi/Astra-Zeneca, Sanofi, Puma Biotechnology, Phillips; and research funding to the institution: Genentech/Roche, Immunomedics, Novartis, Pfizer, Merck, Radius Health, Sanofi, Mersana.
ORCID iD: Aditya Bardia  https://orcid.org/0000-0003-4885-1157
https://orcid.org/0000-0003-4885-1157
Contributor Information
Aiko Nagayama, Department of Surgery, Keio University School of Medicine, Shinjuku, Tokyo, Japan.
Neelima Vidula, Massachusetts General Hospital Cancer Center, Harvard Medical School, Boston, MA, USA.
Leif Ellisen, Massachusetts General Hospital Cancer Center, Harvard Medical School, Boston, MA, USA.
Aditya Bardia, Massachusetts General Hospital Cancer Center, Harvard Medical School, 10 North Grove Street, Boston, MA 02114-2621, USA.
References
- 1. Waks AG, Winer EP. Breast cancer treatment: a review. JAMA 2019; 321: 288–300. [DOI] [PubMed] [Google Scholar]
- 2. Schmid P, Adams S, Rugo HS, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med 2018; 379: 2108–2121. [DOI] [PubMed] [Google Scholar]
- 3. Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975; 256: 495–497. [DOI] [PubMed] [Google Scholar]
- 4. Schwartz RS. Paul Ehrlich’s magic bullets. N Engl J Med 2004; 350: 1079–1080. [DOI] [PubMed] [Google Scholar]
- 5. Sievers EL, Larson RA, Stadtmauer EA, et al. Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukemia in first relapse. J Clin Oncol 2001; 19: 3244–3254. [DOI] [PubMed] [Google Scholar]
- 6. Bross PF, Beitz J, Chen G, et al. Approval summary: gemtuzumab ozogamicin in relapsed acute myeloid leukemia. Clin Cancer Res 2001; 7: 1490–1496. [PubMed] [Google Scholar]
- 7. Labrijn AF, Buijsse AO, van den Bremer ET, et al. Therapeutic IgG4 antibodies engage in fab-arm exchange with endogenous human IgG4 in vivo. Nat Biotechnol 2009; 27: 767–771. [DOI] [PubMed] [Google Scholar]
- 8. Castaigne S, Pautas C, Terré C, et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): a randomised, open-label, phase 3 study. Lancet 2012; 379: 1508–1516. [DOI] [PubMed] [Google Scholar]
- 9. Beck A, Goetsch L, Dumontet C, et al. Strategies and challenges for the next generation of antibody–drug conjugates. Nat Rev Drug Discov 2017; 16: 315–337. [DOI] [PubMed] [Google Scholar]
- 10. Younes A, Gopal AK, Smith SE, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J Clin Oncol 2012; 30: 2183–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pro B, Advani R, Brice P, et al. Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: results of a phase II study. J Clin Oncol 2012; 30: 2190–2196. [DOI] [PubMed] [Google Scholar]
- 12. Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 2012; 367: 1783–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat Rev Immunol 2010; 10: 317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Okeley NM, Miyamoto JB, Zhang X, et al. Intracellular activation of SGN-35, a potent anti-CD30 antibody–drug conjugate. Clin Cancer Res 2010; 16: 888–897. [DOI] [PubMed] [Google Scholar]
- 15. Ogitani Y, Hagihara K, Oitate M, et al. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody–drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci 2016; 107: 1039–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Junttila TT, Li G, Parsons K, et al. Trastuzumab-DM1 (T-DM1) retains all the mechanisms of action of trastuzumab and efficiently inhibits growth of lapatinib insensitive breast cancer. Breast Cancer Res Treat 2011; 128: 347–356. [DOI] [PubMed] [Google Scholar]
- 17. Lipinski M, Parks DR, Rouse RV, et al. Human trophoblast cell-surface antigens defined by monoclonal antibodies. Proc Natl Acad Sci USA 1981; 78: 5147–5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ripani E, Sacchetti A, Corda D, et al. Human Trop-2 is a tumor-associated calcium signal transducer. Int J Cancer 1998; 76: 671–676. [DOI] [PubMed] [Google Scholar]
- 19. Lin H, Huang JF, Qiu JR, et al. Significantly upregulated TACSTD2 and Cyclin D1 correlate with poor prognosis of invasive ductal breast cancer. Exp Mol Pathol 2013; 94: 73–78. [DOI] [PubMed] [Google Scholar]
- 20. Shvartsur A, Bonavida B. Trop2 and its overexpression in cancers: regulation and clinical/therapeutic implications. Genes Cancer 2015; 6: 84–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goldenberg DM, Stein R, Sharkey RM. The emergence of trophoblast cell-surface antigen 2 (TROP-2) as a novel cancer target. Oncotarget 2018; 9: 28989–29006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McDougall AR, Tolcos M, Hooper SB, et al. Trop2: from development to disease. Dev Dyn 2015; 244: 99–109. [DOI] [PubMed] [Google Scholar]
- 23. Bardia A, Mayer IA, Diamond JR, et al. Efficacy and safety of anti-trop-2 antibody drug conjugate sacituzumab govitecan (IMMU-132) in heavily pretreated patients with metastatic triple-negative breast cancer. J Clin Oncol 2017; 35: 2141–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pommier Y. DNA topoisomerase I inhibitors: chemistry, biology, and interfacial inhibition. Chem Rev 2009; 109: 2894–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goldenberg DM, Cardillo TM, Govindan SV, et al. Trop-2 is a novel target for solid cancer therapy with sacituzumab govitecan (IMMU-132), an antibody–drug conjugate (ADC). Oncotarget 2015; 6: 22496–22512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bardia A, Mayer IA, Vahdat LT, et al. Sacituzumab govitecan-hziy in refractory metastatic triple-negative breast cancer. N Engl J Med 2019; 380: 741–751. [DOI] [PubMed] [Google Scholar]
- 27. Taylor KM, Morgan HE, Johnson A, et al. Structure-function analysis of LIV-1, the breast cancer-associated protein that belongs to a new subfamily of zinc transporters. Biochem J 2003; 375: 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sussman D, Smith LM, Anderson ME, et al. SGN-LIV1A: a novel antibody–drug conjugate targeting LIV-1 for the treatment of metastatic breast cancer. Mol Cancer Ther 2014; 13: 2991–3000. [DOI] [PubMed] [Google Scholar]
- 29. Taylor KM, Hiscox S, Nicholson RI. Zinc transporter LIV-1: a link between cellular development and cancer progression. Trends Endocrinol Metab 2004; 15: 461–463. [DOI] [PubMed] [Google Scholar]
- 30. Modi S, Pusztai L, Forero A, et al. Phase 1 study of the antibody–drug conjugate SGN-LIV1A in patients with heavily pretreated triple-negative metastatic breast cancer. Clin Cancer Res 2018; 78(Suppl.): abstract PD3-14. [Google Scholar]
- 31. Han HS, Alemany CA, Brown-Glaberman UA, et al. SGNLVA-002: single-arm, open label phase Ib/II study of ladiratuzumab vedotin (LV) in combination with pembrolizumab for first-line treatment of patients with unresectable locally advanced or metastatic triple-negative breast cancer. J Clin Oncol 2019; 37(Suppl.): TPS1110. [Google Scholar]
- 32. Yardley D, Abu-Khalaf M, Boni V, et al. MORPHEUS: a phase Ib/II trial platform evaluating the safety and efficacy of multiple cancer immunotherapy combinations in patients with hormone receptor–positive and triple-negative breast cancer. Cancer Res 2019; 79(Suppl.): abstract OT2-06-04. [Google Scholar]
- 33. Moasser MM. The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene 2007; 26: 6469–6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 1989; 244: 707–712. [DOI] [PubMed] [Google Scholar]
- 35. Ogitani Y, Aida T, Hagihara K, et al. DS-8201a, a novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin Cancer Res 2016; 22: 5097–5108. [DOI] [PubMed] [Google Scholar]
- 36. Shiose Y, Ochi Y, Kuga H, et al. Relationship between drug release of DE-310, macromolecular prodrug of DX-8951f, and cathepsins activity in several tumors. Biol Pharm Bull 2007; 30: 2365–2370. [DOI] [PubMed] [Google Scholar]
- 37. Modi S, Ohtani S, Lee CC, et al. A phase III, multicenter, randomized, open label trial of [fam-] trastuzumab deruxtecan (DS-8201a) versus investigator’s choice in HER2-low breast cancer. J Clin Oncol 2019; 37(Suppl.): TPS1102. [Google Scholar]
- 38. Thwaites MJ, Figueredo R, Tremblay G, et al. AVID100 is an anti-EGFR ADC that promotes DM1-meditated cytotoxicity on cancer cells but not on normal cells. Cancer Res 2019; 79(Suppl.): abstract 218. [Google Scholar]
- 39. Lakhani N, Chandana S, Tolcher A, et al. A phase Ia/IIa trial of AVID100, an anti-EGFR antibody–drug conjugate. Cancer Res 2019; 79(Suppl.): abstract CT056. [Google Scholar]
- 40. Hashimoto Y, Koyama K, Kamai Y, et al. A novel HER3-targeting antibody-drug conjugate, U3-1402, exhibits potent therapeutic efficacy through the delivery of cytotoxic payload by efficient internalization. Clin Cancer Res 2019; 25: 7151–7161. [DOI] [PubMed] [Google Scholar]
- 41. Shi F, Telesco SE, Liu Y, et al. ErbB3/HER3 intracellular domain is competent to bind ATP and catalyze autophosphorylation. Proc Natl Acad Sci USA 2010; 107: 7692–7697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Xia W, Petricoin EF, III, Zhao S, et al. An heregulin-EGFR-HER3 autocrine signaling axis can mediate acquired lapatinib resistance in HER2+ breast cancer models. Breast Cancer Res 2013; 15: R85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lyu H, Han A, Polsdofer E, et al. Understanding the biology of HER3 receptor as a therapeutic target in human cancer. Acta Pharm Sin B 2018; 8: 503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ocana A, Vera-Badillo F, Seruga B, et al. HER3 overexpression and survival in solid tumors: a meta-analysis. J Natl Cancer Inst 2013; 105: 266–273. [DOI] [PubMed] [Google Scholar]
- 45. Yonemori K, Masuda N, Takahashi S, et al. 151OSingle agent activity of U3-1402, a HER3-targeting antibody–drug conjugate, in HER3-overexpressing metastatic breast cancer: updated results from a phase I/II trial. Ann Oncol 2019; 30(Suppl. 3): iii48. [Google Scholar]
- 46. Bayerlová M, Menck K, Klemm F, et al. Ror2 signaling and its relevance in breast cancer progression. Front Oncol 2017; 7: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sharp LL, Chang C, Frey G, et al. Anti-tumor efficacy of BA3021, a novel conditionally active biologic (CAB) anti-ROR2 ADC. Cancer Res 2018; 78(Suppl.): abstract 833. [Google Scholar]
- 48. Trombe M, Caron A, Tellier A, et al. Preclinical activity of an antibody drug conjugate targeting tumor specificmuc1 structural peptide-glycotope. Cancer Res 2019; 79(Suppl.): abstract 235. [Google Scholar]
- 49. Gomez-Roca CA, Boni V, Moreno V, et al. A phase I study of SAR566658, an anti CA6-antibody drug conjugate (ADC), in patients (Pts) with CA6-positive advanced solid tumors (STs)(NCT01156870). J Clin Oncol 2016; 34(Suppl. 15): 2511. [Google Scholar]
- 50. Rose AA, Grosset AA, Dong Z, et al. Glycoprotein nonmetastatic B is an independent prognostic indicator of recurrence and a novel therapeutic target in breast cancer. Clin Cancer Res 2010; 16: 2147–2156. [DOI] [PubMed] [Google Scholar]
- 51. Yardley DA, Weaver R, Melisko ME, et al. EMERGE: a randomized phase II study of the antibody-drug conjugate glembatumumab vedotin in advanced glycoprotein NMB-expressing breast cancer. J Clin Oncol 2015; 33: 1609–1619. [DOI] [PubMed] [Google Scholar]
- 52. King GT, Eaton KD, Beagle BR, et al. A phase 1, dose-escalation study of PF-06664178, an anti-Trop-2/Aur0101 antibody–drug conjugate in patients with advanced or metastatic solid tumors. Invest New Drugs 2018; 36: 836–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cardillo TM, Sharkey RM, Rossi DL, et al. Synthetic lethality exploitation by an anti-trop-2-SN-38 antibody-drug conjugate, IMMU-132, plus PARP inhibitors in BRCA1/2-wild-type triple-negative breast cancer. Clin Cancer Res 2017; 23: 3405–3415. [DOI] [PubMed] [Google Scholar]
- 54. Del Bano J, Florès-Florès R, Josselin E, et al. A bispecific antibody-based approach for targeting mesothelin in triple negative breast cancer. Front Immunol 2019; 10: 1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Andreev J, Thambi N, Perez Bay AE, et al. Bispecific antibodies and antibody-drug conjugates (ADCs) bridging HER2 and prolactin receptor improve efficacy of HER2 ADCs. Mol Cancer Ther 2017; 16: 681–693. [DOI] [PubMed] [Google Scholar]