Abstract
Objective:
To provide an overview of immune checkpoint inhibitor (ICI) therapy-associated immune-related adverse events (irAEs) and their management, focusing on the key responsibilities for pharmacists in recognizing, distinguishing, and treating irAEs and in educating patients about irAEs and their management.
Data Sources:
Literature published from January 2000 to March 2018 available from online sources.
Study Selection and Data Extraction:
Relevant English-language studies, guidelines, and articles.
Data Synthesis:
ICI therapies have been approved for the treatment of several cancers as single-agent therapies, combined ICI therapies, or in combination with other agents. ICI therapies increase the activity of the immune system and consequently can have autoimmune-like adverse effects that are often termed irAEs. irAE management can be challenging as irAEs can vary in their frequency and severity among patients, according to the specific agent, and can occur at any time during treatment or after therapy discontinuation. Additionally, for patients treated with ICI therapies in combination with other therapies, ICI-associated irAEs must be distinguished from adverse events associated with chemotherapy or targeted therapies, which often require different management. Pharmacists can provide essential support to diagnose and manage irAEs.
Relevance to Patient Care and Clinical Practice:
Early and accurate diagnosis and prompt management of irAEs by pharmacists are critical to reduce the risk of severe or life-threatening complications and prevent premature ICI discontinuation.
Conclusions:
Pharmacists have a key role in the recognition, monitoring, and management of irAEs and in educating patients about irAEs associated with ICI therapies and the agents used to manage them.
Keywords: adverse drug reactions, corticosteroids, immunosuppressants, monoclonal antibodies, oncology
Introduction
Immuno-oncology treatments enhance the ability of a patient’s immune system to recognize and mount an attack against a variety of malignancies.1 In recent years, the US Food and Drug Administration (FDA) has approved several indications for immuno-oncology agents known as immune checkpoint inhibitor (ICI) therapies (Table 1).2-8 ICI therapies can increase antitumor immune responses by modifying the activity of immune checkpoint molecules, such as cytotoxic T-lymphocyte antigen-4 (CTLA-4), programmed death-1 (PD-1), and programmed death-ligand 1 (PD-L1), which regulate immune responses.1,9 Blocking CTLA-4 has been shown to enhance T-cell activation and increase T-cell proliferation within lymphoid tissues.10,11 PD-1/PD-L1 blockade has been shown to restore effector T-cell activity in peripheral tissues and in the tumor microenvironment.9,10
Table 1.
FDA-Approved Indications for ICI Single-Agent and Combined Therapies.a
| ICI Therapy (Immune Target) | Therapy Strategy | FDA-Approved Indicationsb |
|---|---|---|
| Atezolizumab6 (PD-L1) | Monotherapy |
|
| + Bevacizumab + paclitaxel + carboplatin |
|
|
| + Carboplatin + etoposide |
|
|
| + Paclitaxel |
|
|
| Avelumab5 (PD-L1) | Monotherapy |
|
| Cemipilimab8 (PD-1) | Monotherapy |
|
| Durvalumab2 (PD-L1) | Monotherapy |
|
| Ipilimumab3 (CTLA-4) | Monotherapy |
|
| Nivolumab3,4 (PD-1) | Monotherapy |
|
| + Ipilimumab |
|
|
| Pembrolizumab7 (PD-1) | Monotherapy |
|
| + Pemetrexed + carboplatin |
|
Abbreviations: cHL, classical Hodgkin lymphoma; CRC, colorectal cancer; CSCC, cutaneous squamous cell carcinoma; CTLA-4, cytotoxic T-lymphocyte antigen-4; dMMR, deficient mismatch repair; ES-SCLC, extensive-stage small-cell lung cancer; FDA, Food and Drug Administration; GEJ, gastroesophageal junction; HCC, hepatocellular carcinoma; ICI, immune checkpoint inhibitor; MCC, Merkel cell carcinoma; MSI, microsatellite instability; NSCLC, non-small-cell lung carcinoma; NSQ, nonsquamous; PD-1, programmed death-1; PD-L1, programmed death-ligand 1; PMBLC, primary mediastinal large B-cell lymphoma; RCC, renal cell carcinoma; SCCHN, squamous cell carcinoma of the head and neck; SCLC, small-cell lung cancer; TNBC, triple-negative breast cancer; UC, urothelial carcinoma.
a As of July 18, 2019.
b Advanced disease unless otherwise stated.
ICI therapies have demonstrated significant patient benefit, with regard to overall survival, progression-free survival, and durability of response, across multiple tumor types.10 However, the antitumor effects of ICI therapies can be accompanied by immune-related adverse events (irAEs) that present similarly to autoimmune-like disorders, reflecting their immune-based mechanisms of action.1,12 As the clinical use of ICI therapies increases so does the need for practitioners, such as pharmacists, to be cognizant of the irAE profiles specific to these therapies. Early recognition and appropriate management of irAEs are key to reducing morbidity and ensuring that patients can continue receiving ICI treatment.13-15
This review provides pharmacists with a guide to key ICI-associated irAEs and how they might be treated and summarizes the critical role that pharmacists have in identifying, monitoring, managing, and educating patients about irAEs.
Data Sources
A comprehensive search of the literature was performed by collating relevant English-language literature published between January 2000 and November 2018 and online sources. Literature was included in this manuscript if it discussed ICI-associated irAEs and their management. The types of literature included randomized clinical trials, retrospective review studies, professional society guidelines and recommendations, and drug manufacturer resources. PubMed and the American Society of Clinical Oncology (ASCO), the Society for Immunotherapy of Cancer (SITC), and the European Society for Medical Oncology (ESMO) Congress publication libraries were the primary sources of information; key search terms were “adverse event,” “toxicity,” “immune-related,” and “immunotherapy/immune checkpoint inhibitor”. The Hematology/Oncology Pharmacy Association (HOPA), the National Comprehensive Cancer Network® (NCCN®), ASCO, SITC, and ESMO websites and publications were consulted for recommendations and best practices on how to manage irAEs. Further recommendations, guidelines, and checklists were sourced from drug manufacturer websites and package inserts for the 7 currently FDA-approved ICI agents: nivolumab, ipilimumab (Bristol-Myers Squibb Co, Princeton, NJ), pembrolizumab (Merck & Co Inc, Whitehouse Station, NJ), atezolizumab (Genentech Inc, South San Francisco, CA), durvalumab (AstraZeneca Pharmaceuticals LP, Wilmington, DE), avelumab (EMD Serono Inc, Rockland, MA), and cemiplimab (Regeneron Pharmaceuticals Inc, Tarrytown, NY).
ICI-Associated irAEs
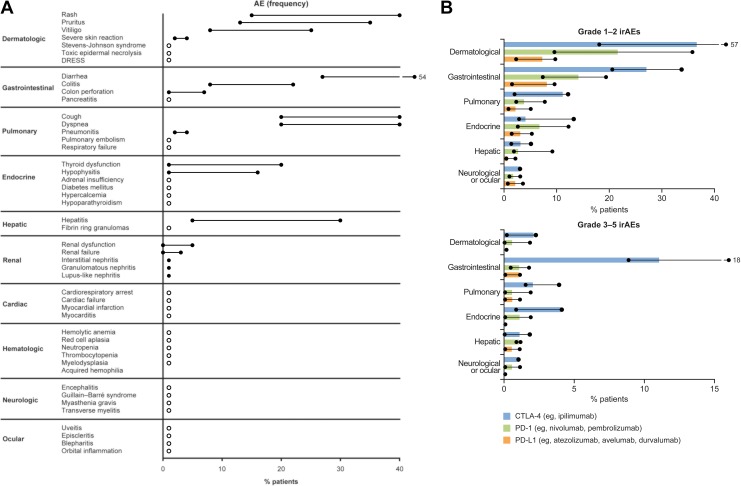
ICI therapies can induce a wide range of irAEs that are often inflammatory in nature, mostly resulting from the infiltration of hyperactivated CD4+ and CD8+ T cells into normal tissues and organs.1 The exact mechanisms through which irAEs manifest remain unknown; however, the release of cytokines, as well as antigen cross-reactivity between the tumor and normal tissue, may contribute to their onset.1 Data from phase 2 and 3 clinical studies consistently show that irAEs can affect any organ system but are most commonly dermatologic, gastrointestinal, pulmonary, endocrine, or hepatic in nature and include rash, pruritus, vitiligo, diarrhea, colitis, cough, dyspnea, thyroid dysfunction, and hepatitis. Rare irAEs that have been observed include those affecting the ocular system (uveitis and orbital inflammation) and hematological, cardiac, and other neurological irAEs (Figure 1A).12-14,16
Figure 1.
Frequency of irAEs for ICI monotherapya by (A) organ system (range) and (B) class of ICI and gradeb (median and range). Circles and lines represent the range of frequency. White circles in (A) represent <1%. Solid bars in (B) represent the median frequency. aMonotherapies include nivolumab, pembrolizumab, and ipilimumab. birAE grading according to National Cancer Institute Common Terminology Criteria for Adverse Events. (A) Data collated from prospective and retrospective phase 2 and 3 clinical studies summarized and cited in the study by Pardoll et al, Buchbinder et al, Tarhini et al, Haanen et al, and Puzanov et al.9–11,13,14 The number of patients in clinical studies ranged from 14 to 1685. (B) Adapted from Michot et al, where the phase 2 and 3 clinical studies from which the data were summarized are cited.12 The number of patients in these studies ranged from 135 to 799. CTLA-4, cytotoxic T lymphocyte antigen-4; DRESS, drug rash with eosinophilia and systemic symptoms; GI, gastrointestinal; ICI, immune checkpoint inhibitor; irAE, immune-related adverse event; PD-1, programmed death-1; PD-L1, programmed death-ligand 1.
The majority of all irAEs are mild to moderate in severity, although some can become severe or even life-threatening if not promptly identified and appropriately managed.12,14,17 For instance, cardiac, hematologic, or neurologic irAEs, although rare, can be fatal if diagnosis and treatment are delayed.16,17 Grade 3 to 5 irAEs are most commonly severe diarrhea and can include severe skin reactions (defined as papules and/or rash covering >30% body surface area), pneumonitis, pancreatitis, cardiac arrest, diabetes mellitus, encephalitis, Guillain-Barré syndrome, and neuropathy.12,14,16,17 The frequency and severity of irAEs differ among patients and can vary across tumor types and between different classes of ICI therapies (Figure 1B).18 An increased rate of any-grade irAEs has been observed in patients treated with PD-1 inhibitors compared with those treated with PD-L1 inhibitors (Figure 1B).14,18 In general, treatment with CTLA-4 inhibitors, such as ipilimumab, is associated with more frequent and severe irAEs than with PD-1/PD-L1 inhibitors (Figure 1B). Anti-CTLA-4 antibody administration has been associated with increased rates of gastrointestinal irAEs compared with PD-1/PD-L1 inhibitors, potentially because of the distinct immunopathological mechanisms in gastrointestinal immunity.19 Also, the higher rate of hypophysitis seen with CTLA-4 blockade is likely due to ectopic expression of CTLA-4 in the pituitary gland.20
The manifestation of irAEs may also be influenced by tumor type and ICI dose. For instance, a higher frequency of anti-PD-1-associated colitis, pruritus, diarrhea, and rash has been observed in patients with advanced melanoma compared with those who have non-small cell lung cancer or renal cell carcinoma.18 Studies across tumor types have shown that incidence and grade of irAEs increased with increased doses of anti-CTLA-4 therapy. However, irAE rates and severities appeared similar among anti-PD-1/PD-L1 therapies regardless of dose.16
In addition to monotherapy, some ICI therapies have been FDA approved for use in combination with other ICI therapies or with chemotherapy for specific tumor types (Table 1).2-8 Combined ICI therapies have demonstrated enhanced T-cell function and greater antitumor activity than monotherapy treatment.19,21 However, there is evidence that irAEs are more frequent or severe for patients treated with ICI combinations than with single-agent ICI.21-24 As with ICI monotherapies, the most frequent irAEs for combined therapies are dermatologic, gastrointestinal, hepatic, pulmonary, and endocrine related, which vary by individual patient, tumor type, and dosing regimen.21-24 Symptoms of irAEs may overlap with toxicities associated with chemotherapy or targeted therapy (eg, diarrhea and colitis) but have different underlying causes and therefore require different management.25
Key Considerations for irAE Diagnosis
Attributing Adverse Events as Immune-Related
A critical consideration for irAE management is the early identification and appropriate referral of patients with potential irAEs for accurate diagnosis and appropriate intervention.14,26 Due to the complex nature of irAEs and the variable safety profile of each ICI, there is often difficulty in distinguishing irAEs from adverse events associated with other therapies or underlying disease. Adverse events from chemotherapy generally present earlier than irAEs from immunotherapy, which can have a delayed onset and prolonged duration.14 Because the timing of irAEs differs from those typically observed with other treatments such as chemotherapy, knowledge of irAE presentation timelines may help distinguish irAEs in patients receiving combined therapies.
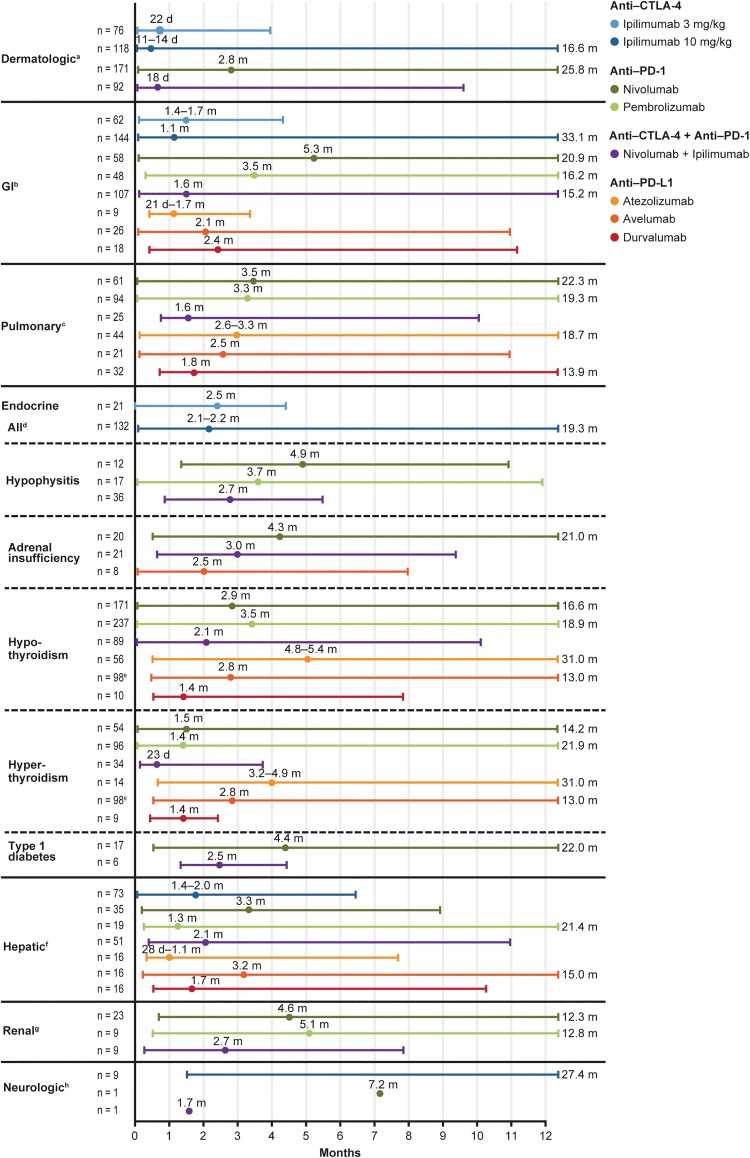
The time to onset of irAEs can vary between ICI monotherapies and combined therapies and among specific ICI classes. IrAEs resulting from CTLA-4 inhibitors tend to present sooner than those from PD-1/PD-L1 inhibitors, and pembrolizumab-associated irAEs generally manifest earlier than those with nivolumab.26,27 IrAEs resulting from ICI combinations typically present earlier than for monotherapies and persist for longer periods (Figure 2).13,27 Occasionally, irAEs arise several months after initiation of therapy and can even occur up to a year after treatment completion or discontinuation, leading to delay in irAE recognition and treatment.12,14,26 Stringent monitoring of patients for symptoms of potential irAEs is therefore important at ICI treatment initiation, during treatment, and throughout an extended follow-up period.26,28
Figure 2.
Time to onset (median and range) of irAEs by organ affected and ICI monotherapy or combined therapy. Circles represent the median time to onset. Lines represent the range for time to onset; where the range extends further than 12 months, this is indicated numerically. aDermatitis in ipilimumab studies; immune-mediated rash in nivolumab and nivolumab + ipilimumab studies. bEnterocolitis in ipilimumab studies; colitis in nivolumab, pembrolizumab, avelumab, and nivolumab + ipilimumab studies; colitis or diarrhea in atezolizumab and durvalumab studies. cPneumonitis. dIncludes hypopituitarism, adrenal insufficiency, hypothyroidism, hyperthyroidism, hypogonadism, thyroiditis, Cushing’s syndrome, and Graves’ ophthalmopathy. eHypothyroidism and hyperthyroidism are combined for avelumab. fHepatitis. gNephritis or renal dysfunction in nivolumab and nivolumab + ipilimumab studies; nephritis in pembrolizumab studies. hNeuropathy in ipilimumab studies and encephalitis in nivolumab and nivolumab + ipilimumab studies. CTLA-4, cytotoxic T-lymphocyte antigen-4; FDA, Food and Drug Administration; GI, gastrointestinal; ICI, immune checkpoint inhibitor; irAE, immune-related adverse event; PD-1, programmed death-1; PD L1, programmed death-ligand 1. Adapted from Davies et al, used under CC BY-NC 3.0 (reordered from the original), which summarizes data collated from the package inserts of FDA-approved CTLA-4 (ipilimumab), PD-1 (nivolumab, pembrolizumab), and PD-L1 (atezolizumab, avelumab, and durvalumab) ICIs at the time of publication.2–8
Patient Populations at High Risk of irAEs
Some patients may be more susceptible to certain irAEs because of their overall health condition and comorbidities or previous ICI therapy.13,15,29 For example, sarcopenia or low muscle mass has been associated with high-grade irAEs in ipilimumab-treated patients with melanoma.30 Currently, most of the clinical trials investigating ICI therapies exclude patients at high risk of developing irAEs, such as those with a history of autoimmune disease (eg, ulcerative colitis, Crohn’s disease, lupus, active rheumatoid arthritis), allogeneic stem cell or solid organ transplant, previous viral infections, or concomitant use of therapies with known autoimmune toxicities, such as interferons.31 Limited retrospective studies have examined the use of anti-PD-1 agents in patients with preexisting autoimmune disorders, mostly rheumatologic or dermatologic in nature, or prior irAEs from anti-CTLA-4 therapy. Results showed that flares of autoimmune disease were common; however, most events were mild and successfully managed with corticosteroids.32 Interestingly, data also indicate that patients who have autoimmune disease comorbidity or prior CTLA-4-induced irAEs can be treated with anti-PD-1 therapies and experience typical rates of irAEs that can be effectively managed.33 Although extensive research has been performed to establish biomarkers of efficacy for ICI therapies, limited data exist on biomarkers to identify patients likely to experience particular irAEs.16 It is important that pharmacists screen patients for predisposing conditions so that patients at high risk can be monitored more rigorously, preferably in close collaboration with organ specialists.
Strategies for Managing irAEs
Some irAEs (eg, rash, diarrhea) may prompt over-the-counter treatment; however, many irAEs require treatment with corticosteroids and other immunosuppressants.12 Reflecting the increased use of ICI therapies in clinical practice over recent years, several groups have published guidelines for the management of irAEs associated with ICI therapies, including ESMO, SITC, and NCCN in collaboration with ASCO.13-15 These guidelines are based on expert consensus and built on systematic evaluation of observational data primarily derived from retrospective studies and case reports. For mild irAEs, ICI therapy may often be able to continue with the use of supportive-care agents.12,14,15 For moderate-to-severe irAEs, dose reduction of ICI therapies should be avoided; instead, temporary or permanent discontinuation of therapy is recommended and treatment with corticosteroids and other immunosuppressants may be required.14,15 The general approach to irAE management is summarized in Table 2.
Table 2.
General Summary of NCCN and SITC Guidelines for irAE Management (Consult guidelines for recommendations for each specific irAE).
| Grade (Severity) | Guidelines14,15 |
|---|---|
| 1 (mild) | Continue ICI therapy with close monitoringa
• Corticosteroids are not usually required |
| 2 (moderate) | Temporarily discontinue ICI therapy • Initiate low-dose corticosteroids (0.5-1 mg/kg/d prednisone or equivalent) • Consult the relevant disease specialists (eg, dermatologists or pulmonary consultants) • If no improvement in 2 to 3 days, increase corticosteroid dose to 2 mg/kg/d prednisone or equivalent • Gradually taper corticosteroid dose over at least 4 to 6 weeks once symptoms improve to ≤grade 1 • Provide supportive treatment/care as needed ˆ Readminister ICI therapy when symptoms improve and/or laboratory values decrease to ≤grade 1 and/or corticosteroid dose has been reduced to <10 mg prednisone or equivalent ˆ For steroid-refractory cases and/or when steroid sparing is desirable, management should be coordinated with disease specialists |
| 3 (severe) | Temporarily discontinue ICI therapy
|
| 4 (life-threatening) | Permanently discontinue ICI therapy, except for endocrinopathies controlled with hormone replacement
|
Abbreviations: ICI, immune checkpoint inhibitor; irAE, immune-related adverse event; NCCN, National Comprehensive Cancer Network; SITC, Society for Immunotherapy of Cancer.
a For some neurologic, hematologic, and cardiac irAEs, ICI therapy should be discontinued at any grade of toxicity until the nature of the irAE and symptom progression is defined.
Although the severity and frequency of irAEs vary between ICI therapies, the recommendations regarding immunosuppressive treatment regimens for irAE clinical management are similar for all types of ICI therapies, including ICI combinations.12-16,27 irAEs associated with monotherapy and combined therapies are generally well managed with immunosuppressive agents.28 Resolution rates for grade 3 or 4 irAEs treated with steroids were 80% to 100% for most organ categories in a phase 2 clinical study of patients with melanoma receiving nivolumab plus ipilimumab.34 The decision to resume ICI therapy at irAE resolution, however, may differ among ICI regimens. Readministration of anti-PD-1 and anti-PD-L1 therapies is often well tolerated. In contrast, readministration of anti-CTLA-4 therapy is usually not recommended after high-grade irAEs.15 For example, for patients who experience grade 3 diarrhea or colitis, those receiving nivolumab monotherapy should have therapy temporarily withheld and later resumed at irAE resolution. For patients receiving nivolumab plus ipilimumab, combined therapy should be permanently discontinued at the occurrence of high-grade irAEs. Upon recovery, those patients may resume treatment with nivolumab monotherapy only.14,15
Essentially, the risk of irAE recurrence must be weighed against the benefit of ICI resumption. For instance, readministration of ICI therapy is generally not warranted if the ICI therapy is used in the adjuvant setting because the risk of irAE recurrence is not justified in patients with no evidence of active cancer. Hence, early recognition and management of an irAE before it leads to discontinuation of therapy is considered best practice.15
The society guidelines are generally applicable; however, there are some exceptions. For example, low-grade cardiac and neurologic irAEs should be considered serious and treatment with ICI therapies should be discontinued immediately. Steroid taper longer than the typical 4 to 6 weeks may be warranted for pneumonitis. Furthermore, not all moderate-to-severe irAEs necessitate treatment with high-dose corticosteroids. Endocrine irAEs usually do not require high-dose prednisone but are often managed with endocrine-directed agents. For example, adrenal insufficiency, hypothyroidism, and hypophysitis are treated with hormone replacement therapy, and ICI therapy can be resumed after high-grade immune-related endocrinopathies when patients are stable on hormone therapies.
Steroid Treatment Management
Proper steroid dosing, and timing of and approach to tapering, is essential to promote prompt irAE resolution, prevent irAE relapse, and minimize steroid-related adverse events.13,15 The recommended dose of corticosteroids, with higher dose used for more severe irAE grade, is largely extrapolated from the management of autoimmune disease counterparts and based on experts’ opinions.12 In general, the recommended steroid dose is weight-based without an upper limit.
Early administration of steroids (within 5 days from the onset of symptoms) has been associated with faster resolution of diarrhea and colitis compared with delayed steroid initiation (more than 5 days from the onset of symptoms).35
Corticosteroids should be used until irAE symptoms are controlled, and tapering begins once this is achieved to ensure safety.36 Currently, there are no specific guidelines for steroid taper strategy; thus, clinical practices vary.27,36,37 Generally, a minimum tapering duration of 4 to 6 weeks is suggested, since irAE recurrence and/or worsening may occur with rapid steroid taper.13,27 The duration of tapering should be guided by the type and severity of irAEs. Due to the increased risk of irAE recurrence during the tapering period, frequent monitoring is necessary.37
Challenges for irAE Management
Steroids should be used as first-line treatment of irAEs where indicated due to low cost, easy accessibility, and known adverse event profile. Steroid-refractory irAEs can be treated with other immunosuppressants, such as infliximab, mycophenolate mofetil, calcineurin inhibitors, intravenous immunoglobulin, and plasmapheresis.14 Use of additional immunosuppressants is typically based on institutional and patient-specific factors such as formulary availability, patient comorbidities, and cost. It is important to keep in mind that immunosuppression may impact the response to ICI therapies and that immunosuppressive agents also have their own toxicities.12 Although a number of retrospective studies have shown that immunosuppressive regimens administered in the setting of irAEs did not appear to compromise the outcomes of ICI therapies, it is still prudent to minimize systemic immunosuppression.38,39 Rigorous monitoring may allow for early recognition of symptoms and the use of more localized therapy, such as topical or intra-articular steroid administration, potentially sparing the need for systemic immunosuppression. The use of immunosuppressants with a narrower spectrum of activity is another approach to reduce the degree of systemic immunosuppression. For example, vedolizumab, an anti-integrin that prevents the homing of lymphocytes to the gastrointestinal tract, may offer targeted immunosuppression localized to the gut when compared with infliximab in the management of immune-related enterocolitis.40,41 Since prolonged immunosuppression, especially with corticosteroids, can cause debilitating toxicities, such as opportunistic infections, diabetes mellitus, mood disorders, myopathy, and osteoporosis, efforts to mitigate these adverse events with prophylactic agents, such as proton pump inhibitors, must also be an integral part of irAE management.25,42
Other challenges and/or barriers to early irAE detection and optimal management are summarized in Table 3.12,14,15,43 Further to these factors, there are still uncertainties regarding the best treatment strategies, in terms of dosing and logistics, for irAEs treated with immunomodulatory therapies other than steroids, or pulse steroids used to treat neurologic or hematologic irAEs.14
Table 3.
Common Challenges for irAE Management.
| Stage | Description12,14,15,43 |
|---|---|
| Diagnosis |
|
| Monitoring | • Oncologists do not intervene until moderate/severe symptoms are present • Lack of resources to call patients in between treatment cycles to assess toxicities may lead to a delay in irAEs being recognized and treated • Lack of evidence-based, cost-effective monitoring strategies for rare but life-threatening irAEs (eg, myocarditis) may delay diagnosis and treatment |
| Follow-up |
|
Abbreviations: ICI, immune checkpoint inhibitor; irAE, immune-related adverse event.
Role of Pharmacists in Managing irAEs
Patient Education
A crucial role of pharmacists in irAE management is educating patients and their caregivers about irAEs by providing them with the necessary information to promptly identify potentially serious irAEs, thereby ensuring early detection and reporting to enable swift action to address the symptoms. At the initial education session, pharmacists should discuss several key points with patients. Patients should know that ICI therapies have a different adverse event profile compared with other anticancer treatments that patients may have previously received. Information about the specific signs and symptoms of potential irAEs must be provided. It is critical to stress to patients the need to pay attention to the symptoms of potential irAEs and to report them immediately. The mechanism of symptom reporting (ie, who to call and what number to call during both on and off hours) should then be specified to patients and caregivers. Pharmacists should also reassure patients that, if promptly and appropriately managed, irAEs generally resolve and ICI treatment may continue.27 At the end of the education session, it is recommended to provide patients with Oncology Nursing Society Immunotherapy Patient Wallet Cards (https://www.ons.org/sites/default/files/2019-01/IO%20Card%201-sided_Vertical.pdf), summarizing ICI therapy information, potential signs and symptoms of irAEs, and contact details for the oncology team. The wallet card can also serve as helpful communication tools with nononcology practitioners or emergency department practitioners.44 Patients should be advised to carry these wallet cards and other resources at all times, even after treatment discontinuation.
A study found that a pharmacist-led patient education program, which involved regular assessment of toxicities and continued education on the signs and symptoms of irAEs and when to seek medical attention, was associated with improved patient education, early recognition and management of irAEs, and improved outcomes.36 These data reinforce that patient education by pharmacists should be a continuous process, and pharmacists should assess and enhance patients’ knowledge regarding irAE symptom recognition and reporting at regular patient–pharmacist encounters in person or by telephone. Moreover, through patient education, patient-reported outcome (PRO) methods become better validated as patient knowledge and awareness of irAEs increases.
Monitoring of Patients
To ensure early diagnosis and effective management of irAEs, patients should be actively monitored by pharmacists for signs and symptoms of irAEs and regularly undergo both clinical and laboratory examinations during ICI therapy initiation, throughout the duration of treatment, and during an extended follow-up period (minimum of 1 year) after therapy discontinuation.28,29 The NCCN Clinical Practice Guidelines In Oncology (NCCN Guidelines®) recommend routine monitoring at 4- to 6-week intervals, with monitoring frequency increasing up to every 1 to 2 days depending on the type of irAE.15
Most irAEs initially present with nonspecific symptoms (eg, fatigue, weakness, headache, nausea, fever, electrolyte disturbance) that can overlap with adverse events observed during disease progression; therefore, pharmacists should be vigilant if such symptoms persist or worsen and discuss the suspicion with the wider oncology team to determine the underlying cause and/or consider specialist referral.14 The symptoms patients experience at the initiation of ICI therapy, and how those symptoms have or have not changed while on treatment, should be considered. Furthermore, pharmacists must remain aware of the potential for late-onset irAEs beyond the therapy administration period.
PRO measures also provide an opportunity for early intervention of irAEs, as many symptoms are subjective and may impact patients more than what clinicians perceive, similar to other adverse events such as fatigue, nausea, and pain. Asking patients specific questions when evaluating treatment tolerance and ensuring patients pay attention to changes in symptoms are helpful in identifying potential irAEs. A comprehensive irAE symptom checklist is available on the HOPA website, and agent-specific patient monitoring checklists can be found on the drug manufacturer’s website.45-47 By assessing toxicities between therapy cycles in person or by telephone and using specific questions such as those provided in the recommended checklists, pharmacists can improve the likelihood of early diagnosis and effective management of irAEs.44
IrAE Management
Pharmacists’ knowledge of the mechanisms of action, safety profiles, and efficacy data of immunosuppressive agents facilitates risk–benefit evaluation and individualization of immunosuppressive regimens for irAE management. For instance, vedolizumab is recommended for the treatment of infliximab-refractory severe diarrhea or colitis15, and may therefore be recommended for treatment of these irAEs in patients with congestive heart failure or elevated liver enzymes, conditions in which the use of infliximab is generally considered contraindicated.13 One of the most important contributions of pharmacists in managing irAEs is designing an appropriate steroid taper schedule. Guidelines published by ASCO, NCCN, SITC, and ESMO provide guidance on steroid taper for specific irAEs, and a general example regimen is outlined in Table 4.13-15 Implementation of a pharmacist-led irAE management program promoted interventions and improved outcomes, and protocols are being developed at certain US centers to give pharmacists the authority to order relevant tests, manage steroid taper, refill steroid prescription, and initiate medications for supportive care in close collaboration with other members of the oncology team.36
Table 4.
Example of Steroid Tapering Guidance for irAEs >Grade 1.
| Steroid Tapering Guidancea | |
|---|---|
Oral steroid:
|
IV steroid:
|
Abbreviations: irAE, immune-related adverse event; IV, intravenous.
a Example based on guidance provided by American Society of Clinical Oncology, National Comprehensive Cancer Network, Society for Immunotherapy of Cancer, European Society for Medical Oncology, and a specific center in the United Kingdom.13–15,37 Clinical practice varies across centers, and taper should be designed on an individual case basis.
b Prednisone or equivalent.
With expertise in pharmacokinetics, pharmacists can also assist with monitoring of immunosuppressants, such as calcineurin inhibitors, to ensure efficacy and avoid toxicities in patients with steroid-refractory irAEs. As thyroid dysfunction is a common immune-related endocrinopathy, pharmacists can help with monitoring thyroid function tests, identifying patients in need of hormone supplements, and initiating levothyroxine therapy. Likewise, pharmacists can optimize care by monitoring patients with irAEs to prevent and treat the adverse events associated with prolonged immunosuppression, especially with long-term steroid use.
Relevance to Patient Care and Clinical Practice
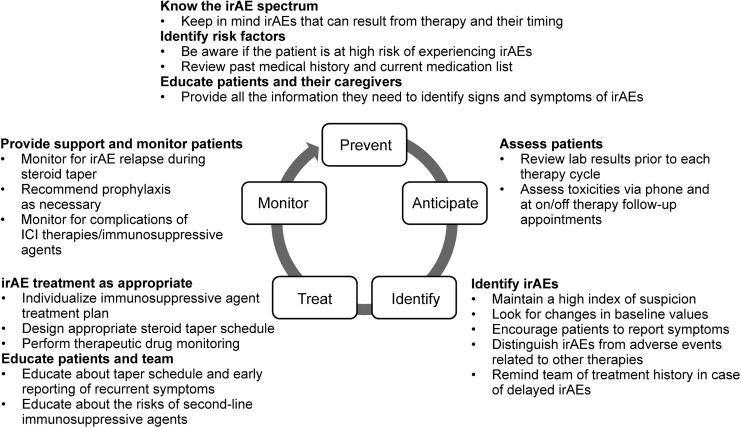
As an integral part of the oncology team, pharmacists are well positioned to participate in the management of ICI-associated irAEs. It is therefore important for pharmacists to be aware of which therapies an individual patient is receiving and understand the best practices for early detection, accurate attribution, and optimal treatment of irAEs. Continuous monitoring of patients is recommended at ICI treatment initiation, during therapy, and during the post-therapy follow-up period, with a focus on early recognition and diagnosis of irAEs so that patients can avoid potentially life-threatening toxicities and remain on therapy to achieve maximum clinical benefit. Pharmacists also have an important role in engaging patients with education, including helping patients recognize symptoms of irAEs to support early recognition and reporting and prompt intervention. Figure 3 provides general guidance for pharmacists on the management of irAEs. ESMO, NCCN, and SITC guidelines provide more detailed guidance on how to assess and manage a number of specific irAEs,13-15 and ICI package inserts provide detailed guidance on how to manage irAEs that are experienced during or after treatment with the specific agent.2-8
Figure 3.
Guidance for pharmacists on the management of ICI-associated irAEs, based on published guidelines.13–15 ICI, immune checkpoint inhibitor; irAE, immune-related adverse event. Adapted from Champiat et al.
Conclusions
ICI therapies are used to treat multiple tumors as single-agent therapy, or in combination with other agents, and can induce a wide range of irAEs that vary in frequency and severity according to tumor type, dose, and combination of agents. Pharmacists must know the irAE profiles of ICI therapies and be familiar with best practices for irAE management as recommended in published guidelines to recognize and treat irAEs in a timely manner, ensure proper use of corticosteroids with/without additional immunosuppressants, and recommend appropriate ancillary supportive care measures. Pharmacists have a key role in the effective management of irAEs through continuous patient education and monitoring. As more patients are treated with current and emerging immuno-oncology therapies, the roles of pharmacists in irAE management will continue to evolve.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: R.D.H. reports grants from AstraZeneca, Bristol-Myers Squibb, Merck, Novartis, and Regeneron, and personal consulting fees from Genentech. K.D.J. reports personal fees from Amgen, Bristol-Myers Squibb, Genentech, INSYS, and TESARO. PM and VAT have nothing to disclose.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Medical writing support and editorial assistance were provided by Amrita Dervan, MSc, and Jay Rathi, MA, of Spark Medica Inc, funded by Bristol-Myers Squibb, according to Good Publication Practice guidelines.
References
- 1. Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378(2):158–168. doi:10.1056/NEJMra1703481 [DOI] [PubMed] [Google Scholar]
- 2. IMFINZI® (Durvalumab) [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2019. [Google Scholar]
- 3. YERVOY® (ipilimumab) [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2019. [Google Scholar]
- 4. OPDIVO® (nivolumab) [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2019. [Google Scholar]
- 5. BAVENCIO® (avelumab) [package insert]. Rockland, MA: EMD Serono Inc; 2019. [Google Scholar]
- 6. TECENTRIQ® (atezolizumab) [package insert]. South San Francisco, CA: Genentech Inc; 2019. [Google Scholar]
- 7. KEYTRUDA® (pembrolizumab) [package insert]. Whitehouse Station, NJ: Merck & Co Inc; 2019. [Google Scholar]
- 8. LIBTAYO® (cemiplimab) [package insert]. Tarrytown, NY: Regeneron Pharmaceuticals Inc; 2018. [Google Scholar]
- 9. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi:10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Buchbinder EI, Desai A. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol. 2016;39(1):98–106. doi:10.1097/COC.0000000000000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tarhini A, Lo E, Minor DR. Releasing the brake on the immune system: ipilimumab in melanoma and other tumors. Cancer Biother Radiopharm. 2010;25(6):601–613. doi:10.1089/cbr.2010.0865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139–148. doi:10.1016/j.ejca.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 13. Haanen J, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl 4):iv119–iv142. doi:10.1093/annonc/mdx225. [DOI] [PubMed] [Google Scholar]
- 14. Puzanov I, Diab A, Abdallah K, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5(1):95 doi:10.1186/s40425-017-0300-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Management of Immunotherapy-Related Toxicities, Version 2.2019. © National Comprehensive Cancer Network, Inc. 2019. All rights reserved. Accessed October 19, 2019. To view the most recent and complete version of the guideline, go online to NCCN.org. (NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.)
- 16. Kumar V, Chaudhary N, Garg M, et al. Current diagnosis and management of immune related adverse events (irAEs) induced by immune checkpoint inhibitor therapy. Front Pharmacol. 2017;8:49 doi:10.3389/fphar.2017.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hu Y-B, Zhang Q, Li H-J, et al. Evaluation of rare but severe immune related adverse effects in PD-1 and PD-L1 inhibitors in non-small cell lung cancer: a meta-analysis. Transl Lung Cancer Res. 2017;6(suppl 1):S8–S20. doi:10.21037/tlcr.2017.12.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Khoja L, Day D, Wei-Wu C, et al. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol. 2017;28(10):2377–2385. doi:10.1093/annonc/mdx286. [DOI] [PubMed] [Google Scholar]
- 19. Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377:1345–1356. doi:10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Caturegli P, Di Dalmazi G, Lombardi M, et al. Hypophysitis secondary to cytotoxic T-lymphocyte-associated protein 4 blockade: insights into pathogenesis from an autopsy series. Am J Pathol. 2016;186(12):3225–3235. doi:10.1016/j.ajpath.2016.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(13):23–34. doi:10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hammers HJ, Plimack ER, Infante JR, et al. Safety and efficacy of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma: the CheckMate 016 study. J Clin Oncol. 2017;35(34):3851–3858. doi:10.1200/jco.2016.72.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rizvi NA, Hellmann MD, Brahmer JR, et al. Nivolumab in combination with platinum-based doublet chemotherapy for first-line treatment of advanced non–small-cell lung cancer. J Clin Oncol. 2016;34(25):2969–2979. doi:10.1200/JCO.2016.66.9861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Antonia SJ, López-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol. 2016;17(7):883–895. doi:10.1016/S1470-2045(16)30098-5. [DOI] [PubMed] [Google Scholar]
- 25. Villadolid J, Amin A. Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities. Transl Lung Cancer Res. 2015;4(5):560–575. doi:10.3978/j.issn.2218-6751.2015.06.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sosa A, Lopez Cadena E, Simon Olive C, et al. Clinical assessment of immune-related adverse events. Ther Adv Med Oncol. 2018;10:1758835918764628 doi:10.1177/1758835918764628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Davies M, Duffield EA. Safety of checkpoint inhibitors for cancer treatment: strategies for patient monitoring and management of immune-mediated adverse events. Immunotargets Ther. 2017;6:51–71. doi: 10.2147/ITT.S141577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Eigentler TK, Hassel JC, Berking C, et al. Diagnosis, monitoring and management of immune-related adverse drug reactions of anti-PD-1 antibody therapy. Cancer Treat Rev. 2016;45:7–18. doi:10.1016/j.ctrv.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 29. Champiat S, Lambotte O, Barreau E, et al. Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann Oncol. 2016;27(4):559–574. doi:10.1093/annonc/mdv623. [DOI] [PubMed] [Google Scholar]
- 30. Daly LE, Power DG, O’Reilly Á, et al. The impact of body composition parameters on ipilimumab toxicity and survival in patients with metastatic melanoma. Br J Cancer. 2017;116(3):310–317. doi:10.1038/bjc.2016.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Baik CS, Rubin EH, Forde PM, et al. Immuno-oncology clinical trial design: limitations, challenges, and opportunities. Clin Cancer Res. 2017;23(17):4992–5002. doi:10.1158/1078-0432.CCR-16-3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Johnson DB, Sullivan RJ, Ott PA, et al. Ipilimumab therapy in patients with advanced melanoma and preexisting autoimmune disorders. JAMA Oncol. 2016;2(2):234–240. doi:10.1001/jamaoncol.2015.4368. [DOI] [PubMed] [Google Scholar]
- 33. Menzies AM, Johnson DB, Ramanujam S, et al. Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann Oncol. 2017;28(2):368–376. doi:10.1093/annonc/mdw443. [DOI] [PubMed] [Google Scholar]
- 34. Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372(21):2006–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. O’Day S, Weber JS, Wolchok JD, et al. Effectiveness of treatment guidance on diarrhea and colitis across ipilimumab studies. J Clin Oncol. 2011;29:8554–8554. doi:10.1200/jco.2011.29.15_suppl.8554. [Google Scholar]
- 36. Bosworth T. Tapering protocols needed to manage checkpoint inhibitor AEs; 2018. http://www.clinicaloncology.com/Current-Practice/Article/04-15/Tapering-Protocols-Needed-To-Manage-Checkpoint-Inhibitor-AEs-/48704. Accessed July 16, 2018.
- 37. Upton J. Steroid tapering guidance; 2017. https://www.clatterbridgecc.nhs.uk/application/files/1314/9086/9715/Steroid_Tapering_and_supportive_treatment_Guidance_V1.0.pdf. Accessed July 15, 2018.
- 38. Horvat TZ, Adel NG, Dang T-O, et al. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol. 2015;33(28):3193–3198. doi:10.1200/jco.2015.60.8448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Weber JS, Hodi FS, Wolchok JD, et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol. 2017;35(7):785–792. doi:10.1200/jco.2015.66.1389. [DOI] [PubMed] [Google Scholar]
- 40. Abu-Sbeih H, Ali FS, Alsaadi D, et al. Outcomes of vedolizumab therapy in patients with immune checkpoint inhibitor–induced colitis: a multi-center study. J Immunother Cancer. 2018;6(1):142 doi:10.1186/s40425-018-0461-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bergqvist V, Hertervig E, Gedeon P, et al. Vedolizumab treatment for immune checkpoint inhibitor-induced enterocolitis. Cancer Immunol Immunother. 2017;66(5):581–592. doi:10.1007/s00262-017-1962-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liu D, Ahmet A, Ward L, et al. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin Immunol. 2013;9(1):30–30. doi:10.1186/1710-1492-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Flaherty K, Sharma P. Both Patients and Clinicians Face Challenges in Recognizing and Reporting Immune-Related Adverse Events; 2018. http://www.ascopost.com/issues/may-25-2018/challenges-in-recognizing-and-reporting-iraes/. Accessed July 15, 2018.
- 44. Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36(17):1714–1768. doi:10.1200/jco.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hematology/Oncology Pharmacy Association. Immune-related adverse events symptom checklist. http://hoparx.org/resources/guidelines-standards-summaries. Accessed October 23, 2019.
- 46. Bristol-Myers Squibb. Yervoy® patient monitoring checklist. 2018. http://www.hcp.yervoy.com/servlet/servlet.FileDownload?file=00Pi000000PHxB9EAL. Accessed July 16, 2018.
- 47. Bristol-Myers Squibb. OpdivoTM patient monitoring checklist; 2017. http://www.opdivohcp.com/servlet/servlet.FileDownload?file=00Pi000000ijs1lEAA. Accessed July 16, 2018.