Abstract
With the advancement of computed tomography pulmonary angiography, differentiating between acute and chronic thrombus in pulmonary embolism has become more feasible. However, whether pulmonary embolism with chronic thrombus contributes to a higher mortality than pulmonary embolism with acute thrombus remains undetermined. Additionally, the clinical features of patients with chronic thrombus are largely unknown. Herein, we aimed to investigate the incidence and outcomes of patients with pulmonary embolism and chronic thrombus. This retrospective study included patients with pulmonary embolism from 2008 to 2016 at National Cheng Kung University Hospital. After excluding patients with tumor emboli or other etiologies and a lack of computed tomography images, we identified 205 patients with acute thrombus and 58 patients with chronic thrombus. Patients with chronic thrombus initially presented mainly with dyspnea, and the etiology was not related to recent surgery. Patients with chronic thrombus had a significantly higher incidence of elevated right ventricular systolic pressure detected by echocardiography and a higher incidence of subsequent events due to residual pulmonary embolism. Despite no differences in clinically recurrent pulmonary embolism, patients with chronic thrombus presented with a higher risk of all-cause and pulmonary embolism-related mortality than patients with acute thrombus. Chronic thrombus (hazard ratio: 2.03, p = 0.03), simplified pulmonary embolism severity index, anticoagulant use, and body mass index were the independent factors for all-cause mortality. Our findings suggest that using computed tomography pulmonary angiography for identifying patients with pulmonary embolism and chronic thrombus, which was associated with a higher risk of mortality, is pivotal for early intervention in addition to anticoagulant use.
Keywords: chronic pulmonary thromboembolism, chronic pulmonary embolism (PE), computed tomography (CT)
Introduction
Chronic thromboembolic pulmonary hypertension (CTEPH) is believed to arise from multiple endothelialized pulmonary emboli that do not resolve, and leads to pulmonary hypertension (PH) due to the mechanical obstruction of thrombi undergoing fibrotic transformation in the pulmonary artery.1 The incidence of CTEPH after acute pulmonary embolism (PE) has been reported to be between 0.1% and 9.1%.1–3 With the vigorous development of treatment for CTEPH, including riociguat4 and balloon pulmonary angioplasty (BPA),5 the prognosis of patients has gradually improved.
Furthermore, patients with chronic thromboembolic disease (CTED) with no definite PH show significant improvements in functional status and quality of life after pulmonary endarterectomy.6 Therefore, it may be accepted that BPA is beneficial in patients with CTED. CTED has also been increasingly recognized as a potential cause of symptoms and exercise limitations in patients with previous PE. However, the incidence of CTED and the outcome of patients with CTED are unclear.
Computed tomography (CT) pulmonary angiography has become the imaging method of choice for suspected acute PE.7 Among all imaging modalities, CT pulmonary angiography plays a key role in acute and chronic PE.8–10 However, the features of CTED or CTEPH are subtle and often difficult to diagnose by CT. Most CTEPH studies are from the perspective of PH, which is defined as a mean pulmonary artery pressure greater than 25 mmHg according to right heart catheterization, but articles from the perspective of chronic PE or CTED are lacking. The epidemiology and presentation of patients with chronic thrombus are also unclear. Therefore, we conducted this observational study to investigate the incidence and outcome of patients with PE and chronic thrombus with CT.
Materials and methods
Study design
This retrospective study included patients with PE according to the International classification of disease, ninth revision between January 2008 and March 2016 at the National Cheng Kung University Hospital in Tainan. This study adhered to the Declaration of Helsinki and received approval. We excluded patients with tumor emboli, tumor invasion or encasement, septic emboli, fat emboli, or stump thrombosis as well as patients without CT imaging or with no evidence of emboli according to CT imaging. Clinical comorbidity and blood sample data were obtained by careful review of each patient's medical records. The initial presentation was mainly based on the chief complaint of the patients in the admission note, and the events of PE during hospitalization were carefully judged by specialists according to the medical record. If the presentation of PE could be explained by other major diseases, then it was determined to be an incidental finding. The use of anticoagulations was defined as the patient receiving anticoagulants within three months after a PE event. The simplified Pulmonary Embolism Severity Index (PESI), which is a prognostic assessment of patients with PE, was also recorded, and the items of the simplified PESI included the following: age >80 years, cancer, chronic heart failure or chronic pulmonary disease, pulse rate >110 bpm, systolic blood pressure <100 mmHg, and arterial oxyhemoglobin saturation <90%.11,12
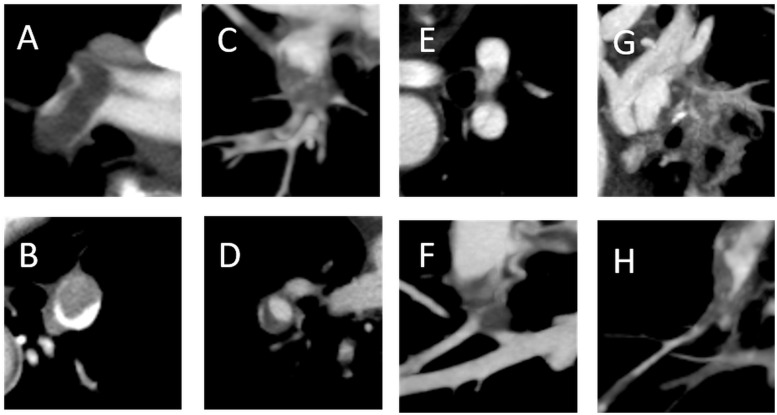
To distinguish between acute and chronic thrombus, we evaluated the most central and largest emboli in the bilateral pulmonary arteries. Well-defined central filling defects and eccentric filling defects with acute angles within the vessel walls were defined as acute thrombus; pouch defects, filling defects with obtuse angles within the vessel walls, band lesions, web lesions, and abrupt vessel narrowing with irregular intima were defined as chronic thrombus (Fig. 1 and Table 1).8,9,13–15 All images were manually reviewed by two blinded specialists on our PE team. If there was a discrepancy in interpretation, further discussions were held in an unblinded fashion until consensus was obtained.
Fig. 1.
Acute thrombus (a and b) and chronic thrombus (c–h) according to CT. (a) Well-defined central filling defect; (b) eccentric filling defect with acute angles within the vessel wall; (c) pouch defect; (d) filling defect with obtuse angle within the vessel wall (crescent shape); (e) band lesion; (f and g) web lesion with calcification; and (h) abrupt vessel narrowing with irregular intima.
Table 1.
Vascular and thrombus features of acute and chronic pulmonary embolism.
| Vascular and thrombus features | Key image findings | References | |
|---|---|---|---|
| Acute | Complete obstruction with expansive vessel | Expansion of diameter of involved vessel distal to point of obstruction | 8, 15 |
| Central filling defect | Well-defined central thrombus; “polo mint” or “railroad track” sign (Fig. 1a) | 8, 9 | |
| Eccentric filling defect with acute angles | Form acute angles (<90°) with arterial walls (Fig. 1b) | 8, 9, 15 | |
| Chronic | Complete obstruction with contractionary thrombus | Abrupt decrease in arterial diameter and absence of distal opacification | 8, 13, 15 |
| Eccentric filling defect with obtuse angles | Form obtuse angles (>90°) with arterial walls; pouch defect (Fig. 1c); crescent shape on axial image (Fig. 1d) | 8, 13–15 | |
| Band | Linear structure; slit appearance (Fig. 1e) | 8, 9, 13–15 | |
| Web | Multiple branching bands; laminated or recanalized thrombus (Fig. 1f and g) | 9, 13–15 | |
| Abrupt vessel narrowing with irregular intima | Abrupt convergence of contrast material leading to thin column of intravascular contrast material; intima irregularities and post stenotic dilatation | 8, 9, 13–15 | |
| Calcification | High attenuation foci within filling defects | 13–15 |
According to the European Society of Cardiology guidelines of PE,7 patients with shock or hypotension were defined as having high clinical risk; patients with signs of right ventricle (RV) dysfunction or enlargement were defined as having intermediate clinical risk; and others were defined as having low clinical risk. RV dysfunction or enlargement was defined as the following: a bedside echocardiography present in the electronic records; a formal echocardiography report within one week of the PE event; and a ratio of the RV:left ventricle greater than 1.1:1 in CT.9 PH was defined according to echocardiography as a peak tricuspid regurgitation velocity greater than 2.9 m/s.16
Endpoints
Because most of the patients did not receive a right heart catheter exam, which is a standard exam for PH, we used a peak tricuspid regurgitation velocity greater than 2.9 m/s according to echocardiography more than three months after the PE event as the endpoint. In patients with other etiologies of PH, including left ventricular dysfunction, etc., the assessment of PH was not included unless PH was significantly increased after the PE event. All chest CTs performed for any reason after the PE event were carefully reviewed for residual thromboembolism to confirm the resolution of CTED. Recurrent PE, death from PE or RV failure, and death due to other causes were also reviewed from the electronic medical records until May 2019.
Statistical analysis
All statistical analyses were performed with SPSS software version 21.0 (IBM, Armonk, NY, USA). Continuous data are presented as the mean ± standard deviation or as the median (interquartile range), depending on the normality of the distribution. Dichotomous data are presented as numbers and percentages. Comparisons were conducted using either Student's t-test or the Mann–Whitney U test for continuous variables with normal or nonparametric distributions, respectively. The chi-square test or Fisher's exact test was used for categorical variables as appropriate. The Kaplan–Meier method was used with the log-rank test to compare the survival rates between strata. Univariate Cox regression analysis was performed to evaluate factors associated with all-cause mortality. Factors with p < 0.1 in the univariate analysis were used in the multivariable Cox regression analysis to investigate risk factors for all-cause mortality. In the Cox regression model, cancer and heart rate were not included because they were items from the simplified PESI.
Results
Baseline characteristics and clinical presentations of the enrolled patients
This study retrospectively screened a total of 326 patients with PE, and a total of 263 patients (65 ± 15 years old, 40% male) were included in the final analysis. Fifty-eight patients (22%) had chronic thrombus. The only difference in the baseline characteristics between patients with acute thrombus and those with chronic thrombus was that chronic thrombus was not caused by recent surgery (Table 2). The initial presentation of patients with acute thrombus was more diverse than that of patients with chronic thrombus, but the symptoms in patients with chronic thrombus were mainly dyspnea or progressed dyspnea for months (Table 3).
Table 2.
Baseline characteristics of patients with different types of thrombus according to CT.
| Acute thrombus | Chronic thrombus | ||
|---|---|---|---|
| (n = 205) | (n = 58) | p Values | |
| Age (years old) | 65 ± 15 | 66 ± 17 | 0.83 |
| Sex (Male) | 121 (59.0%) | 36 (62.1%) | 0.68 |
| BMI (kg/m2) | 25.2 ± 5.1 | 24.4 ± 4.1 | 0.38 |
| Clinical risk: high | 12 (5.9%) | 3 (5.2%) | 0.37 |
| Intermediate | 69 (33.7%) | 25 (43.1%) | |
| Low | 124 (60.5%) | 30 (51.7%) | |
| Etiology: cancer | 82 (40.0%) | 19 (32.8%) | 0.32 |
| APS and autoimmune disease | 8 (3.9%) | 2 (3.4%) | 1 |
| Protein C or S deficiency | 2 (1.0%) | 0 (0%) | NA |
| Recent surgery | 21 (10.2%) | 0 (0%) | NA |
| Simplified PESI | 1.1 ± 0.9 | 1.1 ± 0.8 | 0.81 |
| Deep vein thrombosisa | 46/61 | 13/18 | 0.79 |
| Treatment: thrombolysis | 12 (5.9%) | 2 (3.4%) | 0.74 |
| Heparin or LMWH | 81 (39.5%) | 20 (34.5%) | 0.52 |
| Anticoagulant: warfarin | 132 (64.4%) | 34 (58.6%) | 0.174 |
| NOAC | 18 (8.8%) | 3 (5.2%) | |
| Platelet (103/µL) | 225 ± 118 | 230 ± 101 | 0.81 |
Data are presented as the mean ± SD or n (%).
There were many missing data, and they are presented as positive number/total number.
APS: antiphospholipid syndrome; BMI: body mass index; LMWH: low molecular weight heparin; NOAC: novel oral anticoagulant; PESI: pulmonary embolism severity index.
Table 3.
Initial presentation of patients with different types of thrombus according to CT.
| Acute thrombus | Chronic thrombus | ||
|---|---|---|---|
| (n = 205) | (n = 58) | p Values | |
| Dyspnea | 85 (41.5%) | 26 (44.8%) | <0.01 |
| Progressed dyspnea for months | 0 (0%) | 13 (22%) | |
| Syncope | 15 (7.3%) | 1 (1.7%) | |
| Sudden collapse | 4 (2.0%) | 1 (1.7%) | |
| Hypoxia | 18 (8.8%) | 1 (1.7%) | |
| Chest pain | 20 (9.8%) | 2 (3.4%) | |
| Leg swelling | 14 (6.8%) | 4 (6.8%) | |
| Hemoptysis | 4 (2.0%) | 0 (0%) | |
| Incidental finding | 45 (22.4%) | 10 (17%) |
Data are presented as n (%).
Increasing PH and residual thromboembolism in patients with chronic thrombus
Only 125 patients (48%) received echocardiography to evaluate RV function and PH within one week of PE, and only 122 patients (46%) received echocardiography to detect the development of PH several months after PE. The patients with chronic thrombus had a significantly higher incidence of PH than the patients with acute thrombus (73% vs. 5%, p < 0.01, Table 4). While the incidence of patients with CTED, which was defined as residual PE according to CT more than three months after the initial scan, was high (38% in acute thrombus; 89% in chronic thrombus, p < 0.01, Table 4), only 2–4% of all patients had any history of recurrent PE following the original scan.
Table 4.
Outcome of patients with different types of thrombus according to CT.
| Acute thrombus | Chronic thrombus | ||
|---|---|---|---|
| (n = 205) | (n = 58) | p Values | |
| Echocardiography within one week | 105 | 20 | |
| Velocity of TR >2.9 (m/s) | 40 (20%) | 15 (75%) | <0.01 |
| Echocardiography >3 months later | 100 | 22 | |
| Velocity of TR >2.9 (m/s) | 5 (5%) | 16 (73%) | <0.01 |
| Chest CT >3 months later | 80 | 19 | |
| Residual PE | 30 (38%) | 17 (89%) | <0.01 |
| Recurrent PE | 8 (4%) | 1 (2%) | 0.69 |
| All-cause mortality | 75 (37%) | 32 (55%) | 0.01 |
| Death due to PE or RV failure | 5 (2%) | 5 (9%) | 0.03 |
| Death due to other etiology | 70 (34%) | 27 (47%) | 0.03 |
CT: computed tomography; PE: pulmonary embolism; RV: right ventricle; TR: tricuspid regurgitation.
Increasing all-cause and PE-related mortality in patients with chronic thrombus
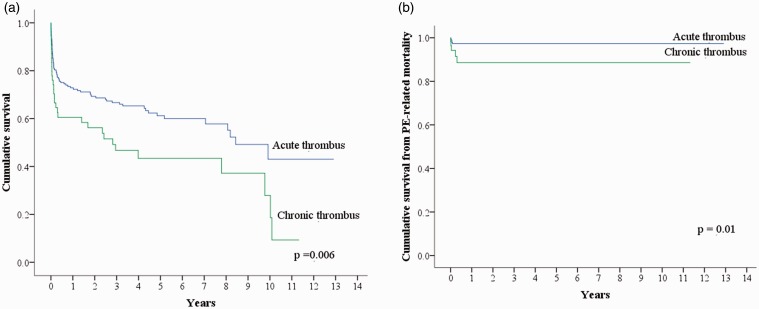
During follow up, 107 (41%) patients died (Supplemental table 1). Fig. 2 shows that the patients with chronic thrombus had higher rates of all-cause mortality (p = 0.006) and death due to PE (p = 0.01) than the patients with acute thrombus. Most of the deaths due to PE occurred within one month. According to multivariable analysis, chronic thrombus, a higher simplified PESI score, a lower body mass index (BMI), and a lack of anticoagulant use were the independent factors for all-cause mortality (Table 5).
Fig. 2.
Kaplan–Meier curve of cumulative survival. (a) Compared with patients with acute thrombus, patients with chronic thrombus had a higher risk of all-cause mortality (p = 0.006). (b) Compared with patients with acute thrombus, patients with chronic thrombus had a higher risk of death due to PE (p = 0.01).
Table 5.
Multivariable Cox regression model to identify factors associated with all-cause mortality.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Variables | Hazard ratio (95% CI) | p Values | Hazard ratio (95% CI) | p Values |
| Chronic thrombus | 1.78 (1.18–2.70) | <0.01 | 2.03 (1.07–3.87) | 0.03 |
| Simplified PESI | 2.21 (1.76–2.78) | <0.01 | 2.07 (1.52–2.80) | <0.01 |
| Anticoagulant use | 0.24 (0.16–0.36) | <0.01 | 0.23 (0.12–0.43) | <0.01 |
| BMI (kg/m2) | 0.85 (0.79–0.90) | <0.01 | 0.89 (0.83–0.96) | <0.01 |
| Clinical risk | 0.97 (0.70–1.35) | 0.86 | NA | NA |
Note: Backward LR method.
BMI: body mass index; PESI: pulmonary embolism severity index.
Outcome of the patients with different clinical risks of PE
Table 6 shows that 10% of the patients with intermediate clinical risk and PE with acute thrombus and 93% of the patients with intermediate clinical risk and PE with chronic thrombus developed PH. In total, 42% of the patients with high clinical risk and PE with acute thrombus and 100% of the patients with high clinical risk and PE with chronic thrombus died from PE or RV failure. All patients with intermediate clinical risk and PE with chronic thrombus developed CTEPH. Interestingly, two patients with low clinical risk and PE with chronic thrombus had no residual thromboemboli after anticoagulant therapy.
Table 6.
Outcome of patients with different risks of PE.
| Acute thrombus |
Chronic thrombus |
|||||
|---|---|---|---|---|---|---|
| Low | Intermediate | High | Low | Intermediate | High | |
| (n = 124) | (n = 69) | (n = 12) | (n = 30) | (n = 25) | (n = 3) | |
| Echocardiography within one week | 63 | 38 | 4 | 5 | 15 | 0 |
| Velocity of TR >2.9 (m/s) | 14 (22%) | 25 (66%) | 1 (25%) | 0 (0%) | 15 (100%) | 0 (0%) |
| Echocardiography >3 months later | 57 | 39 | 4 | 7 | 15 | 0 |
| Velocity of TR >2.9 (m/s) | 1 (2%) | 4 (10%) | 0 (0%) | 2 (29%) | 14 (93%) | 0 (0%) |
| Chest CT >3 months later | 46 | 33 | 1 | 7 | 11 | 1 |
| Residual PE | 16 (35%) | 14 (42%) | 0 (0%) | 5 (71%) | 11 (100%) | 1 (100%) |
| Recurrent PE | 4 (3.2%) | 4 (5.8%) | 0 (0%) | 1 (3%) | 0 (0%) | 0 (0%) |
| Death due to PE or RV failure | 0 (0%) | 0 (0%) | 5 (42%) | 0 (0%) | 2 (8%) | 3 (100%) |
| Death due to other etiology | 49 (40%) | 21 (30%) | 0 (0%) | 16 (53%) | 11 (44%) | 0 (0%) |
CT: computed tomography; PE: pulmonary embolism; RV: right ventricle; TR: tricuspid regurgitation.
Discussion
Our data demonstrated that the patients with PE and chronic or acute CT-defined thrombus had different initial presentation and predisposing factors. Patients with chronic thrombus had a higher risk of all-cause mortality and PE-related mortality and a higher incidence of CTEPH or CTED development than patients with acute thrombus.
With the development of treatment for CTEPH, the treatment methods for acute PE and chronic PE are beginning to show some differences. It is important to distinguish between acute and chronic PE early, and many studies on this issue have been published.8,10,14,15 Many vascular features of chronic PE have been shown to be identifiable by pulmonary angiography,8 intravascular ultrasound, intravascular optical coherence tomography,5 and even histological changes.17 This observational study used CT features to distinguish between acute and chronic thrombus8 and demonstrated different etiologies and outcomes in patients with acute CT-defined thrombus vs. those with chronic CT-defined thrombus. First, most patients with chronic thrombus initially presented with dyspnea but not chest pain, hypoxia, or syncope, and this presentation is consistent with the pathogenesis because the disease process is one of chronic fibrotic vascular remodeling.1 Second, the etiologies of the patients with chronic thrombus included cancer and coagulopathy, but recent surgery was not a cause. Third, our data also demonstrated that patients with chronic thrombus had a poorer outcome than patients with acute thrombus. More importantly, 42% of the patients with high clinical risk and acute thrombus died from PE or RV failure, but the patients with high clinical risk and chronic thrombus died within one month. In a limited number of studies,18 rescue BPA has been shown to be effective in patients with acute on chronic PE that was initially diagnosed and treated as acute PE. The results emphasize the importance of distinguishing between acute and chronic thrombus and the need to further develop treatments such as BPA or to utilize a PE response team.19
Other independent factors for all-cause mortality were simplified PESI scores, BMI values, and the presence of anticoagulant use. The simplified PESI has good prognostic accuracy and clinical utility for quantifying 30-day prognosis in patients with PE.11,12 According to our data, the simplified PESI was also associated with long-term mortality, and we supposed that the most important correlation was with cancer. On the other hand, anticoagulant use is the standard treatment for patients with PE, but only 71% of the patients received anticoagulant treatment according to our data. This finding reflects real-world past conditions, as anticoagulants were not used previously because of the bleeding tendency and comorbidities such as cancer. With the popularity of novel oral anticoagulants, which are more convenient and cause less bleeding,20–23 the wider use of anticoagulants in patients with PE is necessary.
During follow-up, 10% of the intermediate clinical risk patients with acute thrombus and only 2% of the low clinical risk patients with acute thrombus developed PH. In previous publications,1–3 the incidence of PH after acute PE had a wide range, ranging from 0.4% to 9.1%. We had published that the presence of right ventricular dysfunction in patients with PE is associated with the development of CTEPH.24 Additionally, in our data, 10% of intermediate clinical risk patients with acute thrombus developed PH, and only one patient with low clinical risk with acute thrombus developed PH. We hypothesized that the wide range of the incidence of CTEPH in previous studies is mainly due to the different ratio of the clinical risk of patients. This finding may indicate that we should more widely screen the development of PH in patients with at least an intermediate risk. However, 93% of the intermediate clinical risk patients with chronic thrombus developed PH even under anticoagulant therapy. This result showed that anticoagulant use was not sufficient in patients with chronic thrombus, and other therapies, including BPA or riociguat, should be considered earlier. In contrast, only 22 patients (38%) with chronic thrombus received echocardiography to assess PH. Considering the high incidence of CTEPH in patients with chronic thrombus, more wide screening of echocardiography is recommended.
In our data, 38% of patients with acute thrombus developed CTED, which was defined as residual PE according to CT more than three months later. This incidence is higher than we expected, and the finding indicates that CTED has been previously underdiagnosed. Among these patients, only 37% underwent a CT examination, and most of the CT examinations were arranged for other purposes. Considering the cost of CT and the use of contrast, we need a more convenient method to follow these patients. In addition, there are many unknown factors in patients with CTED, and further studies are needed.
This study had several limitations. First, it was a retrospective observational study, and it may have allowed some selection bias. For example, the initial presentation cannot be recorded in more detail since it was based on the medical record. Second, approximately half of the patients did not receive CT or echocardiography follow-up. These missing data points may have affected the results. Third, we only evaluated the most central and largest emboli and could not detect acute or chronic PE, which is the most complex condition. Fourth, only approximately 70% of the patients received anticoagulants, which may have led to the worsening of outcomes. Finally, most of the patients died from cancer or during hospice care, reducing the event rate during long-term follow-up, especially in terms of the rate of death from PE or RV failure.
In conclusion, our findings suggest that using CT pulmonary angiography to identify patients with chronic PE, which is associated with a higher risk of mortality, is pivotal for early intervention in addition to anticoagulant use.
Supplemental Material
Supplemental material, sj-pdf-1-pul-10.1177_2045894020905510 for Pulmonary thromboembolism with computed tomography defined chronic thrombus is associated with higher mortality by Hsien-Yuan Chang, Wei-Ting Chang, Po-Wei Chen, Chih-Chan Lin and Chih-Hsin Hsu in Pulmonary Circulation
Ethical approval
This study adhered to the Declaration of Helsinki and received approval from the Human Research and Ethics Committee of National Cheng Kung University Hospital (IRB number: B‐ER‐105‐136).
Author contributions
Conception and design: Chih-Hsin Hsu, Hsien-Yuan Chang, and Chih-Chan Lin.
Analysis and interpretation of data: Hsien-Yuan Chang and Wei-Ting Chang.
Drafting of the manuscript: Hsien-Yuan Chang.
Revising it critically for important intellectual content: Chih-Hsin Hsu, Wei-Ting Chang, and Po-Wei Chen.
Final approval of the manuscript submitted: Chih-Hsin Hsu, Hsien-Yuan Chang, and Chih-Chan Lin.
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Supplemental Material
Supplemental material for this article is available online.
References
- 1.Lang IM, Madani M. Update on chronic thromboembolic pulmonary hypertension. Circulation 2014; 130: 508–518. [DOI] [PubMed] [Google Scholar]
- 2.Ende-Verhaar YM, Cannegieter SC, Vonk Noordegraaf A, et al. Incidence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism: a contemporary view of the published literature. Eur Respir J 2017; 49: 1601792. [DOI] [PubMed] [Google Scholar]
- 3.Guerin L, Couturaud F, Parent F, et al. Prevalence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism. Prevalence of CTEPH after pulmonary embolism. Thromb Haemost 2014; 112: 598–605. [DOI] [PubMed] [Google Scholar]
- 4.Van Thor MCJ, Ten Klooster L, Snijder RJ, et al. Long-term clinical value and outcome of riociguat in chronic thromboembolic pulmonary hypertension. Int J Cardiol Heart Vasc 2019; 22: 163–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lang I, Meyer BC, Ogo T, et al. Balloon pulmonary angioplasty in chronic thromboembolic pulmonary hypertension. Eur Respir Rev 2017; 26: 160119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taboada D, Pepke-Zaba J, Jenkins DP, et al. Outcome of pulmonary endarterectomy in symptomatic chronic thromboembolic disease. Eur Respir J 2014; 44: 1635–1645. [DOI] [PubMed] [Google Scholar]
- 7.Konstantinides SV, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014; 35: 3033–3069. 3069a–3069k. [DOI] [PubMed] [Google Scholar]
- 8.Wittram C, Kalra MK, Maher MM, et al. Acute and chronic pulmonary emboli: angiography-CT correlation. AJR Am J Roentgenol 2006; 186: S421–S429. [DOI] [PubMed] [Google Scholar]
- 9.Ruggiero A, Screaton NJ. Imaging of acute and chronic thromboembolic disease: state of the art. Clin Radiol 2017; 72: 375–388. [DOI] [PubMed] [Google Scholar]
- 10.Kim SS, Hur J, Kim YJ, et al. Dual-energy CT for differentiating acute and chronic pulmonary thromboembolism: an initial experience. Int J Cardiovas Imaging 2014; 30 Suppl 2: 113–120. [DOI] [PubMed] [Google Scholar]
- 11.Jimenez D, Aujesky D, Moores L, et al. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 2010; 170: 1383–1389. [DOI] [PubMed] [Google Scholar]
- 12.Righini M, Roy PM, Meyer G, et al. The Simplified Pulmonary Embolism Severity Index (PESI): validation of a clinical prognostic model for pulmonary embolism. J Thromb Haemost 2011; 9: 2115–2117. [DOI] [PubMed] [Google Scholar]
- 13.Nishiyama KH, Saboo SS, Tanabe Y, et al. Chronic pulmonary embolism: diagnosis. Cardiovasc Diagn Ther 2018; 8: 253–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dogan H, de Roos A, Geleijins J, et al. The role of computed tomography in the diagnosis of acute and chronic pulmonary embolism. Diagn Interv Radiol 2015; 21: 307–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pena E, Dennie C. Acute and chronic pulmonary embolism: an in-depth review for radiologists through the use of frequently asked questions. Semin Ultrasound CT MR 2012; 33: 500–521. [DOI] [PubMed] [Google Scholar]
- 16.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37: 67–119. [DOI] [PubMed] [Google Scholar]
- 17.Kitani M, Ogawa A, Sarashina T, et al. Histological changes of pulmonary arteries treated by balloon pulmonary angioplasty in a patient with chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv 2014; 7: 857–859. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura M, Sunagawa O, Tsuchiya H, et al. Rescue balloon pulmonary angioplasty under veno-arterial extracorporeal membrane oxygenation in a patient with acute exacerbation of chronic thromboembolic pulmonary hypertension. Int Heart J 2015; 56: 116–120. [DOI] [PubMed] [Google Scholar]
- 19.Witkin AS. Acute and chronic pulmonary embolism: the role of the pulmonary embolism response team. Curr Opin Cardiol 2017; 32: 672–678. [DOI] [PubMed] [Google Scholar]
- 20.Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med 2013; 369: 799–808. [DOI] [PubMed] [Google Scholar]
- 21.Hokusai VTEI, Buller HR, Decousus H, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med 2013; 369: 1406–1415. [DOI] [PubMed] [Google Scholar]
- 22.EINSTEIN-PE Investigators; Büller HR, Prins MH, Lensin AWA, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 2012; 366: 1287–1297. [DOI] [PubMed]
- 23.Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009; 361: 2342–2352. [DOI] [PubMed] [Google Scholar]
- 24.Hsu CH, Lin CC, Li WT, et al. Right ventricular dysfunction is associated with the development of chronic thromboembolic pulmonary hypertension but not with mortality post-acute pulmonary embolism. Medicine 2019; 98: e17953. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-pul-10.1177_2045894020905510 for Pulmonary thromboembolism with computed tomography defined chronic thrombus is associated with higher mortality by Hsien-Yuan Chang, Wei-Ting Chang, Po-Wei Chen, Chih-Chan Lin and Chih-Hsin Hsu in Pulmonary Circulation