Abstract
Objective:
There is no up-to-date information regarding the presentation, management, and clinical course of patients with acute myocardial infarction (MI) in Turkey. The TURKMI registry is designed to provide an insight into the characteristics, management from the symptoms onset to the hospital discharge, and outcome of patients with acute MI in Turkey.
Methods:
The TURKMI study, as a nationwide registry, will be conducted in 50 percutaneous coronary intervention-capable centers, selected from each EuroStat NUTS region in Turkey according to their population sampling weight, prioritizing the hospital volume in each region. All consecutive patients with acute MI admitted to the coronary care units within the 48 hours of the symptoms onset will be prospectively enrolled during a predefined 2-week period.
The first step of the study has a cross-sectional design in which baseline information such as symptoms, risk factors, time periods at each step from the symptoms onset to revascularization, way of arrival to hospital, biochemical analysis, and in-hospital management and outcome will be assessed. The second step has a cohort characteristic in which the enrolled patients will be followed-up up to 2 years. Follow-up visits will be conducted at the 1st, 6th, 12th, and 24th month, and predictors and risk of cardiovascular events and implementation of guidelines will be assessed as secondary outcomes.
Conclusion:
The national TURKMI registry is expected to provide important information to improve the national policy regarding diagnosing, management, and outcomes of MI in Turkey.
Keywords: acute myocardial infarction, registry, Turkey, coronary artery disease
Introduction
As death occurs in one half of cases within few hours following the symptoms onset, mortality can only be minimized with rapid diagnosing and treatment of acute myocardial infarction (MI) (1-4). Current treatment guidelines emphasize the importance of blood flow restoration to the jeopardized myocardium as early as possible with subsequent effective prevention measures and attainment of treatment goals (5, 6). However, the implementation of the recommended guidelines is not easy. Many countries have created national/international MI or acute coronary syndrome registries to identify the extent to which the guideline recommendations have been implemented in clinical practice (4, 7, 8).
In general, registries are helpful in collecting data rapidly and efficiently (9). They allow an analysis of a specific disease under real-life conditions. Moreover, registries may also provide a perspective of the acute MI management at a country level and also may allow a comparison of the results with larger reference populations that may lead to an improvement in the quality and consistency of practice (10). Indeed, many countries have revised their national health policies according to the problems encountered in their national MI registries. Furthermore, by repeating their national acute MI database registrations every 2 or 5 years, many countries may have an opportunity to continuously revise their health policies to capture the updated standards.
In Turkey, such a nationwide acute MI registry is currently unavailable. The only national acute MI study (TUMAR) (11) reflecting the country’s population distribution was conducted in 1998/1999 when the acute coronary interventions were not widely available. Moreover, treatment guidelines and treatment modalities all have been improved since that time with more aggressive targets. To fill this gap, a nationwide registry, TURKMI, will be conducted to provide an insight into the real-life management of patients with acute MI in cardiology centers in Turkey.
Methods
TURKMI (clinicaltrials.gov NCT04241770) is designed as a national, multicentric, observational study, and it has two parts. The first (and the main) part has a cross-sectional study design and is planned to assess the primary objective of how patients with acute MI are currently managed in Turkey, and to obtain their clinical characteristics. Also, determining of differences and disparities in the presentation, management, and outcomes of acute MI by age and gender was planned as a part of primary endpoint. The second part of the TURKMI study has a cohort design, and it includes the follow-up of at least 2 years to assess the risk and predictors of cardiovascular (CV) events as secondary endpoints.
Study protocol has been reviewed and approved by the Ethics Committee of University of Health Sciences, İstanbul Meh-met Akif Ersoy Thoracic and Cardiovascular Surgery Training and Research Hospital (No: 2018-46; Date: 09.10.2018). Written informed consent will be obtained from all participants.
Study population
The screened population will consist of all consecutive patients presenting with suspected acute MI in the participating hospitals. Men and women aged ≥18 years will be enrolled if they fulfil the following inclusion criteria: 1) being hospitalized within 48 hours from the onset of symptoms of the index event; 2) had a final (discharge) diagnosis of acute MI [either ST elevation MI (STEMI) or non ST elevation (NSTEMI)] with positive troponin levels, and 3) signed informed consent. Patients unwilling or unable to consent will be excluded. To facilitate consecutive enrolment, all patients hospitalized at each participating site with acute MI will be recruited consecutively during the same 2 consecutive weeks (15 days).
The diagnosis of MI will be made when the level of troponin is elevated and at least one of the following criteria are present (12): symptoms compatible with myocardial ischemia, new or presumed new significant ST-T wave changes or left bundle branch block (LBBB) detected on 12-lead ECG, or a new pathological q-wave on ECG (12). A ST elevation consistent with MI will be defined as a new ST elevation at the J point in at least two contiguous leads with the cut-off value of ≥0.1 mV in all leads except V2 and V3 where the cut-off values were ≥0.2 mV in men ≥40 years; ≥0.25 mV in men <40 years, or ≥0.15 mV in women (12). In patients who meet the MI criteria, STEMI will be diagnosed if the ST elevation criteria or a new or presumed new LBBB is present; otherwise NSTEMI will be diagnosed. Posterior STEMI will be diagnosed if the ST depression in leads V1-V3 is accompanied by the ST elevation in inferior and/or lateral leads, or a total or near total lesion is found in the right coronary artery or circumflex artery in patients who undergo coronary angiography.
All enrolled patients will undergo routine clinical assessments and receive the standard medical care, currently performed in routine clinical practice. Drug prescription and indications to perform diagnostic/therapeutic procedures will be left to participating cardiologists’ decision. As an observational protocol, patients will not receive any experimental intervention or treatment as a consequence of their participation in the study. Patients will not receive additional visits other than a routine follow-up.
Sampling and selection of the centers
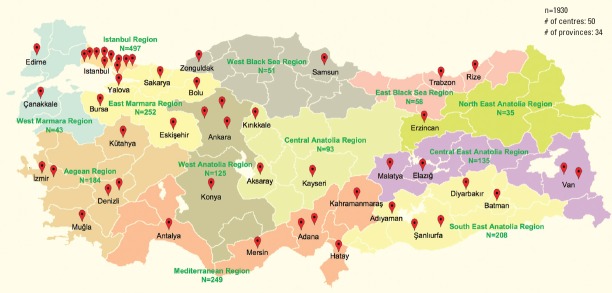
Based on the European Union nomenclature of territorial units for statistics classification (EuroStat NUTS), Turkey has 12 regions (13). The number of people aged ≥18 years living in each region was obtained from the 2018 report of the Turkish Statistical Institute (www.turkstat.gov.tr). Sampling weights were calculated proportionally to the population of each EuroStat NUTS region. Fifty centers were planned to be included in the study, and these centers were selected from the regions according to their sampling weight (Fig. 1). In each region, percutaneous coronary intervention (PCI)-capable hospitals were selected. In this selection, the patient load (i.e., the representativeness) was given the priority.
Figure 1.
Involved centers across the EuroStat NUTS region
This study was planned primarily for descriptive purposes, namely patients’ characteristics, their clinical properties, management from the symptom onset to hospital discharge, and outcome. Therefore, instead of calculating the number of patients required, we a priori planned to include 50 centers due to budget restrictions, and then distributed them proportionally to the population of each region.
Before the enrollment, all centers attended to 2 investigators meeting, conducted to educate the attendees and raise the awareness on the study protocol including inclusion criteria, study procedures, and especially an importance of enrollment of all consecutive patients for the given 15 days. In case of an increased enrolling rate of all consecutive patients, centers and enrollments will be followed on a daily basis, and centers not enrolling will be warned and/or motivated for proper enrollment via a social media communication platform (WhatsApp Group) continually. Moreover, no extra investigational laboratory measurement or procedure will be conducted other than the routine care of acute coronary syndromes.
Definitions and outcomes
Diabetes mellitus is defined as the fasting plasma glucose level of ≥126 mg/dL, a history of diabetes diagnosis/treatment, or glycated hemoglobin (HbA1c) ≥6.5% where available.
Hypertension is defined as the presence of resting systolic blood pressure ≥140 mm Hg, or diastolic blood pressure ≥90 mm Hg on at least two occasions, or a history hypertension diagnosis/treatment.
Hypercholesterolemia is defined as fasting total cholesterol ≥200 mg/dL or LDL cholesterol ≥130 mg/dL or treatment with any lipid-lowering drug, without other underlying conditions that increase cholesterol, such as hypothyroidism or pregnancy. Dyslipidemia is defined as total cholesterol ≥200 mg/dL (or LDL cholesterol ≥130 mg/dL), and/or serum triglycerides ≥150 mg/dL, and/or HDL cholesterol <40 mg/dL in men or <50 mg/dL in women, or diagnosis/treatment of dyslipidemia.
Atrial fibrillation is defined if irregular RR intervals without distinct P waves on ECG, or similar rhythm lasting >30 seconds on Holter records, or a history of atrial fibrillation are present.
Chronic kidney disease is defined as an estimated glomerular filtration rate of <60 mL/min/1.73.
Peripheral arterial disease is defined as prior peripheral arterial revascularization, or a previous diagnosis of peripheral artery disease, or limb claudication along with consistent physical examination findings, or ultrasonographic or angiographic findings of severe arterial disease of peripheral arteries including carotid arteries.
A family history of premature CAD was defined as a history of MI, PCI, or coronary bypass surgery in any first-degree relatives aged <55 for males and <65 years for females.
Following assessments will be made during the follow-up:
-
Death (CV or other reasons), non-fatal MI, stroke, coronary or peripheral revascularization, emergency department visit due to chest pain or dyspnea; hospitalization for heart failure will be assessed at the 1st month and then every 6 months until the 2nd year.
The cause of death will be classified as CV, non-CV, or undetermined cause. The classification will be based on a hierarchy depending on the accessibility: In the first step, the cause of death will be based on the decision of the cardiologists who are taking care of the patients if death occurred during the index admission or at any admission to the cardiology department. In case of problems regarding other organ systems, a shared decision with the cardiologist and their colleagues (neurologist, neurosurgeon, pulmonologist, etc.) will be used. If a consensus cannot be reached, the data will be assessed by a steering committee. In the second step, the reason of death will be based on hospital records if the physicians cannot be reached. In the third step, the cause of death will be determined using the national death record system if the above steps cannot be accessed.
Risk factors will be assessed at each visit to estimate whether modifiable risk factors reach the target levels provided by the current European Society of Cardiology (ESC) guidelines.
Baseline and follow-up data
Investigators will collect the data and complete electronic case report forms (CRFs) for each patient. The steering committee developed the initial CRF, which was subsequently finalized for the field use after pilot testing in five of the participating centers. Table 1 summarizes the variables to be collected at baseline and at each visit. The baseline information will include patient demographic characteristics, medical history, presenting symptoms, clinical characteristics, duration of prehospital delay at each step from the time of onset of acute symptoms to obtaining medical care, electrocardiographic findings, use of cardiac medications, and previous interventional procedures. Each patient’s hospital course will be recorded to ascertain medical or interventional treatments, as well as in-hospital cardiac and extra cardiac outcomes during the index admission. All medications including doses used before (on admission), in-hospital and at discharge will be recorded. All available laboratory values including the lipid profile, fasting blood sugar, creatinine, white blood cell count, hemoglobin, hematocrit, platelet count, triglyceride, HbA1c, thyroid-stimulating hormone, and troponin will also be recorded. ECG, echocardiography, and coronary angiography results will be recorded and uploaded to the electronic data capture program.
Table 1.
Data planned to be collected in the TURKMI registry
| Demographics | Medical background | ECG parameters | Current status including lab, treatment etc. | Laboratory evaluation | |
|---|---|---|---|---|---|
| Baseline | • Date of visit • Date of birth • Gender • Literacy • Occupation • Marital status |
• Patient history (CV comorbidities, cardiac operations/ procedures) • Concomitant diseases • CV risk factors • Hypertension • Hyperlipidemia • Diabetes mellitus • Obesity • Smoking • Menopause • Menstruation details • Alcohol abuse • Stress • Intermittent claudication • Oral contraceptive use • Family history of CVD • Family history of premature CVD |
• LBBB, RBBB • Rhythm • AV block • ST-segment elevation • ST-segment depression • T-wave inversion |
• Presenting symptom • Date of hospitalization • Emergency call • Smoking status • Time-to-onset of symptoms • Diagnosis of hospitalization • Coronary angiography • Primary PCI • Elective PCI • CABG • IABP • Pacemaker/ICD • ECMO • Ventilator |
• Troponin • Peak troponin • FBG, HbA1c • Lipids (LDL, HDL, TG) • Liver enzymes, • TSH • WBC • Hemoglobin • Hematocrit • Platelet count • Creatinine |
| Physical Examination | Echocardiography parameters | Reperfusion Treatment | Medical Treatment | In-Hospital Outcomes | |
| • Waist circumference • Height/weight • Blood pressure • Heart rate |
• On admission all doses • In hospital medication • Discharge treatment |
• Total mortality • CV mortality • Non-cardiac mortality • Adverse events |
|||
| Visit 1 (1st month) | • Physical examination • Blood pressure |
• ECG findings | • Treatment • Adverse Events • CV and non-CV outcomes |
Laboratory evaluation • LDL • TG • HDL • FBG, HbA1c |
|
| Visit 2 (6th month) | • Physical examination • Blood pressure |
• Echocardiography parameters | • ECG findings | • Treatment • Adverse events • CV and non-CV outcomes |
Laboratory evaluation • LDL • TG • HDL • FBG, HbA1c |
| Visit 3 (12th month) | • Physical examination • Blood pressure |
• Echocardiography parameters | • ECG findings | • Treatment • Adverse events • CV and non-CV outcomes |
Laboratory evaluation • LDL • TG • HDL • FBG, HbA1c |
| Visit 4 (24th month) | • Physical examination • Blood pressure |
• Echocardiography parameters | • ECG findings | • Treatment • Adverse events • CV and non-CV outcomes |
Laboratory evaluation • LDL • TG • HDL • FBG, HbA1c |
CVD - cardiovascular disease; LDL - low-density lipoprotein; HDL - high-density lipoprotein; TSH - thyroid-stimulating hormone: FBG- fasting blood glucose; TG- triglyceride;
WBC - white blood cell; LBBB - left bundle branch block; RBBB - right bundle branch block; PCI - percutaneous coronary intervention; CABG - coronary artery bypass graft;
IABP - Intra-aortic balloon pump; ICD - implantable cardioverter-defibrillator; ECMO - extra-corporeal membrane oxygenation
Follow-up outcome measures are planned to be collected at the 1st, 6th, 12th, and 24th month. Data collection for the follow-up will be conducted and retrieved via electronic medical record system. If medical records cannot be reached, then data will be collected by phone calls. In cases lost to follow-up, patient outcomes will be retrieved via the national death record system. At each follow-up visit, information on the current health status, comorbidities, medication regimens, smoking status, alcohol consumption, and any interim cardiac and non-cardiac events will be recorded (Table 1).
Data management
The study data will be uploaded and managed in the OpenClinica (OpenClinica LLC and collaborators, Waltham, MA, USA) hosted by Omega CRO, Ankara, Turkey. OpenClinica is an encrypted, secure, open-source software platform for electronic data capture and clinical data management. Direct remote data entry will be performed by the study centers. The investigators in the study centers will receive training on the use of the mobile devices and the data collection process at the beginning of the study. Data will be extracted from the OpenClinica system into the Excel spreadsheets. These spreadsheets will then be compared for missing data, mismatched data or misrepresented data. If there are any discrepancies, a Query Discrepancy Note will be created about an item that seems incomplete or incorrect and sent to the study centers. Study centers will review that note and respond with information to resolve the query. Using this high-quality data collection system, which is associated with the digital platform, is beneficial in limiting the occurrence of data errors, which limits the number of data queries that may be raised after the study completion.
Statistical analysis
Categorical variables will be presented as the number and percentage, and compared using the chi-squared test, Fisher’s exact test, or the Mantel–Haenszel test between the independent groups such as gender and risk categories. Graphical methods (such as histogram and probability plots) and analytical method (Komogrov–Smirnov test) will be used to assess whether continuous variables have a normal distribution. These variables were given as the mean±standard deviation or median and interquartile range, depending on whether they have normal distribution or not, and compared using an independent t-test or the Mann–Whitney U test. Repeated measures analysis of variance or the Friedman test will be used to assess the differences between the measurements taken at different time periods for continuous variables that have normal or non-normal distribution, respectively, logistic regression was planned to be used in assessing the risk factors for in-hospital cardiovascular outcomes. The Kaplan–Meier and Cox regression analyses will be used for the time-to-event data analysis at the 1-year and 2-year follow-up. Missing values at the follow-up will be assessed in terms of the reason for missing, and if appropriate, they will be entered using a multiple imputation by a chained equation procedure, in which regression methods will be selected based on the type of the dependent variable. Analyses will be performed on the SPSS 18.0 for Windows and STATA, and a p-value of <0.05 will be considered significant.
Discussion
The management of acute MI has been rapidly evolved during the past decades (14, 15). Practice guidelines have also improved with more aggressive targets based on the results of randomized controlled trials. Moreover, adherence to these guidelines is associated with an improvement of care and a significant reduction of adverse clinical outcomes including MI mortality. However, in real life, there are significant gaps between the recommendations by guidelines and their implementation into clinical practice (15). Registries are the easiest way of addressing the efficacy of MI management in terms of revising the national health policies in real-life settings (9).
TURKMI is planned as a snapshot registry to enroll consecutive patients with acute MI to evaluate the burden and variation of MI care and outcomes with regard to adherence to current practice guidelines in Turkey. Its results will provide insights to epidemiology, variation in treatment practice patterns, and in-hospital, post-discharge and 2-year follow-up outcomes of patients with acute MI. It is also expected to give a global picture of how patients with STEMI and NSTEMI are currently managed in Turkey. Moreover, TURKMI findings will identify areas requiring further educational efforts and improved patient care.
For comparison with the previous nationwide acute coronary syndrome registries conducted in Turkey [the TUMAR study and Turkey arm of the EPICOR study (11, 16)], TUMAR study has enrolled a total of 3358 patients diagnosed with acute MI who were hospitalized in coronary intensive care units in the last 24 hours within 24 h of symptom onset in 1998 and 1999. TUMAR covered 52 centers from 23 provinces for a period of 1 year. It was designed as a snapshot registry of 1 month, but the main limitation was the lack of enrolment criteria of consecutive patients. It reported an in-hospital mortality rate of 7.8%. For the follow-up, the mortality rate was 12% during a mean follow-up of 18 ± 5 months. However, loss to follow-up was very high at 54%. As it was conducted over 20 years ago when the primary PCI was restricted and new treatment modalities were not available, its results cannot be compared with current practice. The Turkey arm of the EPICOR study, conducted in years 2010 and 2011, reported the data of 1034 patients hospitalized for ACS within 24 h of symptom onset, who had a final diagnosis of unstable angina, STEMI, or NSTEMI and survived to discharge from 34 centers in Turkey (16). It was not designed to address the epidemiology (not representing Turkey’s population distribution) and mortality data. It was rather a study to evaluate the use of new-generation anti-platelet agents in the management of acute coronary syndromes.
Strengths and limitations
The major strength of the present registry is that the distribution of centers is determined according to the Turkey’s census. Instead of calculating the number of patients with acute MI required for analysis, we preferred to select 50 centers proportional to adult population in each EuroStat NUTS region and to obtain data from these centers within 15 consecutive days. One of the reasons for this preference was the assumption that nearly all patients with acute MI would eventually be directed to PCI-capable centers. Another reason was that it is easily applicable and appropriate for the aim of our study. More centers would have been selected to obtain a more precise estimate; however, we planned to include 50 centers across the country because of budget restrictions. This may reduce the precision of our analyses, but as these centers were selected proportional to the population of each EuroStat NUTS region in Turkey, there will be little concern for accuracy. Also, voluntary centers with primary PCI capability were selected in each region. This may reduce representatives in some regions. On the other hand, data obtained during enrollment and follow-up could not be of high quality if we force non-voluntary centers to enroll the patients. Such limitations are generally a unique feature of acute event registries.
Another important strength of the TURKMI registry is that detailed data with more than 550 variables (552 fields have to be filled in) will be recorded to characterize the patients, their management, and outcomes. Another strength is that we will consecutively enroll the patients and follow them at predefined time intervals prospectively. This may result in obtaining the data with a low bias and missingness compared to retrospective data. Of course, this approach may affect the participating cardiologists to modify their natural practice/behavior toward a more guidelines-concordant approach. However, to minimize this possibility, all centers were requested not to modify their routine therapeutic approach during the registry before the initiation of patient enrolment.
The snapshot nature of the study is also a strength as it allows a comprehensive data collection and monitoring, without exhausting the investigators. It also helps to enroll patients within the same season (time period). However, a snapshot registry enrolling patients within 15 consecutive days will preclude assessing the seasonal variation. Moreover, the success of a 15-day snapshot study is enrolling all consecutive patents. To decrease the probability of missing enrolments, two educational meetings were conducted where detailed information on the importance of consecutive enrolment was emphasized. Moreover, centers’ daily patient enrolment numbers will be monitored online, and centers not enrolling sufficiently will be warned and/or motivated for a proper enrolment via a social media communication platform (WhatsApp group) consistently. Also, the enrolment period will be synchronized as the same 15 days between the centers to increase the likelihood of consecutive enrolments. Despite of all these measures, there could still be missing patients, but social media meetings and consistent contacts probably will decrease this possibility to a minimal level.
Conclusion
In conclusion, TURKMI is designed as a national registry that will provide the current status of clinical management of patients with acute MI in Turkey. Its results will guide the improvement of the national policy of medical care regarding MI in the country.
TURKMI Study Group; Abant İzzet Baysal University: Mehmet İnanır, Osman Yasin Yalçın, Yılmaz Güneş; Adana City Hospital: İbrahim Halil Kurt, Ömer Genç, Abdullah Yıldırım; Adıyaman University: Ramazan Aşoğlu; Aksaray University: Sinan İnci; Ankara Numune Training and Research Hospital: Ender Örnek, Mustafa Çetin, Emrullah Kızıltunç; Ankara University: Mustafa Kılıçkap; Ankara Yüksek İhtisas Training and Research Hospital: Çağrı Yayla, Ahmet Göktuğ Ertem, Mehmet Kadri Akboğa; Antalya Training and Research Hospital: Ahmet Genç, Gülsüm Meral Yılmaz Öztekin; Batman State Hospital: Mesut Gitmez; Bursa Yüksek İhtisas Training and Research Hospital; Burcu Tuncay, Veysi Can, Hasan Arı; Bülent Ecevit University: Fatih Paşa Tatar, Mustafa Umut Somuncu; Çanakkale Onsekiz Mart University; Emine Gazi; Çukurova University; Cuma Yeşildaş, Onur Sinan Deveci; Denizli State Hospital: Okan Er; Diyarbakır Gazi Yaşargil Training and Research Hospital: Önder Öztürk; Ege University: Aytaç Candemir, Meral Kayıkçıoğlu, Oğuz Yavuzgil; Elazığ Training and Research Hospital: Çetin Mirzaoğlu; Erzincan University: Eftal Murat Bakırcı, Hüsnü Değirmenci; Harran University: Feyzullah Beşli; İstanbul Bağcılar Training and Research Hospital: Orhan İnce, Emirhan Hancıoğlu; İstanbul Bakırköy Sadi Konuk Training and Research Hospital: İbrahim Faruk Akürk, Ersan Oflar, Nihan Turhan Çağlar; İstanbul Bezmi-Alem University: Hatice Aylin Yamaç Halaç; İstanbul Haseki Training and Research Hospital: Muhsin Kalyoncuoğlu; İstanbul Kartal Koşuyolu Training and Research Hospital: İsmail Balaban, Mesut Karataş, Cevat Kırma; İstanbul Mehmet Akif Ersoy Training and Research Hospital: Arda Güler, Cemil Can, Ardacan Doğan, Ahmet Arif Yalçın; İstanbul Özel Şişli Kolan Hospital: Mustafa Kemal Erol; İstanbul Siyami Ersek Training and Research Hospital: Can Baba Arın; İstanbul University Institute of Cardiology; Ümit Yaşar Sinan; İzmir Tepecik Training and Research Hospital: Murat Küçükokur, Öner Özdoğan; Kahramanmaraş Sütçü İmam University: Ekrem Aksu, Hakan Güneş; Kayseri Training and Research Hospital: Ziya Şimşek, Eyüp Özkan; Kırıkkale Yüksek İhtisas Training and Research Hospital: Cengiz Şabanoğlu, Yunus Çelik; Kütahya Evliya Çelebi Training and Research Hospital: Taner Şen, Mehmet Ali Astarcıoğlu; Malatya Training and Research Hospital: İbrahim Aktaş, Gökhan Gözübüyük; Marmara University: Mustafa Kürşat Tigen, Murat Sünbül; Mersin University: Ayça Arslan, Ahmet Çelik; Mustafa Kemal University: Oğuz Akkuş; Necmettin Erbakan University: Yakup Alsancak; Osmangazi University: Muhammet Dural, Kadir Uğur Mert; Muğla Yücelen Hospital: Nuri Köse; Pamukkale University: İsmail Doğu Kılıç; Recep Tayyip Erdoğan University: Nadir Emlek; Sakarya University: İbrahim Kocayiğit; Samsun Training and Research Hospital: Ahmet Yanık, Mustafa Yenerçağ; Trabzon Ahi Evran Training and Research Hospital: Ömer Faruk Çıtrakoğlu, İhsan Dursun; Trakya University: Utku Zeybey, Servet Altay; Urfa Mehmet Akif İnan Training and Research Hospital: Sadettin Selçuk Baysal; Van Training and Research Hospital: Nesim Aladağ, Remzi Sarıkaya, Ramazan Düz; Van Yüzüncü Yıl University: Mustafa Tuncer, Haşim Tüner; Yalova State Hospital: İsmail Ünğan; Yıldırım Beyazıt University: Bilge Duran Karaduman, Engin Bozkurt.
Acknowledgments:
TURKMI is an investigator-initiated study, sponsored by the Turkish Society of Cardiology that receives major funding from the Astra-Zeneca Company for this project.
Footnotes
Conflict of interest: Mustafa Kemal Erol has received honoraria (for lectures) from Servier and Pfizer within the last 2 years. Meral Kayıkçıoğlu has received honoraria (for lectures and consultancy) from Abbott and Menarini, and research funding from Amryt Pharma, Amgen, and Sanofi, and has participated in clinical trials with Amgen, Medicines Company, Regenerone, Sanofi, and Pfizer within the last 2 years. Mustafa Kılıçkap is none.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – M.K. Erol, M. Kayıkçıoğlu, M. Kılıçkap; Design – M.K. Erol, M. Kayıkçıoğlu, M. Kılıçkap; Supervision – M.K. Erol, M. Kayıkçıoğlu, M. Kılıçkap; Funding – M.K. Erol; Materials – None; Data collection and/or processing – M.K. Erol, M. Kayıkçıoğlu, M. Kılıçkap; Analysis and/or interpretation – M. Kılıçkap; Literature search – M. Kayıkçıoğlu; Writing – M.K. Erol, M. Kayıkçıoğlu, M. Kılıçkap; Critical review – M.K. Erol, M. Kayıkçıoğlu, M. Kılıçkap.
References
- 1.Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362:2155–65. doi: 10.1056/NEJMoa0908610. [DOI] [PubMed] [Google Scholar]
- 2.Fox KA, Eagle KA, Gore JM, Steg PG, Anderson FA GRACE and GRACE2 Investigators. The Global Registry of Acute Coronary Events 1999 to 2009-GRACE. Heart. 2010;96:1095–101. doi: 10.1136/hrt.2009.190827. [DOI] [PubMed] [Google Scholar]
- 3.Peterson ED, Roe MT, Mulgund J, DeLong ER, Lytle BL, Brindis RG, et al. Association between hospital process performance and outcomes among patients with acute coronary syndromes. JAMA. 2006;295:1912–20. doi: 10.1001/jama.295.16.1912. [DOI] [PubMed] [Google Scholar]
- 4.Chung SC, Gedeborg R, Nicholas O, James S, Jeppsson A, Wolfe C, et al. Acute myocardial infarction:a comparison of short-term survival in national outcome registries in Sweden and the UK. Lancet. 2014;383:1305–12. doi: 10.1016/S0140-6736(13)62070-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. ESC Scientific Document Group 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation:The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2018;39:119–77. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 6.Authors/Task Force Members: Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice:The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention &Rehabilitation (EACPR) Atherosclerosis. 2016;252:207–74. doi: 10.1016/j.atherosclerosis.2016.05.037. [DOI] [PubMed] [Google Scholar]
- 7.Puymirat E, Cayla G, Cottin Y, Elbaz M, Henry P, Gerbaud E, et al. Twenty-year trends in profile, management and outcomes of patients with ST-segment elevation myocardial infarction according to use of reperfusion therapy:Data from the FAST-MI program 1995-2015. Am Heart J. 2019;214:97–106. doi: 10.1016/j.ahj.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Schiele F, Gale CP, Simon T, Fox KAA, Bueno H, Lettino M, et al. Assessment of Quality Indicators for Acute Myocardial Infarction in the FAST-MI (French Registry of Acute ST-Elevation or Non-ST-Elevation Myocardial Infarction) Registries. Circ Cardiovasc Qual Outcomes. 2017;10:e003336. doi: 10.1161/CIRCOUTCOMES.116.003336. pii. [DOI] [PubMed] [Google Scholar]
- 9.Kayıkçıoğlu M, Tokgözoğlu L. The rationale and design of the national familial hypercholesterolemia registries in Turkey:A-HIT1 and A-HIT2 studies. Turk Kardiyol Dern Ars. 2017;45:261–7. doi: 10.5543/tkda.2017.25800. [DOI] [PubMed] [Google Scholar]
- 10.Harikrishnan S, Sanjay G, Ashishkumar M, Menon J, Rajesh G, Kumar RK. Pulmonary Hypertension Registry of Kerala (PROKERALA) - Rationale, design and methods. Indian Heart J. 2016;68:709–15. doi: 10.1016/j.ihj.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enar R. Acute myocardial infarction Thrombocardiology [Turkish] 2nd ed. İstanbul: Nobel Tıp Kitabevi; 2004. [Google Scholar]
- 12.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, et al. Writing Group on the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction Third universal definition of myocardial infarction. Eur Heart J. 2012;33:2551–67. doi: 10.1093/eurheartj/ehs184. [DOI] [PubMed] [Google Scholar]
- 13.Wikipedia contributors NUTS statistical regions of Turkey [Internet] Wikipedia, The Free Encyclopedia 2019 Apr 14, 20:24 UTC. [[cited 2019 Aug 16]]. Available from: URL;https://en.wikipedia.org/w/index.php?title=NUTS_statistical_regions_of_Turkey&oldid=892478048 .
- 14.Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence-based pharmacotherapy and long-term mortality after acute myocardial infarction. JAMA. 2007;297:177–86. doi: 10.1001/jama.297.2.177. [DOI] [PubMed] [Google Scholar]
- 15.Smolina K, Wright FL, Rayner M, Goldacre MJ. Determinants of the decline in mortality from acute myocardial infarction in England between 2002 and 2010:linked national database study. BMJ. 2012;344:d8059. doi: 10.1136/bmj.d8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ertaş FS, Tokgözoğlu L EPICOR Study Group. Pre- and in-hospital antithrombotic management patterns and in-hospital outcomes in patients with acute coronary syndrome:data from the Turkish arm of the EPICOR study. Anatol J Cardiol. 2016;16:900–15. doi: 10.14744/AnatolJCardiol.2016.6755. [DOI] [PMC free article] [PubMed] [Google Scholar]