Abstract
The involvement of serotonin in responses to negative feedback is well established. Acute serotonin reuptake inhibition has enhanced sensitivity to negative feedback (SNF), modelled by behaviour in probabilistic reversal learning (PRL) paradigms. Whilst experiments employing acute tryptophan depletion (ATD) in humans, to reduce serotonin synthesis, have shown no clear effect on SNF, sample sizes have been small. We studied a large sample of healthy volunteers, male and female, and found ATD had no effect on core behavioural measures in PRL. These results indicate that ATD effects can differ from other manipulations of serotonin expected to have a parallel or opposing action.
Keywords: Serotonin, tryptophan depletion, probabilistic reversal learning
Introduction
Serotonin is critically involved in processing aversive outcomes (Bari et al., 2010; Chamberlain et al., 2006; Rygula et al., 2015; Skandali et al., 2018) and adapting behaviour as circumstances change (Park et al., 1994; Rygula et al., 2015). Probabilistic reversal learning (PRL) paradigms model both: individuals learn by trial and error the most adaptive action in an acquisition stage, and this rule changes in a reversal phase (Chamberlain et al., 2006; den Ouden et al., 2013; Evers et al., 2005; Murphy et al., 2002; Rygula et al., 2015; Skandali et al., 2018). Severe serotonin depletion via neurotoxins impairs the ability to update actions upon reversal and increases sensitivity to negative feedback (SNF) in rats (Bari et al., 2010) and monkeys (Rygula et al., 2015). SNF is defined here as switching behaviour following spurious negative feedback (‘lose–shift’), which should be ignored; increased SNF thus causes subjects to choose the less rewarded stimulus, maladaptively. SNF is additionally elevated following single dose administration of selective serotonin reuptake inhibitors (SSRIs) in rats (Bari et al., 2010) and healthy humans (Chamberlain et al., 2006; Skandali et al., 2018). These SSRI data are interpreted as the paradoxical lowering of serotonin (Bari et al., 2010; Chamberlain et al., 2006; Skandali et al., 2018), presumed to parallel the effects following acute tryptophan depletion (ATD), which lowers serotonin synthesis (Evers et al., 2005; Hood et al., 2005; Murphy et al., 2002). Whilst studies of healthy humans have not found effects of ATD on choice in PRL, their samples have been small and studied only one sex: Evers et al. (2005) included 12 males whereas Murphy et al. (2002) enrolled 12 females. Here we studied a large sample of healthy volunteers, male and female, to better determine whether ATD modulates choice behaviour in PRL. Whereas Evers et al. (2005) and Murphy et al. (2002) used within-subjects designs, here we employed a between-subjects design to avoid practice effects and thus better assess learning.
Methods
Power calculation was performed, with α = .05 (two-tailed), power (1-β) set to .8, and mean and standard deviation based on statistically significant PRL results from Skandali et al. (2018). This called for a group size of approximately n = 30. We studied 62 healthy volunteers, free from personal or immediate family psychiatric history. Participants were free from neurological, gastrointestinal or other major medical disorders, and were not taking any medication, besides contraceptives, nor had they taken psychiatric or neurological medication in the past. Volunteers gave informed consent and were paid.
Participants fasted for at least 9 h before the study, then gave a baseline blood sample, and ingested either a tryptophan depletion mixture (n = 30; 14 females) or a placebo mixture, containing tryptophan (n = 32; 15 females). The depletion mixture contained 4.10 g L-alanine, 3.70 g L-arginine, 8.93 g L-aspartic acid, 2.00 g L-cystine, 2.40 g glycine, 2.40 g L-histidine, 6.00 g L-isoleucine, 10.10 g L-leucine, 6.70 g L-lysine, 2.30 g L-methionine, 4.30 g L-phenylalanine, 9.20 g L-proline, 5.20 g L-serine, 4.90 g L-threonine, 3.00 g L-tyrosine, 6.70 g L-valine. The placebo mixture was identical but contained 5.20 g of L-tryptophan. These quantities were derived from Worbe et al. (2014) and the mixtures were manufactured by metaX Institut fur Diatetik GmbH. After approximately 4.5 h, participants gave a second blood sample to verify depletion, and completed the PRL task. The task (Chamberlain et al., 2006; Murphy et al., 2002; Skandali et al., 2018) contained 80 trials: 40 during acquisition and 40 following reversal. For the first 40 trials, one option yielded positive feedback on 80% of trials, the other option on 20% of trials. These contingencies reversed for the latter 40 trials. Eight consecutive correct responses fulfilled the learning criterion.
SNF was our primary outcome measure, defined as the observed probability of behaviour switching away from the correct stimulus, following the delivery of spurious negative feedback. We likewise conducted planned comparisons on proportions for win–stay and lose–shift separately for spurious and veracious feedback, and for each phase (Skandali et al., 2018) reported in Table 1. We calculated two measures of perseveration: immediately following reversal (Murphy et al., 2002) and across the reversal phase (den Ouden et al., 2013).
Table 1.
Top: Summary of measures of choice behaviour, planned contrasts assessed by t-test. Bottom: Blood results.
| Phase | Measure | Feedback | Placebo | Depletion | p | Cohen’s d |
|---|---|---|---|---|---|---|
| Acquisition | Correct responses | 38.63 (3.2) | 39.27 (1.82) | .34 | .25 | |
| Trials to criterion | 10.59 (6.76) | 9.83 (4.63) | .61 | .13 | ||
| Win–stay | Veracious | .97 (.07) | .98 (.04) | .326 | .18 | |
| Lose–shift | Spurious | .06 (.16) | .03 (.1) | .478 | .22 | |
| Reversal | Correct responses | 33.44 (4.7) | 34.13 (4.06) | .536 | .16 | |
| Trials to criterion | 16.63 (8.57) | 15.23 (7.6) | .502 | .17 | ||
| Win–Stay | Veracious | .93 (.13) | .96 (.1) | .331 | .26 | |
| Lose–Shift | Spurious | .11 (.19) | .12 (.17) | .889 | .06 | |
| Perseveration, immediate | 3.69 (2.1) | 3.97 (2.0) | .594 | .137 | ||
| Perseveration, phase | 3.81 (3.68) | 3.67 (3.0) | .865 | .042 | ||
| Blood | TRP:LNAA (1) | .11 (.02) | .10 (.02) | .239 | .50 | |
| TRP:LNAA (2) | .11 (.03) | .005 (.004) | 4.96 × 10 –20 | 4.9 | ||
| Valine (1) | 225 (45.10) | 230 (46.56) | .662 | .11 | ||
| Valine (2) | 841.30 (238.69) | 820.86 (218.96) | .733 | .09 | ||
| Methionine (1) | 29.60 (4.98) | 27.62 (6.44) | .191 | .34 | ||
| Methionine (2) | 67.70 (23.97) | 63.07 (20.71) | .431 | .21 | ||
| Isoleucine (1) | 64.60 (17.47) | 66.14 (17.66) | .738 | .09 | ||
| Isoleucine (2) | 218.13 (92.84) | 218.34 (129.83) | .994 | .002 | ||
| Leucine (1) | 128.23 (27.82) | 126.76 (30.26) | .846 | .05 | ||
| Leucine (2) | 344.37 (134.99) | 342.90 (137.30) | .967 | .01 | ||
| Tyrosine (1) | 67.27 (12.50) | 66.45 (18.53) | .843 | .05 | ||
| Tyrosine (2) | 170.40 (41.242) | 196.55 (82.14) | .132 | .40 | ||
| Phenylalanine (1) | 59.33 (8.53) | 58.93 (9.54) | .865 | .04 | ||
| Phenylalanine (2) | 111.07 (43.94) | 120.07 (63.98) | .530 | .16 | ||
| Tryptophan (1) | 61.97 (9.63) | 59.21 (9.80) | .280 | .28 | ||
| Tryptophan (2) | 201.53 (77.05) | 7.83 (5.33) | 2.68 × 10 –14 | 3.55 |
Note: (1) signifies baseline values; (2) denotes results from sample taken after approximately 4.5 h. TRP:LNAA is the ratio between tryptophan and all large neutral amino acids (listed in table), thought to be most reflective of brain serotonin (Hood et al., 2005). Emboldened values indicate statistical significance at p < .05.
Results
There were no differences between groups in age, years of education, depressive symptoms, or trait anxiety (ps > .05). We achieved a robust depletion of tryptophan (t(49) = −17.726, p = 4.857 × 10–23; degrees of freedom (df) were adjusted after Levene’s test showed unequal variances; we were unable to obtain blood samples from three participants), without affecting mood (t(55) = −1.341, p = .186; data unavailable for five participants). ATD did not affect the core measures of choice behaviour in PRL (Table 1). After placebo, 31/32 participants attained criterion performance in acquisition; after ATD, 30/30 (Fisher’s exact test, p = 1). On placebo, 29/32 participants reached reversal criterion, 28/30 on ATD (p = 1). Analysis of variance with condition (placebo, ATD) and sex (male, female) as the between-subjects factors, and phase (acquisition, reversal) as the within-subjects factor revealed no effects of condition or sex on the number of correct responses (F < 1.60, p > .05, and ηp2 < .03 for all terms involving ATD and sex); trials to criterion (F < 3.10, p > .05, and ηp2 < .055); SNF (F < 3.00, p > .05, and ηp2 < .050), shown in Figure 1; or win–stay to veracious feedback (F < 3.10, p > .05, and ηp2 < .055). Perseveration was unaffected both immediately following reversal (t(60) = .536, p = .594, d = .137) and across the reversal phase (t(60) = −.170, p = .865, d = .042), and neither measure differed between males and females (t(60) = 1.535, p = .130,d = .39; t(60) = .263, p = .793, d = .07, respectively). There were no correlations between the extent of depletion and our key measures of interest: SNF, win–stay to veracious feedback, and either measure of perseveration (ps > .05).
Figure 1.
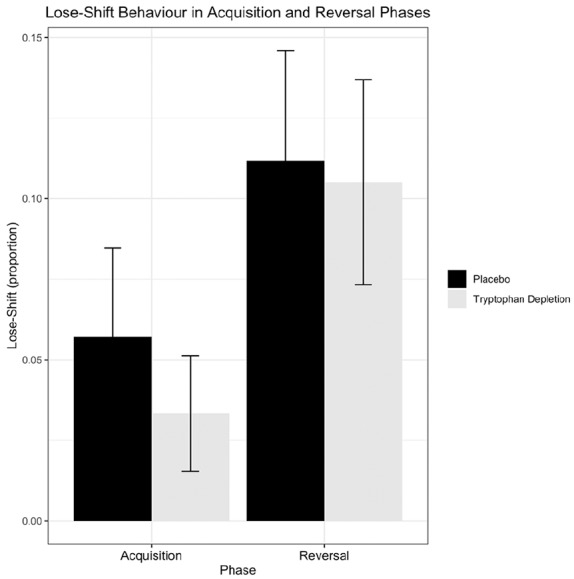
Probabilistic reversal learning task: mean proportion of lose–shift behaviour (probability of shifting) following spurious negative feedback, plotted separately for acquisition and reversal, compared between placebo and ATD groups. Error bars: +/– 1 standard error.
Conclusion
ATD did not affect the core measures of PRL choice behaviour. By nearly tripling the sample size and testing both sexes we considerably extended previous efforts to capture the effects of ATD, and replicated null results on choice (Evers et al., 2005; Murphy et al., 2002). We tested additional measures, beyond those reported in the previous ATD PRL studies, to no avail. This contrasts with other serotonergic challenges that have modulated PRL – and SNF in particular – in healthy humans (Chamberlain et al., 2006; Skandali et al., 2018), rats (Bari et al., 2010) and monkeys (Rygula et al., 2015). The discrepancy is likely to be due to both differences in the magnitude of change – ATD is mild in comparison to neurotoxic depletion via 5,7-dihydroxytryptamine – and the regions preferentially affected. Increased SNF in depression, for instance, is mediated by amygdala hyperactivity (Taylor Tavares et al., 2008), yet Evers et al. (2005) reported no effect of ATD on the amygdala during PRL. However, Rygula et al. (2015) demonstrated that serotonin in both the amygdala and orbitofrontal cortex is required for PRL. It is possible that computational methods, as used in Rygula et al. (2015), could reveal an effect of ATD on the latent mechanisms of PRL. Future studies should additionally employ more salient feedback. The implication of the present results, in conjunction with previous studies, is that under certain task demands ATD does not necessarily produce effects that parallel acute SSRI or neurotoxic serotonin depletion.
Acknowledgments
We would like to thank the staff at the National Institute for Health Research/Wellcome Trust Clinical Research Facility at Addenbrooke’s Hospital, where the study was conducted, and Rachel Kyd of the Cambridge University Hospital Research & Development Office for assistance with study approval. The Cambridge Central Research Ethics Committee granted ethical approval for this study and all participants provided informed written consent.
Footnotes
Declaration of conflicting interests: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: TWR discloses consultancy with Cambridge Cognition, Lundbeck, Mundipharma and Unilever; he receives royalties for CANTAB from Cambridge Cognition and editorial honoraria from Springer Verlag and Elsevier. BJS discloses consultancy with Cambridge Cognition, Greenfield BioVentures, and Cassava Sciences, and receives royalties for CANTAB from Cambridge Cognition. RNC consults for Campden Instruments and receives royalties from Cambridge Enterprise, Routledge, and Cambridge University Press. JWK, FEA, RY, DMC, AMA-S and AP declare no conflicts of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by a Wellcome Trust Senior Investigator Award (104631/Z/14/Z) awarded to TWR. BJS receives funding from the National Institute for Health Research Cambridge Biomedical Research Centre (Mental Health Theme); the views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care. RNC’s research is supported by the UK Medical Research Council (MC_PC_17213). JWK is supported by a Gates Cambridge Scholarship.
ORCID iD: Jonathan W Kanen  https://orcid.org/0000-0002-4095-5405
https://orcid.org/0000-0002-4095-5405
References
- Bari A, Theobald DE, Caprioli D, et al. (2010) Serotonin modulates sensitivity to reward and negative feedback in a probabilistic reversal learning task in rats. Neuropsychopharmacology 35: 1290–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain SR, Müller U, Blackwell AD, et al. (2006) Neurochemical modulation of response inhibition and probabilistic learning in humans. Science 311: 861–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Ouden HEM, Daw ND, Fernandez G, et al. (2013) Dissociable effects of dopamine and serotonin on reversal learning. Neuron 80: 1090–1100. [DOI] [PubMed] [Google Scholar]
- Evers EAT, Cools R, Clark L, et al. (2005) Serotonergic modulation of prefrontal cortex during negative feedback in probabilistic reversal learning. Neuropsychopharmacology 30: 1138–1147. [DOI] [PubMed] [Google Scholar]
- Hood SD, Bell CJ, Nutt DJ. (2005) Acute tryptophan depletion. Part I: Rationale and methodology. Aust N Z J Psychiatry 39: 558–564. [DOI] [PubMed] [Google Scholar]
- Murphy F, Smith K, Cowen PJ, et al. (2002) The effects of tryptophan depletion on cognitive and affective processing in healthy volunteers. Psychopharmacology (Berl) 163: 42–53. [DOI] [PubMed] [Google Scholar]
- Park SB, Coull JT, McShane RH, et al. (1994) Tryptophan depletion in normal volunteers produces selective impairments in learning and memory. Neuropharmacology 33: 575–588. [DOI] [PubMed] [Google Scholar]
- Rygula R, Clarke HF, Cardinal RN, et al. (2015) Role of central serotonin in anticipation of rewarding and punishing outcomes: Effects of selective amygdala or orbitofrontal 5-HT depletion. Cereb Cortex 25: 3064–3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skandali N, Rowe JB, Voon V, et al. (2018) Dissociable effects of acute SSRI (escitalopram) on executive, learning and emotional functions in healthy humans. Neuropsychopharmacology 43: 2645–2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor Tavares JV, Clark L, Furey ML, et al. (2008) Neural basis of abnormal response to negative feedback in unmedicated mood disorders. Neuroimage 42: 1118–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worbe Y, Savulich G, Voon V, et al. (2014) Serotonin depletion induces ‘waiting impulsivity’ on the human four-choice serial reaction time task: Cross-species translational significance. Neuropsychopharmacology 39: 1519–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]


