Abstract
Spinal cord injury (SCI) induces significant reorganization in the sensorimotor cortex. Trunk motor control is crucial for postural stability and propulsion after low thoracic SCI and several rehabilitative strategies are aimed at trunk stability and control. However little is known about the effect of SCI and rehabilitation training on trunk motor representations and their plasticity in the cortex. Here, we used intracortical microstimulation to examine the motor cortex representations of trunk in relation to other representations in three groups of chronic adult complete low thoracic SCI rats: chronic untrained, treadmill trained (but ‘non-stepping’) and robot assisted treadmill trained (but ‘non-stepping’) and compared with a group of normal rats. Our results demonstrate extensive and significant reorganization of trunk motor cortex after chronic adult SCI which includes (1) Expansion and rostral displacement of trunk motor representations in the cortex, with the greatest significant increase observed for rostral (to injury) trunk, and slight but significant increase of motor representation for caudal (to injury) trunk at low thoracic levels in all spinalized rats. (2) Significant changes in coactivation and the synergy representation (or map overlap) between different trunk muscles and between trunk and forelimb. No significant differences were observed between the groups of transected rats for the majority of the comparisons. However, (3) the treadmill and robot-treadmill trained groups of rats showed a further small but significant rostral migration of the trunk representations, beyond the shift caused by transection alone. We conclude that SCI induces significant reorganization of trunk motor cortex, which is not qualitatively altered by non-stepping treadmill training or non-stepping robot assisted treadmill training, but is shifted further from normal topography by the training. This shift may potentially make subsequent rehabilitation with stepping longer or less successful.
Keywords: Spinal Cord Injury, Motor Cortex, Plasticity, Reorganization, Trunk, Robot Rehabilitation, Activity/Exercise induced plasticity
INTRODUCTION
Injuries such as limb amputation (Sanes et al., 1990, Cohen et al., 1991, Ojemann and Silbergeld, 1995, Pascual-Leone et al., 1996, Schieber and Deuel, 1997, Wu and Kaas, 1999) peripheral nerve transection (Sanes et al., 1988, Sanes et al., 1990, Toldi et al., 1996, Rijntjes et al., 1997, Franchi, 2000), stroke (Nudo and Friel, 1999, Nishibe et al., 2010, Chelette et al., 2013, Harrison et al., 2013) or spinal cord injury (SCI) (Jain et al., 1997, Ghosh et al., 2009, Kokotilo et al., 2009, Nardone et al., 2013) can alter the somatotopic organization in the sensory and motor cortex. Alterations occur on both rapid (Sanes et al., 1988, Aguilar et al., 2010) and longer timescales (Sanes et al., 1990, Toldi et al., 1996, Franchi, 2000, Aguilar et al., 2010, Tandon et al., 2013). It is known that in animals and humans, following a complete low thoracic SCI, there is an expansion of the sensory map of spared proximal areas into the deafferented cortex (McKinley and Smith, 1990, Chau and McKinley, 1991, Endo et al., 2007, Aguilar et al., 2010, Henderson et al., 2011) and a shift in motor representations of proximal muscles in the motor cortex (Topka et al., 1991, Bruehlmeier et al., 1998, Laubis-Herrmann et al., 2000, Lotze et al., 2006). Trunk control and representations are likely to be fundamental for highly skilled motions (Anders et al., 2007, Bronner, 2012, Sung et al., 2012). Trunk motor control is also crucial for postural stability and propulsion after SCI in both humans and animals (Yang et al., 2006, Giszter et al., 2008, Bjerkefors et al., 2009, Desroches et al., 2013). Several gait rehabilitative strategies are aimed at trunk stability and control in animals (Udoekwere et al., 2006, Dominici et al., 2012) and humans (Dobkin et al., 2003, Hidler and Sainburg, 2011, Hussain et al., 2011, Dobkin and Duncan, 2012). However little is known about the effect of SCI and rehabilitation on trunk motor representations and their plasticity in the cortex. In clinically complete low thoracic SCI patients, Topka et al. observed that transcranial magnetic stimulation (TMS) activated a large fraction of motorneuron pools and evoked motor evoked potentials (MEPs) with shorter latencies from a large number of scalp positions in abdominal muscles immediately rostral to the level of SCI (Topka et al., 1991). Cariga et al. observed that TMS elicited motor responses in paravertebral muscles in all segments above the lesion and also in varying range of segments below the lesion in clinically complete thoracic SCI patients (Cariga et al., 2002). However, a systematic examination of how the trunk motor cortex reorganizes after adult thoracic SCI and rehabilitation of the paralyzed adults in an animal model is missing from the current literature.
Plastic changes in cortex can arise spontaneously after injury or depend on use and skill (e.g. (Dancause and Nudo, 2011)). Prolonged exercise training increases blood flow to the cortex, induces angiogenesis (Swain et al., 2003, Seifert and Secher, 2011) and results in upregulation of neurotrophic factors, and these can promote neuronal survival and differentiation in the cortex (Klintsova et al., 2004, Vaynman and Gomez-Pinilla, 2005). However, representational changes in motor areas are usually thought to instead be associated with skill acquisition and precisely practiced improvements, not with endurance practice of an existing skill (Kleim et al., 1998, Plautz et al., 2000, Kleim et al., 2002, Kleim et al., 2004, Perez et al., 2004). It is known that treadmill training of adult rats spinalized as neonates (Kao et al., 2009, Kao et al., 2011) or passive hindlimb bike exercise of adult SCI rats (Graziano et al., 2013) induces plasticity in the somatosensory cortex. However, it is not clear whether prolonged exercise training when coupled with the effects of potentially differing skills, strength and endurance alters, improves, or exacerbates, plastic changes in the motor cortex after adult SCI.
Our goal in this study was first to examine the effect of adult SCI on trunk motor cortex representations and second to test whether prolonged treadmill or robot assisted treadmill training executed without any induced hindlimb stepping (i.e., ‘non stepping’ treadmill training) can influence trunk motor cortex plasticity, and if so, how. Given that central or peripheral nerve injury results in a significant cortical expansion of proximal (to injury) regions we hypothesized that chronic adult SCI results in significant reorganization and expansion of trunk motor cortex and given plasticity of motor cortex is typically associated with novel skill learning we further hypothesized that non stepping treadmill and robot training that does not induce any functional recovery will not alter cortical reorganization on top of that caused by adult SCI alone. To test this, we used intracortical microstimulation to examine the motor cortex representations in three groups of chronic adult SCI rats after completion of three treatments: untrained, treadmill trained (but ‘non-stepping’) and robot assisted treadmill trained (but ‘non-stepping’). All transected groups were also compared with a group of normal rats. The results we will present show that adult complete low thoracic SCI induces a significant reorganization of trunk motor cortex compared to normal, which remains largely similar across the three tested SCI groups. However, we also saw these changes were further exaggerated by the non-stepping treadmill or robot assisted treadmill training, changes which might then impact any other subsequent rehabilitation treatments.
MATERIALS AND METHODS
Overview
A total of 44 adult female Sprague-Dawley rats (250–325 g) were used in this study. 36 rats received complete spinal cord transection at T9/10 level (ATX). Rats were subdivided into four groups: Normal (uninjured), Spinalized-untrained (ATX-U), Spinalized-treadmill trained (ATX-TM) and Spinalized-Robot assisted treadmill trained (ATX-R). The latter trained groups were not given any special step promoting interventions such as epidural stimulation or pharmacotherapy, and as adult spinal complete rats thus showed little to no stepping (Rossignol and Frigon, 2011). We used intracortical microstimulation (ICMS) to examine the representation of motor cortex in all rats. All surgical and experimental procedures were carried out with Institutional Animal Care and Use Committee (IACUC) guidelines and approvals.
Adult complete spinal transection at T9–10 (ATX)
36 rats received complete spinal cord transection at T9/10 level similar to that described in (Udoekwere et al., 2006, Hsieh and Giszter, 2011). Rats were anesthesized intraperitoneally with 1.0 ml/kg KXA cocktail (2.0 ml Ketamine (100 mg/kg):1.0 ml Xylazine (20 mg/kg): 0.15 ml Acepromazine (10 mg/kg)). Supplemental doses of KXA (0.38 ml/kg) were administered intraperitoneally as needed to maintain deep level of anesthesia throughout the procedure. A mid dorsal skin incision spanning approximately from T6–T12 segments was made. Thoracic vertebrae were exposed; fat pad and paraspinal muscles were deflected. Laminectomy was performed on arches T9/11. A full segment of spinal cord was gently removed at T9/10 using iridectomy scissors and aspiration after opening a slit in the dura. Cavity was filled with gelfoam and the incision was closed in layers. Core body temperature was maintained at 37°C throughout the surgery using a heating pad.
Pelvic orthosis implantation
12 rats received pelvic orthosis implantation surgery along with spinal transection. Orthosis design and surgical procedure were similar to that described in (Udoekwere et al., 2006, Hsieh and Giszter, 2011, Song and Giszter, 2011, Udoekwere et al., 2014). Briefly, after spinal transection a sterile pelvic orthosis was inserted by making bilateral angled incisions (45 degrees, approximately 1 cm caudal to the iliac crest) separating the gluteus muscle through blunt dissection and with minimal tissue damage. The orthosis was then clamped to the iliac processes on both sides of the rat’s pelvis and the sides of the implant were fastened with screws and epoxy cement (J-B weld) was applied to the joint where the pelvic implant parts were connected.
Post-operative care
Post operatively rats were given 1.0 ml/kg of 0.05 mg/ml buprenorphine subcutaneously for analgesia, every 8–12 hours for 48 hours, 0.5 ml/kg prophylactic antibiotics (dilute 3.4 ml of sterile water with 1g of ampicillin vial) subcutaneously once a day for 7 days and 5–10 ml lactated ringer subcutaneously for 7 days. Spinalized rats were monitored twice daily for skin lesions, autophagia or other health concerns. Bladders were expressed at least twice daily until automatic voiding returned, as happens routinely in spinal rats.
Training
Spinalized rats (ATX) were divided into three groups. Untrained (U), Treadmill trained (TM) and Robot trained (R). Untrained rats were left sedentary in their cages but handled and checked twice daily. Other two groups began training 7–10 days post-surgery. Training did not involve any additional (e.g., perineal) stimulation that would actively recruit locomotor pattern generators, as our goal was to examine cortical plasticity due to training with non-stepping hindlimbs. The treadmill trained group was trained (unassisted) daily on a motorized treadmill at 8–12 cm/s for 20 minutes/day, 5 days/week for 4–5 weeks. Periodically rats were also video recorded using a digital camera for assessment of locomotor function. Robot trained rats were trained on a robot that applied elastic forces during treadmill locomotion for 20 minutes/day, 5 days/week for 4–5 weeks similar to that described in (Udoekwere, Ramakrishnan et al. 2006, Hsieh and Giszter 2011). Briefly, a cantilevered phantom haptic robot (Sensable Devices Inc.) is connected via a gimbal to the pelvic orthosis. Isotropic elastic force fields were applied (kx = ky = kz = 45 N/m) with equilibrium position set such that the trunk posture and carriage height of the spinalized rat is roughly similar to that of a normal adult intact rat. There was a 5 s ramp period before the robot force peaked to its maximum value at training onset. Robot data (position, force, velocity for X, Y and Z directions) sampled at 1 kHz and video was recorded continuously throughout all trials and saved on local hard disk for analysis.
Locomotor assessment
Hindlimb stepping was assessed from the recorded video using a modified BBB (Basso et al., 1995) type scale designed by Antri, Orsal and Barthe (AOB scale) (Antri et al., 2002). The scale scores rats on frequency, amplitude and coordination of hindlimb stepping motions. The scale is divided into four levels. Level 1 (score 0–1) corresponds to animals totally unable to support their body weight with their hindlimbs and unable to walk. Level 2 (score 2–9) corresponds to rhythmic movements of hindlimbs without body weight support. Level 3 (score 10) corresponds to consistent rhythmic movements with dorsal foot placement and occasional body weight support. Level 4 (score 11–22) corresponds to rhythmic movements of hindlimbs with plantar paw placement with the possibility of body weight support. Trained rats were scored for the first 5–10 minutes at the start and last 5–10 minutes at the end of training days. Robot trained rats were also evaluated by examining changes in percentage body weight support provided by the robot over the duration of training which was calculated as vertical force data from desired segment of locomotion converted to Newtons (N) and normalized to weight (N) of the animal. In other studies (Miya et al., 1997) we have used a percentage of weight supported stepping measures, but in this study no rats achieved significant numbers of weight supported steps, due to the absence of perineal or other stimulation or intervention to induce stepping.
Cortical mapping
We used Intracortical microstimulation (ICMS) to map the motor cortex of all rats using techniques similar to those described in (Giszter et al., 1998, Giszter et al., 2008). All spinalized rats were mapped 5–7 weeks after transection. Dexamethasone (5mg/kg) was administered intraperitoneally several hours before the surgery to control blood pressure and brain swelling. Rats were anesthesized intraperitoneally with 1.0 ml/kg KXA cocktail (2.0 ml Ketamine (100 mg/kg):1.0 ml Xylazine (20 mg/kg): 0.15 ml Acepromazine (10 mg/kg)) followed by supplemental doses of ketamine (0.24 ml/kg) only. A skin incision was made on the ventral (abdominal) and dorsal (back) aspects of the trunk. Nine bipolar patch electrodes were sutured to the abdominal muscles covering the rectus abdominus and the right and left external oblique muscles at three levels: Mid thoracic (T5–7); Low thoracic (T12–13); Lumbar (L2–3). Six pairs of bipolar ball electrodes were used to record from the longissimus muscle spanning through the same levels as abdominal muscles. Motor pools for these muscles extend from mid thoracic to mid lumbar segments (Miller, 1987). We identified the site of transection by identifying the missing vertebrae using gentle palpation and ensured mid thoracic recording sites were above the injury and all other sites were below the injury. The rat’s head was placed on the stereotaxic frame and bregma was located and noted and the skull surface (10 mm × 8 mm) and dura were removed to expose the cortical surface. Care was taken to electrically isolate the animal. The cortical surface was kept moist with a shallow saline bath and cotton/gauze reservoir. Core body temperature was maintained at 37°C using an overhead heating lamp. Fine stainless steel electrodes (~ 10 MΩ, initial impedance at 1 kHz, shank diameter 125 μm, and tip < 1- μm diameter, exposed tip ~ 5 μm2 FHC) were used. Mapping penetrations were made vertically to the cortical surface and were arrayed across the motor cortex in a continuous 0.5 mm grid starting on the bregma line. Stimuli were applied as 0.2 ms total duration constant current balanced biphasic pulses with cathodal current leading, at 333 Hz in trains of 300-ms duration. Typical current values were between 60 μA to 80 μA and never exceeded 100 μA. Pulse waveforms were monitored with a Tektronix oscilloscope to examine voltage drop across a 10-kΩ resistor interposed in series between the preparation ground and the stimulator. Electrodes were replaced if pulse shapes altered radically or desired currents (voltage drops across resistors) were not observed. At each point in the cortex, the stimulation electrode was first lowered to a depth of about 1500 μm and responses were checked. Slight adjustments (~ +/− 250–400 μm) were made in the depth to identify the strongest response. Prior to stimulating, limbs were fully extended. A site was considered to be positive if we observed consistent ICMS induced motor responses (nonfacial) such as movements of limbs at any joint, neck or EMG muscle responses for trunk. For all positive response sites whether trunk was active or not, trunk EMG data were recorded using differential amplifiers (A-M systems: model-1700 Differential Amplifier) and A/D data acquisition system (Molecular devices: Digidata-1320). EMG signals were amplified with a gain of 1000 and sampled at 1 kHz. At each positive response site all evoked movements and muscle responses were noted. We did not classify forelimb movement across joints. If we encountered more than three negative sites in succession in the presumed nonfacial motor areas we returned to a region close to a previously positive site (approximately within 100–250 μm) and checked for responses. If this site was also non-responsive we terminated the experiment.
ICMS map construction
A binary map was generated for all medial-lateral and rostral-caudal motor cortex coordinates with response types (0-no response; 1-positive response) for forelimb, neck, hindlimb and 15 trunk segments. Sites with two or more response types (ex: - hindlimb and trunk in normal rat) were counted as a contributing area to both representations (1-hindlimb, 1-for all trunk recording sites that were active at that point in cortex). Bregma was noted and used at the coordinate at (0, 0) in the map. Thus a binary map matrix for each rat was created, comprised of the first two columns corresponding to the coordinates in the cortex followed by a matrix of 0’s and 1’s. This allowed us to easily examine the different characteristics of motor cortex representation in each rat (e.g. sparse, converging, diverging, overlapping, center of gravity etc.).
Histology
Following ICMS mapping, spinalized rats were deeply anesthetized with 3ml of Euthasol and perfused intracardially with 0.9% physiological saline followed by 4% buffered paraformaldehyde (PFA) to fix spinal tissue. Following day spinal cord tissue was extracted and blocks were preserved in 4% buffered PFA for 3 days, soaked in 30% sucrose for 1 week and embedded in M1 embedding matrix (Thermo Scientific Shandon) and stored in −75°C refrigerator. Blocks containing the lesion were cut in serial, parasagittal 25 μm sections. Nissl Myelin stain and serotonin (5 HT) immunohistochemical stain with DAB (3–3’ diaminobenzidine tetrahydrochloride) were used to examine the completeness of spinal transection. Typically we observed absence of Nissl body and myelin at the transection site and absence of serotonergic fibers below the transection. All spinalized rats had histologically complete lesions (data not shown) consistent with our previous results (Udoekwere et al., 2006, Hsieh and Giszter, 2011) with similar surgical transection.
Data Analysis
We examined motor cortex representations derived from ICMS mapping in four groups of rats. To analyze map data we first determined the size of the nonfacial motor cortex by calculating total number of sites in the cortex (arrayed in 0.5 mm grid) where we obtained motor response for forelimb (distal or proximal), trunk, or neck movements. We did not consider exclusive hindlimb sites in determining the size of the nonfacial motor cortex in normal rats to avoid the a priori bias to the normalization for normal rats that it would introduce because spinal transected rats have no hindlimb representation. This ensured that there were no significant differences in the size of nonfacial motor cortex sampled in normal and all transected rats after normalization. For several normal and injured rats we mapped both sides of the cortex. However, for statistical comparisons we only considered the side of the cortex with richer (denser and larger) trunk representation in subsequent analysis. The percentage of dual mapped rats was not significantly different among groups. To examine the effects of spinal cord injury on trunk motor cortical reorganization we compared spinalized rats with normal rats using two-tailed unpaired t-test or Mann Whitney ranksum tests. We also compared all the four groups of rats using one-way ANOVA or Kruskal Wallis tests (as applicable) with the Bonferroni correction for any post hoc comparisons. To examine the effects of the non-stepping robot and treadmill trainings after spinal cord injury we also separately compared the 3 groups of spinalized rats using one-way ANOVA or Kruskal Wallis test (as applicable) with Bonferroni corrected post hoc comparisons. Linear regression was used to measure improvements in the BWS functional recovery measure derived from robot data over successive training days in trained groups. Paired t-test was used to compare changes in AOB scores at the beginning and end of training. For all statistical tests a p-value of less than 0.05 was considered significant. All data analysis was done using custom scripts in MATLAB R2009b, Mathworks.
RESULTS
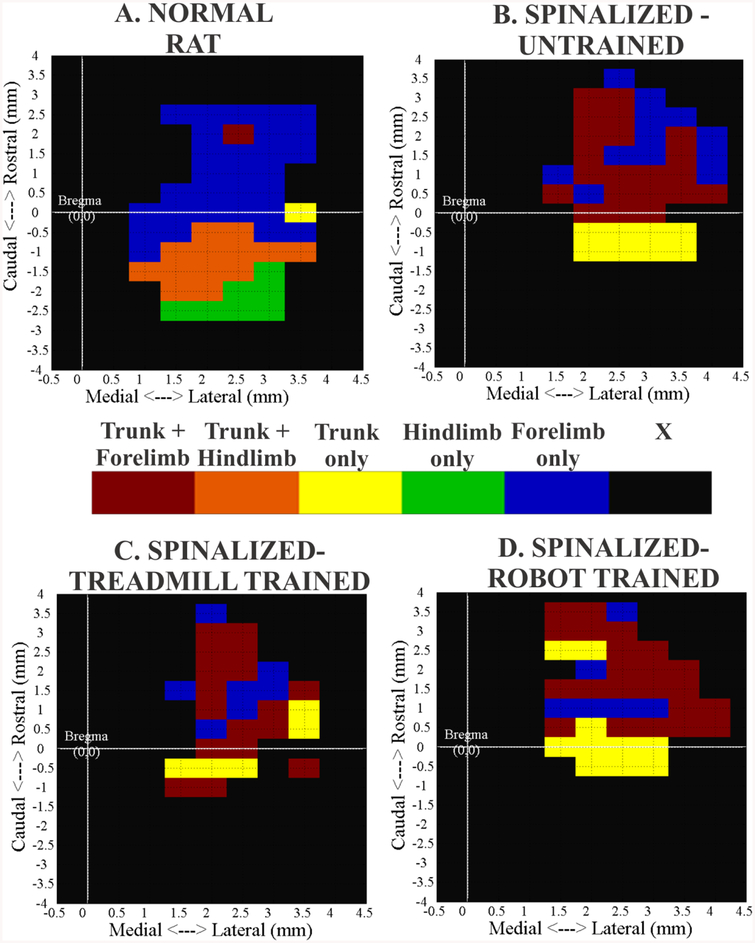
We examined motor cortex representations derived from ICMS mapping in four groups of rats. Data were compared from Normal (n=8), Spinalized-Untrained (ATX-U, n=8), Spinalized-Treadmill trained (ATX-TM, n=8), Spinalized-Robot assisted treadmill trained (ATX-R, n=8) rats. Transected rats were all mapped 5–7 weeks after injury. Currents between 60–80 μA and longer stimulation pulse trains (300 ms) were used with the goal of allowing greatest opportunity for temporal facilitation of activity elicited by microstimulation in the spinal cord and to minimize the possibility of false negatives. With our choice of these stimulation parameters, the general features of motor maps of normal rats that we generated were consistent with others (Donoghue and Wise, 1982, Neafsey et al., 1986, Frost et al., 2013), as shown in figure 1A. Mapping penetrations were made vertically and arrayed across cortex in a continuous 0.5 mm grid. Trunk area in normal rats (n=8) occupied approximately 29% of the total area of the nonfacial motor cortex (including hindlimb areas) with the majority of trunk motor sites located caudal to the bregma and with significant overlap with hindlimbs, i.e., consistent with our previous results (Giszter et al., 1998). Our results for spinal transection effects fall into two main categories: First, we examine reorganization of trunk motor area after spinalization and second, we examine the effect of non-stepping treadmill training or robot assisted treadmill training of spinalized rats on this reorganization of trunk motor area. The training regimes we used, without special measures to induce hindlimb stepping (passive hindlimb), had little to no effect on function.
Figure 1:-.
Comparison of example motor cortex maps from normal and adult spinalized rats. Maps show cortical areas from which forelimb, hindlimb and trunk musculature were recruited at 60–80 μA currents. Rostral is at the top of the map and bregma is at (0, 0). Numbers on the axis represents distance in mm. For comparison purposes all maps are presented as right cortex in the same orientation. Note the different shades for trunk representation as it overlaps with other regions. X – No non-facial motor response. (A) Normal Rat (B) Spinalized-Untrained (C) Spinalized–Treadmill trained (D) Spinalized – Robot trained.
Locomotor recovery with passive hindlimb training
We assessed locomotor recovery of the spinalized groupsusing the AOB scale. All trained rats were scored at the beginning and end of training. We also scored 5 out of 8 untrained rats prior to mapping their motor cortex (equivalent timeline to the end of training in the two trained groups). Consistent with our previous findings (Udoekwere et al., 2006, Hsieh and Giszter, 2011), training in absence of any perineal or epidural stimulation or supply of pharmacological or transplant agents did not induce any significant recovery of stepping function. Most spinalized rats in the two trained groups had a score of 0 or 1 at the beginning of training and most achieved a score of 1 or 2 and in some cases a score of 3 at the end of training on a scale that goes up to 22. Average scores in the trained groups thus reflected a very low level of hindlimb recovery that corresponds to some sporadic movement of the hindlimbs with weak amplitude. Similar AOB scores were observed for the 5 untrained rats and there were no significant differences between the three spinalized groups for AOB scores at 4–6 weeks post SCI (p=0.39, F(2,18) = 0.99, Kruskal Wallis test).
We examined changes in percentage body weight support (% BWS) provided by the robot across training days in the robot trained rats. Daily % BWS was calculated as the average vertical robot interaction force during the entire trial divided by the rat’s weight and expressed as percentage. Average % BWS for all the rats at the first day of training (42.12±0.84 %) and at the last day of training (41.55±0.84 %) were not significantly different (p = 0.58, paired t-test). We also used linear regression to examine changes in % BWS across successive training days. We normalized each rat’s daily % BWS by their % BWS from day 1 and averaged across rats per training day. No significant changes were found in normalized % BWS across successive training days in the robot trained rats (p=0.105). Since there was no intervention to induce active stepping during training (e.g. perineal or epidural stimulation) no adult SCI rats were able to take weight supported steps. Thus non-stepping treadmill training or robot assisted treadmill training were both ineffective in promoting any significant functional recovery, or recovery of stepping (data not shown).
Effect of spinalization on total trunk motor representation
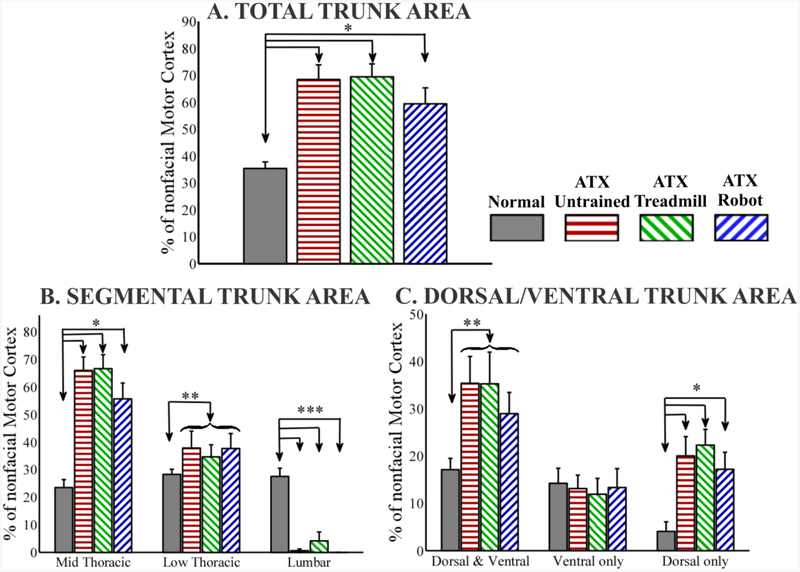
Despite the lack of functional improvement, we observed cortex reorganization and alteration in the rats. Typical motor maps from example rats from all groups are shown in figure 1. To compare the effect of transection on trunk motor representation we calculated motor areas in all groups of rats as a percentage of the nonfacial motor cortex area, which included forelimb (distal and proximal), neck and trunk representations. Since complete transection at T9/10 eliminates all motor cortex output to the hindlimbs we did not consider exclusive hindlimb sites in determining the size of the nonfacial motor cortex in normal rats, as it would bias the normalization as noted in Methods. This ensured that there were no significant differences (p=0.58, F(3,28) = 0.67, 1-way ANOVA) in the absolute size of nonfacial motor cortex compared in normal (34.12 ± 2.24) and transected rats (ATX-U 32.25±3.65; ATX-TM 37.25±3.12; ATX-R 31.75±3.03; mean±SEM response points in cortex). Normalized Trunk area was significantly higher in all the spinalized groups of rats compared to normal rats (p<0.0001, F(3,28) = 10.68, 1-way ANOVA with Bonferroni corrected post hoc comparisons (Figure 2A). No significant differences in normalized trunk area were found between the three spinalized groups (p=0.37, F(2,21) = 1.04, 1-way ANOVA). There was no significant difference in the normalized forelimb area between the 4 groups (data not shown, p=0.39, F(3,28) = 1.05, 1-way ANOVA).
Figure 2:-.
Trunk Motor Area in all groups (A). Normalized Total Trunk Motor Area calculated as the number of sites in the cortex where any trunk response was obtained divided by the total nonfacial motor cortex sites and expressed as percentage (nonfacial motor cortex = total number of sites in the cortex where forelimb, neck, or trunk response was obtained). For normal rats, exclusive hindlimb sites were not included in calculating size of nonfacial motor cortex. Normal group is significantly different than spinalized groups. No significant difference between the three spinalized groups. (B). Normalized segmental Trunk Motor Area for Mid Thoracic (Low Thoracic or Lumbar) calculated as the number of sites in the cortex where any Mid Thoracic (Low Thoracic or Lumbar) response was obtained divided by the size of nonfacial motor cortex and expressed as percentage. Sites with co-activation of multiple segments (e.g. both mid thoracic and low thoracic) were counted as contributing to both representations. Normal group is significantly different than spinalized groups for all segments. No significant difference between the three spinalized groups. (C). Percentage Normalized Trunk Motor Area divided into Dorsal and Ventral overlap, Ventral only and Dorsal only. Normal group is significantly different than spinalized groups for Dorsal and Ventral overlap and Dorsal only. No significant difference between the three spinalized groups. (*p<0.05, 1-way ANOVA with Bonferroni corrected post hoc comparisons, ** p<0.05, t-test normal v/s all spinalized combined, *** p<0.05 1-way KRUSKAL WALLIS with Bonferroni corrected post hoc comparisons, curly brace indicates combined into 1 group, data expressed as mean ± SEM).
Effect of spinalization on segmental trunk representation
We next examined the effect of spinalization on the motor representations more selectively. We looked at representations of trunk segments rostral and caudal to the spinal cord injury. We recorded EMGs from 5 muscles (ipsilateral and contralateral external obliques, ispilateral and contralateral longissimus and from rectus abdominus) at 3 segmental levels – mid thoracic (rostral to the injury), low thoracic (caudal to the injury) and mid lumbar (caudal to the injury). Thus we had a total of 5 EMG recording sites each for mid thoracic, low thoracic and mid lumbar trunk segments. We expressed the area of representation for each segment as a percentage of the nonfacial motor cortex (figure 2B). Motor sites with co-activation of multiple segments (e.g. both mid thoracic and low thoracic) were counted as a contributing area to both representations. We found significant increases in motor area for mid thoracic (rostral) trunk segment in spinalized rats compared to normal rats (p<0.00001, F(3,28) = 17.98, 1-way ANOVA with Bonferroni corrected post hoc comparisons). There were no significant differences for this mid thoracic representation between the 3 groups of transected rats (p=0.27, F(2,21) = 1.36, 1-Way ANOVA).
All transected rats also showed consistent representation of the low thoracic trunk segments below the level of injury. There were again no significant differences between the three transected groups (p=0.89, F(2,21) = 0.11, 1-Way ANOVA). Hence we combined the three groups of transected rats (combined n=24) and compared this combined group with normal rats. We found significant increase in the low thoracic motor area for the transected rats grouped when compared to normal rats (p<0.05, unpaired t-test). We further verified this by individual 1 tailed t-test comparisons between the normal and other groups under the hypothesis that each increased. We found significant differences between the normal and each of the 3 transected groups (p<0.05, 1 tailed t-test). With the exception of 1 rat in the untrained spinalized group and 2 rats in the treadmill trained spinalized group, none of the transected rats had motor representation for the lumbar trunk segments. Hence there were significant differences between normal rats and transected groups for lumbar trunk representation due to its almost complete absence after transection (p<0.0005, F(3,28) = 22.76, Kruskal Wallis with Bonferroni corrected post hoc comparisons).
Effect of spinalization on dorsal and ventral trunk representation
We next examined the effect of spinalization on the balance and type of the expanded motor representations for the represented ventral (abdominal) and dorsal (back) trunk muscles noted in the preceding section. Ventral muscles included ipsilateral and contralateral external obliques and rectus abdominus while dorsal muscles included ipsilateral and contralateral longissimus. As noted already, all recordings were done at 3 segmental levels – mid thoracic, low thoracic and mid lumbar. Thus we had 9 segments at which we recorded ventral trunk EMG activity and 6 segments at which we recorded dorsal trunk EMG activity. We divided trunk motor cortex sites into three components – Ventral only, Dorsal only and Ventral and Dorsal overlap/coactivation and expressed the area of each component as a percentage of nonfacial motor cortex (figure 2C). We found that there were no significant differences between the three groups of transected rats: for ventral and dorsal overlap (p=0.66, F(2,21) = 0.42, 1-Way ANOVA) or for ventral only trunk (p=0.95, F(2,21) = 0.05, 1-Way ANOVA) or for dorsal only trunk (p=0.62, F(2,21) = 0.49, 1-Way ANOVA). There were also no significant differences found between the normal and spinalized groups for ventral only trunk (p=0.97, F(3,28) = 0.08, 1-Way ANOVA). However, when compared to normal rats, spinalized groups all had a significantly larger motor areas for dorsal and ventral overlap (p<0.05, unpaired t-test, normal v/s all spinalized combined) and dorsal only representation (p<0.005, F(3,28) = 5.99, 1-Way ANOVA with Bonferroni corrected post hoc comparisons).
Synergies and coactivation density of trunk motor cortex representation
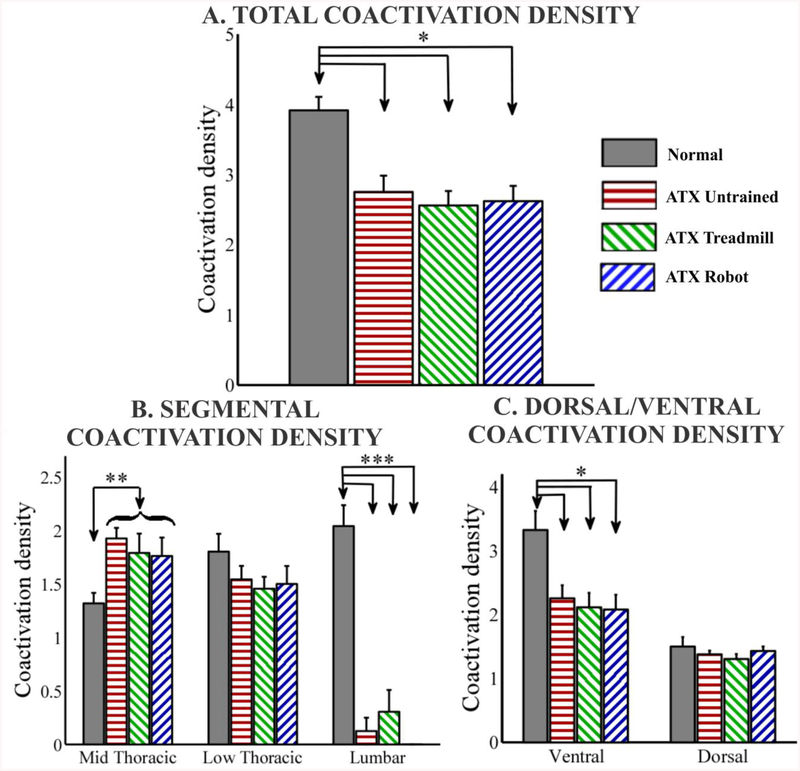
We calculated the total number of trunk EMG channels (trunk segments) co-activated per site in the trunk motor cortex and defined this as the “coactivation density” of trunk motor representations (figure 3). Compared to normal intact rats, all spinalized groups showed a significant decrease in the total number of trunk EMG channels co-activated per site in the trunk motor cortex (p<0.0005, F(3,28) = 9.08, 1-Way ANOVA with Bonferroni corrected post hoc comparisons). There were no significant differences between the three groups of transected rats (p=0.81, F(2,21) = 0.21, 1-Way ANOVA). However, there were clearly the most significant losses of control below the lesion in transected rats in the lumbar segments. We therefore removed the lumbar sites from consideration even in normal rats. After this manipulation we found no significant differences between normal and all transected rats (p=0.55, F(3,28) = 0.71, 1-Way ANOVA).
Figure 3:-.
Coactivation density is defined as the total number of trunk EMG channels (trunk segments) co-activated per site (x,y) in the trunk motor cortex. (A). Total trunk coactivation density. Normal group is significantly different than spinalized groups. No significant difference between the three spinalized groups. (B). Segmental coactivation density for each segment. Normal group is significantly different than spinalized groups for Mid thoracic and Lumbar. No significant difference between the three spinalized groups. (C). Dorsal and Ventral coactivation density. Normal group is significantly different than spinalized groups for Ventral. No significant difference between the three spinalized groups. (*p<0.05, 1-way ANOVA with Bonferroni corrected post hoc comparisons, ** p<0.05, t-test normal v/s all spinalized combined, *** p<0.05, 1-way KRUSKAL WALLIS with Bonferroni corrected post hoc comparisons, curly brace indicates combined into 1 group, data expressed as mean ± SEM).
We also calculated the coactivation density of trunk representations by segmental level. To calculate this measure, we only considered trunk motor sites where a particular segmental representation was present (e.g. to get low thoracic coactivation density we count only those EMG channels co-activated where at least 1 low thoracic trunk EMG channel was also active). In effect, if there was no representation for low thoracic muscles anywhere in the motor cortex, the low thoracic density count could only be 0. We found that the coactivation density for mid thoracic representations was not significantly different between the three groups of spinalized rats (p=0.73, F(2,21) = 0.32, 1 Way ANOVA). We also found that there were no significant differences between the spinalized groups and the normal rats for the low thoracic coactivation density (p=0.34, F(3,28) = 1.16, 1 Way ANOVA) or between the three spinalized groups for low thoracic coactivation density (p=0.9, F(2,21) = 0.1, 1 Way ANOVA. Hence to further probe any changes we combined all spinalized rats (combined n=24) and compared this combined group with normal rats at each segmental level. We found the combined spinalized group to have significantly higher mid thoracic coactivation density than normal rats (p<0.05, unpaired t-test). Combining all spinalized rats into a single group did not reveal any low thoracic differences. Given the lack of lumbar representation in the spinalized rats, as noted, lumbar coactivation density was significantly lower for spinalized rats compared to normal rats (p<0.0001, 1-way Kruskal Wallis with Bonferroni corrected post hoc comparisons).
We next examined the coactivation densities of dorsal and ventral trunk representations. Coactivation density of ventral trunk representation was significantly lower for the spinalized groups compared to normal rats (p<0.005, F(3,28) = 5.95, 1-Way ANOVA with Bonferroni corrected post hoc comparisons) whereas there were no significant differences between any of the groups for dorsal trunk coactivation density (p=0.54, F(3,28) = 0.74, 1 Way ANOVA). Further there were no significant differences when restricting analysis to only among the spinalized groups for either ventral coactivation density (p=0.83, F(2,21) = 0.18, 1 Way ANOVA) or dorsal coactivation density (p=0.45, F(2,21) = 0.83, 1 Way ANOVA). Alterations in coactivation densities in spinalized adult rats compared to normal rats thus were focused primarily in upper thoracic trunk muscles (which were increased), and in ventral muscles (which were decreased), and were unaltered among spinalized rats by training.
Effect of spinalization on location of trunk motor representation
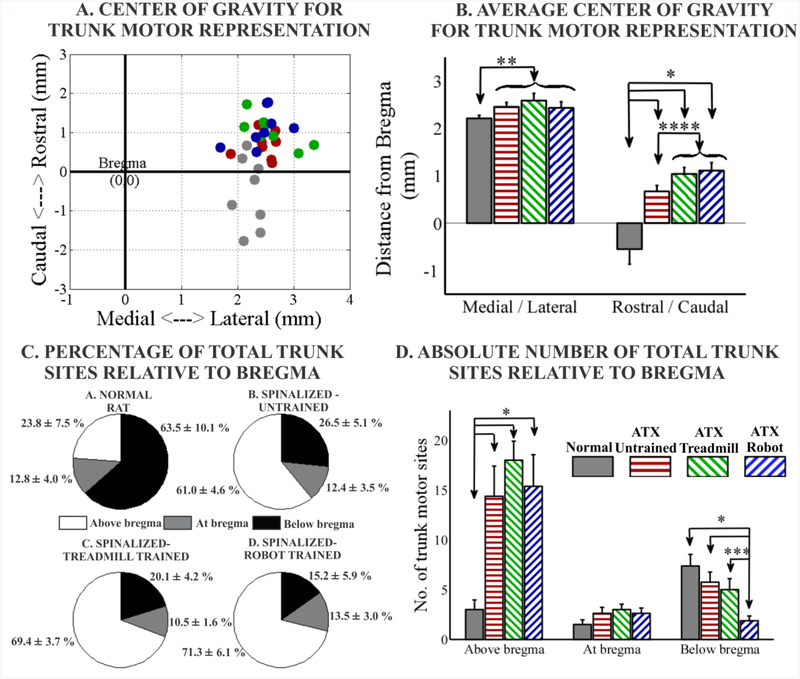
We next examined the location in cortex of the trunk motor representations in the different groups of rats. In normal rats the majority of trunk motor sites are located caudal to the bregma landmark and within 2–3 mm of the midline (medial<->lateral) (Donoghue and Wise, 1982, Neafsey et al., 1986). We calculated the center of gravity (spatial mean location of trunk sites) of the total trunk motor representation for each rat and compared this across groups (figures 4A, 4B). There were no significant differences between the three groups of transected rats for the medial/lateral center of gravity (p=0.66, F(2,21) = 0.42, 1 way ANOVA). However, if we combined all spinalized rats (combined n=24) and compared this group with normal rats, we found the combined spinalized group to have small but significant lateral shift in the center of gravity for trunk representation (p=0.04, unpaired t-test). All transected rat groups also showed significant displacement in the rostral direction in the motor cortex compared to the normal rats (p<0.00001, F(3,28) = 14.58, 1-way ANOVA with Bonferroni corrected post hoc comparisons). There were no significant differences between the two trained spinal groups (robot + treadmill and treadmill alone) in rostro-caudal center of gravity (treadmill v/s robot p=0.74, two-tailed corrected t-test). We next explored trained versus untrained. We found study did not have the statistical power to distinguish between the untrained and the two trained groups individually using a two-tailed corrected t-test. The p value for two-tailed corrected t-tests comparing the rostral center of gravity for untrained v/s treadmill trained was 0.06, for untrained v/s robot trained was 0.05. However, use of one-tailed tests under the hypothesis of rostral shift with training were individually significantly different from untrained in both trained groups individually. Further, we found significant rostral shift in the trained rats compared to the untrained group (p<0.05, unpaired t-test), if we combined both trained groups into 1 group (combined n=16) and compared this combined group with the spinalized untrained rats using a two-tailed test. Additionally, we found significant concomitant rostral shifts for forelimb motor representation centers of gravity in the trained spinalized rats (1.38 ± 0.11 mm) compared to the untrained spinalized group (1.01 ± 0.13 mm) (p=0.04, unpaired t-test, data not shown). All rat groups showed a significant correlation (p<0.05) between the rostro-caudal center of gravities for trunk and forelimb representations within each group, suggesting small map register shifts existed. However, we also observed there was a significant difference in the intercept of the linear fits based on these correlations, between the combined trained spinal group and the untrained spinal group, thus supporting the notion that the overall rostral shifts observed were systematic changes due to the effect of training, and not random map registration shifts relative to bregma (data not shown).
Figure 4:-.
(A). Scatter plot showing center of gravity for trunk motor representation for each rat. For comparison purposes all points are presented as right cortex in the same orientation. Rostral is at the top of the map and bregma is at (0, 0). (B). Average center of gravity (medial/lateral and rostral/caudal). For medial/lateral location - No significant difference between the three spinalized groups for medial/lateral location. Normal group is significantly different than combined spinalized group for medial/lateral location. For rostral/caudal location - Normal group is significantly different than all spinalized groups. Spinalized untrained is significantly different than spinalized trained combined. Trunk motor sites divided based on relative location from bregma and expressed as (C) percentage of total trunk motor sites or (D) absolute number of sites. Normal group is significantly different than spinalized groups for absolute and percentage of trunk motor sites above bregma. No significant difference between the three spinalized groups for above bregma. No significant difference between the groups for trunk sites at bregma. Normal group is significantly different than spinalized groups for absolute and percentage of trunk motor sites below bregma. No significant difference between the three spinalized groups for percentage of trunk sites below bregma. For absolute trunk sites below bregma, spinalized untrained and spinalized treadmill trained significantly different than spinalized robot trained. (*p<0.05, 1-way ANOVA with Bonferroni corrected post hoc comparisons, ** p<0.05, t-test normal v/s all spinalized combined, *** p<0.05, t-test, **** p<0.05, t-test spinalized untrained v/s combined trained spinalized, curly brace indicates combined into 1 group data expressed as mean ± SEM).
To investigate further the location variations in topography of trunk motor sites, we divided trunk motor sites into three regions for each rat: At bregma line, above bregma and below bregma and show them as absolute number of sites (figure 4D) and also express them as a percentage of total trunk motor sites (figure 4C).
Above bregma, compared to normal, all spinalized groups had significantly greater absolute number of trunk motor sites (p<0.05, F(3,28) = 7.38, 1 Way ANOVA with Bonferroni corrected post hoc corrections) and greater percentage of total trunk motor sites (p<0.00005, F(3,28) = 15.31, 1 Way ANOVA with Bonferroni corrected post hoc corrections). There were no significant differences between the three spinalized groups for above bregma absolute trunk sites (p=0.64, F(2,21) = 0.46, 1 Way ANOVA) or above bregma percentage of total trunk sites (p=0.31, F(2,21) = 1.22, 1 Way ANOVA).
At the bregma line, no significant differences were found between the groups for absolute trunk motor sites (p=0.24, F(3,28) = 1.48, 1 Way ANOVA) or when expressed as percentage of total trunk motor sites (p=0.92, F(3,28) = 0.16, 1 Way ANOVA).
Below bregma, compared to normal, all spinalized groups had significantly lower percentage of total trunk motor sites (p<0.00005, F(3,28) = 10.63, 1 Way ANOVA with Bonferroni corrected post hoc corrections) and no significant differences were found between the three spinalized groups for below bregma trunk when expressed as a percentage of total trunk (p=0.3, F(2,21) = 1.23, 1 Way ANOVA). However when we compared the absolute trunk motor sites below bregma between the groups we observed significant differences. Not surprisingly we saw differences between the normal group and the spinalized robot trained (p<0.05, F(3,28) = 5.57, 1 Way ANOVA with Bonferroni corrected post hoc corrections). However, there were also significant differences between the spinalized untrained and the spinalized robot trained (p<0.05, F(2,21) = 5.13, 1 Way ANOVA with Bonferroni corrected post hoc corrections) and between the spinalized treadmill trained and the spinalized robot trained (p<0.05, unpaired t-test). These data suggest a reduction or withdrawal of trunk representations from below bregma as a result of training with the robot.
Thus two effects may have contributed to the rostral shift in center of gravity of total trunk representation in the trained groups compared to the untrained: slightly larger (but not significant) number of trunk motor sites above bregma in the treadmill trained group (18±1.9) compared to the untrained (14.37±3.03) together with the significantly lower number of trunk motor sites below bregma in the robot trained group (1.87±0.48) compared to the untrained (5.75±1), together acting to cause the significant rostral shift in total trunk motor representations in the combined trained group compared to the untrained group.
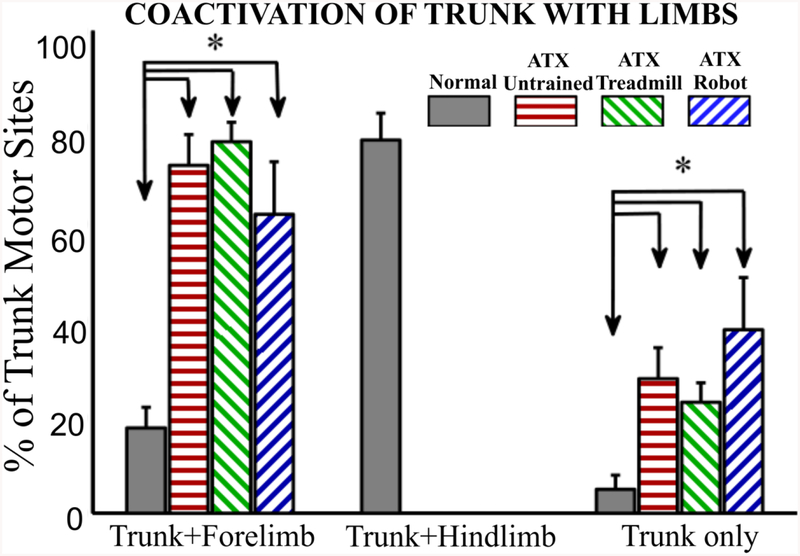
Overlap of trunk and forelimb motor representation
Given that transected rats showed significant displacement of trunk motor representation in the rostral direction without a significant change in the overall size of the nonfacial motor cortex or forelimb motor area, this suggests an increase in motor overlap and coactivation between trunk and forelimb. To demonstrate this we next divided total trunk motor sites in each rat into three categories - sites where trunk overlaps with hindlimb, sites where trunk overlaps with forelimb and sites where trunk does not overlap with either limb and we expressed these as a percentage of total trunk representation (figure 5). As shown previously, a significant amount of trunk motor representation overlaps with hindlimb representation in normal rats. However all transected rats showed significant increases in both the percentage of trunk that is co-activated with forelimb (p<0.00001, F(3,28) = 15.15, 1 Way ANOVA with Bonferroni corrected post hoc comparisons) and percentage of trunk that is not co-activated with either limb (p<0.05, each unpaired t-test). Trunk representation that overlapped with forelimb included trunk sites at mid thoracic as well as low thoracic segments. There were no significant differences between the individual or trained/untrained groups of spinalized rats in these pronounced forelimb coactivation effects.
Figure 5:-.
Trunk motor sites divided based on coactivation with forelimb or hindlimb and expressed as percentage of total trunk sites. Normal group is significantly different than spinalized groups for forelimb coactivation and no coactivation (trunk only). No significant differences between the spinalized groups. Spinalized rats have no motor representation for hindlimb. (*p<0.05, 1-way ANOVA with Bonferroni corrected post hoc comparisons, data expressed as mean ± SEM).
DISCUSSION
The purpose of the present study was twofold. First, we examined the effect of adult spinal cord injury (SCI) on detailed trunk motor cortex representation in a rat model. Second, we assessed the extent of reorganization, due to increased physical activity (in the form of treadmill training or robot assisted treadmill training in absence of induced hindlimb stepping) to see how this can enhance plasticity. The main finding of the present study is that the trunk motor cortex undergoes an extensive reorganization after chronic adult SCI as we had hypothesized. This includes an expansion and rostral displacement of trunk motor representations in the cortex, with the greatest increase observed for rostral (to injury) trunk, and slight but significant increase of motor representation for caudal (to injury) trunk at low thoracic levels in all spinalized rats. In parallel there were changes in coactivation and the synergy representation (or map overlap) between different trunk muscles. There were no significant differences between the groups of transected rats for the majority of the comparisons. However, in contrast to what we had hypothesized the treadmill and robot-treadmill trained groups of rats showed further small but significant rostral migration of the trunk representations, beyond that caused by transection alone. Therefore, we conclude that SCI induces significant reorganization of trunk motor cortex, and although this is not qualitatively altered by non-stepping treadmill training or non-stepping robot assisted treadmill training, it is shifted further from normal topography by the training.
Mechanisms of cortical plasticity
Reorganization of motor cortex can involve multiple steps working at different time scales. Expansion of motor maps into the deafferented cortex (e.g. forelimb expansion into vibrissae motor area after facial nerve injury) is attributed to unmasking (reduction of GABAergic inhibition) of the latent intracortical connections between the two regions (Jacobs and Donoghue, 1991, Huntley, 1997). This can happen on a very short time scale. Subsequently in the long term, activity dependent plastic changes such as long term potentiation (Hess and Donoghue, 1994, Hess et al., 1996, Hess and Donoghue, 1996), growth of new horizontal connection (Florence et al., 1998) and synaptogenesis (Kleim et al., 2002, Kleim et al., 2004) are also possible. Several other studies examining plasticity specifically sought to investigate the underlying neural substrates of plasticity after amputations (Ziemann et al., 1998) or skill learning (Adkins et al., 2006) have identified cortex as the primary substrate. Our data and technique does not allow us to attribute plastic changes to any particular timescale of plasticity mechanism, since the timescale of our study allows for both short term changes that can stabilize with time, or long term rewiring. However, the overall map changes manifest and explored here were partly driven by injury and rehabilitation techniques used and thus are important in relation to rehabilitation and recovery of function.
Expansion of trunk area
Motor cortex reorganization following nerve injuries and amputations usually involves both the loss of motor representation for denervated musculature and the enlargement of adjacent (in cortex or periphery) motor areas into the denervated cortex. Such changes are observed within hours after nerve damage (Sanes et al., 1988) and remain stable over a longer period after the lesion or amputations (Sanes et al., 1990, Toldi et al., 1996, Tandon et al., 2013). Similar observations such as a shift in proximal arm motor representation towards the de-efferented cortical area have been made in clinically complete thoracic SCI patients (Bruehlmeier et al., 1998, Laubis-Herrmann et al., 2000, Lotze et al., 2006). Topka et al. observed that transcranial magnetic stimulation (TMS) activated a large fraction of motorneuron pools and evoked motor evoked potentials (MEPs) with shorter latencies from a large number of scalp positions in abdominal muscles immediately rostral to the level of SCI in clinically complete thoracic SCI patients (Topka et al., 1991). The expansion of trunk in our rats was also rostral and also involved a withdrawal from below bregma (the trunk overlap cortex), exacerbated by non-step training. Our results are consistent with the other reports of motor reorganization and provide additional insights into the nature of the trunk motor reorganization possible.
The biomechanics needed by the injured subjects may dominate the changes in cortical organization and the coordinative patterns represented. While Topka et al. observed expansion of abdominal motor representation in bipedal man, our results also suggest an expansion of back muscle and an increase in overlap between abdominal and back muscle representation in the cortex in the quadrupedal rat when made paraplegic. However, similar to their results we here observed a significant increase in motor area for trunk segments rostral to the injury (~ mid thoracic recording sites). We also observed that the number of trunk segments (longitudinal and intermuscle coactivation) represented per cortex site was higher for rostral trunk. Thus there was not only an expansion of area but an increase in what we have termed coactivation density (or synergy degrees of coactivation) of rostral trunk motor representations. These specific adaptations to a new paraplegic function in the injured rats supported the novel biomechanical actions of pulling the body along with forelimbs and managing passive haunches.
In addition to expansion of rostral trunk we also observed a slight but significant increase in motor area devoted to trunk segments caudal to the anatomically defined segmental level of the injury (~ lower thoracic recording sites). Activations of trunk muscles caudal to the injury level have also been observed in clinically complete thoracic SCI patients in response to TMS (Cariga et al., 2002) and voluntary actions or balance perturbations (Bjerkefors et al., 2009), and seen in our laboratory in neonatally transected weight supporting rats (Giszter et al., 1998).
Clinically complete SCI in human patients may not be anatomically complete and there have been reports of some residual function in complete SCI patients (Sherwood et al., 1992). However all our lesions were histologically complete and were at similar spinal levels, providing a well controlled analysis and description of this phenomenon. There are multiple possibilities that could explain this recruitment of trunk segments below the level of lesion. First, we were recording from the same abdominal and back muscles at different segmental levels above and below the injury and hence there is mechanical interaction between them. Voluntary contractions of segments above the injury might lead to stretch reflex activation of the caudal segments. Trunk muscles below the lesion may be hyper reflexive due to loss of supraspinal input. Second, we know that trunk muscles have broadly distributed motor pools with multi segmental innervations (Miller, 1987, Calguner et al., 2006). A recent study from our laboratory showed that stimulation of T9 motor nerve evoked relatively short latency motor responses in trunk muscles at multiple segmental levels and this activation pattern did not change after an acute SCI at T10 (Udoekwere, 2010). Thus it is possible that the low thoracic trunk recruitment from the cortex that we observed could be due to the multi segmental distribution of peripheral motor nerves exiting from the spinal cord above the lesion. Additionally, we did not observe any cortical recruitment of lumbar trunk. This is not surprising since these SCI rats were unable to step with our training paradigm.
Representation in de-efferented cortex
Studies with peripheral nerve injury or amputations have observed enlargement of adjacent motor regions, these entering into the de-efferented cortex (Sanes et al., 1988, Donoghue et al., 1990, Sanes et al., 1990, Wu and Kaas, 1999). Similarly after incomplete SCI which targets descending pathways (e.g. bilateral lesions to corticospinal tract), stimulation in the de-efferented hindlimb cortex evoked forelimb, whisker and trunk responses (Fouad et al., 2001). Also in complete SCI, TMS and neuroimaging studies have revealed a displacement of arm representations towards the de-efferented area (Lotze et al., 1999, Lotze et al., 2006). However, our results demonstrate a significant expansion of trunk motor representation into the rostral motor cortex (i.e., away from the de-efferented cortex) and this was coupled with reduced total motor sites in the de-efferented caudal cortex (due to loss of hindlimb representation) in transected groups. The de-efferented cortex is sensory motor overlap cortex and hence can be more vulnerable to silencing, as reported in other studies (Donoghue and Wise, 1982, Hummelsheim and Wiesendanger, 1985). Reduced total motor response in de-efferented has been shown to correlate with time after injury in clinically complete thoracic SCI patients (Lotze et al., 2006) and was also observed in rats with incomplete cervical (Tandon et al., 2013) or thoracic (Fouad et al., 2001) lesions. Finally, there could be ongoing anatomical changes such as increasing fiber loss, demyelination etc. in the de-efferented cortex (Wrigley et al., 2009) resulting in some of the observed lack of motor response in the de-efferented cortex. However, non-stepping training with the robot applied in the rats here caused significant further reduction of representation below bregma, on matching time scales with the other groups, despite similar activity levels in treadmill alone, and thus the change was not simple progression of time. There is a real possibility that the lack of response in de-efferented overlap cortex and subsequent migration of representations into the rostral cortex following training has functional implications (see below).
Rostral displacement and Functional overlap of trunk with forelimb
Interestingly, we observed a significant trunk motor expansion in the rostral cortex which overlapped significantly with forelimb motor representation. In a normal rat, a majority of trunk motor representation is found in the caudal portion of motor cortex (post-bregma) where it overlaps significantly with hindlimb. There is also a small region of upper trunk in the rostral cortex, as observed also in our intact mapping here (see figure 1A). Overlap between trunk and forelimb motor representation in our SCI rats cannot be because of direct current spread due to larger currents used during ICMS. Even with 90 μA current, a generous estimate of the spread of current effective for direct stimulation of soma and axons is only 0.6 mm while the expansion and overlap reported here extended to several mm. Further, similar currents (60–80 μA current) were used across normal and transected rats used in the study. Finally, in 2 normal control rats in which we changed stimulation currents to 100 μA we still did not observe any additional activation of trunk in forelimb motor regions. The vulnerability of motor representations below bregma (de-efferented cortex) in spinalized rats may be due to the sensory motor overlap there, as noted above.
An emerging view of the motor cortex is that it controls muscle synergies and feedback controls subserving movements in an integrated manner (e.g., (Kargo and Nitz, 2003), and for a debate on this topic see (Tresch and Jarc, 2009)). Central control signals jointly and proportionately activate all muscles in the synergy allowing a simplified control of a particular motor structure within biomechanical constraints and task demands (e.g. (Kargo and Giszter, 2000, d’Avella and Bizzi, 2005, Kargo and Giszter, 2008)). However when the task demand changes, control signals must modulate activation levels of these synergies, and optimal synergy compositions may often alter (Torres-Oviedo et al., 2006). Primary motor cortex likely orchestrates activation of such cortically represented muscle synergies (Holdefer and Miller, 2002) with modules residing in the spinal cord (Kargo and Giszter, 2000, Bizzi et al., 2002, Tresch et al., 2002, Kargo and Giszter, 2008, Hart and Giszter, 2010, Giszter and Hart, 2013). Electrical microstimulation (cortex or spinal) can reveal these synergies (Giszter et al., 1993, Tresch and Bizzi, 1999, Graziano et al., 2002, Ramanathan et al., 2006, Cheung et al., 2012, Overduin et al., 2012). Injury or sensorimotor experience can add to, merge, fractionate or simply preserve these synergies (e.g. (Cheung et al., 2012)). ICMS with longer pulse trains and larger currents evokes complex coordinated movements – “ethologically” and physiologically and behaviorally meaningful movements (Graziano et al., 2002, Ramanathan et al., 2006, Overduin et al., 2012). Likewise, we also observed complex overlapping representations for hindlimb and trunk in a normal rat and forelimb and trunk (which involved both mid thoracic and low thoracic segments) in the SCI rats. In the context of our study and the techniques used, we define muscle synergy as divergent projections and overlapping representations of muscles by cortical motor neurons at a site to activate synergy muscle groups (co-activation). These representations were approximated and derived using ICMS. Our results with SCI rats suggest that once hindlimb representation and cortical hindlimb control is lost, trunk muscle representation merges with forelimb to form a set of novel synergies. These synergies can play a role in the altered biomechanics, locomotion and postural control necessary in SCI rats. The synergies needed in the rat without hindlimb stepping are different from those in stepping SCI rats such as weight supporting neonatal spinalized rats which have hindlimbs that often continue to step (Giszter et al., 1998). The training processes used here altered task demands for the non-stepping rats in a mass training paradigms (20 minutes/day, 5 days/week). This training might have had the effect of further consolidating the representations used for non-stepping functions, or extending representations in new directions. In practice we observed the former. Indeed, the center of gravity of trunk representations was moved forward by training, and away from the intact representation locations. Presumably, this forward movement of representations improved or consolidated the compensated patterns of forelimb stepping and trunk integration for non-stepping biomechanics of trunk and haunches with forelimbs in the paraplegic SCI rats here. We speculate that these larger changes mean that such training of novel paraplegic coordination has the potential to interfere with any subsequent rehabilitation to re-integrate hind-limb stepping into effective autonomous patterns of function. Additionally, our other work (manuscript under preparation) shows that successful robot rehabilitation training of adult rats spinalized as neonates actually shifts trunk motor representations in the cortex towards the normal topography. Therefore we speculate that the shift away from normal topography in the cortex seen after training here may have a negative impact on subsequent recovery, though it is adaptive in the context of the paraplegic function of the rats as tested here.
Overlap of different trunk muscles
We observed development of novel balances of muscle synergies between different trunk segments in the motor cortex of transected rats. These included significant motor expansion of ventral and dorsal overlapping representations and dorsal only representations in the trunk motor cortex. We also observed that the number of co-activated trunk segments represented per cortex site was higher for mid thoracic in transected rats, but lower elsewhere. The lowering of overall richness matches the reduced motor repertoire of the SCI rats. Increase in co-contraction or synergy between different trunk muscle segments in mid thoracic regions can enhance stiffness and action ranges and hence stability of trunk in its altered biomechanical modes of use in the injured rats. Dorsal muscles also play an important role in propulsion. During wheelchair propulsion task, able bodied subjects with surface trunk EMG recordings, showed significantly higher co-activation of abdominal and back muscles with back muscle activation significantly higher than abdominal activation (Yang et al., 2006). Development of novel postural muscle synergies for stabilization and control has been shown in human thoracic SCI subjects (Seelen et al., 1998, Seelen et al., 1998). Our data corroborates these findings by providing cortical correlates of such rearrangement and refocusing of muscle activation patterns in an animal SCI model.
Effect of training on plasticity
As expected, non-stepping treadmill training or robot assisted treadmill training did not promote any explicit new functional recovery using our measures. We speculate that the training might have enhanced control strictly within the limited function possessed by the injured rats, i.e., it may have helped their dragging the haunches subject to their paralysis. This was not directly examined. Prior to our study it was conceivable that some cortical changes in our robot rehabilitation might be unrelated to novel function achieved with the robots. However, there were no significant differences in many of the cortical plasticity measures between untrained and trained SCI groups here when no new functional improvement occurred. Nonetheless, there were changes: the center of gravity of trunk motor representation for trained rats was displaced still further in the rostral direction, and significantly so, compared to the untrained SCI rats. This was probably influenced by addition of rostral (above bregma) sites in the treadmill trained group and loss of caudal (below bregma) sites in the robot trained group. These results suggest that non-step training exacerbates some SCI plastic changes by moving representations further away from the normal topography. Our current understanding in spinal transected rats is that quadrupedal function involves a quite different cortical organization from these changes. Conceivably then, the training adaptations here might worsen subsequent rehabilitation prospects. Rehabilitation and quadrupedal weight support function in rats associate with the trunk representations developed at or below bregma as we detailed above. Given the value of exercise and maintaining the physical plant below the injury after SCI, the ideal mix of early exercise and active rehabilitation will depend of the therapy options, their serial interactions in time, and the therapeutic timelines available. Our results suggest to us that serial interactions in time of the mechanical demands and their management in SCI rats could be important to subsequent outcomes. However, we do not know with certainty if either type of plastic cortical process observed here (addition of rostral sites or loss of caudal sites) would interfere with or slow subsequent alternative rehabilitation enabled by a stepping intervention. It seems that at least some of the new representations and synergies we measured here would need to be reorganized and altered or dismantled by plastic processes during rehabilitation in order to achieve the organizations of cortex observed in functionally autonomous stepping rats (Giszter et al., 1998, Giszter et al., 2008) and in our other rehabilitation work (e.g., in adult rats spinalized as neonates, manuscripts in preparation). This perspective is also consistent with (Ramanathan et al., 2006) who showed that effective rehabilitation training following brain injury reverses the loss of complex motor representations due to injury, and also showed that cortical reorganization with rehabilitation correlates significantly with the degree of recovery of function. In the future it will therefore be important to induce stepping in adult SCI rats with pharmacological interventions or perineal stimulation during training and examine the plastic changes in the motor cortex in such stepping rats after adult SCI, either preceded by the training used here, or after cage rest alone.
Conclusion
Our study demonstrates that complete low thoracic SCI induces significant reorganization of trunk motor cortex. This results in an expansion of trunk motor representation in the rostral cortex and the formation of new balances of motor synergies between trunk and forelimb and between different trunk segments. Non-stepping rehabilitation training does not induce any further measurable plastic changes in trunk motor cortex synergies but induces an additional rostral shift in these trunk motor representations formed after SCI. This training effect might indicate consolidation and further reinforcement of the SCI patterns of use. Such changes could potentially exacerbate subsequent locomotor recovery if hindlimb stepping were restored. Plasticity of trunk motor cortex and its ease of reversibility or limitations should thus be considered while designing rehabilitative strategies for spinal cord injury, in the context of preserving opportunities for possible future improvements in SCI therapies and the ability to use them effectively.
Highlights:-.
Trunk motor cortex representations compared after SCI and non stepping training
Chronic SCI results in significant expansion of trunk motor area in rostral cortex
Chronic SCI changes overlap between trunk segments and trunk and forelimb
Non stepping training did not alter the majority of representations except
Non stepping training induced small but significant rostral migration of trunk
Acknowledgements
This work was supported by NIH NS54894, NIH NS726251, Neilsen Foundation support and the Drexel University College of Medicine Neuroengineering Strategic Plan. We thank Kavon Noorbehesht for assistance with training, animal care and surgical support and Sarah Kumar for partial assistance with robot training. We thank Dr. Ubong Ime Udoekwere for assistance in setting up surgical and robot training protocols. We thank Dr. Tim Himes for assistance with histology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adkins DL, Boychuk J, Remple MS,Kleim JA, 2006. Motor training induces experience-specific patterns of plasticity across motor cortex and spinal cord. J Appl Physiol 101, 1776–1782. [DOI] [PubMed] [Google Scholar]
- Aguilar J, Humanes-Valera D, Alonso-Calvino E, Yague JG, Moxon KA, Oliviero A,Foffani G, 2010. Spinal Cord Injury Immediately Changes the State of the Brain. Journal of Neuroscience 30, 7528–7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders C, Wagner H, Puta C, Grassme R, Petrovitch A,Scholle HC, 2007. Trunk muscle activation patterns during walking at different speeds. J Electromyogr Kinesiol 17, 245–252. [DOI] [PubMed] [Google Scholar]
- Antri M, Orsal D,Barthe JY, 2002. Locomotor recovery in the chronic spinal rat: effects of long-term treatment with a 5-HT2 agonist. Eur J Neurosci 16, 467–476. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS,Bresnahan JC, 1995. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 12, 1–21. [DOI] [PubMed] [Google Scholar]
- Bizzi E, D’Avella A, Saltiel P,Tresch M, 2002. Modular organization of spinal motor systems. Neuroscientist 8, 437–442. [DOI] [PubMed] [Google Scholar]
- Bjerkefors A, Carpenter MG, Cresswell AG,Thorstensson A, 2009. Trunk muscle activation in a person with clinically complete thoracic spinal cord injury. J Rehabil Med 41, 390–392. [DOI] [PubMed] [Google Scholar]
- Bronner S, 2012. Differences in segmental coordination and postural control in a multi-joint dance movement: developpe arabesque. J Dance Med Sci 16, 26–35. [PubMed] [Google Scholar]
- Bruehlmeier M, Dietz V, Leenders KL, Roelcke U, Missimer J,Curt A, 1998. How does the human brain deal with a spinal cord injury? Eur J Neurosci 10, 3918–3922. [DOI] [PubMed] [Google Scholar]
- Calguner E, Erdogan D, Elmas C, Bahcelioglu M, Gozil R,Ayhan MS, 2006. Innervation of the rat anterior abdominal wall as shown by modified Sihler’s stain. Med Princ Pract 15, 98–101. [DOI] [PubMed] [Google Scholar]
- Cariga P, Catley M, Nowicky AV, Savic G, Ellaway PH,Davey NJ, 2002. Segmental recording of cortical motor evoked potentials from thoracic paravertebral myotomes in complete spinal cord injury. Spine (Phila Pa 1976) 27, 1438–1443. [DOI] [PubMed] [Google Scholar]
- Chau CW,McKinley PA, 1991. Chronological observations of primary somatosensory cortical maps in kittens following low thoracic (T12) spinal cord transection at 2 weeks of age. Somatosens Mot Res 8, 355–376. [DOI] [PubMed] [Google Scholar]
- Chelette KC, Carrico C, Nichols L,Sawaki L, 2013. Long-term cortical reorganization following stroke in a single subject with severe motor impairment. NeuroRehabilitation. [DOI] [PubMed] [Google Scholar]
- Cheung VC, Turolla A, Agostini M, Silvoni S, Bennis C, Kasi P, Paganoni S, Bonato P,Bizzi E, 2012. Muscle synergy patterns as physiological markers of motor cortical damage. Proc Natl Acad Sci U S A 109, 14652–14656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen LG, Bandinelli S, Findley TW,Hallett M, 1991. Motor reorganization after upper limb amputation in man. A study with focal magnetic stimulation. Brain 114 (Pt 1B), 615–627. [DOI] [PubMed] [Google Scholar]
- d’Avella A,Bizzi E, 2005. Shared and specific muscle synergies in natural motor behaviors. Proc Natl Acad Sci U S A 102, 3076–3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancause N,Nudo RJ, 2011. Shaping plasticity to enhance recovery after injury. Prog Brain Res 192, 273–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desroches G, Gagnon D, Nadeau S,Popovic MR, 2013. Effects of sensorimotor trunk impairments on trunk and upper limb joint kinematics and kinetics during sitting pivot transfers in individuals with a spinal cord injury. Clin Biomech (Bristol, Avon) 28, 1–9. [DOI] [PubMed] [Google Scholar]
- Dobkin BH, Apple D, Barbeau H, Basso M, Behrman A, Deforge D, Ditunno J, Dudley G, Elashoff R, Fugate L, Harkema S, Saulino M,Scott M, 2003. Methods for a randomized trial of weight-supported treadmill training versus conventional training for walking during inpatient rehabilitation after incomplete traumatic spinal cord injury. Neurorehabil Neural Repair 17, 153–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobkin BH,Duncan PW, 2012. Should body weight-supported treadmill training and robotic-assistive steppers for locomotor training trot back to the starting gate? Neurorehabil Neural Repair 26, 308–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici N, Keller U, Vallery H, Friedli L, van den Brand R, Starkey ML, Musienko P, Riener R,Courtine G, 2012. Versatile robotic interface to evaluate, enable and train locomotion and balance after neuromotor disorders. Nature Medicine 18, 1142–+. [DOI] [PubMed] [Google Scholar]
- Donoghue JP, Suner S,Sanes JN, 1990. Dynamic organization of primary motor cortex output to target muscles in adult rats. II. Rapid reorganization following motor nerve lesions. Exp Brain Res 79, 492–503. [DOI] [PubMed] [Google Scholar]
- Donoghue JP,Wise SP, 1982. The motor cortex of the rat: cytoarchitecture and microstimulation mapping. J Comp Neurol 212, 76–88. [DOI] [PubMed] [Google Scholar]
- Endo T, Spenger C, Tominaga T, Brene S,Olson L, 2007. Cortical sensory map rearrangement after spinal cord injury: fMRI responses linked to Nogo signalling. Brain 130, 2951–2961. [DOI] [PubMed] [Google Scholar]
- Florence SL, Taub HB,Kaas JH, 1998. Large-scale sprouting of cortical connections after peripheral injury in adult macaque monkeys. Science 282, 1117–1121. [DOI] [PubMed] [Google Scholar]
- Fouad K, Pedersen V, Schwab ME,Brosamle C, 2001. Cervical sprouting of corticospinal fibers after thoracic spinal cord injury accompanies shifts in evoked motor responses. Curr Biol 11, 1766–1770. [DOI] [PubMed] [Google Scholar]
- Franchi G, 2000. Reorganization of vibrissal motor representation following severing and repair of the facial nerve in adult rats. Exp Brain Res 131, 33–43. [DOI] [PubMed] [Google Scholar]
- Frost SB, Iliakova M, Dunham C, Barbay S, Arnold P,Nudo RJ, 2013. Reliability in the location of hindlimb motor representations in Fischer-344 rats: laboratory investigation. J Neurosurg Spine 19, 248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Sydekum E, Haiss F, Peduzzi S, Zorner B, Schneider R, Baltes C, Rudin M, Weber B,Schwab ME, 2009. Functional and anatomical reorganization of the sensory-motor cortex after incomplete spinal cord injury in adult rats. J Neurosci 29, 12210–12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giszter S, Davies MR, Ramakrishnan A, Udoekwere UI,Kargo WJ, 2008. Trunk sensorimotor cortex is essential for autonomous weight-supported locomotion in adult rats spinalized as P1/P2 neonates. Journal of Neurophysiology 100, 839–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giszter SF,Hart CB, 2013. Motor primitives and synergies in the spinal cord and after injury--the current state of play. Ann N Y Acad Sci 1279, 114–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giszter SF, Kargo WJ, Davies M,Shibayama M, 1998. Fetal transplants rescue axial muscle representations in M1 cortex of neonatally transected rats that develop weight support. Journal of Neurophysiology 80, 3021–3030. [DOI] [PubMed] [Google Scholar]
- Giszter SF, Mussa-Ivaldi FA,Bizzi E, 1993. Convergent force fields organized in the frog’s spinal cord. J Neurosci 13, 467–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano A, Foffani G, Knudsen EB, Shumsky J,Moxon KA, 2013. Passive exercise of the hind limbs after complete thoracic transection of the spinal cord promotes cortical reorganization. PLoS One 8, e54350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano MS, Taylor CS,Moore T, 2002. Complex movements evoked by microstimulation of precentral cortex. Neuron 34, 841–851. [DOI] [PubMed] [Google Scholar]
- Harrison TC, Silasi G, Boyd JD,Murphy TH, 2013. Displacement of sensory maps and disorganization of motor cortex after targeted stroke in mice. Stroke 44, 2300–2306. [DOI] [PubMed] [Google Scholar]
- Hart CB,Giszter SF, 2010. A neural basis for motor primitives in the spinal cord. J Neurosci 30, 1322–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson LA, Gustin SM, Macey PM, Wrigley PJ,Siddall PJ, 2011. Functional Reorganization of the Brain in Humans Following Spinal Cord Injury: Evidence for Underlying Changes in Cortical Anatomy. Journal of Neuroscience 31, 2630–2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess G, Aizenman CD,Donoghue JP, 1996. Conditions for the induction of long-term potentiation in layer II/III horizontal connections of the rat motor cortex. J Neurophysiol 75, 1765–1778. [DOI] [PubMed] [Google Scholar]
- Hess G,Donoghue JP, 1994. Long-term potentiation of horizontal connections provides a mechanism to reorganize cortical motor maps. J Neurophysiol 71, 2543–2547. [DOI] [PubMed] [Google Scholar]
- Hess G,Donoghue JP, 1996. Long-term potentiation and long-term depression of horizontal connections in rat motor cortex. Acta Neurobiol Exp (Wars) 56, 397–405. [DOI] [PubMed] [Google Scholar]
- Hidler J,Sainburg R, 2011. Role of Robotics in Neurorehabilitation. Top Spinal Cord Inj Rehabil 17, 42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdefer RN,Miller LE, 2002. Primary motor cortical neurons encode functional muscle synergies. Exp Brain Res 146, 233–243. [DOI] [PubMed] [Google Scholar]
- Hsieh FH,Giszter SF, 2011. Robot-driven spinal epidural stimulation compared with conventional stimulation in adult spinalized rats. Conference proceedings : … Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Conference 2011, 5807–5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummelsheim H,Wiesendanger M, 1985. Is the hindlimb representation of the rats cortex a sensorimotor amalgam. Brain Research 346, 75–81. [DOI] [PubMed] [Google Scholar]
- Huntley GW, 1997. Correlation between patterns of horizontal connectivity and the extend of short-term representational plasticity in rat motor cortex. Cereb Cortex 7, 143–156. [DOI] [PubMed] [Google Scholar]
- Hussain S, Xie SQ,Liu G, 2011. Robot assisted treadmill training: mechanisms and training strategies. Med Eng Phys 33, 527–533. [DOI] [PubMed] [Google Scholar]
- Jacobs KM,Donoghue JP, 1991. Reshaping the cortical motor map by unmasking latent intracortical connections. Science 251, 944–947. [DOI] [PubMed] [Google Scholar]
- Jain N, Catania KC,Kaas JH, 1997. Deactivation and reactivation of somatosensory cortex after dorsal spinal cord injury. Nature 386, 495–498. [DOI] [PubMed] [Google Scholar]
- Kao T, Shumsky JS, Knudsen EB, Murray M,Moxon KA, 2011. Functional role of exercise-induced cortical organization of sensorimotor cortex after spinal transection. Journal of Neurophysiology 106, 2662–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao T, Shumsky JS, Murray M,Moxon KA, 2009. Exercise Induces Cortical Plasticity after Neonatal Spinal Cord Injury in the Rat. Journal of Neuroscience 29, 7549–7557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kargo WJ,Giszter SF, 2000. Rapid correction of aimed movements by summation of force-field primitives. J Neurosci 20, 409–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kargo WJ,Giszter SF, 2008. Individual premotor drive pulses, not time-varying synergies, are the units of adjustment for limb trajectories constructed in spinal cord. J Neurosci 28, 2409–2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kargo WJ,Nitz DA, 2003. Early skill learning is expressed through selection and tuning of cortically represented muscle synergies. Journal of Neuroscience 23, 11255–11269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleim JA, Barbay S, Cooper NR, Hogg TM, Reidel CN, Remple MS,Nudo RJ, 2002. Motor learning-dependent synaptogenesis is localized to functionally reorganized motor cortex. Neurobiol Learn Mem 77, 63–77. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Barbay S,Nudo RJ, 1998. Functional reorganization of the rat motor cortex following motor skill learning. Journal of Neurophysiology 80, 3321–3325. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Cooper NR,VandenBerg PM, 2002. Exercise induces angiogenesis but does not alter movement representations within rat motor cortex. Brain Res 934, 1–6. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Hogg TM, VandenBerg PM, Cooper NR, Bruneau R,Remple M, 2004. Cortical synaptogenesis and motor map reorganization occur during late, but not early, phase of motor skill learning. J Neurosci 24, 628–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klintsova AY, Dickson E, Yoshida R,Greenough WT, 2004. Altered expression of BDNF and its high-affinity receptor TrkB in response to complex motor learning and moderate exercise. Brain Res 1028, 92–104. [DOI] [PubMed] [Google Scholar]
- Kokotilo KJ, Eng JJ,Curt A, 2009. Reorganization and preservation of motor control of the brain in spinal cord injury: a systematic review. J Neurotrauma 26, 2113–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laubis-Herrmann U, Dichgans J, Bilow H,Topka H, 2000. Motor reorganization after spinal cord injury: evidence of adaptive changes in remote muscles. Restorative Neurology and Neuroscience 17, 175–181. [PubMed] [Google Scholar]
- Lotze M, Laubis-Herrmann U,Topka H, 2006. Combination of TMS and fMRI reveals a specific pattern of reorganization in M1 in patients after complete spinal cord injury. Restorative Neurology and Neuroscience 24, 97–107. [PubMed] [Google Scholar]
- Lotze M, Laubis-Herrmann U, Topka H, Erb M,Grodd W, 1999. Reorganization in the primary motor cortex after spinal cord injury - A functional Magnetic Resonance (fMRI) Study. Restorative Neurology and Neuroscience 14, 183–187. [PubMed] [Google Scholar]
- McKinley PA,Smith JL, 1990. Age-dependent differences in reorganization of primary somatosensory cortex following low thoracic (T12) spinal-cord transection in cats. Journal of Neuroscience 10, 1429–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AD, 1987. Localization of motoneurons innervating individual abdominal muscles of the cat. J Comp Neurol 256, 600–606. [DOI] [PubMed] [Google Scholar]
- Miya D, Giszter S, Mori F, Adipudi V, Tessler A,Murray M, 1997. Fetal transplants alter the development of function after spinal cord transection in newborn rats. Journal of Neuroscience 17, 4856–4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardone R, Holler Y, Brigo F, Seidl M, Christova M, Bergmann J, Golaszewski S,Trinka E, 2013. Functional brain reorganization after spinal cord injury: systematic review of animal and human studies. Brain Res 1504, 58–73. [DOI] [PubMed] [Google Scholar]
- Neafsey EJ, Bold EL, Haas G, Hurley-Gius KM, Quirk G, Sievert CF,Terreberry RR, 1986. The organization of the rat motor cortex: a microstimulation mapping study. Brain Res 396, 77–96. [DOI] [PubMed] [Google Scholar]
- Nishibe M, Barbay S, Guggenmos D,Nudo RJ, 2010. Reorganization of motor cortex after controlled cortical impact in rats and implications for functional recovery. J Neurotrauma 27, 2221–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudo RJ,Friel KM, 1999. Cortical plasticity after stroke: implications for rehabilitation. Rev Neurol (Paris) 155, 713–717. [PubMed] [Google Scholar]
- Ojemann JG,Silbergeld DL, 1995. Cortical stimulation mapping of phantom limb rolandic cortex. Case report. J Neurosurg 82, 641–644. [DOI] [PubMed] [Google Scholar]
- Overduin SA, d’Avella A, Carmena JM,Bizzi E, 2012. Microstimulation activates a handful of muscle synergies. Neuron 76, 1071–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Leone A, Peris M, Tormos JM, Pascual AP,Catala MD, 1996. Reorganization of human cortical motor output maps following traumatic forearm amputation. Neuroreport 7, 2068–2070. [DOI] [PubMed] [Google Scholar]
- Perez MA, Lungholt BK, Nyborg K,Nielsen JB, 2004. Motor skill training induces changes in the excitability of the leg cortical area in healthy humans. Exp Brain Res 159, 197–205. [DOI] [PubMed] [Google Scholar]
- Plautz EJ, Milliken GW,Nudo RJ, 2000. Effects of repetitive motor training on movement representations in adult squirrel monkeys: role of use versus learning. Neurobiol Learn Mem 74, 27–55. [DOI] [PubMed] [Google Scholar]
- Ramanathan D, Conner JM,Tuszynski MH, 2006. A form of motor cortical plasticity that correlates with recovery of function after brain injury. Proceedings of the National Academy of Sciences of the United States of America 103, 11370–11375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijntjes M, Tegenthoff M, Liepert J, Leonhardt G, Kotterba S, Muller S, Kiebel S, Malin JP, Diener HC,Weiller C, 1997. Cortical reorganization in patients with facial palsy. Ann Neurol 41, 621–630. [DOI] [PubMed] [Google Scholar]
- Rossignol S,Frigon A (2011). Recovery of Locomotion After Spinal Cord Injury: Some Facts and Mechanisms. Annual Review of Neuroscience, Vol 34. S. E. J. T. M. S. C. J. S. C. F. Z. H. Y. Hyman. 34: 413–440. [DOI] [PubMed] [Google Scholar]
- Sanes JN, Suner S,Donoghue JP, 1990. Dynamic organization of primary motor cortex output to target muscles in adult rats. I. Long-term patterns of reorganization following motor or mixed peripheral nerve lesions. Exp Brain Res 79, 479–491. [DOI] [PubMed] [Google Scholar]
- Sanes JN, Suner S, Lando JF,Donoghue JP, 1988. Rapid reorganization of adult rat motor cortex somatic representation patterns after motor nerve injury. Proc Natl Acad Sci U S A 85, 2003–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieber MH,Deuel RK, 1997. Primary motor cortex reorganization in a long-term monkey amputee. Somatosens Mot Res 14, 157–167. [DOI] [PubMed] [Google Scholar]
- Seelen HA, Potten YJ, Adam JJ, Drukker J, Spaans F,Huson A, 1998. Postural motor programming in paraplegic patients during rehabilitation. Ergonomics 41, 302–316. [DOI] [PubMed] [Google Scholar]
- Seelen HA, Potten YJ, Drukker J, Reulen JP,Pons C, 1998. Development of new muscle synergies in postural control in spinal cord injured subjects. J Electromyogr Kinesiol 8, 23–34. [DOI] [PubMed] [Google Scholar]
- Seifert T,Secher NH, 2011. Sympathetic influence on cerebral blood flow and metabolism during exercise in humans. Prog Neurobiol 95, 406–426. [DOI] [PubMed] [Google Scholar]
- Sherwood AM, Dimitrijevic MR,McKay WB, 1992. Evidence of subclinical brain influence in clinically complete spinal cord injury: discomplete SCI. J Neurol Sci 110, 90–98. [DOI] [PubMed] [Google Scholar]
- Song W,Giszter SF, 2011. Adaptation to a cortex-controlled robot attached at the pelvis and engaged during locomotion in rats. J Neurosci 31, 3110–3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung PS, Lee KJ,Park WH, 2012. Coordination of trunk and pelvis in young and elderly individuals during axial trunk rotation. Gait Posture 36, 330–331. [DOI] [PubMed] [Google Scholar]
- Swain RA, Harris AB, Wiener EC, Dutka MV, Morris HD, Theien BE, Konda S, Engberg K, Lauterbur PC,Greenough WT, 2003. Prolonged exercise induces angiogenesis and increases cerebral blood volume in primary motor cortex of the rat. Neuroscience 117, 1037–1046. [DOI] [PubMed] [Google Scholar]
- Tandon S, Kambi N, Mohammed H,Jain N, 2013. Complete reorganization of the motor cortex of adult rats following long-term spinal cord injuries. Eur J Neurosci. [DOI] [PubMed] [Google Scholar]
- Toldi J, Laskawi R, Landgrebe M,Wolff JR, 1996. Biphasic reorganization of somatotopy in the primary motor cortex follows facial nerve lesions in adult rats. Neurosci Lett 203, 179–182. [DOI] [PubMed] [Google Scholar]
- Topka H, Cohen LG, Cole RA,Hallett M, 1991. Reorganization of corticospinal pathways following spinal cord injury. Neurology 41, 1276–1283. [DOI] [PubMed] [Google Scholar]
- Torres-Oviedo G, Macpherson JM,Ting LH, 2006. Muscle synergy organization is robust across a variety of postural perturbations. J Neurophysiol 96, 1530–1546. [DOI] [PubMed] [Google Scholar]
- Tresch MC,Bizzi E, 1999. Responses to spinal microstimulation in the chronically spinalized rat and their relationship to spinal systems activated by low threshold cutaneous stimulation. Exp Brain Res 129, 401–416. [DOI] [PubMed] [Google Scholar]
- Tresch MC,Jarc A, 2009. The case for and against muscle synergies. Curr Opin Neurobiol 19, 601–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tresch MC, Saltiel P, d’Avella A,Bizzi E, 2002. Coordination and localization in spinal motor systems. Brain Res Brain Res Rev 40, 66–79. [DOI] [PubMed] [Google Scholar]
- Udoekwere UI, 2010. Identifying the Substrate for Successful Robot Rehabilitation in Adult Rats Spinalized as Neonates: The Role of the Trunk in Locomotor Recovery after Complete Low-Thoracic Transection.. Dissertation Thesis Drexel University, PA. [Google Scholar]
- Udoekwere UI, Oza CS,Giszter SF, 2014. A pelvic implant orthosis in rodents, for spinal cord injury rehabilitation, and for brain machine interface research: construction, surgical implantation and validation. J Neurosci Methods 222, 199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udoekwere UI, Ramakrishnan A, Mbi L,Giszter SF, 2006. Robot application of elastic fields to the pelvis of the spinal transected rat: a tool for detailed assessment and rehabilitation. Conference proceedings : … Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Conference 1, 3684–3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaynman S,Gomez-Pinilla F, 2005. License to run: exercise impacts functional plasticity in the intact and injured central nervous system by using neurotrophins. Neurorehabil Neural Repair 19, 283–295. [DOI] [PubMed] [Google Scholar]
- Wrigley PJ, Gustin SM, Macey PM, Nash PG, Gandevia SC, Macefield VG, Siddall PJ,Henderson LA, 2009. Anatomical changes in human motor cortex and motor pathways following complete thoracic spinal cord injury. Cereb Cortex 19, 224–232. [DOI] [PubMed] [Google Scholar]
- Wu CW,Kaas JH, 1999. Reorganization in primary motor cortex of primates with long standing therapeutic amputations. J Neurosci 19, 7679–7697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YS, Koontz AM, Triolo RJ, Mercer JL,Boninger ML, 2006. Surface electromyography activity of trunk muscles during wheelchair propulsion. Clin Biomech (Bristol, Avon) 21, 1032–1041. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Hallett M,Cohen LG, 1998. Mechanisms of deafferentation-induced plasticity in human motor cortex. J Neurosci 18, 7000–7007. [DOI] [PMC free article] [PubMed] [Google Scholar]