Abstract
Background:
Celiac disease (CD) is a common gastrointestinal pathology; however, prevalence and comorbidities are unknown in collegiate athletics.
Hypotheses:
(1) Athletes will have similar odds of CD as general population estimates (approximately 1 in 141) based on self-report and signs and symptoms, (2) athletes scoring higher on the Celiac Symptom Index (CSI) will have lower self-reported quality of life (QoL), (3) athletes scoring higher on the CSI will have higher depression scores, and (4) athletes scoring higher on the CSI will have higher perceived stress scores.
Study Design:
Epidemiological cross-sectional study.
Level of Evidence:
Level 4.
Methods:
The CSI, WHO Quality of Life-BREF, Beck Depression Inventory, and Perceived Stress Scale were used to assess patients’ signs and symptoms of CD and psychosocial measures/QoL in male and female National Collegiate Athletic Association (all divisions) athletes (N = 141). Participants also self-reported a formal diagnosis of CD. Chi-square analyses determined CD prevalence. Odds ratios determined risk for either being diagnosed with CD or reporting more symptoms than the general population. Correlational analyses determined whether symptoms correlated with QoL and psychosocial measures.
Results:
Athletes were 3.85 times (95% CI, 0.42-34.89) more likely to report a CD diagnosis and were 18.36 times (95% CI, 2.40-140.48) more likely to report a high degree of CD symptoms than the general population. Athletes with more symptoms had worse physical, psychological, social, and environmental QoL indicators and higher depression and perceived stress scores.
Conclusion:
Athletes may be a higher risk population for experiencing CD and report greater signs/symptoms compared with general population estimates. Additionally, athletes with higher CD symptom scores also reported poorer QoL.
Clinical Relevance:
Allied health care professionals should be aware of the diversity of CD symptoms and be prepared to refer athletes when gastrointestinal symptoms persist to ensure proper care and unhampered performance.
Keywords: collegiate, diet, gastrointestinal, gluten, nutrition, performance
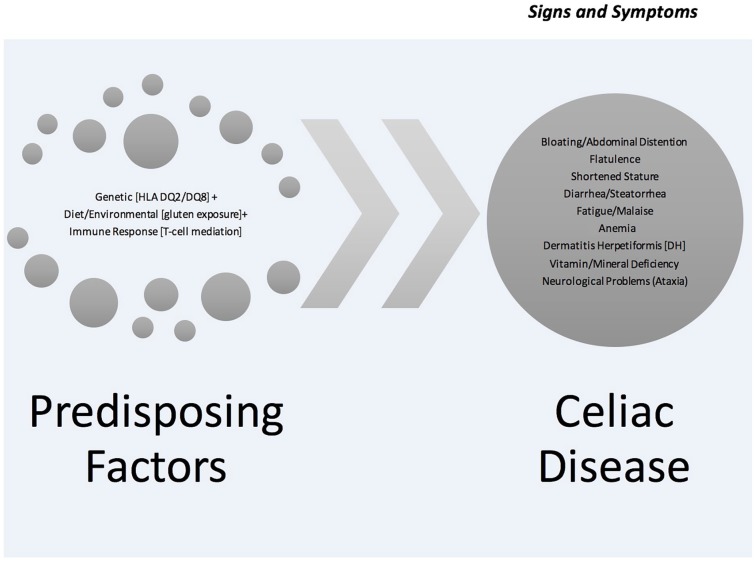
Celiac disease (CD) affects approximately 1% of the general population worldwide.4,13,21,34 However, CD is likely underdiagnosed due to lack of awareness and the varied symptomatology and clinical presentation along with the heterogeneity of genetic variation in the United States.40 Unlike many gastrointestinal disorders (Crohn’s disease, irritable bowel syndrome, etc), the etiology of CD is well known (gluten).11,20 People can become symptomatic once gluten is introduced into the diet or it may be triggered by stressful life events, such as environmental exposure, dose, or trauma.25,40 While the disease presents a myriad of signs and symptoms (Table 1), people with CD are often unaware of their status for a variety of reasons, including lack of awareness/education about the disease, diverse signs and symptoms often confused with other gastrointestinal problems, denial, and subclinical symptoms that people often grow accustomed to handling.13,19,37,40 Table 1 and Figure 1 detail the diversity of CD.
Table 1.
| Disease/Pathology | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Celiac disease | × | × | × | × | × | × | × | × | × | × | × | × | × | |||||
| Nonceliac gluten sensitivity | × | × | × | × | × | × | × | × | × | × | × | × | ||||||
| Wheat allergy | × | × | × | × | × | × | × | × | ||||||||||
| Crohn’s disease | × | × | × | × | × | × | × | × | × | × | ||||||||
| Irritable bowel syndrome | × | × | × | × | × | × | ||||||||||||
| Sjrögren syndrome | × | × | × | × |
The signs and symptoms are as follows: 1, abdominal pain; 2, diarrhea; 3, constipation; 4, vomiting; 5, nausea; 6, headache; 7, gas/flatulence/bloating; 8, musculoskeletal pain/arthralgia; 9, brain “fog”; 10, fatigue; 11, ataxia; 12, paresthesia in limbs (numbness/tingling); 13, dermatitis herpetiformis; 14, weight loss; 15, eczema; 16, asthma; 17, diabetes; 18, rash.
Figure 1.
The etiological process of celiac disease.
CD is one such pathology that is yet to be formally studied in athletes, particularly its prevalence and symptomatology and how it may affect training and performance. CD poses unique barriers for athletes because of their increased metabolic demand and nutritional needs that exceed the general population.5 People with CD may not be able to absorb most of these nutrients and subsequently meet the demands of their physical activity.19,37 The fact that psychological and/or physical stress could activate CD in subclinical cases,23 coupled with the demands of sports,10,23 may plausibly be enough of a trigger to activate CD in some athletes. Additionally, athletes with CD may be more likely to be depressed, experience neurological changes due to nutrient and hormonal problems, and may struggle with a fear of food that makes them sick.38 These issues may provoke some to experience struggles with food and possibly eating disorders.16
Symptoms of CD in athletes are diverse and often remain misdiagnosed or underdiagnosed.6,18,25 Lack of prevalence estimates in athletes makes it difficult to meet their needs because services and awareness may not coincide with resource allocation. Moreover, the disease itself is not very well defined in the United States versus other countries,6,7,18,36 again making identification, diagnosis, and treatment a challenge in sports. Therefore, the purpose of this article was to examine relative prevalence of CD in athletes as well as symptoms and the physical and psychological correlates in collegiate athletes as they continue to be an understudied population. Better understanding of the unique challenges and needs of athletes with CD symptoms may lead to greater awareness, allocation of resources, greater return on investment, performance, and ultimately, advancement of the standard of care.
The following 4 hypotheses were derived: (1) Athletes will have similar odds of CD as general population estimates (approximately 1 in 141)36 based on self-report and signs and symptoms, (2) athletes scoring higher on the Celiac Symptom Index (CSI) will have lower self-reported quality of life (QoL), (3) athletes scoring higher on the CSI will have higher depression scores, and (4) athletes scoring higher on the CSI will have higher perceived stress scores.
Methods
All participants provided informed consent by linking to the online survey. This study was approved by the institutional review board of Bridgewater State University.
This was a level 4 ecological, epidemiological, cross-sectional correlational research design. The dependent variables consisted of reported CD diagnosis and related symptoms. Given that CD often goes undiagnosed, the validated CSI was used as a proxy as to whether athletes were experiencing CD symptoms. We examined relationships between self-reported CD symptoms, as measured by the CSI, and other variables, including QoL, perceived stress, and depression. Well-known validated measures of each construct were implemented in this study. Given the scaled nature of each questionnaire, they were appropriate for implementation in a correlational analysis.
Patients
An a priori power analysis using G*Power18 was conducted to determine a sample size. Sainsbury et al32 found that severity of symptoms of CD at diagnosis has a weak to moderate relationship with quality of life (r = 0.22; P < 0.01). This correlation was used to determine the necessary minimum sample size (N = 126) to obtain 70% power. After review of missing data from the initial sample (N = 171), described in the results section, data were analyzed from a convenience sample of 141 participants, all of whom indicated they were collegiate athletes, with the most prevalent sports represented being track and field (33.3%), football (21.3%), basketball (14.2%), and baseball (10.6%) (Table 2).
Table 2.
Participant demographic data (N = 141)
| Variable | Mean/Frequency | SD/% | Range |
|---|---|---|---|
| Sex | |||
| Male | 84 | 40% | |
| Female | 57 | 60% | |
| Age, y | 20.60 | 1.65 | 18-27 |
| Height, m | 1.76 | 0.12 | 1.40-2.23 |
| Weight, kg | 81.14 | 19.61 | 45.00-128.25 |
| BMI | 26.01 | 4.72 | 17.58-43.97 |
| Competition level | |||
| NCAA Division I | 77 | 55% | |
| NCAA Division II | 7 | 5% | |
| NCAA Division III | 45 | 32% | |
| NAIA | 2 | 1% | |
| Other | 8 | 6% | |
| Not reported | 2 | 1% | |
| Race/ethnicity | |||
| White/Caucasian | 99 | 70% | |
| Black/African American | 29 | 20% | |
| Hispanic/Latino | 6 | 4% | |
| Asian/Pacific Islander | 5 | 4% | |
| Multiracial | 2 | 1% | |
BMI, body mass index; NAIA, National Association of Intercollegiate Athletics; NCAA, National Collegiate Athletic Association.
Measures
We used the following measures to test our hypotheses. Each measure was selected for its capability to detect both the physical signs and symptoms consistent with CD and psychoemotional and health-related QoL factors.
Demographic Questionnaire: A 13-item demographic questionnaire was developed to establish a basic profile of each student-athlete. Sex, age, height/weight, National Collegiate Athletic Association (NCAA) status and division, sport type, race/ethnicity, and employment status were assessed.
Beck Depression Inventory (BDI)39: Athletes’ psychological status as it relates to depression was assessed via the BDI. The BDI is a well-known, valid, and reliable self-report measure of attitudes and symptoms of depression. The 21-item inventory takes approximately 10 minutes to complete. Each item is scored on a 0 to 3 scale. Total scores are summed, yielding a range between 0 and 63. The BDI has demonstrated good internal consistency ranging from 0.73 to 0.92, with a mean of 0.86.28 In our study we found α = 0.87.
Perceived Stress Scale (PSS-14)12: The PSS-14 is a widely used psychological instrument measuring the perception of stress, specifically the degree to which situations (unpredictability, uncontrollability, and overload) in one’s life are appraised as stressful. The 14-item scale takes approximately 5 minutes to complete. Each item is evaluated on a 0 to 4 scale and is sum totaled. Scores range from 0 to 56, with norms from the United States being 19.62.24 In our study, we found a mean value of 25.19 (SD, 6.46). Cronbach’s alpha was 0.72.
Assessment of Quality of Life (WHOQOL-BREF)8: We used the WHO Quality of Life–BREF questionnaire to measure health-related QoL. This self-report measure is composed of 26 items that assess domains of physical and psychological health, social relationships, and functioning in the environment and takes approximately 15 minutes to complete. Scoring of the WHOQOL-BREF is conducted by scoring the items for each of the 4 domains and transforming them onto a 0 to 100 scale. Internal consistency, item total correlations, discriminant validity, and construct validity indicate that the WHOQOL-BREF is a psychometrically sound, cross-culturally valid assessment of QoL.15 In our study, we found α = 0.72, 0.85, 0.84, and 0.88 for the physical, psychological, social relations, and environment domains, respectively.
CSI4: We used the CSI to establish symptoms consistent with CD. The CSI is a 16-item self-report measure that takes approximately 5 minutes to complete. The survey covers domains consistent with CD-related symptoms (eg, gastrointestinal, neurological, and general body systems) and determines the frequency at which a person experiences symptoms on a 5-point Likert scale (none of the time to all of the time). The CSI is scored by summing the 16-item scale. The scale has excellent psychometric properties that correlated well with patients with confirmed CD via testing (biopsy). We found the internal consistency to be α = 0.93. We also specifically asked whether patients had confirmed CD (by a physician) to better establish prevalence of CD in our sample.
Procedures
After institutional review board approval, we solicited NCAA athletes through their athletic directors, strength and conditioning coaches, and athletic trainers based on random regional targeting of NCAA programs as well as word of mouth (ie, convenience) based on the authors’ networks. A formal letter introducing the study, goals, and survey link were emailed from December 2014 until December 2015. Athletic directors, athletic trainers, and coaches were asked to forward the email (study introduction) and link to their athletes. If respondents agreed to participate, they clicked the survey link, which outlined the study rationale and their role and gave them access to the survey platform (SurveyMonkey). Accessing the survey via the link assured informed consent. Presentation of surveys was rotated to control for any ordering effects bias. Recruitment continued until enough usable surveys were completed for meaningful data analysis and interpretation. Surveys took approximately 20 to 30 minutes to complete and were cleaned, coded, and prepared for analysis.
Statistical Analyses
One-way chi-square analyses were conducted to compare (1) frequencies of diagnosed CD in the sample of athletes with the estimated population prevalence and (2) frequencies of a greater number of CD symptoms in the sample athletes with prevalence of a greater number of CD symptoms in the population. Odds ratios were calculated to determine whether athletes had a higher likelihood of being diagnosed with CD or reporting a greater number of symptoms of CD than the general population. Leffler et al20 reported that patients with a score of less than 30 on the CSI reported a high QoL and high gluten-free diet (GFD) adherence while participants with score greater than 45 reported relatively lower QoL and low GFD adherence. CSI scores were transformed into 2 conservative categories: low CD symptoms (<45) and high CD symptoms (≥45). Correlational analyses were conducted to determine whether degree of CD symptoms was correlated with the WHOQOL-BREF, BDI, and the PSS-14. An a priori alpha level of 0.05 was set. Surveys were analyzed using SPSS Version 23 (IBM Corp.).
Results
Prior to conducting inferential statistics, data were screened for missing items, outliers, normality, and homoscedasticity. Initially, 171 respondents opened the survey; 21 athletes were removed for having completely missing data or only completing the initial demographic data. An additional 8 athletes were removed for not completing 3 of the 4 surveys, making their data uninterpretable. Respondent scores missing over 10% of a survey were not analyzed. Missing survey scores existed, but the largest amount of missing data for a single scale or survey was less than 9% of the total sample. Therefore, for the remainder of the analyses, listwise deletion was used to handle missing data. Sample sizes for each analysis are reported. Data were visually analyzed for outliers and normality. Skewness and kurtosis statistics were calculated to determine normality. No drastic deviations from normality or extreme outliers were found; therefore, data from the remaining 141 participants were deemed usable. When reviewing scatterplots for the correlational analyses, the basic assumption of homoscedasticity was met.
One-way chi-square analyses were conducted to determine whether athletes reported being diagnosed with CD more than the general population. Based on a review of literature, we concluded that 1:125 (0.8%) was a conservative estimate of prevalence of being diagnosed with CD in the typical population31; prevalence values for diagnosed (1) and nondiagnosed (125) were entered as expected values in the chi-square analysis. Athletes were significantly more likely to report being diagnosed (n = 4) than not being diagnosed (n = 130) than the expected population values (χ2(1) = 8.17; P < 0.01). Athletes were 3.85 times (95% CI, 0.42-34.89) more likely to report being diagnosed with CD than the general population. Using the categorical variable developed from the CSI, a chi-square analysis was conducted to determine whether athletes reported a higher degree of CD symptoms than the general population, using the same expected n values reported by Rubio-Tapia et al31 (median age, 45 years; interquartile range, 23-66 years). Athletes were significantly more likely to report a higher degree of symptoms (n = 16) than a low degree of symptoms (n = 122) when compared with general population values (χ2(1) = 234.04; P < 0.001). Athletes were 18.36 (95% CI, 2.40-140.48) times more likely to report having a high degree of CD symptoms.
Pearson product moment correlational analyses were used to test the remaining 3 hypotheses. CSI scores were correlated with the 4 transformed domains of the WHOQOL-BREF to determine whether there was a significant linear relationship between reported CD symptoms and QoL. The CSI was significantly negatively correlated with the following domains: physical (r = −0.49; 95% CI, −0.61 to −0.35; P < 0.05; n = 132; r2 = 0.24), psychological (r = −0.53; 95% CI, −0.64 to −0.39; P < 0.05; n = 129; r2 = 0.28), social relations (r = −0.38; 95% CI, −0.52 to −0.26; P < 0.05; n = 133; r2 = 0.14), and environment (r = −0.36; 95% CI, −0.50 to −0.20; P < 0.05; n = 126; r2 = 0.13). While a statistical association was found between CD symptoms and QoL, the percentage of explained variance ranges from moderate to large.41 Two additional correlational analyses were run. It was hypothesized that athletes scoring higher on the CSI also would score higher on the BDI. There was a significant positive relationship between CSI scores and BDI scores (r = 0.62; 95% CI, 0.50-0.71; P < 0.05; n = 138; r2 = 0.38). The PSS-14 also was significantly positively correlated with the CSI (r = 0.53; 95% CI, 0.39-0.65; P < 0.05; n = 125; r2 = 0.28). The CSI explained large portions of both the BDI and PSS-14. Results for all correlational analyses are presented in Table 3.
Table 3.
Pearson correlation coefficients of measures with the CSI
| Measures | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| 1. CSI | — | −0.49** | −0.53** | −0.38** | −0.36** | 0.62 | 0.53 |
| 2. WHOQOL-BREF Physical domain | — | 0.65** | 0.55** | 0.62** | −0.52** | −0.50** | |
| 3. WHOQOL-BREF Psychological domain | — | 0.70** | 0.67** | −0.65** | −0.52** | ||
| 4. WHOQOL-BREF Social relations domain | — | 0.62** | −0.52** | −0.40** | |||
| 5. WHOQOL-BREF Environment domain | — | −0.43** | −0.39** | ||||
| 6. BDI | — | 0.61** | |||||
| 7. PSS-14 | — |
BDI, Beck Depression Inventory; CSI, Celiac Symptom Inventory; PSS-14, Perceived Stress Inventory; WHOQOL-BREF, WHO Quality of Life–Brief version.
P < 0.05. **P < 0.01.
Discussion
This study was an initial attempt at establishing prevalence estimates of CD and related symptoms in a sample of NCAA collegiate athletes. While we were able to establish foundational knowledge in this nascent area of research, we implore readers to interpret our findings keeping in mind the small sample size and the lack of comparison with like (collegiate) populations. Ultimately, the value of this research is to build a foundational knowledge in NCAA athletics to improve recognition and management of CD in athletes.
Our first hypothesis proposed that athletes would have similar odds of having CD as the general population (roughly 1 in 141) or approximately 1%.36 Our results suggest that NCAA athletes had higher prevalence of CD than the general population. This was surprising given the current US prevalence (1 in 125).36 It is possible that athletes diagnosed with CD were more likely to respond given the nature of this study, introducing a form of response bias. Athletes also tend to have more regular contact with an interdisciplinary team of health care providers and undergo yearly preparticipation physicals; therefore, they have additional opportunity to discuss health changes.
Additionally, athletes in our sample were 18.36 times more likely than the general population to report symptoms consistent with CD. This may be due to athletes being more attuned with their bodies33 or due to dietary factors. An athlete’s diet, typically high in carbohydrates, is a factor as to why they may experience signs and symptoms similar to CD.27 Considering these results, we rejected our first hypothesis. Having good prevalence estimates in athletes can help providers recognize the magnitude of CD and better allocate resources and accommodate unique dietary and physical needs, possibly leading to better performance.
We accepted our second hypothesis that athletes with symptoms consistent with CD would experience lower QoL scores than individuals with fewer symptoms. High scores on the CSI were significantly negatively correlated with the physical, psychological, social, and environmental domains of health-related QoL. These results were not surprising, as established research has demonstrated CD is a disorder that affects nearly all body systems.13,14 Psychological QoL also was lower in our sample reporting higher CSI scores. Athletic identity often is predicated on mental readiness, physicality, body competence, and ability.17 Given the reciprocal relationship between mental well-being and physical performance, the persistent physical effects of CD may create lower perceived psychological QoL due to challenges to one’s athletic identity.
Social and environmental domains of QoL also were negatively correlated with higher CSI scores in our sample. Social QoL may be negatively affected by CD signs and symptoms due to manifestations of the disease that affect how people engage in activities of daily living. Athletes have intense schedules, and the cumulative effects of the aforementioned physical and psychological challenges related to CD tend to affect social QoL. Because of the diversity of CD signs and symptoms, athletes may experience uncertainty concerning how their body will feel, function, and perform; therefore, they may become more withdrawn, thereby negatively affecting social QoL. The environmental QoL domain encompasses areas such as accessibility, security, participation, and resources. The following areas were relevant in our sample: financial resources, home environment, and opportunities for recreation and leisure. Participants with higher CSI scores may have experienced greater financial constraints particularly as it relates to food and medication/treatments.35 Additionally, food options in cafeterias may not offer accessibility to gluten-free items or may be subject to cross-contamination via food preparation practices. Opportunities for recreation and leisure also may be negatively affected in people reporting higher CSI scores. As such, the everyday demand of athletic competition creates a challenging schedule. Athletes with CD may have compounded issues physically and psychologically that may limit other forms of social, recreational, and leisure engagements, thus leading to lower perception of environmental QoL.
Our third hypothesis confirmed that athletes who had higher scores consistent with CD also reported higher depression scores. Mental health is greatly influenced by one’s relative health status.10 Athletes typically are subjected to a variety of physical and psychological stressors such as competition, injuries, success, expectations, and scholarships.3 Being a CD patient alone increases the likelihood of depression due to symptoms,29 but additional stressors from athletics can compound physical and mental wear on the body.2 Athletes in our sample who scored higher on the CSI also reported greater physical and emotional challenges, thereby confirming previous findings.23,26 With the close association between mental health and physical performance, it is essential that allied health care professionals work closely with athletes to help improve mental and physical well-being via education, training, recovery, and nutritional protocols.2
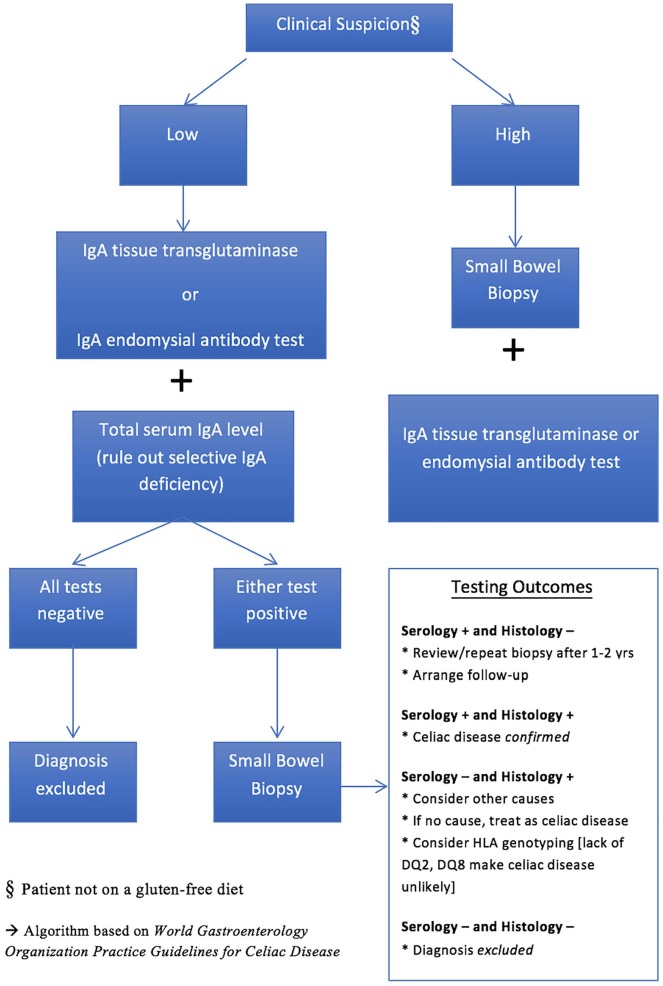
Our fourth and final hypothesis was also supported. Athletes with symptoms consistent with CD also reported greater perceived stress. Athletes may perceive stress differently from the general population in that stress scores may be higher due to increased physical demands, competition, and injuries.3 People with a chronic ailment such as CD also may experience greater perceived stress than the general population due to the variety of omnipresent physical and psychological challenges.10,30 Perceived stressors likely are compounded given the stress of being a student-athlete and having CD and may lead to negative health consequences. This finding demonstrates the diversity of CD and speaks to the need to view such diseases with a broader lens in health care and tailor health (nutritional) and training programs with unique athlete needs in mind (see Figure 2).
Figure 2.
Algorithm for clinical diagnosis of celiac disease.28
Limitations
Our research is limited by its correlational nature, meaning that we cannot infer causality. In the current study, we used a convenience sample of athletes. The NCAA athletic population consists of approximately 380,000 athletes, and it should be noted that our sample represents only a small slice (<1%) of the collegiate population, which was largely drawn from the Midwest (48%) and Northeast (24%) regions. Additionally, a greater limitation is that we used general population estimates of CD when comparing with our sample. While athletes are certainly a cross section of the general population, future research may wish to compare NCAA athletes with a college population. Athletes in our sample who had a diagnosis of CD simply could have been coincidental. A form of selection bias may have been present in this study, in that athletes come into contact with health care providers and coaches more frequently (eg, athletic trainers, dieticians) and therefore may be more likely to be referred for assessment and screening, thereby increasing possibility of a CD diagnosis. Also, athletes with CD might have been more interested in participating in this study because of the nature of the topic (ie, response bias). Additionally, there may be regional dietary differences that can influence the presentation of CD signs and symptoms. This factor can influence what people consume and in what quantities, thereby possibly masking (or exacerbating) CD expression. We attempted to account for this by asking from what region athletes hailed so as to note any oversampling from a particular area (no meaningful differences were noted). Research has confirmed that certain racial/ethnic groups may be more predisposed (both genetically and environmentally) to having CD (ie, Irish, Scandinavian, Italian).25 To account for any sample selection bias, we surveyed a general cross section of athletes from various racial/ethnic backgrounds. Future research may wish to draw from a more regionally, racially, and NCAA level balanced sample. Also, we did not assess actual CD diagnosis via clinical (ie, biopsy) or serological tests; rather, we explored the relationship between athletes who scored higher on a symptoms inventory to the known prevalence estimates in the US population.36 We also asked directly whether a physician made a formal diagnosis of CD. Because we did not assess CD via clinical testing, our results were subject to self-report; we caution that elevated rates may reflect this. Last, we were interested in a specific population (ie, NCAA athletes); therefore, our results may have underrepresented signs and symptoms of CD being that athletes often are in good physical health versus the general population,22 thus possibly introducing a form of a “healthy worker effect” and potentially masking the true extent of CD symptoms in this population.
Conclusion
Athletes in our sample had nearly a 4 times greater likelihood of being diagnosed with CD than the general population. Additionally, athletes reported an 18 times greater likelihood of having signs and symptoms consistent with CD. These physical signs and symptoms also negatively affected athlete health–related QoL, depression, and perceived stress, possibly compromising athletic performance. These results can help allied health care professionals leverage this information in advocating for better awareness and coordinated, integrated care of athletes with unique dietary needs as in the case of CD.
Our study provides new insight into the literature concerning CD in that much of the scope and focus of CD studies pertain to the general population. The latter has led to little or no information on the prevalence of CD in athletes, particularly NCAA athletes. This research helps to establish an emerging epidemiology of CD signs and symptoms in NCAA athletes, particularly physical issues and psychoemotional stresses, so as to advance the standard of care (ie, physically, emotionally, nutritionally). Allied health care professionals often are perceptive of their athletes’ patterns and needs and are poised to appropriately respond with better information and enhanced awareness as to how CD may manifest in this population. Ultimately, allied health care providers are essential links in improving athletic QoL and performance while in competition and across the lifespan.
Clinical Relevance
Describing and bringing attention to a commonly overlooked disease was a primary goal of this study. Studies have noted an average time to diagnosis for CD at approximately 8 to 11 years.9,31 The main goals of an interdisciplinary health care team are primary prevention and advancing athlete care and performance.1 With proper awareness and surveillance, better allocation of resources and access to services (eg, testing, medical care, nutritional counseling, dietary options) is possible. Ivarsson et al17 noted that there are clear benefits to early identification and treatment, particularly in terms of financial return on investment. This may amount to less time lost to disease/injury (morbidity), better use of medical resources, and perhaps better athletic performance. Having better prevalence estimates and awareness of CD, allied health care professionals can play a crucial role in bridging the gap of athletes to professionals who can assist in guiding them through managing CD (Figure 2), truly a team-based, interdisciplinary approach, as recently advocated by Ralphs and Piper.29 Athletes may be overlooked, as coaches and allied health care providers might assume an inherent health bias. Athletes also may normalize their pain and discomfort and “play through the pain.” Our findings bring to light a condition that may affect athletes at higher rates than once presumed; therefore, extra vigilance should be taken when approaching gastrointestinal issues. Moreover, discussing CD at conferences and as part of a student’s professional preparation (eg, sports nutrition courses) may help better identify a disease that presents with a high level of diversity. Last, as CD can be successfully treated with a strict GFD, earlier access to an interdisciplinary health care team may help alleviate the distress of some athletes with CD.
Acknowledgments
We would like to sincerely thank all the study participants, athletic directors, and athletic trainers that helped to facilitate this research. We also extend our gratitude to the work, insights, and guidance of Daniel A. Leffler, MD, of Beth Israel Deaconess Medical Center, particularly in his consulting role in measuring celiac disease signs and symptoms.
Footnotes
The authors report no potential conflicts of interest in the development and publication of this article.
References
- 1. Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: 25 years of evaluation. Clin Psychol Rev. 1998;8:77-100. [Google Scholar]
- 2. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561-571. [DOI] [PubMed] [Google Scholar]
- 3. Byass P, Kahn K, Ivarsson A. The global burden of childhood coeliac disease: a neglected component of diarrhoeal mortality? PLoS One. 2011;6:e22774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Catassi C, Elli L, Bonaz B, et al. Diagnosis of non-celiac gluten sensitivity (NCGS): the Salerno Experts’ criteria. Nutrients. 2015;7:4966-4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chao A, Grilo CM, White MA, Sinha R. Food cravings mediate the relationship between chronic stress and body mass index. J Health Psychol. 2015;20:721-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ciacci C, Siniscalchi M, Bucci C, Zingone F, Morra I, Iovino P. Life events and the onset of celiac disease from a patient’s perspective. Nutrients. 2013;5:3388-3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cohen S, Williamson G. Perceived stress in a probability sample of the United States. In: Spacapan S, Oskamp S, eds. The Social Psychology of Health. Newbury Park, CA: Sage; 1988:31-67. [Google Scholar]
- 8. Cosh S, Tully PJ. “All I have to do is pass”: a discursive analysis of student athletes’ talk about prioritising sport to the detriment of education to overcome stressors encountered in combining elite sport and tertiary education. Psychol Sport Exerc. 2014;15:180-189. [Google Scholar]
- 9. Currie S, Hadjivassiliou M, Clark M, et al. Should we be “nervous” about coeliac disease? Brain abnormalities in patients with coeliac disease referred for neurological opinion. J Neurol Neurosurg Psychiatry. 2012;83:1216-1221. [DOI] [PubMed] [Google Scholar]
- 10. Di Sabatino A, Corazza GR. Coeliac disease. Lancet. 2009;373:1480-1493. [DOI] [PubMed] [Google Scholar]
- 11. Fasano A, Catassi C. Current approaches to diagnosis and treatment of celiac disease: an evolving spectrum. Gastroenterology. 2001;120:636-651. [DOI] [PubMed] [Google Scholar]
- 12. Ford RP. The gluten syndrome: a neurological disease. Med Hypotheses. 2009;73:438-440. [DOI] [PubMed] [Google Scholar]
- 13. Garatachea N, Santos-Lozano A, Sanchis-Gomar F, et al. Elite athletes live longer than the general population: a meta-analysis. Mayo Clinic Proc. 2014;89:1195-1200. [DOI] [PubMed] [Google Scholar]
- 14. Hadjivassiliou M, Boscolo S, Davies-Jones GA, et al. The humoral response in the pathogenesis of gluten ataxia. Neurology. 2002;58:1221-1226. [DOI] [PubMed] [Google Scholar]
- 15. Hawley JA, Leckey JJ. Carbohydrate dependence during prolonged, intense endurance exercise. Sports Med. 2015;45(suppl 1):5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Husby S, Koletzko S, Korponay-Szabó IR, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54:136-160. [DOI] [PubMed] [Google Scholar]
- 17. Ivarsson A, Myléus A, Norström F, et al. Prevalence of childhood celiac disease and changes in infant feeding. Pediatrics. 2013;131:e687-e694. [DOI] [PubMed] [Google Scholar]
- 18. Joy E, Kussman A, Nattiv A. Update on eating disorders in athletes: a comprehensive narrative review with a focus on clinical assessment and management. Br J Sports Med. 2016;50:154-162. [DOI] [PubMed] [Google Scholar]
- 19. Lebwohl B, Michaëlsson K, Green PHR, Ludvigsson J. Persistent mucosal damage and risk of fracture in celiac disease. J Clin Endocrinol Metabol. 2014;99:609-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Leffler DA, Dennis M, Edwards-George J, et al. A validated disease specific symptom index for adults with celiac disease. Clin Gastroenterol Hepatol. 2009;7:1328-1334. [DOI] [PubMed] [Google Scholar]
- 21. Lionetti E, Catassi C. New clues in celiac disease epidemiology, pathogenesis, clinical manifestations, and treatment. Int Rev Immunol. 2011;30:219-231. [DOI] [PubMed] [Google Scholar]
- 22. Lohi S, Mustalahti K, Kaukinen K, et al. Increasing prevalence of coeliac disease over time. Aliment Pharmacol Ther. 2007;26:1217-1225. [DOI] [PubMed] [Google Scholar]
- 23. Ludvigsson JF, Leffler DA, Bai JC, et al. The Oslo definitions for coeliac disease and related terms. Gut. 2013,62:43-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lundin KE, Alaedini A. Non-celiac gluten sensitivity. Gastrointest Endosc Clin N Am. 2012;22:723-734. [DOI] [PubMed] [Google Scholar]
- 25. Mancini LA, Trojian T, Mancini AC. Celiac disease and the athlete. Curr Sports Med Rep. 2011;10:105-108. [DOI] [PubMed] [Google Scholar]
- 26. Margaritte-Jeannin P, Babron MC, Bourgey M, et al. HLA-DQ relative risks for coeliac disease in European populations: a study of the European genetics cluster on coeliac disease. Tissue Antigens. 2004;63:562-567. [DOI] [PubMed] [Google Scholar]
- 27. Mustalahti K, Catassi C, Reunanen A, et al. The prevalence of celiac disease in Europe: results of a centralized, international mass-screening project. Ann Med. 2010;42:587-595. [DOI] [PubMed] [Google Scholar]
- 28. Not T, Horvath K, Hill ID, et al. Celiac disease risk in the USA: high prevalence of antiendomysium antibodies in healthy blood donors. Scand J Gastroenterol. 1998;33:494-498. [DOI] [PubMed] [Google Scholar]
- 29. Ralphs DE, Piper TJ. Celiac disease: A review for the athlete and interdisciplinary team. Strength Condit J. 2016;38(4):66-71. [Google Scholar]
- 30. Ringel Y, Williams RE, Kalilani L, Cook SF. Prevalence, characteristics, and impact of bloating symptoms in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2009;7:68-72. [DOI] [PubMed] [Google Scholar]
- 31. Rubio-Tapia AR, Kyle RA, Kaplan EL, et al. Increased prevalence and mortality in undiagnosed celiac disease. Gastroenterol. 2009;137:88-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sainsbury K, Mullan B, Sharpe L. Reduced quality of life in coeliac disease is more strongly associated with depression than gastrointestinal symptoms.J Psychosom Res. 2013;75:135-141. [DOI] [PubMed] [Google Scholar]
- 33. Shah S, Akbari M, Vanga R, et al. Patient perception of treatment burden is high in celiac disease compared with other common conditions. Am J Gastroenterol. 2014;109:1304-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Smith DF, Gerdes LU. Meta-analysis on anxiety and depression in adult celiac disease. Acta Psychiatr Scand. 2012;125:189-193. [DOI] [PubMed] [Google Scholar]
- 35. Stevens L, Rashid M. Gluten-free and regular foods: a cost comparison. Can J Dietetic Prac Res. 2008;69:147-150. [DOI] [PubMed] [Google Scholar]
- 36. The WHOQOL Group. Development of the World Health Organization WHOQOL-BREF quality of life assessment. Psychol Med. 1998;28:551-558. [DOI] [PubMed] [Google Scholar]
- 37. Torres-McGehee TM, Pritchett KL, Zippel D, Minton DM, Cellamare A, Sibilia M. Sports nutrition knowledge among collegiate athletes, coaches, athletic trainers, and strength and conditioning specialists. J Athl Train. 2012;47:205-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. United States Preventative Services Task Force. Draft recommendation statement: celiac disease screening. http://www.uspreventiveservicestaskforce.org/Page/Document/draft-recommendation-statement150/celiac-disease-screening. Published May 2016. Accessed December 17, 2017.
- 39. Volta U, Caio G, Stanghellini V, De Giorgio R. The changing clinical profile of celiac disease: a 15-year experience (1998-2012) in an Italian referral center. BMC Gastroenterol. 2014;14:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wagner G, Zeiler M, Berger G, et al. Eating disorders in adolescents with celiac disease: influence of personality characteristics and coping. Eur Eat Disord Rev. 2015;23:361-370. [DOI] [PubMed] [Google Scholar]
- 41. Warner R. Applied Statistics: From Bivariate Through Multivariate Techniques. 2nd ed. Thousand Oaks, CA: Sage; 2013. [Google Scholar]