Abstract
Background:
Bone marrow contusions are common after an acute anterior cruciate ligament (ACL) injury. It is unknown whether the severity of this initial bruise can predict the potential of developing chondral changes even after ACL reconstructive surgery (ACLR).
Purpose:
To investigate whether the initial bone bruise area could be predictive of progressive chondral defects.
Study Design:
Cohort study; Level of evidence, 3.
Methods:
A retrospective chart review was performed to capture patients with an acute ACL injury with pre- and post-ACLR magnetic resonance imaging (MRI) between January 2000 and December 2017. Lesion areas were measured on initial MRI, and chondral wear was graded on final imaging by use of the modified Outerbridge classification. An ordinal model was created to determine whether the initial area was a significant predictor for future chondral degeneration.
Results:
A total of 40 patients with a mean age of 34.5 ± 12.6 years were included for analysis. All patients underwent ACLR at a mean 139 ± 64 days from initial injury. A lateral tibial and femoral bone bruise was most commonly present in patients (77.5% and 62.5%, respectively). A medial femoral bone bruise was found in only 12.5% (5/40) of patients. The initial contusion area significantly correlated with increasing chondral wear over time in the tibia and lateral femoral condyle (P < .001). Patients with a bone bruise encompassing 100% of the lateral femoral compartment on MRI had a 74% chance of having grade 3 or 4 chondral changes at 5 years (P = .001). Absence of a bone bruise on initial MRI was the greatest predictor of no cartilage wear at 5 years in all compartments (P < .001). The presence of a concomitant lateral meniscal injury increased the risk of developing type 3 or 4 chondral wear in the lateral tibial plateau (P = .012) but did not pose increased risk of femoral wear (P = .23).
Conclusion:
A significant relationship between area of initial bone bruise at the time of injury and progressive posttraumatic chondral disease was found in the tibial and lateral femoral compartments.
Keywords: ACL, bone bruise, MRI, osteoarthritis, chondral wear, Outerbridge
Acute tears of the anterior cruciate ligament (ACL) most commonly occur due to noncontact twisting injuries, resulting in a traumatic pivot-shift mechanism within the knee joint.8,10 This high impact between the articular surfaces of the femur and tibia can create subchondral edema, or “bone bruising,” demonstrated on magnetic resonance imaging (MRI) in more than 80% of acute ACL injuries.4,18,19,27 Although the presence of a bone bruise has been associated with concomitant articular and meniscal injuries, its relationship to the development of osteoarthritis is poorly understood.5,6,10
Although surgical reconstruction of the ACL is able to restore mechanical stability, progression of chondral degeneration and osteoarthritis continues to be significant.1,15 The rate of progression to osteoarthritis after an isolated ACL tear has been reported to range from 0% to 13% and can be as high as 48% when combined with meniscal injury at 10 years.15 Inability of surgical reconstruction to reduce this unwanted sequela may be secondary to the initial trauma sustained at the time of injury.7,11 In a histologic evaluation of bone bruises in humans, Johnson et al11 found that extensive damage was sustained with these contusions, involving large areas of chondrocyte degeneration in the overlying articular cartilage and osteocyte necrosis in the subchondral bone. These changes suggest not just localized chondral injury but also disruption of the entire process of articular cartilage homeostasis.
First described by Mink and Deutsch,14 bone bruises can be measured on MRI by various methods.10,22 The bruise typically presents with a high, intense signal on T2-weighted imaging in the area representing the mechanism of the ACL rupture.2 Potter et al17 tracked cartilage loss compared with the size of the initial bone bruise and found no significant correlation after 3 years of injury. However, their study focused on only the lateral compartment and not the medial side, which may be affected in higher energy mechanisms. This report was later supported by van Meer et al,24 who found that a bone bruise in the medial tibiofemoral compartment was a significant risk factor for degenerative changes as early as 2-year follow-up.
Although the severity of the bone bruise has been associated with concomitant injuries during an acute ACL tear, the relationship of bone bruise to progression of osteoarthritis is not fully understood. The purpose of this study was to retrospectively measure the area of the initial bone bruise on MRI in patients with acute ACL ruptures and assess its relationship to progression of chondral degeneration on repeat MRI. We hypothesized that an increasing bone bruise area would be a predictor for greater chondral degeneration at long-term follow-up.
Methods
Data Collection
A retrospective chart review was performed to capture all patients with an acute ACL tear who underwent MRI within 6 weeks of injury and then a repeat MRI at subsequent follow-up. The entire study period included was between January 2000 and December 2017. Institutional review board approval was obtained before the start of the study (IRB No. 19-084-2). Inclusion criteria included any patient who was diagnosed with an acute ACL tear and underwent MRI within 6 weeks of injury. Patients were excluded if they had previous surgery at the index knee, chronic tears, multiligamentous injuries or fractures, or previous infection or osteomyelitis or if MRI was obtained more than 6 weeks after injury. Nonoperative treatment was not an exclusion criterion. Patients were obtained from the practice of 4 sports fellowship–trained orthopaedic surgeons at a single institution. We searched for electronic medical records with the diagnosis of an ACL tear by using an International Classification of Diseases, Ninth Revision code (844.2) in addition to MRI Current Procedural Terminology codes (73721, 73222, 73723) to find patients with at least 2 MRI scans of the same knee.
Using the abovementioned criteria, we initially obtained 83 patients. After a thorough chart review, we excluded 29 patients who had chronic tears, 10 patients who did not have initial MRI scans available or whose scan was obtained more than 6 weeks after injury, and 4 patients who did not have ACL injuries. This left 40 patients (17 males, 23 females) with a mean ± SD age of 34.5 ± 12.6 years for study inclusion.
Bone Bruise Area
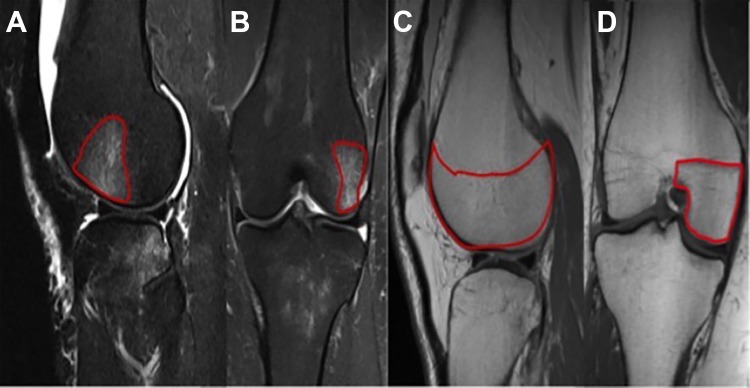
All MRI procedures were performed by use of a 1.5-T magnet machine with standard knee sequences.10 MRI scans were independently reviewed on a dedicated workstation by a senior radiology resident (E.G.) and a musculoskeletal fellowship–trained radiologist (R.P.) using a standardized method for bone bruise measurements. When reviewing study participants, both reviewers were blinded to clinical and intraoperative findings as well as to the patients’ initial imaging. All images were analyzed by use of PACS Intellispace (Philips Corp) software. The initial (injury) MRI scans were evaluated to measure each individual compartment. For the femur, the medial and lateral compartments were divided by a line down the center of the intercondylar fossa on the coronal view. Compartments were defined proximally by the physeal scar and distally to the subchondral surface (Figure 1). Similarly, the medial and lateral tibial spines were used to delineate their respective compartments on the coronal plane. Tibial compartments were confined distally from the tibial tubercle and proximally to the articular tibial plateau. The area of the bone bruise was measured in the compartments through use of sagittal and coronal slices with the highest area of bone bruise on T2-weighted, fat-suppressed slices and then multiplied. Bone bruises were reported as a percentage of the area of the individual compartment (Figure 1). Chondral changes were then evaluated on terminal MRI through use of the modified Outerbridge classification (Table 1).12 This classification was applied to each compartment on initial and then final MRI scans on a fat-suppressed proton density sequence.
Figure 1.
Marked area (red outline) of the bone bruise on (A) sagittal and (B) coronal T2-weighted MRI. The bone bruise area was measured in relation to the total area (the above example measures 45%) of the compartment as measured on (C) sagittal and (D) coronal T1-weighted slices.
Table 1.
Modified Outerbridge Classification
| Grade | Magnetic Resonance Imaging Characteristics |
|---|---|
| 0 | Normal articular cartilage |
| 1 | Focal areas of hyperintensity within the normal contour |
| 2 | Fissure or blister-like swelling of articular cartilage extending to the surface |
| 3 | Focal ulcerations of the articular cartilage |
| 4 | Full-thickness cartilage loss with underlying bone reactive changes |
Statistical Analysis
Descriptive statistics were calculated as mean or frequency and proportion where appropriate. An ordinal logistic regression model was used to predict chondral changes over time based on initial bone bruise size. P values < .05 were considered statistically significant. Odds ratios were used to compute the relative odds of chondral damage based on initial bone bruise area. A subgroup analysis was then repeated with patients who had meniscal injuries, comparing those patients who had meniscal injuries and those without. All statistical analysis was performed using Stata 12 (StataCorp LP).
Results
Patients
All 40 patients underwent arthroscopic ACL reconstruction at mean 139 ± 64 days after injury with either bone–patellar tendon–bone or hamstring autograft fixation. Mean time from initial to final MRI was 5.1 years (range, 0.7-10.4 years). No patient had documented visible chondral injury during diagnostic arthroscopy at the time of ACL reconstruction. We noted that 3 patients underwent concomitant high tibial osteotomy and 9 patients had additional meniscal (4 medial, 5 lateral) repairs or debridement at the time of ACL reconstruction. Osteotomies were performed at the discretion of the surgeon to correct varus alignment. Reasons for repeat MRIs included new pain (n = 16), stiffness (n = 7), concern for ACL retear (n = 7), and concern for new meniscal injury (n = 10). No patient had additional surgeries between the index reconstruction and time of repeat MRI. With the exception of 3 instances, patients with a medial or lateral meniscal injury had a concomitant bone bruise in that compartment (85%). Although the presence of a bruise was associated with a meniscal injury, no correlation was found between the size of the tibial bone bruise and the presence of additional injuries in each tibial compartment (P > .05, respectively). At the time of repeat MRI, 8 patients demonstrated increased laxity compared with what was seen immediately following surgery. All patients were found to have 1+ Lachman grade and negative pivot shift immediately after surgery. Of the 8 patients with increased laxity, 6 patients had 2+ Lachman grade with a negative pivot shift, whereas 2 patients were graded as a 3+ Lachman and positive pivot shift. Return to sport was recorded in only 18 patients, all of whom returned to sport by 1.5 years. We found that 2 patients had repeat rupture of the ACL on follow-up MRI, whereas 3 other patients had a meniscal tear.
Bone Bruise Measurement
A lateral tibial bone bruise was present in 77.5% (31/40) of patients, whereas a lateral femoral bone bruise was present in 62.5% (25/40). Mean area of the bone bruise was 75% ± 37% for the lateral tibia compared with 54% ± 29% for the lateral femur. A medial tibial bone bruise was found in roughly half of the patients (19/40), encompassing on average 80% ± 39% of its area. We noted that 12 patients had bone bruises measuring more than 100% of the area. This occurred when the edema extended more proximally into the metaphysis. Only 12.5% (5/40) patients were found to have a medial femoral bone bruise with a mean area of encompassing 9% ± 6.6% of the medial femur.
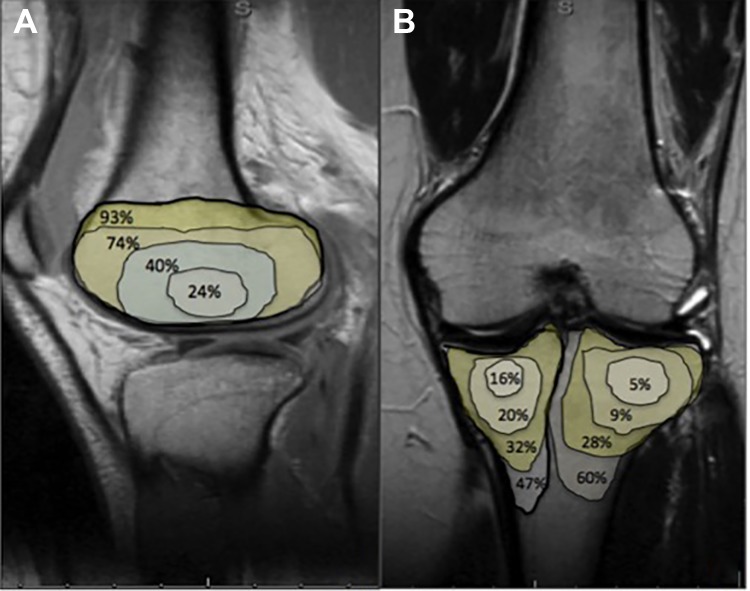
The area of initial bone bruise and Outerbridge classification at follow-up were used to determine whether a predictive model could be created. The low incidence of bruising in the medial femoral compartment limited creation of a model in that compartment. Patients with a bone bruise encompassing more than 100% of the lateral femoral compartment had a 74% chance of having grade 3 or 4 chondral changes on the lateral femur at 5 years (P = .001). Absence of a bone bruise in the lateral femur or in both tibial compartments was the most protective factor for not having chondral changes in these compartments (P > .001) (Tables 2 -4). Having no bone bruise in the medial tibial compartment was the most predictive factor for not developing chondral changes at the medial tibia over time (85%; P > .001). Figure 2 demonstrates the risk of developing grade 3 or 4 chondral changes at 5 years based on the incremental size of the bone bruise in the tibial and lateral femoral compartment. A predictive model was not used for the medial femoral compartment given the low incidence. When adjusting for time from initial injury, we found that increased laxity at follow-up did not significantly change the chance of having osteoarthritic changes (P = .42).
Table 2.
Lateral Tibial Bone Bruise Area Predictive Values for Chondral Wear at the Lateral Tibia
| Area of Bone Bruise, % | Outerbridge Grade at 5 Years | Probability, % | 95% CI | P Value |
|---|---|---|---|---|
| 0 | 0 | 62 | 41 to 83 | <.001 |
| 1 or 2 | 26 | 11 to 41 | .001 | |
| 3 or 4 | 12 | 0 to 23 | .05 | |
| 50 | 0 | 47 | 31 to 62 | <.001 |
| 1 or 2 | 33 | 17 to 48 | <.001 | |
| 3 or 4 | 20 | 8 to 33 | .001 | |
| 100 | 0 | 32 | 12 to 52 | .002 |
| 1 or 2 | 36 | 19 to 52 | <.001 | |
| 3 or 4 | 32 | 13 to 52 | .001 | |
| 150 | 0 | 20 | –4 to 44 | .10 |
| 1 or 2 | 33 | 15 to 51 | <.001 | |
| 3 or 4 | 47 | 13 to 81 | .006 |
Table 3.
Lateral Femoral Bone Bruise Area Predictive Values for Chondral Wear at the Lateral Femur
| Area of Bone Bruise, % | Outerbridge Grade at 5 Years | Probability, % | 95% CI | P Value |
|---|---|---|---|---|
| 0 | 0 | 56 | 37 to 75 | <.001 |
| 1 or 2 | 31 | 16 to 46 | <.001 | |
| 3 or 4 | 13 | 2 to 25 | .02 | |
| 50 | 0 | 23 | 4 to 42 | .01 |
| 1 or 2 | 37 | 21 to 54 | <.001 | |
| 3 or 4 | 40 | 15 to 65 | .002 | |
| 100 | 0 | 6 | –8 to 20 | .39 |
| 1 or 2 | 20 | –11 to 50 | .20 | |
| 3 or 4 | 74 | 31 to 118 | .001 | |
| 150 | 0 | 1 | –4 to 7 | .61 |
| 1 or 2 | 6 | –14 to 26 | .56 | |
| 3 or 4 | 93 | 68 to 100 | <.001 |
Table 4.
Medial Tibial Bone Bruise Area Predictive Values for Chondral Wear at the Medial Tibia
| Area of Bone Bruise, % | Outerbridge Grade at 5 Years | Probability, % | 95% CI | P Value |
|---|---|---|---|---|
| 0 | 0 | 85 | 71 to 99 | <.001 |
| 1 or 2 | 12 | 1 to 24 | .03 | |
| 3 or 4 | 2 | –1 to 6 | .19 | |
| 50 | 0 | 59 | 41 to 78 | <.001 |
| 1 or 2 | 32 | 14 to 49 | <.001 | |
| 3 or 4 | 9 | 0 to 18 | .05 | |
| 100 | 0 | 43 | 22 to 64 | <.001 |
| 1 or 2 | 41 | 20 to 62 | <.001 | |
| 3 or 4 | 16 | 2 to 30 | .02 | |
| 150 | 0 | 9 | –5 to 23 | .21 |
| 1 or 2 | 31 | 4 to 59 | .02 | |
| 3 or 4 | 60 | 23 to 96 | .001 |
Figure 2.
Prediction of developing grade 3 or 4 chondral changes at 5 years with increasing (25%, 50%, 100%, 150%) bone bruise area on the (A) lateral femur and (B) tibia.
Meniscal Injury
A subgroup analysis was performed to compare the risk of developing grade 3 or 4 chondral degeneration for patients with a concomitant meniscal tear that required either fixation or debridement compared with patients who did not have meniscal injury. Although the size of the bone bruise alone was a significant predictor of chondral damage, when analyzing the lateral tibial compartment, we found that a concomitant lateral meniscal tear significantly increased the patients’ chances of developing grade 3 or 4 changes (P = .012), whereas there was no significant difference for the lateral femoral cartilage (P = .23). Similarly, having a medial meniscal tear was not found to increase risk of degenerative changes over time compared with patients who did not have a tear in either the medial femoral (P = .19) or the tibial (P = .22) cartilage.
Discussion
The most important finding of this study was that the area of initial bone bruise in the tibial and lateral femoral compartments was a significant predictor of chondral changes on MRI at 5 years. The low incidence of bone bruising in the medial femoral compartment did not allow for a predictive model to reach significance. Having a concomitant meniscal tear in the lateral side did increase a patient’s risk for further damage; however, having a bone bruise alone was still an independent risk factor for chondral degeneration. This study demonstrates the potential damage that may occur from the initial impact from injury during an ACL tear.
Similar to previous studies, our study showed that bone bruises had the highest prevalence in the lateral tibial condyl and lateral femoral condyle after an acute ACL injury.2,22 The clinical relevance of these bone contusions has varied in the literature.9,20 Hanypsiak et al9 reported on clinical and radiographic outcomes at 12-year follow-up after ACL reconstruction. At final follow-up, the investigators found no significant difference in outcome scores in patients who presented with or without a bone bruise. Although those investigators found articular lesions and meniscal injuries present in compartments with a bone bruise, further correlation could not be established because the contusion was not quantified. Van Ginckel et al23 performed a systematic review assessing cartilage adaptations on MRI scans after an acute ACL injury. They found macroscopic cartilage changes at 2 years, showing that damage correlated with bone marrow lesions and time from injury. However, the severity of the lesion was calculated in only 4 of the 12 studies examined by Van Ginckel et al.23
The predictive model in the current study used MRI scans from various time points in order to predict chondral changes over time. The absence of a visible bone bruise on the initial MRI scan demonstrated that a patient had a significant chance of not having chondral changes at a mean of 5 years. Theologis et al22 quantified bone marrow lesions using a volume computational algorithm that differentiated between the bruised and normal marrow. Those authors found increased chondral signaling in both tibial compartments and the medial femoral condyle despite near resolution of the surrounding marrow at only 1 year. Recent technological advances, including the use of 3.0-T magnets and T2-weighted time quantification, may provide an improved means for earlier identification of chondral changes.
More recent research has focused on the use of ultrashort echo time T2-weighted mapping MRI, which can track articular cartilage matrix degeneration.25 Using this technology, Williams et al25 found evidence of changes to the deep articular cartilage matrix structure at only 2 years compared with non–ACL injured knees. Despite several methods for quantifying marrow lesions, a direct relationship to their progression to osteoarthritis has not been fully established.
The association of bone marrow lesions progressing to osteoarthritis has been described in the atraumatic, degenerative condition.21,26 Although the underlying cause of the bone marrow lesion may be different, the relationship to the subsequent progression of osteoarthritis may be similar. The presence of a bone marrow lesion in healthy, asymptomatic middle-aged men was a significant risk factor for cartilage degeneration after only 2 years.26 Tanamas et al21 measured bone marrow lesions in patients with atraumatic knee osteoarthritis and found that severity of the lesion was positively associated with the risk of knee joint replacement at 4 years. Furthermore, even in lesions that improved, regression was not shown to indicate improvement of osteoarthritis.3
Limitations
The current study has several limitations. First, this was a radiographic study without correlation with clinical and functional outcomes.20 Second, although both reviewers had complete agreement, intrarater reliability was not determined, and such information may have altered whether they were asked to repeat area measurements and Outerbridge classification. Third, the retrospective nature of the study and concomitant injuries that can occur with an ACL tear may be a confounding variable. For instance, meniscal injuries sustained at the time of injury may have played a greater role in the development as it has been shown to be a risk factor for the progression of osteoarthritis in ACL tears.13 Fourth, not all patients were standardized to the same treatment, type of reconstruction, or timing of surgery. Pinczewski et al16 found that patients with patellar tendon reconstruction had increased radiographic evidence of osteoarthritic changes at 10-year follow-up compared with patients who underwent hamstring tendon reconstruction. Fifth, the patients included in the study were those who had repeat MRI for various reasons, including pain, stiffness, concern for ACL retear, or a new meniscal injury. Although the results may be influenced by selection bias, our study examines chondral changes in patients shown by repeat MRI ranging from 1 to 9 years postoperatively, which is limited in the literature.2,20,22
Conclusion
In our study cohort, a significant relationship between severity of initial bone bruise at the time of injury and chance of later chondral degeneration was found in the tibial and lateral femoral compartments. The findings support that the natural course of the knee after an acute ACL injury may be partially dictated by the initial trauma.
Footnotes
Final revision submitted February 23, 2020; accepted March 10, 2020.
One or more of the authors has declared the following potential conflict of interest or source of funding: R.A.A. has received educational funding from Arthrex and Don-Joy; consulting fees from Biorez, Biomet, and DePuy; and has stock options in Biorez. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from UConn Health Institutional Review Board (ref No. 012553).
References
- 1. Chalmers PN, Mall NA, Moric M, et al. Does ACL reconstruction alter natural history? A systematic literature review of long-term outcomes. J Bone Joint Surg Am. 2014;96(4):292–300. [DOI] [PubMed] [Google Scholar]
- 2. Costa-Paz M, Muscolo DL, Ayerza M, Makino A, Aponte-Tinao L. Magnetic resonance imaging follow-up study of bone bruises associated with anterior cruciate ligament ruptures. Arthroscopy. 2001;17(5):445–449. [DOI] [PubMed] [Google Scholar]
- 3. Driban JB, Price LL, Lo GH, et al. Evaluation of bone marrow lesion volume as a knee osteoarthritis biomarker—longitudinal relationships with pain and structural changes: data from the osteoarthritis initiative. Arthritis Res Ther. 2013;15(5):R112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dunn WR, Spindler KP, Amendola A, et al. Which preoperative factors, including bone bruise, are associated with knee pain/symptoms at index anterior cruciate ligament reconstruction (ACLR)? A Multicenter Orthopaedic Outcomes Network (MOON) ACLR cohort study. Am J Sports Med. 2010;38(9):1778–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Faber KJ, Dill JR, Amendola A, Thain L, Spouge A, Fowler PJ. Occult osteochondral lesions after anterior cruciate ligament rupture. Am J Sports Med. 1999;27(4):489–494. [DOI] [PubMed] [Google Scholar]
- 6. Fang C, Johnson D, Leslie MP, Carlson CS, Robbins M, Cesare PED. Tissue distribution and measurement of cartilage oligomeric matrix protein in patients with magnetic resonance imaging-detected bone bruises after acute anterior cruciate ligament tears. J Orthop Res. 2001;19(4):634–641. [DOI] [PubMed] [Google Scholar]
- 7. Frobell RB. Change in cartilage thickness, posttraumatic bone marrow lesions, and joint fluid volumes after acute ACL disruption: a two-year prospective MRI study of sixty-one subjects. J Bone Joint Surg Am. 2011;93(12):1096–1103. [DOI] [PubMed] [Google Scholar]
- 8. Griffin LY, Agel J, Albohm MJ, et al. Noncontact anterior cruciate ligament injuries: risk factors and prevention strategies. J Am Acad Orthop Surg. 2000;8(3):141–150. [DOI] [PubMed] [Google Scholar]
- 9. Hanypsiak BT, Spindler KP, Rothrock CR, et al. Twelve-year follow-up on anterior cruciate ligament reconstruction: long-term outcomes of prospectively studied osseous and articular injuries. Am J Sports Med. 2008;36(4):671–677. [DOI] [PubMed] [Google Scholar]
- 10. Illingworth KD, Hensler D, Casagranda B, Borrero C, van Eck CF, Fu FH. Relationship between bone bruise volume and the presence of meniscal tears in acute anterior cruciate ligament rupture. Knee Surg Sports Traumatol Arthrosc. 2014;22(9):2181–2186. [DOI] [PubMed] [Google Scholar]
- 11. Johnson DL, Urban WP, Caborn DN, Vanarthos WJ, Carlson CS. Articular cartilage changes seen with magnetic resonance imaging-detected bone bruises associated with acute anterior cruciate ligament rupture. Am J Sports Med. 1998;26(3):409–414. [DOI] [PubMed] [Google Scholar]
- 12. Jungius K-P, Schmid MR, Zanetti M, Hodler J, Koch P, Pfirrmann CW. Cartilaginous defects of the femorotibial joint: accuracy of coronal short inversion time inversion-recovery MR sequence. Radiology. 2006;240(2):482–488. [DOI] [PubMed] [Google Scholar]
- 13. Keays SL, Newcombe PA, Bullock-Saxton JE, Bullock MI, Keays AC. Factors involved in the development of osteoarthritis after anterior cruciate ligament surgery. Am J Sports Med. 2010;38(3):455–463. [DOI] [PubMed] [Google Scholar]
- 14. Mink JH, Deutsch AL. Magnetic resonance imaging of the knee. Clin Orthop Relat Res. 1989;244:29–47. [PubMed] [Google Scholar]
- 15. Øiestad BE, Engebretsen L, Storheim K, Risberg MA. Knee osteoarthritis after anterior cruciate ligament injury: a systematic review. Am J Sports Med. 2009;37(7):1434–1443. [DOI] [PubMed] [Google Scholar]
- 16. Pinczewski LA, Lyman J, Salmon LJ, Russell VJ, Roe J, Linklater J. A 10-year comparison of anterior cruciate ligament reconstructions with hamstring tendon and patellar tendon autograft: a controlled, prospective trial. Am J Sports Med. 2007;35(4):564–574. [DOI] [PubMed] [Google Scholar]
- 17. Potter HG, Jain SK, Ma Y, Black BR, Fung S, Lyman S. Cartilage injury after acute, isolated anterior cruciate ligament tear: immediate and longitudinal effect with clinical/MRI follow-up. Am J Sports Med. 2012;40(2):276–285. [DOI] [PubMed] [Google Scholar]
- 18. Speer KP, Spritzer CE, Bassett FH III, Feagin JA, Jr, Garrett WE., Jr Osseous injury associated with acute tears of the anterior cruciate ligament. Am J Sports Med. 1992;20(4):382–389. [DOI] [PubMed] [Google Scholar]
- 19. Speer KP, Warren RF, Wickiewicz TL, Horowitz L, Henderson L. Observations on the injury mechanism of anterior cruciate ligament tears in skiers. Am J Sports Med. 1995;23(1):77–81. [DOI] [PubMed] [Google Scholar]
- 20. Szkopek K, Warming T, Neergaard K, Jørgensen H, Christensen H, Krogsgaard M. Pain and knee function in relation to degree of bone bruise after acute anterior cruciate ligament rupture. Scand J Med Sci Sports. 2012;22(5):635–642. [DOI] [PubMed] [Google Scholar]
- 21. Tanamas SK, Wluka AE, Pelletier J-P, et al. Bone marrow lesions in people with knee osteoarthritis predict progression of disease and joint replacement: a longitudinal study. Rheumatology. 2010;49(12):2413–2419. [DOI] [PubMed] [Google Scholar]
- 22. Theologis AA, Kuo D, Cheng J, et al. Evaluation of bone bruises and associated cartilage in anterior cruciate ligament–injured and –reconstructed knees using quantitative T1ρ magnetic resonance imaging: 1-year cohort study. Arthroscopy. 2011;27(1):65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Van Ginckel A, Verdonk P, Witvrouw E. Cartilage adaptation after anterior cruciate ligament injury and reconstruction: implications for clinical management and research? A systematic review of longitudinal MRI studies. Osteoarthritis Cartilage. 2013;21(8):1009–1024. [DOI] [PubMed] [Google Scholar]
- 24. van Meer BL, Oei EH, Meuffels DE, et al. Degenerative changes in the knee 2 years after anterior cruciate ligament rupture and related risk factors: a prospective observational follow-up study. Am J Sports Med. 2016;44(6):1524–1533. [DOI] [PubMed] [Google Scholar]
- 25. Williams AA, Titchenal MR, Do BH, Guha A, Chu CR. MRI UTE-T2* shows high incidence of cartilage subsurface matrix changes 2 years after ACL reconstruction. J Orthop Res. 2019;37(2):370–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wluka AE, Wang Y, Davies-Tuck M, English DR, Giles GG, Cicuttini FM. Bone marrow lesions predict progression of cartilage defects and loss of cartilage volume in healthy middle-aged adults without knee pain over 2 yrs. Rheumatology. 2008;47(9):1392–1396. [DOI] [PubMed] [Google Scholar]
- 27. Yoon KH, Yoo JH, Kim K-I. Bone contusion and associated meniscal and medial collateral ligament injury in patients with anterior cruciate ligament rupture. J Bone Joint Surg Am. 2011;93(16):1510–1518. [DOI] [PubMed] [Google Scholar]