Abstract
Background: We describe cancer patients with coronavirus disease-2019 (COVID-19) infection treated at the Piacenza’s general hospital (north Italy). Materials & methods: Twenty-five cancer patients infected by COVID-19 admitted at the Piacenza’s general hospital from 21 February to 18 March 2020. Outcome from the infection were compared with infected noncancer patients. Results: Twenty patients (80%) were treated with antiviral therapy and hydroxychloroquine and five (20%) received hydroxychloroquine alone. Nine (36%) patients died, while 16 (64%) overcome the infection. In the control group the mortality was 16.13% and the overcome from infection was 83.87%. Conclusion: Mortality for COVID-19 was greater in cancer patients when compared with noncancer patients, worse prognosis for older age, women and patients treated with hydroxychloroquine alone. However, the comparisons did not reach statistical significance in most cases. This could be due to the small sample size that is the main limitation of the study.
Keywords: : cancer patients and COVID-19, cobicistat, darunavir, hydroxychloroquine, infection and cancer, lopinavir, oncology and coronavirus, ritonavir
In December 2019, a new pathogen enveloped RNA betacoronavirus has been identified and named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) which causes severe pulmonary disease in 14% of infected people [1,2]. The WHO has declared coronavirus disease 2019 (COVID-19), caused by SARS-CoV-2, a public health emergency of international concern and recently declared pandemic the COVID-19 [3]. In Italy, the most involved regions by COVID-19 are Lombardy, Emilia Romagna and Veneto. The city of Piacenza (Emilia Romagna region) is very near to the epicenter of the outbreak of COVID-19, and the catastrophic nature of Lombardy’s outbreak has been widely published [4].
Oncologic patients are considered more susceptible to infections than individuals without cancer, due to systemic immunosuppressive state caused by cancer itself and/or anticancer treatments such as chemotherapy, radiotherapy and surgery [5–8]. Furthermore, these patients carry poorer prognosis in case of infective complications and might be at increased risk of viral infections such as COVID-19. However, it must be emphasized that data on cancer patients affected by COVID-19 are fragmentary and very poor, 18 patients with cancer and COVID-19 infection have been previously reported from Wuhan by Liang et al. [9] and 20 by Yu et al. [10]. In this report we describe 25 cancer patients with COVID-19 infection treated in a western country.
Materials & methods
Patients
In this report, we analyzed data of 25 consecutive cancer patients, with a previous diagnosis of cancer, affected by SARS-CoV-2 and hospitalized between 21 February and 18 March 2020, at the Piacenza’s general hospital (north Italy). In all cases there was evidence of contacts with subjects infected by COVID-19. All the cases were diagnosed with laboratory-confirmed SARS-CoV-2 infection, with reverse-transcription polymerase chain reaction in nasal-pharyngeal swabs, which caused the respiratory illness COVID-19, defined as an oxygen saturation (SaO2) of 94% or less while they were breathing ambient air, or a ratio of the partial pressure of oxygen (PaO2) to the fraction of inspired oxygen (FiO2) of less than 300 mmHg [11]. A total of 13 cases (52%) had history of smoking for more than 20 years. The majority of patients, 19 (76%), had several comorbidities (in particular diabetes mellitus [32%], hypertension [64%] and chronic obstructive pulmonary disease [28%]). The medical records of patients were analyzed by a team of oncologists, data were obtained with data collection forms from electronic medical records. In addition, survival and overcome from infection of cancer patients COVID-19-positive were evaluated compared with a control group of patients COVID-19-positive hospitalized in the same period comparable and matched by age, sex, pneumonia and antiviral treatment.
Data analysis
Data were collected using Microsoft Excel (Microsoft Office version 2010). For all patients, registered with a unique recognition code, we recorded: age, sex, region, type of cancer, stage, comorbidities, history of smoking, cancer therapy ongoing at the time of COVID-19 infection, hospitalization date, radiological examinations, presence of interstitial pneumonia and laterality, antiviral therapy administered and outcome.
The quantitative variables are described by mean ± standard deviation, the qualitative ones by absolute and percentage frequencies. Categorical variables were compared using the Fisher’s exact test (due to the low number of observation) and, once the symmetry parameters were verified, we performed the Mann–Whitney test to compare continuous variables. p-value of <0.05 was considered significant. Rstudio-1.2.1335 program was used for the analysis.
Treatment
Hydroxychloroquine with or without antiviral treatment has been incorporated in our regional guideline to treat COVID-19 [12] as previously reported [13,14].
During the hospitalization, 20 patients (80%) were treated with combination therapy: 10 (50%) received lopinavir and ritonavir tablets (800/200 mg daily) plus hydroxychloroquine tablets (400 mg daily) for seven days, while the other 10 (50%) patients received darunavir and cobicistat (800/150 mg daily) plus hydroxychloroquine tablets (400 mg daily). Among these, additional intravenous methylprednisolone (40 mg daily) and large spectrum antibiotic therapy (ceftriaxone and azithromycin) were administered. The remaining five patients (20%) received hydroxychloroquine alone. Moreover, for all patients oxygen therapy was used to relieve dyspnea and one patient needed noninvasive ventilator support (continuous positive airway pressure-C-PAP) in intensive care unit (ICU).
Laboratory tests
Nasopharyngeal swab specimens were collected according to the Center for Disease Control and Prevention Guidelines [15]. Diagnostic kit for IgM antibodies to Mycoplasma pneumoniae, Chlamydia pneumoniae and Legionella pneumophila was used for detecting three kinds of common respiratory pathogens. Other laboratory tests were implemented as well such as lymphocytes count and CRP. Radiologic examinations were performed by chest x-ray or computerized thomography. All the patients received a radiological evaluation of the chest: three (12%) with a chest x-ray and 22 (88%) with a chest CT scan.
Results
Patients characteristic are reported in Table 1. The mean age of these patients was 71.64 ± 10.08 years (range 50–84 years). A total of seven (28%) patients were younger or equal to 65 years of age, seven (28%) between 66 and 75 years of age and 11 (44%) patients older than or equal to 76 years of age. Male patients were prevalent (male/female 20/5), accounting for 80% of 25 patients, while in our center the majority of cancer patients are women (55%). All these patients were Italian. The most common tumor site was lung (eight patients, 32%) followed by gastroenteric tumor (six patients, 24%), genitourinary tumor (six patients, 24%), breast cancer (two patients, 8%), hematologic tumor (two patients, 8%) and undefined tumor site (one patient, 4%). A total of 19 (76%) showed an advanced/metastatic disease (stage III and IV).
Table 1. Clinical/radiological characteristics, treatment and outcome of the 25 patients with cancer and coronavirus disease-2019 infection.
| Variable | Total patients (n = 25) | Dead patients (n = 9) | Alive patients (n = 16) | p-value |
|---|---|---|---|---|
| Age (years), mean (range) | 71.64 ± 10.08 (50–84) | 74.44 ± 7.21 (64–84) | 68.38 ± 10.16 (50–83) | 0.02 |
| – ≤65 (%) | 7 (28) | 1 (11.11) | 6 (37.5) | 0.25 |
| – 66–75 (%) | 7 (28) | 2 (22.22) | 5 (31.25) | |
| – ≥76 (%) | 11 (44) | 6 (66.67) | 5 (31.25) | |
| Sex | ||||
| – Male (%) | 20 (80) | 5 (55.56) | 15 (93.75) | 0.04 |
| – Female (%) | 5 (20) | 4 (44.44) | 1 (6.25) | |
| Tumor site | ||||
| – Breast (%) | 2 (8) | 2 (22.22) | 0 (0) | 0.02 |
| – Gastroenteric (%) | 6 (24) | 0 (0) | 6 (37.5) | |
| – Genitourinary (%) | 6 (24) | 3 (33.34) | 3 (18.75) | |
| – Hematologic (%) | 2 (8) | 2 (22.22) | 0 (0) | |
| – Lung (%) | 8 (32) | 2 (22.22) | 6 (37.5) | |
| – Undefined (%) | 1 (4) | 0 (0) | 1 (6.25) | |
| Stage | ||||
| – IV (%) | 19 (76) | 6 (66.66) | 13 (81.25) | 0.63 |
| – NED (%) | 6 (24) | 3 (33.34) | 3 (18.75) | |
| Comorbidity | ||||
| Yes (%) | 19 (76) | 6 (66.66) | 13 (81.25) | |
| – COPD (%) | 7 (28) | 3 (33.34) | 4 (25) | 0.67 |
| – Diabetes (%) | 8 (32) | 2 (22.22) | 6 (37.5) | 0.66 |
| – Hypertension (%) | 16 (64) | 5 (55.56) | 11 (68.75) | 0.67 |
| No (%) | 6 (24) | 3 (33.34) | 3 (18.75) | |
| Smoking | ||||
| Yes (%) | 13 (52) | 4 (44.44) | 9 (56.25) | 0.69 |
| Cancer therapy | ||||
| Yes (%) | 12 (48) | 3 (33.33) | 9 (56.25) | |
| – Chemotherapy (%) | 8 (66.67) | 3 (100) | 5 (55.56) | 0.39 |
| – Immunotherapy (%) | 4 (33.33) | 0 (0) | 4 (44.44) | |
| No (%) | 13 (52) | 6 (66.67) | 7 (43.75) | |
| Radiological examination | ||||
| – Chest x-ray (%) | 3 (12) | 1 (11.11) | 2 (12.5) | 1 |
| – Chest CT scan (%) | 22 (88) | 8 (88.89) | 14 (87.5) | |
| Interstitial pneumonia | ||||
| – Bilateral (%) | 22 (88) | 9 (100) | 13 (81.25) | 0.28 |
| – Unilateral (%) | 3 (12) | 0 (0) | 3 (18.75) | |
| Laboratory values, mean (range) | ||||
| – Lymphocyte (×103/Ml) | 1.51 ± 3.21 (0.05–16.5) | 2.67 ± 5.32 (0.05–16.5) | 0.85 ± 0.41 (0.2–1.92) | 0.55 |
| – CRP (mg/dl) | 11.30 ± 6.41 (3–29) | 14.82 ± 7.54 (4.9–29) | 9.33 ± 4.87 (3–17) | 0.047 |
| Treatment | ||||
| – Lopinavir and ritonavir + hydroxychloroquine (%) | 10 (40) | 3 (33.33) | 7 (43.75) | 0.12 |
| – Darunavir and cobicistat + hydroxychloroquine (%) | 10 (40) | 2 (22.22) | 8 (50) | |
| – Hydroxychloroquine (%) | 5 (20) | 4 (44.45) | 1 (6.25) |
Note: Significant p-values reported in bold terms.
COPD: Chronic obstructive pulmonary disease; NED: No evidence of disease.
At the time of the hospitalization 12 (48%) patients were treated with anticancer therapy: eight (66.67%) with chemotherapy and four (33.33%) with immunotherapy; 13 patients (52%) were not on active anticancer treatment.
Ten out of 25 patients were in general good clinical conditions before hospitalization. Before treatment, various clinical symptoms were reported. All patients presented with fevers (range from 37.5 to 39.8°C) and slight to severe cough without phlegm. All patients presented also moderate to severe fatigue. No patients reported chest pain, diarrhea or other gastrointestinal symptoms. The majority of patients had basically normal or slightly decreased white blood cell count (except the two patients with hematological malignancies), and absolute neutrophil count was normal throughout the clinical course.
The absolute lymphocyte count was low and CRP was increased in all cases before treatment, that gradually reduced after treatment in responding patients. Patients were also tested for three kinds of common respiratory pathogens and they were all negative.
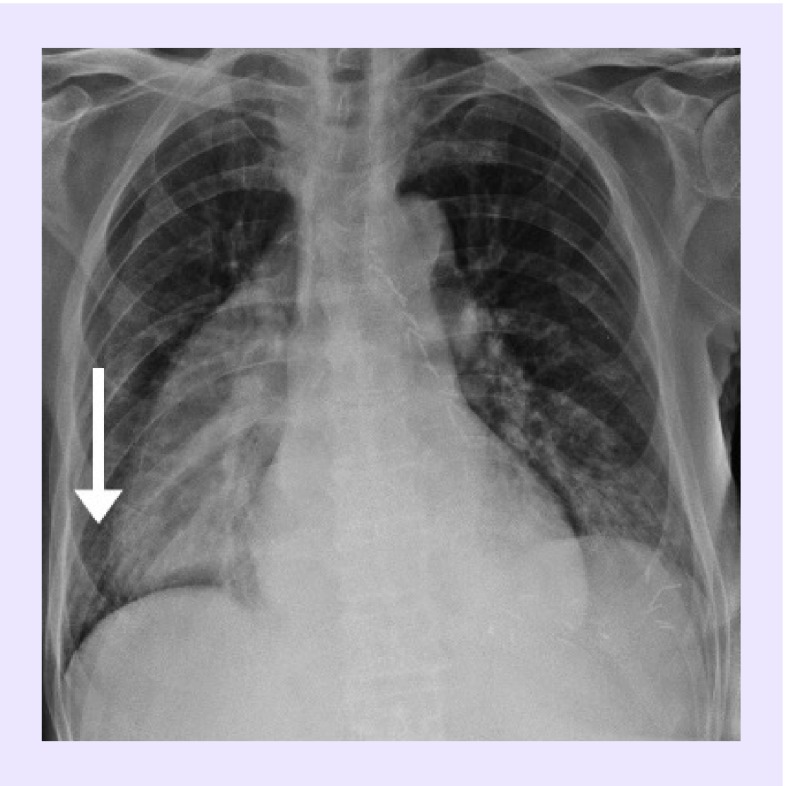
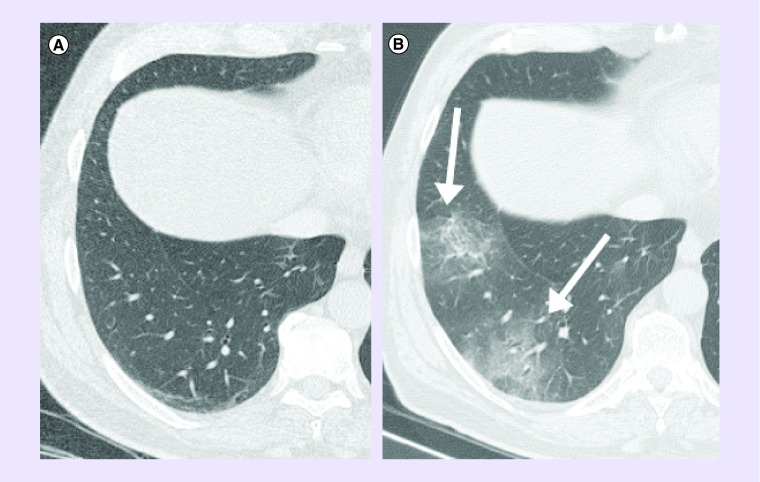
Radiological evaluation revealed in 25 patients interstitial pneumonia, 22 (88%) bilateral and three (12%) unilateral. Common radiological findings were ground-glass opacities, shadowing, interstitial abnormalities and ‘crazy paving’. Cases of typical lung lesions are shown in Figures 1 and 2.
Figure 1. Bilateral interstitial pneumonia in a patient with esophageal cancer.
Chest x-ray of a 70-year-old male with advanced esophageal cancer receiving FOLFIRI chemotherapy and COVID-19 infection: presence of bilateral interstitial thickening at the middle and lower pulmonary fields, particularly on the right side (arrow).
Figure 2. Bilateral pneumonia in a patient with lung cancer.
A 64-year-old male with lung squamous cell carcinoma treated with pembrolizumab and COVID-19 infection: (A) chest CT performed 14-days before infection shows no infiltrates, while (B) CT at the admission shows bilateral rounded ground-glass opacities (arrows).
Prognostic factors are reported on Table 2: older age, mean 74.44 ± 7.21 years for dead patients versus 68.38 ± 10.16 years for alive patients, p = 0.02, female sex, four of five women (80%) died compared with male where only five (25%) died p = 0.04, while in the control group the mortality was higher in men 20% as compared with female 12.5%. Tumor site: all the patient with gastroenteric cancer and 75% of patients with lung cancer overcome the infection and are alive. In addition, CRP value showed prognostic factor, higher CRP mean value, 14.82 ± 7.54 (mg/dl) in the dead group versus 9.33 ± 4.87 (mg/dl) in the alive group, p = 0.047. In addition, combination antiviral therapy with hydroxychloroquine shows better response and seems improve survival when compared with hydroxychloroquine alone. It must be emphasized that all the patients treated with immunotherapy for lung cancer were alive.
Table 2. . Patients with cancer and coronavirus disease-2019 infection – prognostic factors.
| Variable | Dead patients (n = 9) | Alive patients (n = 16) | p-value |
|---|---|---|---|
| Age (years), mean (range) | 74.44 ± 7.21 (64–84) | 68.38 ± 10.16 (50–83) | 0.02 |
| Sex | |||
| – Male (%) | 5/20 (25) | 15/20 (75) | 0.04 |
| – Female (%) | 4/5 (80) | 1/5 (20) | |
| Tumor site | |||
| – Breast (%) | 2/2 (100) | 0/2 (0) | 0.02 |
| – Gastroenteric (%) | 0/6 (0) | 6/6 (100) | |
| – Genitourinary (%) | 3/6 (50) | 3/6 (50) | |
| – Hematologic (%) | 2/2 (100) | 0/2 (0) | |
| – Lung (%) | 2/8 (25) | 6/8 (75) | |
| – Undefined (%) | 0/1 (0) | 1/1 (100) | |
| CRP (mg/dl) | 14.82 ± 7.54 (4.9–29) | 9.33 ± 4.87 (3–17) | 0.047 |
| Treatment | |||
| – Lopinavir and ritonavir+ hydroxychloroquine (%) | 3/10 (30) | 7/10 (70) | 0.12 |
| – Darunavir and cobicistat + hydroxychloroquine (%) | 2/10 (20) | 8/10 (80) | |
| – Hydroxychloroquine (%) | 4/5 (80) | 1/5 (20) |
Note: Significant p-values reported in bold terms.
Of the 25 CoV-2-infected cancer patients, at the date of 18 March 2020, nine (36%) are dead and 16 (64%) are alive, with improvement from pneumonia, in the control group of patients hospitalized and treated with the same protocol in the same period, 16.13% are dead and 83.87% are alive p = 0.12. In the control group the mortality is higher in male (20%; p = 1) while in oncologic patients the mortality is higher in women (80%; p = 0.01; Table 3).
Table 3. Survival differences between cancer patients and control group (noncancer patients) infected by coronavirus disease-2019.
| Group | Dead patients | p-value |
|---|---|---|
| Cancer patients (n = 25) (%) | 9 (36) | 0.12 |
| Control group (n = 31) (%) | 5 (16.13) | |
| Cancer patients-male (n = 20) (%) | 5/20 (25) | 1 |
| Control group-male (n = 15) (%) | 3/15 (20) | |
| Cancer patients-female (n = 5) (%) | 4/5 (80) | 0.01 |
| Control group-female (n = 16) (%) | 2/16 (12.5) |
Note: Significant p-values reported in bold terms.
Conclusion
In this report, we described the first series of cancer patients with COVID-19 treated in western country. Italy has been the first nation in Europe experiencing an outbreak of COVID-19 and Piacenza is very near to the epicenter of the Lombardy’s outbreak (only 10 km). We are aware our results provide some descriptive informations, but the prognostic role of these findings is still potential and should be clarified by further and large-scale studies.
These 25 cancer patients with COVID-19 infection manifested respiratory symptoms including fever, cough and shortness of breath, no gastrointestinal symptoms were recorded.
A total of 20 patients had a long history (more than 20 years) of smoking, however this finding does not seem to correlate with a worse prognosis. This fact should obviously be confirmed by other case studies and larger series.
According to the Guideline for Diagnosis and Treatment of Novel Coronavirus-infected Pneumonia (Trial version 7), patients were clinically classified into four categories including mild, moderate, severe and critical types [16,17]. In our study, there were 12 severely ill patients and 13 moderate patients. However, we observed that the disease of most patients showed a moderate course. In particular only four out of the nine recorded deaths were primarily ascribable to COVID-19; for the remaining cases death was related also to the progression of the cancer other than the COVID-19 infection.
In our patient series, lung cancer had the highest frequency; four of these patients receiving immunotherapy (pembrolizumab) experienced a more moderate and favorable course of the infection. In keeping with previous study [18].
According with the current literature, also in our case series patients laboratory tests showed lymphopenia and increase PCR [19].
As described in the available literature, radiologic findings were bilateral interstitial abnormalities at chest x-ray or ground-glass opacities, consolidation and crazy-paving pattern at chest CT scan [20].
After discharge, our patients underwent follow-up: daily phone control and oximeter examination. Chest CT scan, nasopharyngeal swab and clinical examination were repeated after 15 days from the discarge.
Prognostic factors in these series seem to be: age (worse prognosis in older patients), sex (worse prognosis for women) and type of antiviral treatment. Immunotherapy in lung cancer seems to improve prognosis.
However, we are aware that this series is limited, though some informations can be available for cancer patients and COVID-19 infection.
Future perspective
COVID-19 diffusion represent a great harm for cancer patients that are at greater risk of infection. Oncologist and their patients are eagerly waiting for a vaccine against COVID-19, and we must urgently know its impact among cancer patients, to protect them from this infection and to treat precociously when infection is present. We believe that heavely affected countries as Italy can serve to find knowledge overcome this pandemic.
Summary points.
Cancer patients may be more susceptible to infection respect to noncancer patients.
We reported data about the first 25 coronavirus disease 2019 (COVID-19) cancer patients from a western country.
Mortality for COVID-19 is greater in cancer patients when compared with noncancer patients.
COVID-19 infection seems higher in men and mortality higher in women.
Combinations antiviral therapy plus hydroxychloroquine seems superior to hydroxychloroquine alone.
The majority of cancer patients seems to overcome COVID-19 infection.
Older age and female sex are unfavorable prognostic factors.
Further and large-scale studies are needed.
Author contributions
L Cavanna, EM Stroppa, I Toscani, C Citterio drafted the work or revised it critically for important intellectual content. Final approval of the version to be published was given by L Cavanna, EM Stroppa, I Toscani, C Citterio, E Anselmi, E Zaffignani and M Codeluppi. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved was under L Cavanna, EM Stroppa, I Toscani, C Citterio drafted the work or revised it critically for important intellectual content.
Financial & competing interests disclosure
L Cavanna performed consulting or advisory role for AstraZeneca, Merck. Travel, accommodation, expenses by Celgene, Pfizer, Ipsen. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Lu R, Zhao X, Li J. et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 395(10224), 565–574 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72? 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 323(13), 1239–1242 (2020). [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Coronavirus disease (COVID-19) outbreak (2020). www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 ; • Contains the WHO declaration of coronavirus disease 2019 (COVID-19) pandemic.
- 4.Horowitz J. Italy’s healthcare system groans under coronavirus- a warning to the world. New York Times; (2020). www.nytimes.com/2020/03/12/world/europe/12italy-coronavirus-health-care.html [Google Scholar]; • Contains data about Italy’s Health Care System.
- 5.Kamboj M, Sepkowitz KA. Nosocomial infections in patients with cancer. Lancet Oncol. 10(6), 589–597 (2009). [DOI] [PubMed] [Google Scholar]
- 6.Li JY, Duan XF, Wang LP. et al. Selective depletion of regulatory T cell subsets by docetaxel treatment in patients with non-small-cell lung cancer. J. Immunol. Res. 2014, 286170 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Longbottom ER, Torrance HD, Owen HC. et al. Features of postoperative immune suppression are reversible with interferon gamma and independent of interleukin-6 pathways. Ann. Surg. 264(2), 370–377 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Sica A, Massarotti M. Myeloid suppressor cells in cancer and autoimmunity. J. Autoimmun. 85, 117–125 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Liang W, Guan W, Chen R. et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 21(3), 335–337 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Contains first data about patients with cancer and COVID-19 infection.
- 10.Yu J, Ouyang W, Chua MLK. et al. SARS-CoV-2 transmission in cancer patients of a tertiary hospital in Wuhan. JAMA Oncol. (2020) (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao B, Wang Y, Wen D. et al. A trial of lopinavir-ritonavir in adults hospitalized with severe COVID-19. N. Engl. J. Med. (2020) (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Società Italiana di Malattie Infettive e Tropicali SEZIONE REGIONE LOMBARDIA (SIMIT). Vademecum per la cura delle persone con malattia da COVID-19 (2020). www.simit.org/medias/1569-covid19-vademecum-13-03-202.pdf
- 13.Time. The Italian doctor flattening the curve by treating COVID-19 patients in their homes (2020). http://time.com/5816874/italy-coronavirus-patients-treating-home/
- 14.Time. Heroes of the frontline [collection] (2020). https://time.com/collection/coronavirus-heroes/
- 15.Centers for Disease Control and Prevention. Interim guidelines for collecting, handling, and testing clinical specimens from patients under investigation (PUIs) for 2019 novel coronavirus (2019-nCoV) (2020). www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html ; • Contains guidelines for collecting, handling and testing clinical specimens from patients under investigation for COVID-19.
- 16.National Health Commission. Guideline for diagnosis and treatment of novel coronavirus-infected pneumonia (trial version 7) (2020). www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989/files/ce3e6945832a438eaae415350a8ce964.pdf
- 17.Luzzani A, Polati E, Dorizzi R. et al. Comparison of procalcitonin and C-reactive protein as markers of sepsis. Crit. Care Med. 31(6), 1737–1741 (2003). [DOI] [PubMed] [Google Scholar]
- 18.Bersanelli M. Controversies about COVID-19 and anticancer treatment with immune checkpoint inhibitors. Immunotherapy (2020) (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silverstein WK, Stroud L, Cleghorn GE. et al. First imported case of 2019 novel coronavirus in Canada, presenting as mild pneumonia. Lancet 395(10225), P734 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Contains data abut COVID-19 patients laboratory test.
- 20.Shi H, Han X, Jiang N. et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect. Dis. 20(4), 425–434 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Explains radiologic findings in patients with COVID-19.