Abstract
Study Design:
Ambispective study with propensity matching.
Objective:
To assess the impact of cervical spondylolisthesis (CS) on clinical presentation and surgical outcome in patients with degenerative cervical myelopathy (DCM).
Methods:
A total of 458 magnetic resonance images (MRIs) from the AOSpine CSM-NA and CSM-I studies were reviewed and CS was identified. Patients with DCM were divided into 2 cohorts, those with CS and those without, and propensity matching was performed. Patient demographics, neurological and functional status at baseline and 2-year follow-up were compared.
Results:
Compared with nonspondylolisthesis (n = 404), CS patients (n = 54) were 8.8 years older (P < .0001), presented with worse baseline neurological and functional status (mJOA [modified Japanese Orthopaedic Association Assessment Scale], P = .008; Nurick, P = .008; SF-36-PCS [Short Form–36 Physical Component Score], P = .01), more commonly presented with ligamentum flavum enlargement (81.5% vs 53.5%, P < .0001), and were less commonly from Asia (P = .0002). Surgical approach varied between cohorts (P = .0002), with posterior approaches favored in CS (61.1% vs 37.4%). CS patients had more operated levels (4.3 ± 1.4 vs 3.6 ± 1.2, P = .0002) and tended to undergo longer operations (196.6 ± 89.2 vs 177.2 ± 75.6 minutes, P = .087). Neurological functional recovery was lower with CS (mJOA [1.5 ± 3.6 vs 2.8 ± 2.7, P = .003]; Nurick [−0.8 ± 1.4 vs −1.5 ± 1.5, P = .002]), and CS was an independent predictor of worse mJOA recovery ratio at 2 years (B = −0.190, P < .0001). After propensity matching, improvement of neurological function was still lower in CS patients (mJOA [1.5 ± 3.6 vs 3.2 ± 2.8, P < .01]; Nurick [−0.8 ± 1.4 vs −1.4 ± 1.6, P = .02]).
Conclusions:
CS patients are older, present with worse neurological/functional impairment, and receive surgery on more levels and more commonly from the posterior. CS may indicate a more advanced state of DCM pathology and is more likely to result in a suboptimal surgical outcome.
Keywords: cervical spondylotic myelopathy, compressive myelopathy, MRI, listhesis, spinal cord injury, surgical decision making, imaging
Introduction
Degenerative cervical myelopathy (DCM) encompasses age-related degenerative changes to the cervical spine, including disc disease, vertebral restructuring, hypertrophy and ossification of intraspinal ligaments, and spondylolisthesis. Together, this set of changes represent the most common cause of spinal cord impairment in adults in industrialized countries.1 There has been considerable interest to investigate the influence of specific pathology, such as ossification of the posterior longitudinal ligament (OPLL), on the clinical presentation, surgical outcome, and surgical approach of patients with DCM in comparison with other pathologies2,3; however, there has been little research investigating patients with cervical spondylolisthesis (CS), which presents in about 12% of patients with DCM4 (Figure 1). Outside of DCM, the population prevalence of CS has been estimated to be between 4% and 20%, most commonly occurring at C4-5.5-9 Although there are subtle differences in the definition of CS, it is identified by anterior or posterior displacement of a rostral vertebra in relation to the adjacent caudal vertebra on static or dynamic radiographic imaging or magnetic resonance imaging (MRI), with translation of 3.0 to 3.5 mm typically representing more severe spondylolisthesis.4,5,10 This displacement can result in considerable instability and may predispose patients to movement dependent or dynamic injury.11 While movement dependent spondylolisthesis and the extent of such movements are typically measured using flexion-extension radiographs or MRI, conventional MRI remains useful in assessing spondylolisthesis in the neutral position.
Figure 1.
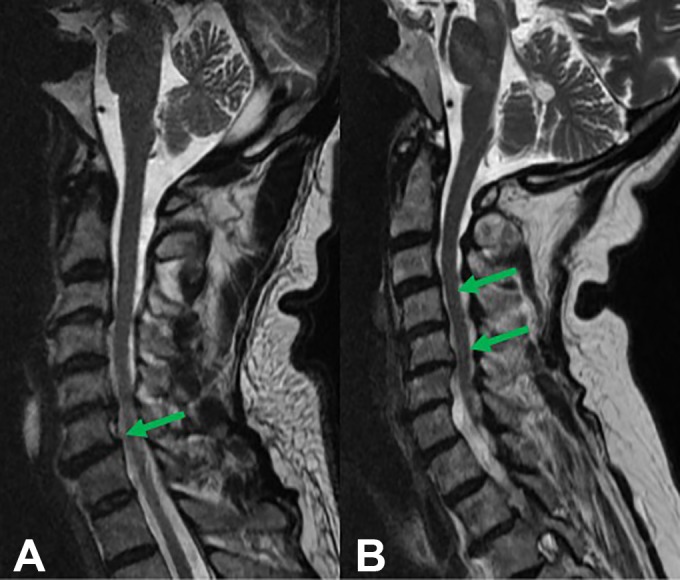
Sagittal T2-weighted magnetic resonance images of patients with degenerative cervical myelopathy (DCM) and spondylolisthesis. (A) The patient is presenting with an anterolisthesis of C6. (B) The patient is presenting with a retrolisthesis of C4 and C5.
Recently, the prevalence and spectrum of MRI pathologic features in patients surgically treated for DCM was reported using the prospective and multicenter AOSpine North America and International studies.4 Using this data, it is the objective of the current study to report on the clinical presentation and surgical outcome of patients that were identified with CS.
Materials and Methods
Study Data
The CSM-NA (12 sites in North America) and CSM-I (16 sites in 4 continents: 6 Asian Pacific sites, 5 European sites, 2 sites North America, and 3 sites in South America) were prospective multicenter studies including patients receiving surgical treatment for DCM, together representing a cohort of 757 patients. The primary study results, which focused on the safety and efficacy of surgical treatment, were previously published.12,13 From the cohort, 458 patients had MRIs available for assessment. Patients were enrolled if they were ≥18 years of age, had clinical signs and symptoms of myelopathy, and did not have prior surgery. Neurological and functional impairment was assessed by the mJOA (modified Japanese Orthopaedic Association Assessment Scale),14 Nurick,15 NDI (Neck Disability Index),16 and SF-36 (Short Form–36)17 measures preoperatively and up to 2 years postoperatively. Assessment was supervised by the attending surgeon at each site and the type of surgical treatment and approach was at the discretion of the attending surgeon. External monitors were used to ensure the integrity of the study data, and research ethics board approval was obtained at all participating sites.
MRI Analysis
MRI analysis of both DICOM (digital imaging and communication in medicine) and JPEG files were performed using OsiriX (http://www.osirix-viewer.com; Pixmeo, Geneva, Switzerland). Given the multicentered global nature of the study, imaging acquisition techniques differed slightly between sites. However, images were typically acquired using 1.5-T MRI machines with typical slice thickness of 3.0 mm for sagittal imaging and 4.0 mm for transverse imaging on T2-weighted images. Analysis to identify the prevalence of pathology was conducted by the primary author, and the diagnostic criteria and results were previously reported4 (Table 1). The modified K-line measurement was described by Taniyama et al18 and was measured by SK.
Table 1.
Diagnostic Criteria Used to Identify Specific Pathology on Magnetic Resonance Imaging.a
| Diagnosis | Criteria |
|---|---|
| Isolated disc pathology | Single-level disc herniation/bulging disc, with no other disc pathology contributing to spinal cord compression or other levels |
| Multilevel disc pathology with or without bone changes (spondylosis) | Spinal cord compression at multiple levels due to multilevel cervical spine degeneration with two or more degenerated discs, with or without associated bone changes |
| Ossification of the posterior longitudinal ligament (OPLL) | OPLL appears hypointense on both T1W1 and T2W1. Effacement of the cerebrospinal fluid (CSF) anterior to the cord on T2W1 as well as spinal cord compression that is contiguous across multiple levels, or in the absence of spondylotic changes, is highly suggestive of ligament pathology |
| Ligamentum flavum buckling, hypertrophy, calcification, or ossification | Any posterior enlargement of the ligamentum flavum contributing to stenosis of the cervical canal |
| Spondylolisthesis or subluxation | Anterior or posterior displacement of the vertical body/bodies in relation to adjacent levels on sagittal imaging |
| Klippel-Feil syndrome | Vertebral levels without a complete disc and a wasp-waist sign. Absent discs due to degenerative autofusion were disregarded |
| Craniocervical junction abnormalities | Abnormal structural pathologies resulting in spinal cord or brain stem compression |
| Congenital stenosis | Patients with a spinal cord occupation ratio (SCOR) of ≥70% in the spinal canal at nonpathological sites |
Spondylolisthesis was identified when there was visible anterior or posterior displacement of a rostral vertebra in relation to the adjacent caudal vertebra on MRI, since lateral or flexion/extension radiographs were not available for all patients. Listhesis was considered present when the entire vertebral body was displaced, rather than solely the edge of the vertebral endplate, to differentiate listhesis from osteophytes. We recognize that utilizing MRI rather than flexion extension radiographs likely underreports patients with movement-dependent spondylolisthesis.
Statistical Analysis
Patients with DCM were separated into groups comprising those with and without spondylolisthesis. Continuous variables are presented as means and were compared using independent t tests. Categorical variables are presented as proportions and were assessed using chi-square and Fischer’s exact tests. Additionally, patients were propensity matched (2 nonspondylolisthesis:1 spondylolisthesis) by age, baseline mJOA, and surgical approach (ie, anterior vs posterior vs combined). This was done because age and baseline mJOA have been shown to be independent predictors of surgical outcome.19,20 In terms of surgical approach, while both anterior and posterior surgery are effective treatment options for DCM, many surgeons prefer a posterior approach for multilevel or more severe disease.3 Surgical approach (anterior vs posterior vs combined) and geographic region were compared using chi-square tests and post hoc pairwise comparisons with Bonferroni correction. A last observation carry-forward approach was used to impute missing data for follow-up at 2 years. After imputation, 436 patients had mJOA, 436 had Nurick, 428 had SF-36, and 363 had NDI outcome data available. Measures of neurological and functional impairment between the groups were compared at baseline and 2 years postoperatively (mean difference from baseline) using independent t tests. Multiple linear regression was used to assess the presence of spondylolisthesis, while controlling for age and baseline severity (mJOA), on mJOA recovery ratio. These factors were included as they are known to independently predict surgical outcome.19,21 The mJOA recovery ratio was previously described by Hirabayashi et al22 and is calculated using the formula: ([postoperative mJOA score – preoperative mJOA score]/(18 – preoperative mJOA score]) × 100%. Statistical significance was considered at P ≤ .05 and tendency toward significance was considered at .05 < P ≤ .10.
Results
Demographics and Clinical Presentation
From the cohort of 458 patients (North America, n = 200; Asia, n = 107; Europe, n = 93; Latin America, n = 58), 54 patients presented with CS and 404 had other forms of DCM. The patient demographics in each group are presented in Table 2. Patients with CS were observed to be older by an average of 8.8 years (P < .0001), and presented with worse baseline neurological function as assessed by both the mJOA (11.72 ± 3.05 vs 12.79 ± 2.74, P = .008) and Nurick (2.78 ± 1.59 vs 1.74 ± 1.57, P = .008), and with worse SF-36 Physical Component Score (PCS) (35.22 ± 9.24 vs 41.23 ± 11.17, P = .01). The prevalence of spondylolisthesis varied significantly between regions (P = .002, as previously reported),4 and post hoc pairwise comparison after Bonferroni correction showed that spondylolisthesis was significantly less common in Asia (P = .0003). Sex, duration of symptoms, SF-36 Mental Component Score (MCS), and NDI were not significantly different (P > .05). On MRI, the prevalence of pathology was mostly comparable between patients presenting with or without CS. However, ligamentum flavum enlargement was considerably more common (81.5% vs 53.5%, P < .0001), the number of compressed levels tended to be more common (3.48 vs 3.08, P = .052), and OPLL tended to be less common in patients with spondylolisthesis (3.7% vs 11.4%, P = .098).
Table 2.
Characteristics of DCM Patients With and Without CS.
| Spondylolisthesis (n = 54) | Nonspondylolisthesis (n = 404) | P a | |
|---|---|---|---|
| Clinical Presentation | |||
| Age (years) | 63.9 ± 11.2 | 55.1 ± 11.4 | <.0001 |
| Sex (male) | 57.40% | 62.90% | .437 |
| Duration of symptoms (months) | 22.8 ± 35.6 | 27.7 ± 35.2 | .33 |
| mJOA | 11.72 ± 3.05 | 12.79 ± 2.74 | .008 |
| Nurick grade | 3.61 ± 1.24 | 3.18 ± 1.12 | .008 |
| NDI | 40.32 ± 18.02 (n = 32) | 38.04 ± 20.56 (n = 336) | .46 |
| SF-36 Mental Component Score | 42.06 ± 15.04 (n = 53) | 40.21 ± 13.32 (n = 399) | .35 |
| SF-36 Physical Component Score | 31.49 ± 9.82 (n = 53) | 35.06 ± 9.37 (n=399) | .01 |
| Region | |||
| North America | 13.5% (n = 27) | 86.5% (n = 173) | .002 |
| Asiab | 1.9% (n = 2) | 98.1% (n = 105) | |
| Europe | 18.3% (n = 17) | 81.7% (n = 76) | |
| Latin America | 13.8% (n = 8) | 86.2% (n = 50) | |
| MRI findings | |||
| Disc herniation | 11.1% (6/54) | 9.2% (37/404) | .64 |
| Multilevel disease (spondylosis) | 87.0% (47/54) | 90.1% (364/404) | .48 |
| OPLL | 3.7% (2/54) | 11.4% (46/404) | .098c |
| Ligamentum flavum enlargement | 81.5% (44/54) | 53.5% (216/404) | <.0001 |
| Klippel-Feil syndrome | 1.9% (1/54) | 2.0% (8/404) | 1c |
| CCS/cord-canal mismatch | 2.6% (1/38) | 9.2% (25/272) | .22c |
| Compressed levels | 3.48 (n = 54) | 3.08 (n = 404) | .052 |
| Modified K-line | 4.11 (n = 52) | 4.15 (n = 391) | .94 |
| T2WI hyperintensity | 77.4% (41/53) | 71% (279/393) | .33 |
| T1WI hypointensity | 19.6% (10/51) | 19.4% (72/371) | .97 |
| Surgical factors | |||
| Operative length | 196.6 ± 89.2 | 177.2 ± 75.6 | .087 |
| Levels operated | 4.3 ± 1.4 | 3.6 ± 1.2 | .0002 |
| Surgical approach | |||
| Anterior (n = 265) | 33.3% (18/54) | 61.1% (247/404) | |
| Posterior (n = 184) | 61.1% (33/54) | 37.4% (151/404) | .0002 |
| Combined (n = 9) | 5.6% (3/54) | 1.5% (6/404) | |
| Anterior surgery type | |||
| Discectomy (n = 202) | 29.4% (15/51) | 47.0% (187/398) | .018 |
| Corpectomy (n = 7) | 2.0% (1/51) | 1.5% (6/398) | .81 |
| Discectomy and corpectomy (n = 51) | 3.9% (2/51) | 12.3% (49/398) | .075 |
| Posterior surgery type | |||
| Laminectomy (n = 13) | 11.8% (6/51) | 1.8% (7/398) | <.0001 |
| Laminectomy and fusion (n = 110) | 47.1% (24/51) | 21.6% (86/398) | <.0001 |
| Laminoplasty (n = 61) | 5.9% (3/51) | 14.6% (58/398) | .088 |
Abbreviations: DCM, degenerative cervical myelopathy; CS, cervical spondylolisthesis; OPLL, ossification of the posterior longitudinal ligament; CCS, congenital cervical stenosis; mJOA, modified Japanese Orthopaedic Association Assessment Scale; NDI, Neck Disability Index; SF-36, Short Form–36.
aBoldfaced P values indicate statistical significance (P < .05).
bWas significantly different from other regions (P = .0002).
cDenotes comparisons made using Fischer’s exact test.
After propensity matching, baseline characteristics were comparable between the cohorts with the exception of a marginally statistically significant worse SF-36 PCS among patients with CS (31.5 ± 9.8 vs 34.5 ± 8.4, P = .04) (Table 3). The significant difference in the prevalence of ligamentum flavum hypertrophy was no longer present in the matched cohort.
Table 3.
Characteristics of DCM Patients With and Without CS After Propensity Matching for Age, Baseline mJOA, and Surgical Approach (Anterior, Posterior, Combined).
| Spondylolisthesis (n = 54) | Nonspondylolisthesis (n = 108) | P a | |
|---|---|---|---|
| Clinical Presentation | |||
| Age (years) | 63.9 ± 11.3 | 64.1 ± 10.8 | .90 |
| Sex (male) | 57.41% | 62.04% | .61 |
| Duration of symptoms (months) | 22.8 ± 35.6 | 27.0 ± 37.0 | .49 |
| mJOA | 11.7 ± 3.0 | 11.7 ± 2.6 | .89 |
| Nurick grade | 3.6 ± 1.2 | 3.5 ± 1.2 | .67 |
| NDI | 40.3 ± 18.0 (n = 50) | 37.4 ± 22.0 (n=86) | .43 |
| SF-36 Mental Component Score | 42.1 ± 15.0 (n = 53) | 40.2 ± 14.1 | .44 |
| SF-36 Physical Component Score | 31.5 ± 9.8 (n = 53) | 34.5 ± 8.4 | .04 |
| MRI findings | |||
| Disc herniation | 11.1% (6/54) | 6.5% (7/108) | .36 |
| Multilevel disease (spondylosis) | 87.0% (47/54) | 93.5% (101/108) | .23 |
| OPLL | 3.7% (2/54) | 11.1% (12/108) | .15 |
| Ligamentum flavum enlargement | 81.5% (44/54) | 74.1% (80/108) | .33 |
| Klippel-Feil syndrome | 1.9% (1/54) | 1.9% (2/108) | 1.00 |
| CCS/cord-canal mismatch | 2.6% (1/38) | 8.6% (6/70) | .42 |
| Compressed levels | 3.5 ± 1.4 | 3.3 ± 1.2 | .51 |
| modified K-line | 4.1 ± 3.3 (n = 52) | 4.4 ± 2.4 (n = 104) | .48 |
| T2WI hyperintensity | 77.4% (41/53) | 81.3% (87/107) | .68 |
| T1WI hypointensity | 19.6% (10/51) | 29.1% (30/103) | .24 |
| Surgical factors | |||
| Operative length | 196.6 ± 89.2 | 174.3 ± 7.8 | .11 |
| Levels operated | 4.3 ± 1.4 | 4.0 ± 1.1 | .16 |
| Surgical approach | |||
| Anterior | 33.3% (18/54) | 38.9% (42/108) | NS |
| Posterior | 61.1% (33/54) | 58.3% (63/108) | |
| Combined | 5.6% (3/54) | 2.8% (3/108) | |
| Anterior surgery type | |||
| Discectomy | 29.4% (15/51) | 30.5% (32/105) | NS |
| Corpectomy | 2.0% (1/51) | 1.0% (1/105) | |
| Discectomy and corpectomy | 3.9% (2/51) | 8.6% (9/105) | |
| Posterior surgery type | |||
| Laminectomy | 11.8% (6/51) | 1.0% (1/105 ) | <.01 |
| Laminectomy and fusion | 47.1% (24/51) | 27.6% (29/105) | .02 |
| Laminoplasty | 5.9% (3/51) | 31.4% (33/105) | <.01 |
Abbreviations: DCM, degenerative cervical myelopathy; CS, cervical spondylolisthesis; OPLL, ossification of the posterior longitudinal ligament; CCS, congenital cervical stenosis; mJOA, modified Japanese Orthopaedic Association Assessment Scale; NDI, Neck Disability Index; SF-36, Short Form–36; NS, not significant.
aBoldfaced P values indicate statistical significance (P < .05).
Surgical Approach
Patients with spondylolisthesis were operated on a greater number of levels on average (4.3 ± 1.4 vs 3.6 ± 1.2, P = .0002) and tended to have longer operative times than patients without spondylolisthesis (196.6 ± 89.2 vs 177.2 ± 75.6, P = .087) (Table 2). There was a statistically significant difference in surgical approach (P = .0002), with posterior surgery more common, and anterior surgery less commonly performed in patients with spondylolisthesis than those without. Anterior discectomy was less often chosen for patients with spondylolisthesis (P = .018), and the combination of discectomy and corpectomy tended to be less commonly chosen for spondylolisthesis (P = .075). There was no difference in the choice for corpectomy. Laminectomy and laminectomy and fusion were both more commonly undertaken in patients with spondylolisthesis than those without (P < .0001). Laminoplasty tended to be more commonly undertaken in patients without spondylolisthesis (P = .088).
After propensity matching, the differences in choices for laminectomy (P < .01) and laminectomy and fusion (P = .02) remained the same as prior to matching, with both more commonly done in patients with CS (Table 3). However, the choice for undertaking laminoplasty more commonly for patients without CS became significant (P < .01), and the number of levels operated was no longer significantly different.
Surgical Outcome
Patients with spondylolisthesis had a significantly worse mean neurological improvement in surgical outcome at 2-year follow-up as measured by mJOA (1.5 ± 3.6 vs 2.8 ± 2.7, P = .003) and Nurick (−0.8 ± 1.4 vs −1.5 ± 1.5, P = .002) compared with patients without spondylolisthesis (Table 4). However, while mean differences in surgical outcome based on the NDI (8.1 ± 20.2 vs 12.8 ± 18.9, P = .11), SF-36 PCS (4.1 ± 11.2 vs 6.1 ± 10.8, P = .22) and MCS 3.6 ± 15.2 vs 7.0 ± 14.0, P = .10) were on average lower for patients with spondylolisthesis, these did not reach statistical significance. After patients with and without CS were propensity matched, the mean improvement of neurological function was still lower in patients with CS (mJOA [1.5 ± 3.6 vs 3.2 ± 2.8, P < .01]; Nurick (−0.8 ± 1.4 vs −1.4 ± 1.6, P = .02]). In addition, there was a trend toward worse SF-36 mental recovery among patients with CS (3.6 ± 15.2 vs 8.5 ± 14.4, P = .051).
Table 4.
Difference in Surgical Outcome at 2 Years in Patients With or Without CS Before and After Propensity Matching.
| Mean Difference (at 2 Years) | P a | ||
|---|---|---|---|
| Spondylolisthesis | Nonspondylolisthesis | ||
| Outcome Measure | |||
| mJOA (n = 436) | 1.5 ± 3.6 (n = 51) | 2.8 ± 2.7 (n = 385) | .003 |
| Nurick grade (n = 436) | −0.8 ± 1.4 (n = 51) | −1.5 ± 1.5 (n = 385) | .002 |
| NDI (n = 363) | 8.1 ± 20.2 (n = 48) | 12.8 ± 18.9 (n = 315) | .11 |
| SF-36 Mental (n = 428) | 3.6 ± 15.2 (n = 51) | 7.0 ± 14.0 (n = 377) | .10 |
| SF-36 Physical (n = 428) | 4.1 ± 11.2 (n = 51) | 6.1 ± 10.8 (n = 377) | .22 |
| Propensity Matched Cohort | |||
| mJOA | 1.5 ± 3.6 (n = 51) | 3.2 ± 2.8 (n = 101) | <.01 |
| Nurick grade | −0.8 ± 1.4 (n = 51) | −1.4 ± 1.6 (n = 101) | .02 |
| NDI | 8.1 ± 20.2 (n = 48) | −12.1 ± 20.2 (n = 79) | .28 |
| SF-36 Mental | 3.6 ± 15.2 (n = 51) | 8.5 ± 14.4 (n = 101) | .051 |
| SF-36 Physical | 4.1 ± 11.2 (n = 51) | 6.4 ± 11.4 (n = 101) | .24 |
Abbreviations: CS, cervical spondylolisthesis; mJOA, modified Japanese Orthopaedic Association Assessment Scale; NDI, Neck Disability Index; SF-36, Short Form–36.
aBoldfaced P values indicate statistical significance (P < .05).
Multiple linear regression to assess the presence of spondylolisthesis on mJOA outcome, controlling for age and baseline mJOA severity, showed that spondylolisthesis was an independent predictor of a lower mJOA recovery ratio (B = −0.190, P < .0001).
Discussion
CS is relatively common in patients with DCM,5 and was present in nearly 12% of patients on MRI in the present cohort. Clinically, these patients presented approximately 9 years older on average, with worse neurological and general functional impairment, a higher rate of ligamentum flavum enlargement, and tended to have more levels of cervical compression than patients without spondylolisthesis. Given these findings, it is not surprising that patients with spondylolisthesis had nearly 1 more cervical level operated on average (4.3 vs 3.6), were more commonly surgically treated from the posterior, and tended to have longer operations. It was notable that despite having this increased severity spectrum, CS presented on average with a 5-month shorter duration of symptoms, and though not statistically significant, it could suggest that these patients may have a more precipitous course owing to potential instability. It was also notable that, on average, patients with CS did not complain of significantly increased neck disability. This is despite the finding of a previous systematic review that neck pain is the first symptom to occur in most patients with degenerative spondylolisthesis.9 Last, as was previously reported,4 patients from the Asian region presented less commonly with spondylolisthesis. This finding may be partially attributable to the high prevalence of OPLL among Asian populations, differences in spinal column size or possibly genetic factors,4,23 which may confer increased cervical stability. However, these suppositions require further investigation.
Surgical Factors and Outcome
In terms of surgical outcome, patients with CS experienced a lower degree of recovery on all measures, but only neurological outcome measures demonstrated a statistically significant difference. While age and baseline neurological severity are known to affect surgical outcome,19,20,24 these were accounted for in the propensity matched cohort and neurological outcome remained suboptimal in the spondylolisthesis cohort. Furthermore, the presence of spondylolisthesis independently affected neurological recovery despite controlling for age and baseline severity on multivariate analysis. It is not entirely clear what causes this influence, but it is possible that spondylolisthesis may exert a more deleterious force upon the spinal cord when it is unstable compared with other forms of DCM whose forces are typically more static in nature. Alternatively, the presentation of spondylolisthesis may simply represent a generally worse degenerative state—this would be supported by the greater number of levels of pathology and the higher rate of circumferential compression which spondylolisthesis patients presented with. However, in terms of T2 hyperintensity presence, there was no statistically significant difference in the prevalence between patients with and without CS.
Before propensity matching, there were more patients without CS that received a discectomy. However, this is likely because of the fact that this group was much younger, making single level disc disease and therefore an ACDF approach much more likely. Indeed, this difference went away after propensity matching, and there was no difference in the approach (anterior vs posterior vs combined). Of note, patients with CS treated posteriorly more commonly received a laminectomy with or without fusion and less commonly a laminoplasty (when looking at posterior surgery only group, patients with CS received a fusion 72.7% (24/33) of the time, while non-CS patients were fused 46% (29/63) of the time). It is possible that this is due to a number of reasons, including regional variations in treatment and surgeon preference. Interestingly, in a recent survey of AOSpine members examining the influence of MRI cervical pathology on surgical decision making in DCM, respondents indicated a moderate to weak preference for an anterior approach if CS is present in isolation.3 However, degenerative CS is often accompanied by other pathological changes, and thus this may indicate the preference for posterior surgery in the present cohort is also dependent on other accompanying factors.
While it seems reasonable that the presence of spondylolisthesis in patients with DCM can have a negative impact on neurological status and outcome, it is interesting that the presence of spondylolisthesis has also shown to be quite benign in patients without myelopathy. Park et al,8 recently showed that the natural history of both cervical anterolisthesis and retrolisthesis appeared to be stable during a follow-up period of up to 8 years. It is challenging to interpret these findings clearly, but this may suggest that the natural history of CS superimposed with other degenerative pathology may represent a different spectrum of pathology than isolated CS.
Comparison With Lumbar Spondylolisthesis
Compared to lumbar spondylolisthesis, there has been considerably less focus on CS. However, there have been recent efforts to investigate the topic more extensively. A common point of discussion has been how to define spondylolisthesis and what constitutes stable and unstable.5,8 While the Meyerding classification is a commonly used measure to assess the extent of listhesis,25 the grading is more appropriate for lumbar pathology, as the vast majority of patients with degenerative CS are likely to exhibit only grade I listhesis given the differences in anatomy between the spine regions. In terms of stability, there have been various definitions ranging from at least 2 to 3.5 mm of translation on flexion-extension for identification of instability5,8,9; however, there is no well-defined diagnostic criterion.
Like lumbar spondylolisthesis, disc degeneration and facet arthropathy are the most common causes for spondylolisthesis development. In the lumbar spine, the anatomical orientation of the facet joint can also predispose patients to listhesis,26 and it is possible that certain anatomical variations, including congenital anomalies or facet tropism in the cervical region, may likewise affect potential listhesis development. Furthermore, sagittal alignment may have an impact on listhesis development and has been suggested to predispose listhesis in the lumbar region.26 Interestingly, randomized control studies assessing surgical outcomes have been unable to determine whether fusion is definitively better than nonfusion surgery for lumbar stenosis, and therefore it is not clear whether this would be true for the cervical region as well.27,28 Last, lumbar spine stenosis patients with or without spondylolisthesis appear to have similar baseline impairment (Oswestry Disability Index [ODI], EQ5D) and improvement after surgery.27,29 It is challenging to directly compare these results with CS, as the findings of the present study indicate that patients with CS appear to have suboptimal neurological recovery based on myelopathy specific measures, and not EQ5D or ODI. While the CS patients here did have less recovery on the SF scales (which would be the most relevant comparison to EQ5D/ODI), these were not significantly different from non-CS and thus perhaps suggest a marginal but insignificant difference in health quality outcomes, as appears to be the case with lumbar stenosis.
Limitations
We assessed patients for CS using conventional MRIs, as we did not have flexion/extension radiographs for all patients, DICOM formats were not available for some radiographs, and due to inconsistent acquisition techniques among different centers. Also, there was some minor variability in MRI acquisition techniques given that images were obtained from multiple centers, including differences in slice thickness and magnet type (Tesla strength). While this results in subtle differences between the images, we believe that this factor had a relatively minor impact with regard to CS diagnosis. Another limitation to consider is that our findings are restricted to those patients with spondylolisthesis apparent on static imaging in a supine position, which we recognize will miss some cases of dynamic spondylolisthesis. Furthermore, patients were identified for the presence or absence of spondylolisthesis and therefore, we have not reported the degree of translation or whether the patients had anterolisthesis or retrolisthesis. However, most patients had anterolisthesis, and breaking this population down would have not provided enough patients for adequate statistical analysis. Future work will focus on the assessment of spondylolisthesis using flexion and extension radiographs, which offers the opportunity to discover both static and movement dependent vertebra translocation, the degree of translation, and stability. In addition, it would be useful in future analysis to investigate the status of the facet joint in greater detail. Furthermore, only 1 rater reviewed the presence of pathology based on the criteria previously published, and 1 rater reviewed the modified K-line. In terms of the type of pathology in the geographic regions, while multiple centers were involved, it is unclear if inclusion in the study by centers was affected by selection bias, and therefore findings such as low spondylolisthesis occurrence in Asia may have been encountered spuriously.
Conclusion
CS presents more commonly in older patients with DCM and manifests with worse baseline neurological function and quality of life. These patients typically have compression arising from both the anterior and posterior, have more levels of spinal cord compression, and receive surgery on a greater number of cervical levels that is more commonly performed with a posterior approach. Furthermore, while these patients experience an average improvement of about 1.5 points on the mJOA scale at 2-year follow-up, this is significantly lower than the improvement experienced by other DCM patients without spondylolisthesis. Overall, these findings suggest that the presence of CS may indicate a more advanced state of DCM pathology and is more likely to result in a suboptimal surgical outcome. However, further studies are needed to evaluate other potential factors relevant to CS such as facet hypertrophy, fluid signal in the facet joints, and stable and unstable CS.
Acknowledgments
The authors would like to acknowledge AOSpine for supporting this work.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by AOSpine.
ORCID iD: Aria Nouri, MD, MSc  https://orcid.org/0000-0002-4965-3059
https://orcid.org/0000-0002-4965-3059
So Kato, MD  https://orcid.org/0000-0003-0835-2724
https://orcid.org/0000-0003-0835-2724
Michael G. Fehlings, MD, PhD  https://orcid.org/0000-0002-5722-6364
https://orcid.org/0000-0002-5722-6364
References
- 1. Nouri A, Tetreault L, Singh A, Karadimas SK, Fehlings MG. Degenerative cervical myelopathy: epidemiology, genetics, and pathogenesis. Spine (Phila Pa 1976). 2015;40:E675–E693. [DOI] [PubMed] [Google Scholar]
- 2. Nakashima H, Tetreault L, Nagoshi N, et al. Comparison of outcomes of surgical treatment for ossification of the posterior longitudinal ligament versus other forms of degenerative cervical myelopathy: results from the prospective, multicenter AOSpine CSM-International Study of 479 patients. J Bone Joint Surg Am. 2016;98:370–378. [DOI] [PubMed] [Google Scholar]
- 3. Nouri A, Martin AR, Nater A, et al. Influence of magnetic resonance imaging features on surgical decision-making in degenerative cervical myelopathy: results from a Global Survey of AOSpine International Members. World Neurosurg. 2017;105:864–874. [DOI] [PubMed] [Google Scholar]
- 4. Nouri A, Martin AR, Tetreault L, et al. MRI analysis of the combined prospectively collected AOSpine North America and International Data: the prevalence and spectrum of pathologies in a global cohort of patients with degenerative cervical myelopathy. Spine (Phila Pa 1976). 2017;42:1058–1067. [DOI] [PubMed] [Google Scholar]
- 5. Suzuki A, Daubs MD, Inoue H, et al. Prevalence and motion characteristics of degenerative cervical spondylolisthesis in the symptomatic adult. Spine (Phila Pa 1976). 2013;38:E1115–E1120. [DOI] [PubMed] [Google Scholar]
- 6. Kopacz KJ, Connolly PJ. The prevalence of cervical spondylolisthesis. Orthopedics. 1999;22:677–679. [PubMed] [Google Scholar]
- 7. Dean CL, Gabriel JP, Cassinelli EH, Bolesta MJ, Bohlman HH. Degenerative spondylolisthesis of the cervical spine: analysis of 58 patients treated with anterior cervical decompression and fusion. Spine J. 2009;9:439–446. [DOI] [PubMed] [Google Scholar]
- 8. Park MS, Moon SH, Lee HM, et al. The natural history of degenerative spondylolisthesis of the cervical spine with 2- to 7-year follow-up. Spine (Phila Pa 1976). 2013;38:E205–E210. [DOI] [PubMed] [Google Scholar]
- 9. Jiang SD, Jiang LS, Dai LY. Degenerative cervical spondylolisthesis: a systematic review. Int Orthop. 2011;35:869–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kawasaki M, Tani T, Ushida T, Ishida K. Anterolisthesis and retrolisthesis of the cervical spine in cervical spondylotic myelopathy in the elderly. J Orthop Sci. 2007;12:207–213. [DOI] [PubMed] [Google Scholar]
- 11. Nouri A, Martin AR, Mikulis D, Fehlings MG. Magnetic resonance imaging assessment of degenerative cervical myelopathy: a review of structural changes and measurement techniques. Neurosurg Focus. 2016;40:E5. [DOI] [PubMed] [Google Scholar]
- 12. Fehlings MG, Ibrahim A, Tetreault L, et al. A global perspective on the outcomes of surgical decompression in patients with cervical spondylotic myelopathy: results from the prospective multicenter AOSpine International Study on 479 patients. Spine (Phila Pa 1976). 2015;40:1322–1328. [DOI] [PubMed] [Google Scholar]
- 13. Fehlings MG, Wilson JR, Kopjar B, et al. Efficacy and safety of surgical decompression in patients with cervical spondylotic myelopathy: results of the AOSpine North America prospective multi-center study. J Bone Joint Surg Am. 2013;95:1651–1658. [DOI] [PubMed] [Google Scholar]
- 14. Benzel EC, Lancon J, Kesterson L, Hadden T. Cervical laminectomy and dentate ligament section for cervical spondylotic myelopathy. J Spinal Disord. 1991;4:286–295. [DOI] [PubMed] [Google Scholar]
- 15. Nurick S. The pathogenesis of the spinal cord disorder associated with cervical spondylosis. Brain. 1972;95:87–100. [DOI] [PubMed] [Google Scholar]
- 16. Vernon H, Mior S. The Neck Disability Index: a study of reliability and validity. J Manipulative Physiol Ther. 1991;14:409–415. [PubMed] [Google Scholar]
- 17. Ware JE, Jr, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 18. Taniyama T, Hirai T, Yoshii T, et al. Modified K-line in magnetic resonance imaging predicts clinical outcome in patients with nonlordotic alignment after laminoplasty for cervical spondylotic myelopathy. Spine (Phila Pa 1976). 2014;39:E1261–E1268. [DOI] [PubMed] [Google Scholar]
- 19. Nakashima H, Tetreault LA, Nagoshi N, et al. Does age affect surgical outcomes in patients with degenerative cervical myelopathy? Results from the prospective multicenter AOSpine International Study on 479 patients. J Neurol Neurosurg Psychiatry. 2016;87:734–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tetreault LA, Karpova A, Fehlings MG. Predictors of outcome in patients with degenerative cervical spondylotic myelopathy undergoing surgical treatment: results of a systematic review. Eur Spine J. 2015;24(suppl 2):236–251. [DOI] [PubMed] [Google Scholar]
- 21. Tetreault LA, Kopjar B, Vaccaro A, et al. A clinical prediction model to determine outcomes in patients with cervical spondylotic myelopathy undergoing surgical treatment: data from the prospective, multi-center AOSpine North America study. J Bone Joint Surg Am. 2013;95:1659–1666. [DOI] [PubMed] [Google Scholar]
- 22. Hirabayashi K, Miyakawa J, Satomi K, Maruyama T, Wakano K. Operative results and postoperative progression of ossification among patients with ossification of cervical posterior longitudinal ligament. Spine (Phila Pa 1976). 1981;6:354–364. [DOI] [PubMed] [Google Scholar]
- 23. Fujimori T, Le H, Hu SS, et al. Ossification of the posterior longitudinal ligament of the cervical spine in 3161 patients: a CT-based study. Spine (Phila Pa 1976). 2015;40:E394–E403. [DOI] [PubMed] [Google Scholar]
- 24. Holly LT, Matz PG, Anderson PA, et al. Clinical prognostic indicators of surgical outcome in cervical spondylotic myelopathy. J Neurosurg Spine. 2009;11:112–118. [DOI] [PubMed] [Google Scholar]
- 25. Meyerding HW. Spondylolisthesis. Surg Gynecol Obstet. 1932;54:371–377. [Google Scholar]
- 26. Steiger F, Becker HJ, Standaert CJ, et al. Surgery in lumbar degenerative spondylolisthesis: indications, outcomes and complications. A systematic review. Eur Spine J. 2014;23:945–973. [DOI] [PubMed] [Google Scholar]
- 27. Forsth P, Olafsson G, Carlsson T, et al. A randomized, controlled trial of fusion surgery for lumbar spinal stenosis. N Engl J Med. 2016;374:1413–1423. [DOI] [PubMed] [Google Scholar]
- 28. Ghogawala Z, Dziura J, Butler WE, et al. Laminectomy plus fusion versus laminectomy alone for lumbar spondylolisthesis. N Engl J Med. 2016;374:1424–1434. [DOI] [PubMed] [Google Scholar]
- 29. Lonne G, Fritzell P, Hagg O, et al. Lumbar spinal stenosis: comparison of surgical practice variation and clinical outcome in three national spine registries. Spine J. 2019;19:41–49. [DOI] [PubMed] [Google Scholar]


