Abstract
Background:
Immobilization of the ankle joint has been suggested as a key element in the pathogenesis leading to deep vein thrombosis (DVT).
Purpose:
To investigate whether early controlled ankle motion (ECM) could reduce the incidence of DVT compared with immobilization (IM) in the treatment of acute Achilles tendon rupture.
Study Design:
Randomized controlled trial; Level of evidence, 2.
Methods:
Patients aged 18 to 70 years were eligible for inclusion, and treatment was nonoperative. The ECM group performed movements of the ankle 5 times a day from weeks 3 to 8 after rupture. The control group was immobilized for 8 weeks. The outcome measure was DVT diagnosed with color Doppler ultrasound for above- and below-knee DVT at 2 and 8 weeks. The Achilles tendon Total Rupture Score, the heel-rise work test, and the Copenhagen Achilles ultrasonographic Length Measurement were performed at 4-, 6-, and 12-month follow-up.
Results:
A total of 189 patients were assessed for eligibility from February 2014 to December 2016. Of these, 130 were randomized: 68 patients were allocated to the ECM group and 62 to the IM group. All patients participated in follow-up at 8 weeks assessing for DVT. In total, 62 (47.7%) patients were diagnosed with DVT: 33 of 68 (48.5%) in the ECM group and 28 of 61 (46.8%) in the IM group (P = .84). DVT did not affect treatment outcomes at 4, 6, and 12 months. D-dimer had low sensitivity (71%) for detecting DVT.
Conclusion:
We found that 1 in 2 patients presented with DVT in nonoperative treatment of acute Achilles tendon rupture. The ECM protocol revealed no benefit versus IM in reducing the incidence of DVT. DVT did not influence functional and patient-reported outcomes the first year after rupture. D-dimer seems an inappropriate test for detection of DVT in patients with acute Achilles tendon rupture.
Registration:
NCT02015364 (ClinicalTrials.gov identifier).
Keywords: acute Achilles tendon rupture, deep vein thrombosis, DVT, early controlled motion, dynamic rehabilitation
The incidence of deep vein thrombosis (DVT) in the treatment of acute Achilles tendon rupture is reported to be between 0.3% and 50%.14,20,26,29 Acute Achilles tendon rupture has an incidence of 20 to 32 per 100,000 individuals per year.17,19 Patients with DVT have an up to 50% risk of developing postthrombotic syndrome, a condition associated with poor quality of life and significant societal costs.9 Immobilization of the ankle joint has been suggested as a key element in the pathogenesis leading to DVT,11,14 and intermittent pneumatic compression has been highly effective in reducing the incidence of DVT in patients with acute Achilles tendon rupture.14
Treatment of Achilles tendon rupture differs among hospitals, regions, and countries; some use immobilization of the ankle joint for 6 to 10 weeks, while others use early controlled motion (ECM) of the ankle joint.7 It has not been possible to show a clear superiority of one treatment protocol over another.4,15,21,32,33,37 However, the question remains whether ECM of the ankle reduces the incidence of DVT and whether DVT negatively affects outcome after acute Achilles tendon rupture.
The primary objective of this study was to investigate whether ECM of the ankle joint in weeks 3 through 8 would reduce the incidence of DVT in patients with acute Achilles tendon rupture, compared with a treatment protocol where the ankle joint was immobilized for 8 weeks. Patients were treated nonoperatively, and full weightbearing was allowed from day 14 in both groups. A second objective was to determine the impact of DVT on outcomes after nonoperative treatment of acute Achilles tendon ruptures.
The main hypothesis was that ECM of the ankle joint would lead to a reduced risk of DVT. A secondary hypothesis was that DVT would lead to impaired outcome. The null hypotheses were that ECM would have no influence on the incidence of DVT and that DVT would not influence outcome.
Methods
The study was performed as a secondary analysis of a randomized controlled trial (RCT) with patients allocated in a 1:1 ratio to 1 of 2 parallel groups. The main study investigated functional outcomes after acute Achilles tendon rupture.5,6 The present study investigated the risk of developing DVT after acute Achilles tendon rupture. The detailed study design and protocol have been published previously.5 The trial protocol was developed in accordance with the SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials)13 and CONSORT (Consolidated Standards of Reporting Trials)22 guidelines and checklists.
Study Participants
Eligible for inclusion were patients treated for acute Achilles tendon rupture at Copenhagen University Hospital Hvidovre who were age 18 to 70 years, were able to attend rehabilitation and postoperative examinations, and were able to speak and understand Danish. Patients were excluded if they had previous rupture of 1 or both Achilles tendons, had previous surgery on the affected Achilles tendon, were treated with fluoroquinolones or corticosteroids within the past 6 months, had arterial insufficiency in the legs (no palpable pulse in the dorsal pedis artery) or severe medical illness (American Society of Anesthesiologists score ≥3), or had a distance between the rupture and the calcaneus of less than 1 cm.
Patients were screened in the outpatient clinic within 14 days after rupture and, if eligible, were given the opportunity to decide whether they wanted to participate in the trial. Patients who did not participate were treated nonoperatively without ECM of the ankle joint.
Treatment and Intervention
In the emergency department, patients were treated with a posterior plaster splint in approximately 30° of plantarflexion (evaluated visually) and were referred for an appointment in the outpatient clinic within 4 days. In the outpatient clinic, the diagnosis was confirmed by an investigator, and a below-knee full synthetic cast was applied in approximately 30° (evaluated visually) of plantarflexion. No weightbearing was allowed for the first 2 weeks after injury.
After 2 weeks, the cast was removed. Each patient was placed in an Aircast AirSelect Standard orthosis (DJO LLC) with 2 wedges that each provided 1.5 cm of heel lift. Full weightbearing was allowed, but patients were advised to use the crutches for another 1 or 2 weeks. At this point, patients were randomized into 1 of 2 groups:
Early controlled motion (ECM): The patient was instructed to perform early controlled ankle motion exercises from the beginning of week 3 through week 8.
Immobilization (IM): The patient was instructed to wear the orthosis at all times and was not allowed to move the ankle before week 9. To ensure adherence to the protocol, a seal was applied around the orthosis via an 8-mm broad cable strip.
At both 4 and 6 weeks after rupture, patients were seen by the project nurse, and 1 wedge was removed at each visit. In the intervention (ECM) group, the training diary was inspected and patients were encouraged to perform their home exercises. In the control (IM) group, the seal of the boot was broken and the foot gently washed before the seal was reapplied. Both groups were instructed not to remove the orthosis at night. After 8 weeks, the orthosis was removed, and walking was allowed in standard running shoes. Crutches were used as needed, and the orthosis was used in situations of high strain or risk during weeks 9 through 12.
Rehabilitation from weeks 9 to 16 was organized as group exercises twice a week at the hospital and was identical for the 2 groups. A standardized rehabilitation program inspired by Willits et al36 and Nilsson-Helander et al24 was used. Please refer to the protocol paper for a full description of the program.5
DVT Prophylaxis
DVT prophylaxis, in the form of rivaroxaban 10 mg once a day for the first 3 weeks, was to be given to participants at high risk of DVT. The high-risk population was defined as people with previous thromboembolic events, first-degree relatives with previous thromboembolic events, active cancer, body mass index greater than 40 kg/m2, or coagulopathy. Because none of the trial participants fulfilled these criteria, no DVT prophylaxis was given.
Intervention
Participants performed ECM of the ankle joint while sitting on the edge of a table with both legs hanging. After allowing the foot to be pulled downward by gravity, the patient actively flexed the foot upward to a horizontal position (Figure 1). This was performed at least 5 times a day in series of 25 repetitions. The treatment protocols for the 2 groups were similar except for the intervention.
Figure 1.
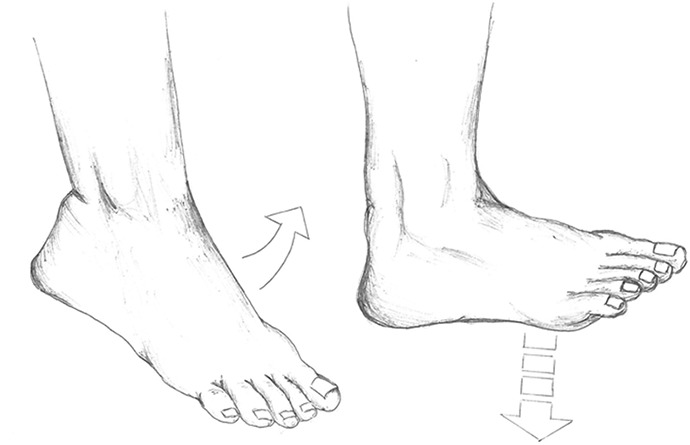
The intervention: early controlled motion of the ankle joint.
Compliance
The patients in the intervention group were equipped with training diaries and were encouraged to record their training for each of the 5 daily training sessions. For the patients in the control group, the orthosis was sealed with an 8-mm broad cable strip that could not be removed without use of industrial tools.
Outcomes
The outcome measure was DVT at 2 or 8 weeks assessed with color Doppler sonography.1 Investigations were performed by use of a GE LOGIQ E9 ultrasound machine with a linear 9-L ultrasound transducer. The color Doppler sonography examination followed a standard procedure covering the external iliac, common, deep and superficial femoral, popliteal, gastrocnemius, paired posterior tibial, and peroneal veins and the proximal part of the anterior tibial and proximal junctions of the greater and lesser saphenous veins.
Vein segments were assessed regarding compressibility and flow. The primary diagnostic criterion was venous incompressibility in the transverse view. Color flow findings were used to clarify the anatomic features and as supportive findings, especially in the venous segments that are less easily viewed and compressed, including the distal femoral vein at the adductor canal and the trifurcation at the proximal calf.
Examinations were performed by 1 of 2 chief radiologists (B.H.O. and P.G.V.) who subspecialized as ultrasound practitioners, both with more than 20 years of experience in venous ultrasonography. At the time of the examination, the 2 radiologists were blinded as to which group each patient was randomized. The interpretation images were recorded, and the radiologists were required to note the presence of proximal DVT (proximal to the popliteal vein) and distal DVT (the popliteal vein or the deep or muscular veins distal to the popliteal vein).
Secondary outcome measures were the Achilles tendon Total Rupture Score (ATRS),16,25 the heel-rise work test,25 elongation of the tendon measured with the Copenhagen Achilles ultrasonographic Length Measurement,8 perimeter of the calf, return to work, and return to sport.
The primary outcome was assessed at 2 and 8 weeks. The secondary outcomes were assessed at 4, 6, and 12 months. The protocol paper provides a full description of the secondary outcomes.5 No changes were made to the outcomes after commencement of the trial and publication of the protocol.
Wells Score
Several structured scoring systems have been developed to predict the risk of DVT; the most well-studied is the Wells score.31,34,35 The simplified clinical model investigates the following signs, symptoms, and risk factors for DVT: active cancer, calf swelling, swollen unilateral superficial veins, unilateral pitting edema, previous documented DVT, swelling of the entire leg, localized tenderness along the deep venous system, being recently bedridden for 3 days or longer, major surgery requiring regional or general anesthetic in the past 12 weeks, and paralysis, paresis, or recent cast immobilization of the lower extremities.35 The patient receives a score from –2 to 9. A score of 3 or higher indicates high probability of DVT (53%; 95% CI, 44%-61%), a score of 1 or 2 indicates moderate probability of DVT (17%; 95% CI, 13%-23%), and a score of 0 or lower indicates low probability of DVT (5.0%; 95% CI, 4%-8%).35
Treatment of DVT
Patients diagnosed with DVT underwent a blood test to investigate D-dimer and plasma electrolytes. All were treated with rivaroxaban 15 mg twice a day for 3 weeks followed by 10 mg once a day for 9 weeks. After 12 weeks, a new blood test for D-dimer was performed. If D-dimer had returned to a normal value (<0.7 FEU/mL), no further follow-up was performed. If plasma levels were above 0.7 mg/L, patients were referred to the coagulation laboratory for further investigation.
Sample Size
Based on the primary outcome of the main study (the ATRS), 130 patients were included.5,6 To estimate the power in the present study, a secondary power calculation was performed before data analysis: 124 patients were required to have a 60% chance of detecting, as significant at the 5% level, a decrease in DVT from 34% in the IM group to 17% in the ECM group.
Randomization
Randomization was computer based following the random allocation rule to ensure balanced group sizes. Patients were allocated 1:1 to either the control or the intervention group. An experienced senior researcher with no other connection to the trial was responsible for generation of the allocation key and a research assistant for creation of numbered, opaque, and sealed envelopes. The allocation key was stored and accessible by only a senior researcher.
Blinding
Patients were enrolled in the study by 1 of 2 designated physiotherapists at the 14-day appointment. After enrollment, the physiotherapist left the room and randomization was performed by the project nurse, who was responsible for treatment in the intervention period. Neither the patients nor the project nurse were blinded to the intervention. The project nurse prepared the patients for color Doppler sonography at the 2- and 8-week assessments. The cable strip was removed, and the patients were instructed not to reveal their allocation. The physician treating the DVTs and the primary investigator (K.W.B.) conducting the data analysis were blinded to the intervention. The allocation key was not revealed until all analyses had been performed.
Statistics
Descriptive statistics were performed regarding demographic parameters. The numbers of patients with DVT in the ECM group and the IM group were compared by use of the Pearson chi-square test. To investigate the sensitivity of D-dimer, the proportion of true-positive cases (patients with elevated D-dimer and DVT) was divided by the number of patients with DVT. To investigate whether DVT influenced treatment outcome after acute Achilles tendon rupture, the population was divided into a group having DVT and a group not having DVT. Between-group differences in functional and patient-reported outcomes were investigated by use of the independent Student t test for normally distributed continuous data. All statistical testing was performed at the 2-sided 5% significance level. Statistical testing was performed using SPSS Statistics (Version 22; IBM).
Results
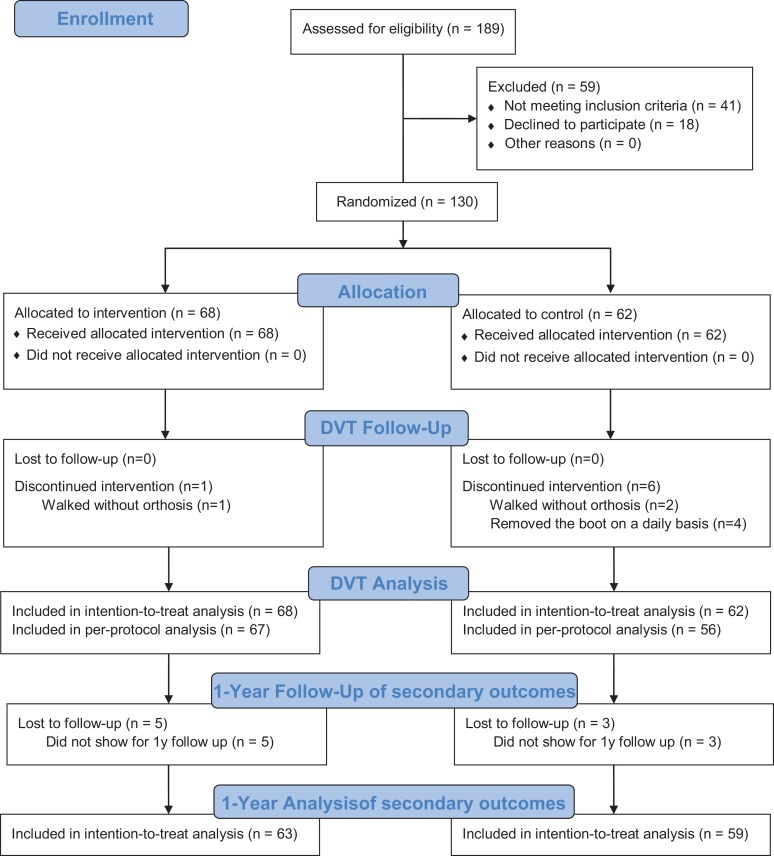
From February 2014 to December 2016, 189 patients were assessed for eligibility (Figure 2). Of these, 130 patients were randomized: 68 were allocated to the intervention (ECM) group and 62 to the control (IM) group. In the intervention group, 67 of 68 patients adhered to the assigned treatment; 1 did not, as he walked without the orthosis. In the control group, 56 of the 62 patients adhered to the assigned treatment; 2 persons walked without the orthosis, and 4 persons removed the cable strip. Baseline data of the population are shown in Table 1. The training diaries were returned by 44 of 68 patients in the intervention group. All returned diaries revealed greater than 80% adherence to the training protocol. No statistically significant differences were found between responders and nonresponders when we analyzed baseline data and outcomes.
Figure 2.
CONSORT (Consolidated Standards of Reporting Trials) flowchart. DVT, deep vein thrombosis.
TABLE 1.
Baseline Dataa
| Intervention and Control Groups | |||
|---|---|---|---|
| Early Controlled Motion | Immobilization | Difference of Means (95% CI) | |
| Age, y | 41.0 ± 9.3 (n = 68) | 42.7 ± 1.7 (n = 62) | –1.7 (–5.3 to 2.0) |
| Weight, kg | 85.5 ± 13.7 (n = 61) | 87.3 ± 14.4 (n = 59) | –1.8 (–6.9 to 3.3) |
| Height, cm | 180.4 ± 7.8 (n = 66) | 178.3 ± 8.0 (n = 61) | 2.1 (–0.6 to 4.9) |
| Female | 12/68 (18) | 11/62 (18) | |
| Diabetes | 2/68 (3) | 0/62 (0) | |
| Elevated blood pressure | 5/68 (7) | 6/62 (10) | |
| Rheumatism | 1/68 (1) | 0/62 (0) | |
| ASA score | 60/68 (87) | 58/62 (94) | |
| Smoker | 18/68 (27) | 15/62 (25) | |
| Patients Who Did and Did Not Develop DVT | |||
| No DVT | DVT | Difference of Means (95% CI) | |
| Age, y | 40.6 ± 10.2 (n = 68) | 43.1 ± 10.8 (n = 62) | –2.5 (–6.1 to 1.2) |
| Weight, kg | 87.1 ± 15.5 (n = 63) | 85.6 ± 12.4 (n = 57) | 1.5 (–3.6 to 6.6) |
| Height, cm | 178.6 ± 7.7 (n = 67) | 180.2 ± 8.2 (n = 60) | –1.5 (–4.3 to 1.3) |
| Female | 14/68 (21) | 9/62 (15) | |
| Diabetes | 0/62 (0) | 2/60 (3) | |
| Elevated blood pressure | 6/68 (9) | 5/62 (8) | |
| Rheumatism | 1/68 (2) | 0/62 (0) | |
| ASA score | 60/68 (87) | 58/62 (94) | |
| Smoker | 19/67 (28) | 14/60 (23) | |
aData are presented as mean ± SD for continuous data and n/N (%) for dichotomous data. Difference of means was tested using the Student independent t test for continuous data and chi-square or Fisher exact test for dichotomous data. ASA, American Society of Anesthesiologists; DVT, deep vein thrombosis.
All patients were available for follow-up at 2 and 8 weeks. At 1 year, 5 patients were lost to follow-up in the ECM group and 3 in the IM group, leaving 63 and 59 patients, respectively (Figure 2). In total, 62 of 130 patients (47.7%) developed DVT, but only 3 patients (3/130; 2.3%) developed above-knee DVT and only 1 patient presented with clinical symptoms of DVT. No patient developed symptomatic pulmonary embolism or other severe complications of DVT. The majority of the below-knee DVTs were situated in the muscle veins. ECM of the ankle did not statistically significantly affect the risk of development of DVT (Table 2).
TABLE 2.
Primary Outcome Analysis for DVTa
| Early Controlled Motion | Immobilization | ||||
|---|---|---|---|---|---|
| n/N | % (range) | n/N | % (range) | P Value | |
| Intention-to-treat analysis | |||||
| DVT at any time | 33/68 | 48.5 (36.7-60.4) | 28/61 | 46.8 (35.7-58.4) | .84 |
| DVT at 2 wk | 23/68 | 33.8 (22.6-45.1) | 23/62 | 37.1 (25.6-48.6) | .7 |
| DVT at 8 wk | 22/58 | 37.9 (26.4-49.5) | 22/61 | 36.1 (24.7-47.5) | .83 |
| Developed DVT from 2 to 8 wk | 10/58 | 17.2 (8.3-26.2) | 7/61 | 11.5 (3.9-19.1) | .37 |
| Above-knee DVT at any time | 1/68 | 1.5 (0-4.3) | 2/62 | 3.2 (0-7.4) | ≥.999 |
| Per-protocol analysis | |||||
| DVT at any time | 33/67 | 49.3 (37.4-61.1) | 26/56 | 46.4 (34.6-58.3) | .76 |
| DVT at 2 wk | 23/67 | 34.3 (23.0-45.6) | 21/56 | 37.5 (26.0-49.0) | .72 |
| DVT at 8 wk | 22/58 | 37.9 (26.4-49.5) | 20/56 | 35.7 (24.3-47.1) | .81 |
| Developed DVT from 2 to 8 wk | 10/58 | 17.2 (8.3-26.2) | 6/56 | 10.7 (3.4-18.1) | .32 |
| Above-knee DVT at any time | 1/67 | 1.5 (0-4.4) | 2/56 | 6.6 (0-8.0) | ≥.999 |
aDVT, deep vein thrombosis. Difference of means was tested using the chi-square or Fisher exact test for dichotomous data.
D-dimer at the time of DVT was available in 52 of the 62 patients diagnosed with DVT. The sensitivity of D-dimer for predicting DVT was 71% (37/52). However, of the 3 patients who had above-knee DVT, only 1 had a D-dimer level above the threshold of 0.7 FEU/mL, implying a sensitivity for diagnosing above-knee DVT of 33% (1/3). Of the 62 patients diagnosed with DVT, 59 had a Wells score of 3 or higher, indicating a high risk of DVT.
Treatment outcome 1 year after rupture was not statistically significantly affected by development of DVT. Neither ATRS, heel-rise work, tendon elongation, or perimeter of the calf showed statistically significant differences at 4, 6, or 12 months after injury (Table 3). Rates of return to work and sport were also comparable between groups, with no statistically significant differences for patients with and without DVT (Table 3). No bleeding incidents were noted in the group treated with rivaroxaban.
TABLE 3.
Secondary Outcome Analysis: Influence of DVT on Patient-Reported and Functional Outcomesa
| No DVT | DVT | Difference of Means (95% CI) | P Value | |
|---|---|---|---|---|
| 4 months | ||||
| ATRS | 56.1 ± 17.7 (n = 63) | 56.5 ± 16.0 (n = 58) | –0.3 (–6.4 to 5.8) | .92 |
| Heel-rise work, LSI % | 16.8 ± 17.3 (n = 61) | 20.1 ± 19.7 (n = 52) | –3.2 (–10.2 to 3.6) | .35 |
| Tendon elongation, mm | 18.9 ± 15.1 (n = 62) | 17.8 ± 16.5 (n = 59) | 1.1 (–4.6 to 6.7) | .71 |
| Perimeter of calf difference, mm | 20.1 ± 12.2 (n = 62) | 20.5 ± 12.8 (n = 58) | –0.4 (–4.9 to 4.1) | .85 |
| 6 months | ||||
| ATRS | 62.9 ± 19.1 (n = 65) | 63.4 ± 19.1 (n = 59) | –0.5 (–7.0 to 6.1) | .89 |
| Heel-rise work, LSI % | 39.4 ± 23.9 (n = 64) | 41.5 ± 26.3 (n = 52) | –2.1 (–11.1 to 6.9) | .64 |
| Tendon elongation, mm | 12.6 ± 12.7 (n = 65) | 15.3 ± 14.4 (n = 58) | –2.8 (–7.6 to 2.1) | .26 |
| Perimeter of calf difference, mm | 18.4 ± 10.5 (n = 63) | 21.1 ± 11.8 (n = 58) | –2.7 (–6.7 to 1.3) | .19 |
| 12 months | ||||
| ATRS | 74.0 ± 18.6 (n = 63) | 74.5 ± 17.8 (n = 59) | –0.6 (–7.1 to 6.0) | .87 |
| Heel-rise work, LSI % | 60.5 ± 21.3 (n = 63) | 57.8 ± 21.1 (n = 59) | 2.7 (–4.9 to 10.3) | .48 |
| Tendon elongation, mm | 15.2 ± 12.7 (n = 65) | 17.3 ± 14.8 (n = 58) | –2.1 (–6.9 to 2.7) | .39 |
| Perimeter of calf difference, mm | 16.4 ± 11.2 (n = 61) | 20.0 ± 12.5 (n = 58) | –3.6 (–7.9 to 7.4) | .1 |
| Return to work, d | 56.9 ± 76.6 (n = 52) | 41.8 ± 60.8 (n = 47) | 15.1 (–12.7 to 42.9) | .28 |
| Return to sport, d | 184.8 ± 88.8 (n = 36) | 192.9 ± 103.1 (n = 46) | –8.2 (–51.2 to 34.8) | .71 |
aData are presented as mean ± SD. Difference of means was tested using the Student independent t test. ATRS, Achilles tendon Total Rupture Score; DVT, deep vein thrombosis; LSI, limb symmetry index.
Discussion
No Benefit of ECM
The most important finding of the study was that ECM exercises, as performed in the present mobilization protocol, revealed no benefit compared with immobilization in reducing the incidence of DVT in the treatment of acute Achilles tendon rupture. This was a rather surprising finding, rejecting the study hypothesis, as early mobilization is considered key in the prevention of DVT.11 However, our finding is in line with a recently published RCT by Aufwerber et al,3 who investigated 150 surgically treated patients. Their intervention took place the first 2 weeks after surgery: The intervention group was encouraged to practice full weightbearing and ankle motion in an orthosis, and the control group was immobilized in a plaster cast. Like us, Aufwerber et al did not find a benefit of ECM.
When interpreting the results of the present study, one should consider the following points: The first is whether the finding is generalizable to early controlled mobilization in general or whether the chosen mobilization protocol was more restrictive than other protocols, thereby rendering this specific protocol inefficient with regard to prevention of DVT. The investigated protocol allowed for only short periods of mobilization during the day, totaling approximately half an hour of mobilization per day. Other mobilization protocols allow for continuous movement of the ankle joint throughout the day by use of hinged orthoses. Also, the investigated protocol allowed only for active dorsiflexion and not for active plantarflexion. However, both active dorsiflexion and plantarflexion have been shown to lead to increased venous return.18 One should be careful to generalize the finding to early mobilization protocols that differ substantially from the one used. The second point is whether a possible effect of mobilization might be masked by other, more important aspects of the treatment protocol. Patients in both groups were allowed early weightbearing. Putting weight on the foot stimulates the venous flow14 and might be more effective in reducing DVT than ECM of the ankle joint in itself. The third point is whether the finding represents a true lack of difference between groups or represents a type II error. A secondary power calculation showed a 40% risk of a type II error when looking for a 50% decrease in DVT rate. Despite the very clear signal found in the present study, the possibility for a type II error must be carefully considered when interpreting the result.
DVT Did Not Affect Treatment Outcome
The second main finding of the study was that DVT had no negative implication for functional and patient-reported outcomes 1 year after rupture. This is in contrast to the study by Arverud et al,2 who found an odds ratio of 0.31 for having a good outcome for patients with DVT. However, in the Arverud et al2 study, the division in the groups with a good and a bad outcome was based on a purpose-made, unvalidated outcome score. None of the validated outcomes show differences between the groups with and without DVT except for heel-rise height. In summary, none of the studies show convincing evidence that DVT influences short-term function of the leg. However, 1 year after DVT, postthrombotic syndrome would not have developed, and hence, negative implications due to postthrombotic syndrome are not accounted for in either study.9
D-Dimer Levels Demonstrated Very Low Sensitivity in Detecting DVT
Guidelines for diagnosis of DVT recommend stratifying patients by risk parameters.10,12 Patients with high risk should undergo color Doppler sonography, whereas patients with low risk can be assessed using D-dimer.10,12 Risk parameters are usually assessed with the Wells score, which is a 3-level scoring of low (≤0 points), moderate (1 or 2 points), or high (≥3 points) probability for DVT. D-dimer testing cannot be used in patients with a high Wells score because a high prevalence of DVT negatively affects the predictive value of D-dimer.10 All patients in the study had moderate to high Wells scores, explaining the low sensitivity of D-dimer in the present population with a prevalence of DVT of 48%. In this population, D-dimer had a sensitivity of 71% for diagnosing DVT, thereby missing 1 in 3 patients. All patients had an estimated Wells score above 1 point, and most of the patients with a color Doppler sonography–verified DVT had an estimated Wells score of 3 points or higher.
Our finding suggests that in patients with acute Achilles tendon rupture, D-dimer testing cannot be used to rule out DVT, as their overall high risk of DVT reduces the negative predictive value of D-dimer. Our data suggest that Wells score and color Doppler sonography are more sensitive and accurate tools for the detection of DVT after acute Achilles tendon rupture, regardless of treatment protocol.
Clinical Relevance of Isolated Distal DVTs
In the present study, 59 of 62 DVTs detected were isolated distal to the knee, all except 1 were asymptomatic, and none entailed pulmonary embolism or other severe complications due to the DVT. This finding raises the question of whether the isolated distal DVTs have clinical relevance in patients with acute Achilles tendon rupture or are casual findings with no clinical relevance.
Isolated distal DVT is a variant of DVT that accounts for 23% to 59% of all DVTs.28 Despite the frequent manifestation of isolated distal DVT, the treatment is diverse with no standard of care.27,30 Some institutions consider distal DVTs to be without clinical relevance and thus limit color Doppler sonography to the proximal veins without extension to the distal veins, a practice that might lead to the underdiagnosis of isolated distal DVT.30 Accordingly, some national clinical guidelines do not recommend treatment for isolated distal DVT because anticoagulants have been shown to increase the risk of bleeding and isolated distal DVTs are thought to be less likely to cause postthrombotic syndrome and embolize to the lungs in comparison with proximal DVTs.30,31 However, the international consensus statement on prevention and treatment of venous thromboembolism indicates that oral anticoagulants should be given to all patients with symptomatic isolated distal DVT for 3 months.23 It remains unknown whether isolated distal DVTs are of clinical relevance, especially if asymptomatic.
Limitations
The study was limited by the outcome being a secondary outcome to a main study. Also, the nature of the intervention is a limiting factor, as blinding of the patients was impossible. The patients could have crossed over between the intervention and control group, but we believe that we controlled for this by using training diaries in the intervention group and sealing the boot in the control group.
Conclusion
The study hypotheses were rejected, as the ECM protocol revealed no benefit versus immobilization in reducing the incidence of DVT. Furthermore, DVT did not seem to negatively affect outcome at 1 year after rupture. ECM of the ankle 5 times a day from weeks 3 to 8 after rupture does not seem to be the key in reducing the risk of DVT. The large difference in incidence of isolated distal DVTs and proximal DVTs raises the question of whether below-knee DVTs are of clinical relevance.
Acknowledgment
The authors thank heads of department Peter Gebuhr, Thue Ørsnes, and Jette Christensen for creating a prosperous research environment and providing full support for the trial. Furthermore, we thank physiotherapists Julie Jenlar, Thomas Budolfsen, and Anne Charlotte Anker-Petersen for inclusion and treatment of patients.
Footnotes
Final revision submitted December 28, 2019; accepted January 21, 2020.
One or more of the authors has declared the following potential conflict of interest or source of funding: Research support for this study was received from DJO Nordic (to K.W.B.) and from Gigtforeningen (the Danish Rheumatism Association). AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from the Regional Science Ethics Committee for the capital region of Denmark (protocol No. H-4-2013-176).
References
- 1. Andrews EJJ, Fleischer AC. Sonography for deep venous thrombosis: current and future applications. Ultrasound Q. 2005;21(4):213–225. [DOI] [PubMed] [Google Scholar]
- 2. Arverud ED, Anundsson P, Hardell E, et al. Ageing, deep vein thrombosis and male gender predict poor outcome after acute Achilles tendon rupture. Bone Joint J. 2016;98-B(12):1635–1641. [DOI] [PubMed] [Google Scholar]
- 3. Aufwerber S, Heijne A, Edman G, Grävare Silbernagel K, Ackermann PW. Early mobilization does not reduce the risk of deep venous thrombosis after Achilles tendon rupture: a randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2020;28(1):312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barfod KW, Bencke J, Lauridsen HB, Ban I, Ebskov L, Troelsen A. Nonoperative dynamic treatment of acute Achilles tendon rupture: the influence of early weight-bearing on clinical outcome: a blinded, randomized controlled trial. J Bone Joint Surg Am. 2014;96(18):1497–1503. [DOI] [PubMed] [Google Scholar]
- 5. Barfod KW, Hansen MS, Holmich P, Troelsen A, Kristensen MT. Efficacy of early controlled motion of the ankle compared with no motion after non-operative treatment of an acute Achilles tendon rupture: study protocol for a randomized controlled trial. Trials. 2016;17(1):564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barfod KW, Hansen MS, Hölmich P, Kristensen MT, Troelsen A. Efficacy of early controlled motion of the ankle compared with immobilisation in non-operative treatment of patients with an acute Achilles tendon rupture: an assessor-blinded, randomised controlled trial [published online October 9, 2019]. Br J Sports Med. doi:10.1136/bjsports-2019-100709 [DOI] [PubMed] [Google Scholar]
- 7. Barfod KW, Nielsen F, Helander KN, et al. Treatment of acute Achilles tendon rupture in Scandinavia does not adhere to evidence-based guidelines: a cross-sectional questionnaire-based study of 138 departments. J Foot Ankle Surg. 2013;52(5):629–633. [DOI] [PubMed] [Google Scholar]
- 8. Barfod KW, Riecke AF, Boesen A, et al. Validation of a novel ultrasound measurement of Achilles tendon length. Knee Surg Sports Traumatol Arthrosc. 2015;23(11):3398–3406. [DOI] [PubMed] [Google Scholar]
- 9. Barnes GD. Predicting the post-thrombotic syndrome: not quite ready for prime time. Thromb Haemost. 2018;118(8):1345–1346. [DOI] [PubMed] [Google Scholar]
- 10. Bates SM, Jaeschke R, Stevens SM, et al. Diagnosis of DVT. Chest. 2012;141(2):e351S–e418S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Calder JDF, Freeman R, Domeij-Arverud E, van Dijk CN, Ackermann PW. Meta-analysis and suggested guidelines for prevention of venous thromboembolism (VTE) in foot and ankle surgery. Knee Surg Sports Traumatol Arthrosc. 2016;24(4):1409–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cassese S, Byrne RA, Schulz S, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35(43):3033–3073. [DOI] [PubMed] [Google Scholar]
- 13. Chan AW, Tetzlaff JM, Gøtzsche PC, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346:e7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Domeij-Arverud E, Labruto F, Latifi A, Nilsson G, Edman G, Ackermann PW. Intermittent pneumatic compression reduces the risk of deep vein thrombosis during postoperative lower limb immobilisation: a prospective randomised trial of acute ruptures of the Achilles tendon. Bone Joint J. 2015;97-B(5):675–680. [DOI] [PubMed] [Google Scholar]
- 15. El-Akkawi AI, Joanroy R, Barfod KW, Kallemose T, Kristensen SS, Viberg B. Effect of early versus late weightbearing in conservatively treated acute Achilles tendon rupture: a meta-analysis. J Foot Ankle Surg. 2018;57(2):346–352. [DOI] [PubMed] [Google Scholar]
- 16. Ganestam A, Barfod K, Klit J, Troelsen A. Validity and reliability of the Achilles tendon total rupture score. J Foot Ankle Surg. 2013;52(6):736–739. [DOI] [PubMed] [Google Scholar]
- 17. Ganestam A, Kallemose T, Troelsen A, Barfod KW. Increasing incidence of acute Achilles tendon rupture and a noticeable decline in surgical treatment from 1994 to 2013: a nationwide registry study of 33,160 patients. Knee Surg Sports Traumatol Arthrosc. 2016;24:3730–3737. [DOI] [PubMed] [Google Scholar]
- 18. Hickey BA, Morgan A, Pugh N, Perera A. The effect of lower limb cast immobilization on calf muscle pump function: a simple strategy of exercises can maintain flow. Foot Ankle Int. 2014;35(5):429–433. [DOI] [PubMed] [Google Scholar]
- 19. Huttunen TT, Kannus P, Rolf C, Felländer-Tsai L, Mattila VM. Acute Achilles tendon ruptures: incidence of injury and surgery in Sweden between 2001 and 2012. Am J Sports Med. 2014;42(10):2419–2423. [DOI] [PubMed] [Google Scholar]
- 20. Makhdom AM, Cota A, Saran N, Chaytor R. Incidence of symptomatic deep venous thrombosis after Achilles tendon rupture. J Foot Ankle Surg. 2013;52(5):584–587. [DOI] [PubMed] [Google Scholar]
- 21. Mark-Christensen T, Troelsen A, Kallemose T, Barfod KW. Functional rehabilitation of patients with acute Achilles tendon rupture: a meta-analysis of current evidence. Knee Surg Sports Traumatol Arthrosc. 2016;24(6):1852–1859. [DOI] [PubMed] [Google Scholar]
- 22. Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Int J Surg. 2012;10(1):28–55. [DOI] [PubMed] [Google Scholar]
- 23. Nicolaides A, Fareed J, Kakkar AK, et al. Prevention and treatment of venous thromboembolism: international consensus statement (guidelines according to scientific evidence). Clin Appl Thromb. 2013;19(2):116–118. [DOI] [PubMed] [Google Scholar]
- 24. Nilsson-Helander K, Silbernagel KG, Thomeé R, et al. Acute Achilles tendon rupture: a randomized, controlled study comparing surgical and nonsurgical treatments using validated outcome measures. Am J Sports Med. 2010;38(11):2186–2193. [DOI] [PubMed] [Google Scholar]
- 25. Nilsson-Helander K, Thomeé R, Silbernagel KG, et al. The Achilles tendon Total Rupture Score (ATRS): development and validation. Am J Sports Med. 2007;35(3):421–426. [DOI] [PubMed] [Google Scholar]
- 26. Nilsson-Helander K, Thurin A, Karlsson J, Eriksson BI. High incidence of deep venous thrombosis after Achilles tendon rupture: a prospective study. Knee Surg Sports Traumatol Arthrosc. 2009;17(10):1234–1238. [DOI] [PubMed] [Google Scholar]
- 27. Palareti G. How I treat isolated distal deep vein thrombosis (IDDVT). Blood. 2014;123(12):1802–1809. [DOI] [PubMed] [Google Scholar]
- 28. Palareti G, Schellong S. Isolated distal deep vein thrombosis: what we know and what we are doing. J Thromb Haemost. 2012;10(1):11–19. [DOI] [PubMed] [Google Scholar]
- 29. Patel A, Ogawa B, Charlton T, Thordarson D. Incidence of deep vein thrombosis and pulmonary embolism after Achilles tendon rupture. Clin Orthop Relat Res. 2012;470(1):270–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Righini M, Galanaud JP, Guenneguez H, et al. Anticoagulant therapy for symptomatic calf deep vein thrombosis (CACTUS): a randomised, double-blind, placebo-controlled trial. Lancet Haematol. 2016;3(12):e556–e562. [DOI] [PubMed] [Google Scholar]
- 31. Royal College of Physicians, National Clinical Guideline Centre (UK). Venous thromboembolic diseases: the management of venous thromboembolic diseases and the role of thrombophilia testing. http://www.ncbi.nlm.nih.gov/pubmed/23638495. Published June 2012. [PubMed]
- 32. Soroceanu A, Sidhwa F, Aarabi S, Kaufman A, Glazebrook M. Surgical versus nonsurgical treatment. J Bone Joint Surg Am. 2012;94(23):2136–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Valkering KP, Aufwerber S, Ranuccio F, Lunini E, Edman G, Ackermann PW. Functional weight-bearing mobilization after Achilles tendon rupture enhances early healing response: a single-blinded randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2017;25(6):1807–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wells P, Hirsh J, Anderson D, et al. Accuracy of clinical assessment of deep-vein thrombosis. Lancet. 1995;345(8961):1326–1330. [DOI] [PubMed] [Google Scholar]
- 35. Wells PS, Owen C, Doucette S, Wells PS, Owen C. Does this patient have deep vein thrombosis? Clinical scenario. JAMA. 2008;295(2):199–207. [DOI] [PubMed] [Google Scholar]
- 36. Willits K, Amendola A, Bryant D, et al. Operative versus nonoperative treatment of acute Achilles tendon ruptures: a multicenter randomized trial using accelerated functional rehabilitation. J Bone Joint Surg Am. 2010;92(17):2767–2775. [DOI] [PubMed] [Google Scholar]
- 37. Wu Y, Lin L, Li H, et al. Is surgical intervention more effective than non-surgical treatment for acute Achilles tendon rupture? A systematic review of overlapping meta-analyses. Int J Surg. 2016;36(2016):305–311. [DOI] [PubMed] [Google Scholar]