Abstract
Background
Transthyretin (TTR) has been described as a predictor for outcomes in medical and surgical patients. However, the association of TTR on admission and over time on outcomes has not yet been prospectively assessed in trauma patients.
Methods
This is a prospective observational study including trauma patients admitted to the intensive care unit (ICU) of a large Level I trauma center 05/2014–05/2015. TTR levels at ICU admission and all subsequent values over time were recorded. Patients were observed for 28 days or until hospital discharge. The association of outcomes and TTR levels at admission and over time was assessed using multivariable regression and generalized estimating equation (GEE) analysis, respectively.
Results
A total of 237 patients with TTR obtained at admission were included, 69 of whom had repeated TTR measurements. Median age was 40.0 years and median ISS 16.0; 83.1% were male. Below-normal TTR levels at admission (41.8%) were independently associated with higher in-hospital mortality (p = 0.042), more infectious complications (p = 0.032), longer total hospital length of stay (LOS) (p = 0.013), and ICU LOS (p = 0.041). Higher TTR levels over time were independently associated with lower in-hospital mortality (p = 0.015), fewer infections complications (p = 0.028), shorter total hospital and ICU LOS (both p < 0.001), and fewer ventilator days (0.004).
Conclusions
In critically ill trauma patients, below-normal TTR levels at admission were independently associated with worse outcomes and higher TTR levels over time with better outcomes, including lower in-hospital mortality, less infectious complications, shorter total hospital and ICU LOS, and fewer ventilator days. Based on these results, TTR may be considered as a prognostic marker in this patient population.
Introduction
Transthyretin (TTR) has been reported as a predictor for worse outcomes in surgical [1–6], burn [7, 8], and critically ill medical [9] patients. Furthermore, TTR has been shown to be a useful marker assessing malnutrition [10, 11] and for tracking the adequacy of in-hospital nutritional support [12–14]. In trauma patients, lower TTR levels at hospital admission correlated with the injury severity [15].
For critically ill patients, changes in TTR levels over time have been shown to interact with C-reactive protein (CRP) levels [16, 17]. This can be explained by the so-called reprioritization of liver synthesis during the acute inflammatory phase in critically ill patients, leading to an increase in positive acute phase proteins, mainly CRP, and a reduction in negative acute phase proteins, including TTR [13]. Thus, the decreasing acute phase response in critically ill patients is reflected by changes in TTR levels over time.
In a recent retrospective study including critically ill trauma patients specifically, lower TTR levels at intensive care unit (ICU) admission have been shown to be independently associated with worse outcomes, including a higher mortality and infectious complication rate, longer ICU and total hospital length of stay (LOS), and increased ventilator days [18].
Although an association between TTR levels and outcomes has been described in previous studies [1–9], TTR has not been prospectively assessed as a maker for outcomes in critically ill trauma patients so far. Furthermore, the impact of TTR values over time on outcomes has not yet been prospectively investigated in this patient population. This is of particular importance, as TTR levels may change over time, depending on the inflammatory response to the trauma and subsequent interventions, as well as the adequacy of nutrition during the hospital course. The aim of the current study was, therefore, to prospectively investigate the association of TTR levels at ICU admission and over time on clinical outcomes in critically ill trauma patients. We hypothesized that higher TTR levels are associated with better clinical outcomes.
Methods
This study was approved by the Institutional Review Board (IRB) of the University of Southern California.
Patient selection
This is a prospective observational study including all trauma patients admitted to the adult surgical Intensive Care Unit (ICU) of the Los Angeles County + University of Southern California (LAC + USC) Medical Center during a 1-year period (05/19/2014 to 05/27/2015) in whom TTR was measured at ICU admission.
Data collection
Included patients were prospectively followed for 28 days or until hospital discharge. Data collection included patient characteristics (age, sex, body mass index [BMI], known diabetes mellitus [DM], chronic obstructive pulmonary disease [COPD], and chronic heart failure [CHF]), injury characteristics (penetrating vs. blunt injury, gastrointestinal tract [GIT] injury, traumatic brain injury [TBI], Abbreviated Injury Scale [AIS], Injury Severity Score [ISS]), Emergency Department (ED) variables (Glasgow Coma Scale [GCS], hypotension [systolic blood pressure <90 mmHg], tachycardia [heart rate >100 bpm], desaturation [arterial oxygen saturation <90%]), surgical procedures (number of procedures performed, laparotomy, thoracotomy, GIT surgery, neurosurgery), daily nutritional intake (enteral nutrition [EN], oral nutrition [ON], total parenteral nutrition [TPN]), laboratory values over time (TTR [normal range 19.0–38.0 mg/dL], high-sensitivity C-reactive protein [hs-CRP, normal range 0.0–7.0 mg/L], Albumin [ALB, normal range 3.5–5.0 g/dL], Lactate [LACT, normal range 0.5–2.2 mmol/L]), infection (positive bacterial cultures, urinary tract infections [UTI], pneumonia, ventilator-associated pneumonia [VAP], surgical site infection [SSI], abscess, pleural empyema, sepsis), total hospital and ICU length of stay (LOS), and in-hospital mortality. The nutritional intake, i.e., EN, ON, and/or TPN, was recorded daily for 28 days or until hospital discharge.
Infectious complications
The following definitions for infectious complications were used: UTI—positive bacterial urinary culture; pneumonia—positive broncho-alveolar lavage with ≥10,000 colony-forming units; VAP—according to the Centers of Disease Control (CDC) guidelines [19]; SSI—according to CDC guidelines [20]; abscess—pus or positive bacterial culture in aspirated or drained fluid collection, or according to computed tomography (CT) scan report; pleural empyema—pus or positive bacterial culture in aspirated or drained fluid from pleural cavity; sepsis—according to the American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference [21].
Laboratory values at ICU admission and over time
TTR, hs-CRP, ALB, and LACT at ICU admission were defined as samples drawn within 24 h prior to or after ICU admission. TTR at ICU admission and over time was obtained at the discretion of the attending trauma surgeon. Indications for repeated TTR measurements were a complicated hospital course or prolonged ICU LOS. All laboratory variables were recorded for 28 days or until hospital discharge. Changes of laboratory variables over time were shown on a scatter plot with individual regression lines.
Statistical analysis
Normality of distribution was assessed using histograms, skewness, and the Shapiro–Wilk test. Variables were reported as medians and interquartile ranges (IQR) or numbers and percentages, as appropriate.
Patients included in the study were compared to all other trauma patients admitted to the ICU during the study period using Fisher’s exact test for categorical and Mann–Whitney U test for continuous variables, respectively.
The change of laboratory variables over time was analyzed using univariable generalized estimating equation (GEE) with an unstructured correlation matrix.
The effect of below-normal TTR levels at ICU admission on clinical outcomes, including in-hospital mortality, infectious complications, total hospital and ICU length of stay (LOS), and ventilator days, was assessed in univariable and multivariable logistic or linear regression analysis, as appropriate.
The association of TTR levels over time, maintained as a continuous variable, on the above-mentioned clinical outcomes was investigated in univariable and multivariable GEE analysis, using an unstructured correlation matrix.
In multivariable regression and GEE analysis, the effect of TTR on clinical outcomes was adjusted for clinically important variables, including male sex, age, BMI, GIT injury, TBI, and the hospital day (analysis over time only). Clinically important variables with a p value ≤0.1 in univariable analysis were entered in multivariable regression and GEE models. Non-normally distributed dependent variables were log10-transformed for linear regression and GEE analyses. Multicollinearity was assessed using the variance inflation factor (VIF). A VIF < 5 was assumed to exclude significant collinearity.
Results were reported as odd ratios (OR) or regression coefficients (RC) with 95% confidence intervals (CI) and p values. p values ≤0.05 were considered statistically significant.
Statistical analyses were performed using SPSS Statistics (IBM Corporation, Armonk, NY).
Results
Baseline characteristics
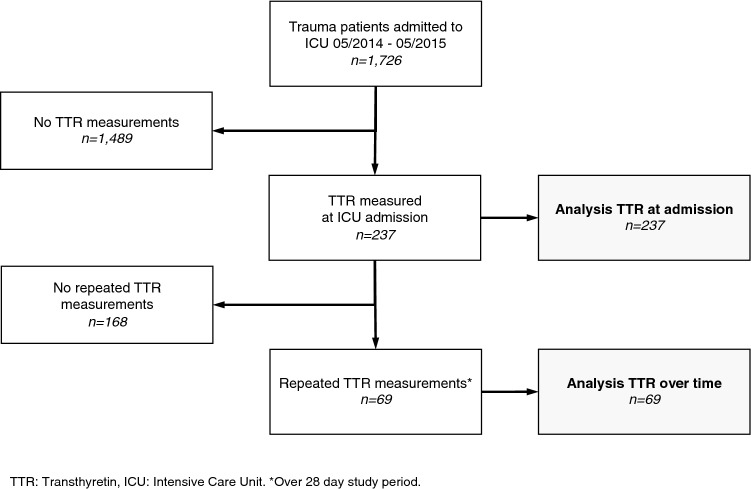
During the 1-year study period, 1726 trauma patients were admitted to the ICU of the LAC + USC Medical Center. Of these, 237 patients with TTR measured at ICU admission were included in the study. A total of 69 patients had repeated TTR measurements (Fig. 1). Patients were predominantly male (83.1%) with a median age of 40.0 years (IQR 31.0), a low incidence of known comorbidities (DM 6.6%, COPD 1.3%, CHF 1.3%), and a median ISS of 16.0 (IQR 13.0). Surgical procedures were performed in 53.6% of included patients. Baseline characteristics are outlined in Table 1.
Fig. 1.
Patients included
Table 1.
Baseline characteristics
| Patients (n = 237) | Patients (n = 237) | ||
|---|---|---|---|
| Patient characteristics | Surgery (cont.) | ||
| Age (years)* | 40.0 (31.0) | Number of procedures | |
| Sex (male/female) | 197/40 (83.1/16.9) | 0 | 110 (46.4) |
| BMI (kg/m2)* | 26.0 (5.6) | 1 | 47 (19.8) |
| Diabetes mellitus | 16 (6.6) | 2 | 46 (19.4) |
| COPD | 3 (1.3) | 3 | 20 (8.4) |
| Chronic heart failure | 3 (1.3) | 4 | 7 (3.0) |
| ≥5 | 7 (3.0) | ||
| Injury characteristics | |||
| Penetrating injury | 51 (21.5) | Infection | |
| GIT injury | 37 (15.6) | Urinary tract infection | 31 (13.1) |
| Traumatic brain injury | 171 (72.2) | Pneumonia overall | 24 (19.0) |
| ISS* | 16.0 (13.0) | VAP | 5 (2.1) |
| AIS head ≥3 | 82 (34.6) | Surgical site infection | 6 (2.5) |
| AIS chest ≥3 | 101 (42.6) | Abscess | 6 (2.5) |
| AIS abdomen ≥3 | 51 (21.5) | Pleural empyema | 2 (0.8) |
| AIS extremities ≥3 | 64 (27.0) | Positive blood culture | 29 (12.2) |
| Sepsis | 3 (1.3) | ||
| ED vital signs | Infection overall | 68 (28.7) | |
| Hypotension (SBP <90 mmHg | 18 (7.6) | ||
| Tachycardia (HR >100 bpm) | 84 (35.4) | Outcomes | |
| Desaturation (aSO2 <90%) | 9 (3.8) | In-hospital mortality | 16 (6.8) |
| Total GCS* | 15.0 (2.0) | Total hospital LOS* | 10.0 (15.0) |
| ICU LOS* | 4.0 (4.0) | ||
| Surgery | Ventilator days* | 3.0 (7.0) | |
| Laparotomy | 55 (23.2) | ||
| Thoracotomy | 9 (3.8) | ||
| GIT surgery | 39 (16.5) | ||
| Neurosurgery | 12 (5.1) |
Values are numbers (valid percentages) unless indicated otherwise. *Values are medians (interquartile ranges)
BMI Body Mass Index, COPD chronic obstructive pulmonary disease, GIT gastrointestinal tract, ISS injury severity score, AIS abbreviated injury scale, SBP systolic blood pressure, HR heart rate, aSO2 arterial oxygen saturation, GCS Glasgow coma scale, ICU intensive care unit, VAP ventilator-associated pneumonia, LOS length of stay
Compared to all other trauma patients admitted to the ICU of the LAC + USC Medical Center during the study period, patients with TTR measured at ICU admission were more frequently male (83.1 vs. 76.8%, p = 0.035), had a higher injury severity (median ISS 16.0 [IQR 13] vs. 11.0 [IQR 14], p < 0.001), suffered more frequently from penetrating injuries (21.5 vs. 12.0%, p < 0.001), were more frequently hypotensive at ED admission (systolic blood pressure <90 mmHg 8.4 vs. 3.4%, p = 0.002), and had a longer total hospital LOS (median 10.0 [IQR 15.0] vs. 7.0 [IQR 11.0] days, p < 0.001].
TTR values at ICU admission and over time
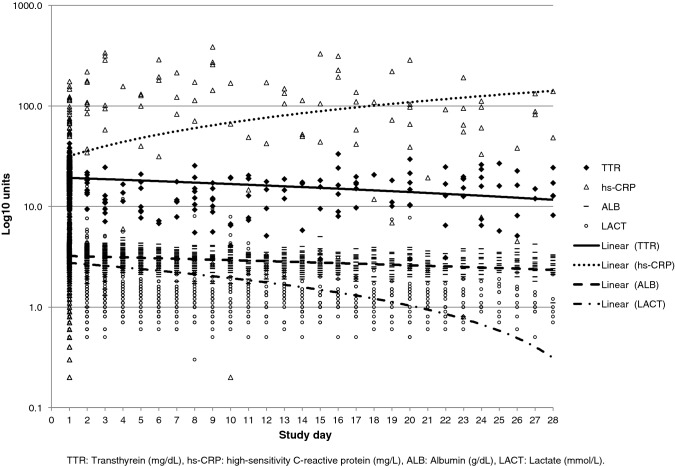
Median TTR at ICU admission was 20.3 (IQR 7.8). Below-normal TTR values (<19.0 mg/dL) at ICU admission were found in 41.8%. The median number of repeated TTR measures was 2.0 (IQR 2.0, range 2–9). TTR, ALB, and LACT levels decreased over time, while CRP levels increased (Table 2, Fig. 2). Higher ISS were associated with significantly lower TTR values at ICU admission (RC −0.160, 95% CI −0.226/−0.094, p < 0.001). In analysis over time, higher ISS and artificial nutrition were associated with significantly lower TTR levels over time (Log10TTR, RC −0.007, 95% CI −0.009/−0.004, p < 0.001 and RC −0.093, 95% CI −0.146/−0.039, p = 0.001).
Table 2.
Laboratory values over time
| Effect of study day | |||
|---|---|---|---|
| RC | 95% CI (lower/upper) | p value | |
| Log10 transthyretin | −0.006 | −0.010/−0.003 | 0.001 |
| Log10 hs C-reactive protein | 0.039 | 0.029/0.049 | <0.001 |
| Log10 albumin | −0.001 | −0.002/0.001 | 0.483 |
| Log10 lactate | −0.021 | −0.027/−0.016 | <0.001 |
RC Regression coefficient, CI confidence interval
*Univariable GEE. GEE performed with unstructured correlation matrix
Fig. 2.
Laboratory values over time
Effect of TTR levels at ICU admission on clinical outcomes
The association of below-normal TTR levels at ICU admission and clinical outcomes are shown in Table 3. Below-normal TTR levels were independently associated with higher in-hospital mortality, more infectious complications, shorter total hospital LOS, and shorter ICU LOS. No significant collinearity was detected between TTR and other independent variables entered in linear regression models. The VIF was smaller than 1.5 for all variables included.
Table 3.
Effect of low TTR at ICU admission on clinical outcomes
| Univariable analysis* | Adjusted multivariable analysis** | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI (lower/upper) | p value | AOR | 95% CI (lower/upper) | p value | |
| In-hospital mortality | 1.201 | 1.117/9.898 | 0.031 | 1.142a | 1.045/9.395 | 0.042 |
| Infectious complications | 1.893 | 1.071/3.344 | 0.028 | 1.901b | 1.058/3.416 | 0.032 |
| Univariable analysis* | Adjusted multivariable analysis** | |||||
|---|---|---|---|---|---|---|
| RC | 95% CI (lower/upper) | p value | ARC | 95% CI (lower/upper) | p value | |
| Log10 total hospital LOS | 0.125 | 0.021/0.229 | 0.019 | 0.133c | 0.028/0.237 | 0.013 |
| Log10 ICU LOS | 0.104 | 0.007/0.202 | 0.036 | 0.102d | 0.004/0.199 | 0.041 |
| Log10 ventilator days | 0.179 | 0.000/0.358 | 0.050 | 0.124e | −0.048/0.295 | 0.156 |
*Univariable logistic and linear regression analysis. **Multivariable logistic and linear regression analysis
RC Regression coefficient, OR odds ratio, ARC adjusted regression coefficient, AOR adjusted odds ratio, ICU intensive care unit, LOS length of stay, TBI traumatic brain injury, BMI body mass index, GIT gastrointestinal tract
aAdjusted for male sex and study day
bAdjusted for male sex, age, TBI, and study day
cAdjusted for BMI, GIT injury, and study day
dAdjusted for male sex, TBI, and study day
eAdjusted for age and study day
Effect of TTR levels over time on clinical outcomes
Results of the GEE analysis on the association of TTR over time and clinical outcomes are outlined in Table 4. Higher TTR levels over time were independently associated with lower in-hospital mortality, fewer infectious complications, shorter total hospital and ICU LOS, and fewer ventilator days.
Table 4.
Effect of TTR over time on clinical outcomes
| Univariable GEE | Adjusted multivariable GEE | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI (lower/upper) | p value | AOR | 95% CI (lower/upper) | p value | |
| In-hospital mortality | 0.940 | 0.895/0.988 | 0.014 | 0.941a | 0.895/0.988 | 0.015 |
| Infectious complications | 0.960 | 0.931/0.990 | 0.008 | 0.966b | 0.937/0.996 | 0.028 |
| Univariable GEE | Adjusted multivariable GEE | |||||
|---|---|---|---|---|---|---|
| RC | 95% CI (lower/upper) | p value | ARC | 95% CI (lower/upper) | p value | |
| Log10 total hospital LOS | −0.010 | −0.015/−0.005 | <0.001 | −0.011c | −0.016/−0.006 | <0.001 |
| Log10 ICU LOS | −0.012 | −0.017/−0.006 | <0.001 | −0.013d | − 0.020/−0.006 | <0.001 |
| Log10 ventilator days | −0.015 | −0.024/−0.005 | 0.002 | −0.015e | −0.025/−0.005 | 0.004 |
TTR maintained as continuous variable. GEE performed with unstructured correlation matrix
TTR Transthyretin, GEE generalized estimating equation, RC regression coefficient, OR odds ratio, ARC adjusted regression coefficient, AOR adjusted odds ratio, ICU intensive care unit, LOS length of stay, TBI traumatic brain injury, BMI body mass index
aAdjusted for male sex and study day
bAdjusted for age and study day
cAdjusted for BMI and study day
dAdjusted for BMI, TBI, and study day
eAdjusted for age, BMI, and study day
Discussion
This study aimed to assess TTR at ICU admission and over time as a marker for clinical outcomes in critically ill trauma patients. Below-normal TTR levels at ICU admission were associated with higher in-hospital mortality, more infectious complications, and longer total hospital and ICU LOS. Analysis over time revealed a significant association of higher TTR levels over the hospital course and better clinical outcomes, including lower in-hospital mortality, less infectious complications, shorter total hospital and ICU LOS, and fewer ventilator days.
We have previously published a retrospective study that revealed lower TTR levels at ICU admission as an independent predictor for worse outcomes in trauma patients [18]. The current prospective study confirmed this finding.
TTR by itself is not known to have an impact on outcomes in critically ill patients. However, as it is affected by both the inflammatory response after trauma [13, 15] and the nutritional state [12–14], TTR can be looked at as a surrogate parameter for the overall clinical condition in critically ill patients that may be used as a marker for outcomes. Higher TTR levels over time reflect a decreasing inflammatory response and adequate nutrition and therefore better outcomes. On the other hand, decreasing TTR levels over time may indicate both, an ongoing inflammatory state, e.g., due to multiple surgical procedures or complications, and inadequate nutritional support. Taking this into account, efforts should be made to treat sources ongoing inflammation and to improve nutrition in critically ill patients with decreasing TTR levels over the hospital course.
Previous studies described lower TTR levels in patients with higher injury severity [15] and inadequate nutritional intake [12–14]. This is in line with the results of the current study that revealed a significant correlation of higher ISS and artificial nutrition, i.e., enteral or parenteral nutrition, with lower TTR levels over time. Artificial nutrition, although it may improve the nutritional state in an individual patient, has been reported as risk factor for malnutrition, due to frequent interruption of the feeding [22, 23].
In the current study, TTR levels significantly decreased over time (Fig. 2, Table 2). Parent et al., in an observational study including 1056 trauma patients admitted to the ICU, reported an increase in TTR levels by the beginning of the third week [14]. The different results of the current study and the study by Parent et al. may be explained by the different inclusion criteria. In the study by Parent et al., patients who required less than 2 days of mechanical ventilation and less than 7 days of ICU care, as well as patients who were expected to die during the first 2 days after admission, were excluded. Consequently, the ICU and total hospital LOS were much longer in the study by Parent et al. than in the current study (14 vs. 4 and 26 vs. 10, respectively). Thus, more time to correct the initial low TTR levels during the hospital course was available in the study by Parent et al., whereas part of the patients with low initial TTR levels included in the current study were discharged early or died during the hospital course.
Not surprisingly, lower TTR levels at ICU admission and over time were associated with infectious complications. Previous studies have shown increased infections in critically ill surgical patients with higher energy deficits [24], a lower incidence of infections in critically ill patients with early enteral nutrition [25], and fewer nosocomial infections in critically ill patients with enteral nutrition and supplemental parenteral nutrition to achieve 100% of the energy target [26]. Although TTR levels are affected not only by nutrition, but also by the inflammatory state [27], persistently low TTR levels, especially in the absence of ongoing surgical procedures, may indicate an insufficient nutritional supply and therefore increased infectious complications. Consequently, adequate nutrition is crucial to avoid infectious complications and may be monitored using TTR over time.
CRP levels significantly increased over time in the current study. Several studies have shown higher CRP levels over time in patients with complications after colorectal surgery [28, 29], esophageal surgery [30, 31], and abdominal surgery overall [32]. Considering these previous studies, increasing CRP levels over time should trigger a search for complications in trauma patients, too.
Analysis over time revealed a faster decrease in lactate levels compared to TTR levels and a nonsignificant slight decrease in albumin levels over time (Fig. 1, Table 2). Albumin has been shown to reflect the nutritional state less accurately than TTR [10, 12]. Lactate has a short biological half-life and has been described as a marker of tissue hypoxia [33], making this metabolite a suitable short-term endpoint of resuscitation [33–35]. TTR, on the other hand, with its longer half-life, may serve as surrogate parameter of the systemic inflammatory response and the nutritional state in critically ill patients over a longer time period, i.e., the ICU and hospital course.
In the current study, TTR at ICU admission and over time was obtained at the discretion of the attending surgeon. This is reflected by the different characteristics of the patients included in the study, i.e., patients with TTR measurements, and all other trauma patients admitted to the ICU during the study period. TTR measurements were more frequently ordered in male patients and patients with penetrating injuries, more severe trauma, and a longer hospital LOS.
The current study has some inherent limitations. As the proportion of patients admitted to the ICU with TTR measurements was relatively small and baseline characteristics of the patients included in the study and all other trauma patients admitted to the ICU during the study period were different, the results may not be valid for critically ill trauma patients in general. The current analysis of TTR levels at ICU admission and over time therefore has to be interpreted with care. Nevertheless, the current study presents the first prospective evaluation of TTR levels at ICU admission and over time as a marker for clinical outcomes in critically ill trauma patients.
In conclusion, in this prospective single-center study including critically ill trauma patients, below-normal TTR levels at ICU admission were independently associated with worse clinical outcomes and higher TTR levels over time with better clinical outcomes, including lower in-hospital mortality, less infectious complications, shorter total hospital and ICU LOS, and less ventilator days. Based on these results, TTR may be considered as a prognostic marker in this patient population. Further research is warranted to confirm the results of this study in larger cohorts of critically ill trauma patients.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest or financial ties to disclose.
Ethical approval
This study was approved by the Institutional Review Board of the University of Southern California.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bae HJ, Lee HJ, Han DS, et al. Prealbumin levels as a useful marker for predicting infectious complications after gastric surgery. J Gastrointest Surg. 2011;15:2136–2144. doi: 10.1007/s11605-011-1719-z. [DOI] [PubMed] [Google Scholar]
- 2.Shum J, Markiewicz MR, Park E, et al. Low prealbumin level is a risk factor for microvascular free flap failure. J Oral Maxillofac Surg. 2014;72:169–177. doi: 10.1016/j.joms.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 3.Yu PJ, Cassiere HA, Dellis SL, et al. Impact of preoperative prealbumin on outcomes after cardiac surgery. J Parenter Enter Nutr. 2014;39:870–874. doi: 10.1177/0148607114536735. [DOI] [PubMed] [Google Scholar]
- 4.Geisler JP, Linnemeier GC, Thomas AJ, et al. Nutritional assessment using prealbumin as an objective criterion to determine whom should not undergo primary radical cytoreductive surgery for ovarian cancer. Gynecol Oncol. 2007;106:128–131. doi: 10.1016/j.ygyno.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Zago L, Dupraz H, Weisstaub A, et al. Indices of protein status as predictors of complications in low risk surgical patients of hernias and lithiasis. Nutr Res. 2000;20:203–213. doi: 10.1016/S0271-5317(99)00153-0. [DOI] [Google Scholar]
- 6.Huang L, Li J, Yan JJ, et al. Prealbumin is predictive for postoperative liver insufficiency in patients undergoing liver resection. World J Gastroenterol. 2012;18:7021–7025. doi: 10.3748/wjg.v18.i47.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang HT, Yim H, Cho YS, et al. Prediction of clinical outcomes for massively-burned patients via serum transthyretin levels in the early postburn period. J Trauma Acute Care Surg. 2012;72:999–1005. doi: 10.1097/TA.0b013e3182413bd8. [DOI] [PubMed] [Google Scholar]
- 8.Yang HT, Yim H, Cho YS, et al. Serum transthyretin level is associated with clinical severity rather than nutrition status in massively burned patients. J Parenter Enter Nutr. 2013;38:966–972. doi: 10.1177/0148607113499588. [DOI] [PubMed] [Google Scholar]
- 9.Devakonda A, George L, Raoof S, et al. Transthyretin as a marker to predict outcome in critically ill patients. Clin Biochem. 2008;41:1126–1130. doi: 10.1016/j.clinbiochem.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 10.Mears E. Outcomes of continuous process improvement of a nutritional care program incorporating serum prealbumin measurements. Nutrition. 1996;12:479–484. doi: 10.1016/S0899-9007(96)91721-9. [DOI] [PubMed] [Google Scholar]
- 11.Robinson MK, Trujillo EB, Mogensen KM, et al. Improving nutritional screening of hospitalized patients: the role of prealbumin. J Parenter Enter Nutr. 2003;27:389–395. doi: 10.1177/0148607103027006389. [DOI] [PubMed] [Google Scholar]
- 12.Bernstein LH, Leukhardt-Fairfield CJ, Pleban W, et al. Usefulness of data on albumin and prealbumin concentrations in determining effectiveness of nutritional support. Clin Chem. 1989;35:271–274. [PubMed] [Google Scholar]
- 13.Raguso CA, Dupertuis YM, Pichard C. The role of visceral proteins in the nutritional assessment of intensive care unit patients. Curr Opin Clin Nutr Metab Care. 2003;6:211–216. doi: 10.1097/00075197-200303000-00010. [DOI] [PubMed] [Google Scholar]
- 14.Parent B, Seaton M, O'Keefe GE (2016) Biochemical markers of nutrition support in critically ill trauma victims. J Parenter Enter Nutr 148607116671768 [DOI] [PMC free article] [PubMed]
- 15.Houston-Bolze MS, Downing MT, Sayed AM, et al. Gender differences in the responses of serum insulin-like growth factor-1 and transthyretin (prealbumin) to trauma. Crit Care Med. 1996;24:1982–1987. doi: 10.1097/00003246-199612000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Davis CJ, Sowa D, Keim KS, et al. The use of prealbumin and C-reactive protein for monitoring nutrition support in adult patients receiving enteral nutrition in an urban medical center. J Parenter Enter Nutr. 2012;36:197–204. doi: 10.1177/0148607111413896. [DOI] [PubMed] [Google Scholar]
- 17.Pinilla JC, Hayes P, Laverty W, et al. The C-reactive protein to prealbumin ratio correlates with the severity of multiple organ dysfunction. Surgery. 1998;124:799–805. doi: 10.1067/msy.1998.91365. [DOI] [PubMed] [Google Scholar]
- 18.Cheng V, Inaba K, Haltmeier T, et al. Serum transthyretin is a predictor of clinical outcomes in critically ill trauma patients. Surgery. 2015;158:438–444. doi: 10.1016/j.surg.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 19.6 Ventilator-associated Pneumonia (VAP) Events - 6pscvapcurrent.pdf. Available from: https://www.cdc.gov/nhsn/pdfs/pscmanual/6pscvapcurrent.pdf
- 20.9 Surgical Site Infection (SSI) Event - 9pscssicurrent.pdf. Available from: https://www.cdc.gov/nhsn/pdfs/pscmanual/9pscssicurrent.pdf.
- 21.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 22.Heydari A, Emami Zeydi A. Is gastric residual volume monitoring in critically ill patients receiving mechanical ventilation an evidence-based practice? Indian J Crit Care Med. 2014;18:259–260. doi: 10.4103/0972-5229.130588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Leary-Kelley CM, Puntillo KA, Barr J, et al. Nutritional adequacy in patients receiving mechanical ventilation who are fed enterally. Am J Crit Care. 2005;14:222–231. doi: 10.4037/ajcc2005.14.3.222. [DOI] [PubMed] [Google Scholar]
- 24.Villet S, Chiolero RL, Bollmann MD, et al. Negative impact of hypocaloric feeding and energy balance on clinical outcome in ICU patients. Clin Nutr. 2005;24:502–509. doi: 10.1016/j.clnu.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 25.Marik PE, Zaloga GP. Early enteral nutrition in acutely ill patients: a systematic review. Crit Care Med. 2001;29:2264–2270. doi: 10.1097/00003246-200112000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Heidegger CP, Berger MM, Graf S, et al. Optimisation of energy provision with supplemental parenteral nutrition in critically ill patients: a randomised controlled clinical trial. Lancet. 2013;381:385–393. doi: 10.1016/S0140-6736(12)61351-8. [DOI] [PubMed] [Google Scholar]
- 27.Ingenbleek Y, Bernstein LH. Plasma transthyretin as a biomarker of lean body mass and catabolic states. Adv Nutr. 2015;6:572–580. doi: 10.3945/an.115.008508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ortega-Deballon P, Radais F, Facy O, et al. C-reactive protein is an early predictor of septic complications after elective colorectal surgery. World J Surg. 2010;34:808–814. doi: 10.1007/s00268-009-0367-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Welsch T, Muller SA, Ulrich A, et al. C-reactive protein as early predictor for infectious postoperative complications in rectal surgery. Int J Colorectal Dis. 2007;22:1499–1507. doi: 10.1007/s00384-007-0354-3. [DOI] [PubMed] [Google Scholar]
- 30.Hoeboer SH, Groeneveld AB, Engels N, et al. Rising C-reactive protein and procalcitonin levels precede early complications after esophagectomy. J Gastrointest Surg. 2015;19:613–624. doi: 10.1007/s11605-015-2745-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kano K, Aoyama T, Nakajima T, et al. Prediction of postoperative inflammatory complications after esophageal cancer surgery based on early changes in the C-reactive protein level in patients who received perioperative steroid therapy and enhanced recovery after surgery care: a retrospective analysis. BMC Cancer. 2017;17:812. doi: 10.1186/s12885-017-3831-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adamina M, Steffen T, Tarantino I, et al. Meta-analysis of the predictive value of C-reactive protein for infectious complications in abdominal surgery. Br J Surg. 2015;102:590–598. doi: 10.1002/bjs.9756. [DOI] [PubMed] [Google Scholar]
- 33.Bakker J, Nijsten MW, Jansen TC. Clinical use of lactate monitoring in critically ill patients. Ann Intensive Care. 2013;3:12. doi: 10.1186/2110-5820-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fuller BM, Dellinger RP. Lactate as a hemodynamic marker in the critically ill. Curr Opin Crit Care. 2012;18:267–272. doi: 10.1097/MCC.0b013e3283532b8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joshi R, de Witt B, Mosier JM. Optimizing oxygen delivery in the critically ill: the utility of lactate and central venous oxygen saturation (ScvO2) as a roadmap of resuscitation in shock. J Emerg Med. 2014;47:493–500. doi: 10.1016/j.jemermed.2014.06.016. [DOI] [PubMed] [Google Scholar]