Abstract
Applying lung protective mechanical ventilation (LPV) during general anaesthesia even in patients with non-injured lungs is recommended. However, the effects of an individual PEEP-optimisation on respiratory mechanics, oxygenation and their potential correlation with the inflammatory response and postoperative complications have not been evaluated have not been compared to standard LPV in patients undergoing major abdominal surgery. Thirty-nine patients undergoing open radical cystectomy were enrolled in this study. In the study group (SG) optimal PEEP was determined by a decremental titration procedure and defined as the PEEP value resulting the highest static pulmonary compliance. In the control group (CG) PEEP was set to 6 cmH2O. Primary endpoints were intraoperative respiratory mechanics and gas exchange parameters. Secondary outcomes were perioperative procalcitonin kinetics and postoperative pulmonary complications. Optimal PEEP levels (median = 10, range: 8–14 cmH2O), PaO2/FiO2 (451.24 ± 121.78 mmHg vs. 404.15 ± 115.87 mmHg, P = 0.005) and static pulmonary compliance (52.54 ± 13.59 ml cmH2O-1 vs. 45.22 ± 9.13 ml cmH2O-1, P < 0.0001) were significantly higher, while driving pressure (8.26 ± 1.74 cmH2O vs. 9.73 ± 4.02 cmH2O, P < 0.0001) was significantly lower in the SG as compared to the CG. No significant intergroup differences were found in procalcitonin kinetics (P = 0.076). Composite outcome results indicated a non-significant reduction of postoperative complications in the SG. Intraoperative PEEP-optimization resulted in significant improvement in gas exchange and pulmonary mechanics as compared to standard LPV. Whether these have any effect on short and long term outcomes require further investigations. Trial registration: Clinicaltrials.gov, identifier: NCT02931409.
Keywords: Lung protective ventilation, Positive end-expiratory pressure, Respiratory mechanics, Procalcitonin, Inflammatory response
Introduction
Ventilator induced lung injury (VILI) is the result of physical and biological injury of the lungs. The former is due to volu-, baro-, atelecto-trauma, the latter is caused by surfactant aggregation and inactivation, harmful local inflammatory response and damage of the pulmonary extracellular matrix. These can lead to postoperative pulmonary and consequent extrapulmonary complications that is a common risk of mechanical ventilation not just in critically ill patients ventilated with injured lung but also during general anaesthesia [1, 2]. Indeed, previously conducted trials over the past decades identified the main surgical, anaesthesia-, and patient-related risk factors and the pathophysiology of VILI resulting postoperative pulmonary complications (PPC) [3–6].
The main pathophysiological risk factors are excessive lung stress due to high transpulmonary and driving pressures (ΔP); extensive lung strain characterized by destructive cyclic closing and opening of small airways; and induction of local and systemic inflammatory response [4]. The main inflammatory cytokines and interleukins (IL) involved in this mechanism are tumor necrosis factor-alpha (TNF-α), nuclear factor kappa-beta (NF-κβ), IL-6, IL-8 and IL-1β, surfactant protein-D, receptor for advanced glycation end-products (RAGE) and club cell secretory protein (CC-16). Measuring the level of these proinflammatory molecules is challenging, cumbersome and expensive, however it has been shown by several studies that these induce procalcitonin (PCT)—a commonly used inflammatory marker -, production and release [7–9]. Therefore, it has some rationale to monitor PCT values in order to evaluate their potential correlation with the development of VILI [10–16].
There is convincing evidence to recommend the use of LPV applying low tidal volumes (TV = 6 ml kg−1 of Ideal Body Weight, IBW), optimal positive end-expiratory pressure (PEEP) and regular alveolar recruitment manoeuvres (ARM) during general anaesthesia even in patients with non-injured lungs [17–21]. Applying individual PEEP titrated during a decremental procedure after an ARM in order to optimize respiratory mechanics is the key to avoid hyperinflation of the lungs and even to prevent or reverse atelectasis and to achieve the so called open lung approach (OLA) [22–25]. The main advantages of protective OLA ventilation are improved respiratory mechanics and gas exchange, and prevention from VILI. These anticipated advantages may also improve postoperative recovery and survival rates, shorten in-hospital stay and reduce healthcare related costs. However, inappropriate PEEP values may lead to decreased pulmonary compliance and gas exchange disorders due to pulmonary atelectasis and/or hyperinflation of the lungs [20]. Additionally, results of recent trials suggested the use of moderate PEEP values (5–6 cmH2O) against low or high PEEP values. However, the effect of applying an individually titrated optimal PEEP (PEEPopt) on respiratory mechanics, oxygenation and even on the inflammatory response, and its correlation with postoperative complications has not entirely been evaluated yet. As radical cystectomy is considered major abdominal surgery and associated with high rates (50–72%) of postoperative complications [26–29] we decided to investigate this patient population. The purpose of this physiological trial was to compare the effects of a standard LPV applying a 6 cmH2O of PEEP with a LPV using an individually titrated PEEPopt on respiratory mechanics and oxygenation.
Methods
This investigator-initiated, double-centre, single-blinded (subject), interventional, prospective, randomized controlled trial (RCT) was approved by the Hungarian Scientific and Medical Research Council Ethics Committee (21,586–4/2016/EKU, on 17 June 2016), the Local Ethics Committee of Péterfy Sándor Hospital Budapest (CO-338–045, on 12 September 2016) and the Regional Ethics Committee of the University of Szeged (149/2016-SZTE, on 19 September 2016). This study was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants prior to inclusion.
Patient selection
Patients with bladder cancer scheduled for open radical cystectomy and urinary diversion (ileal conduit or orthotopic bladder substitute) were screened and recruited during standard institutional perioperative assessment. Patient’s medical history, laboratory, chest X-ray or CT scan results, 12-lead ECG, ASA physical status, body mass index (BMI), risk of postoperative respiratory failure regarding to the Respiratory Failure Risk Index (RFRI), nutritional indicators using the Nutrition Risk Screening 2002 tool and if required results of spirometry, echocardiography and ergometry were evaluated, in order to determine the individual surgical risk and overall eligibility for radical cystectomy.
Inclusion criteria were age over 18 years, scheduled for open radical cystectomy and urinary diversion (ileal conduit or orthotopic bladder substitute) due to bladder cancer and signed consent to participate in the trial. Exclusion criteria were age below 18 years, ASA physical status IV, history of severe restrictive or chronic obstructive pulmonary disease (COPD, GOLD grades III or IV), uncontrolled bronchial asthma, pulmonary metastases, history of any thoracic surgery, need for thoracic drainage before surgery, renal replacement therapy prior to surgery, congestive heart failure (NYHA grades III or IV), extreme obesity (BMI > 35 kg m−2) and lack of patient’s consent. Participants were randomized and allocated to the Study Group (SG) or Control Group (CG) in a ratio of 1:1 using a computer-generated blocked randomization list. Data were recorded on participants’ Case Report Files.
Study arms and assigned intraoperative interventions
Patients randomized into the SG underwent a Cstat directed decremental PEEP titration procedure after induction of anaesthesia: PEEP was decreased from 14 cmH2O by 2 cmH2O every 4 min, until a final PEEP of 6 cmH2O. On each level of PEEP mean Cstat values were recorded and arterial blood gas samples (ABGs) were collected and evaluated. PEEPopt was considered as the PEEP value resulting the highest possible Cstat measured by the ventilator. After PEEP titration procedure, LPV was performed applying PEEPopt. An ARM using the sustained airway pressure by the CPAP method (30 cmH2O PEEP for 30 s) was performed immediately after endotracheal intubation and repeated every 60 min during surgery.
Patients in CG group underwent an ARM immediately after endotracheal intubation followed by low tidal volumes LPV using a PEEP value of 6 cmH2O. ARM were repeated every 60 min during surgery.
The details of perioperative care are summarised in Table 1.
Table 1.
Protocolized perioperative care and procedures
| Preoperative period |
|---|
| Central venous catheter insertion followed by a chest X-ray in order to evaluate catheter position and exclude any insertion-related complications |
| Blood sampling to measure participant’s baseline PCT levels |
| Deep vein thrombosis prophylaxis (enoxaparine) |
| Antimicrobial prophylaxis (ciprofloxacin and metronidazole) |
| Oral carbohydrate loading (maltodextrin) |
| Intraoperative period |
| General anaesthesia combined with lumbar epidural analgesia |
| Lung protective ventilation applying FiO2 of 50% in both groups |
| Continuous invasive arterial blood pressure monitoring |
| Continuous capnography and heart rate monitoring |
| Respiratory mechanics parameters (static pulmonary compliance, airway resistance, dead space fraction) data recording every 15 min |
| Core temperature and train-of-four relaxometry data recording every 15 min |
| Regular ABG and CVBG sampling every 60 min |
| Maintenance fluid: 3 ml kg−1 h−1 of balanced crystalloid solution until the end of surgery |
| Rescue fluid: 200 ml of colloid solution bolus (hydroxyethyl starch) and crystalloid substitution in case of bleeding |
| Transfusion: PRBC transfusion, whenever the attending anaesthetist rendered it necessary |
| Vasopressor treatment: intravenous norepinephrine to maintain MAP above 65 mmHg |
| PCT sampling: 2 and 6 h after surgical incision intraoperatively |
| Postoperative period (POD1-3) |
| Continuous epidural analgesia combined with intravenous analgesics |
| Continuous intraabdominal pressure monitoring |
| Intravenous and oral fluid supplementation and if required, further transfusion |
| Oral clear fluids immediately after surgery |
| Removal of nasogastric tube at the latest on POD1 in the morning |
| Prokinetics and an oral liquid diet from POD1 |
| Active mobilization with the help of a physiotherapist from POD1 |
| Evaluation of patient’s ABG, CVBG, PaO2/FiO2 and dCO2 every 6 h from POD1 to POD3 |
| Evaluation of PCT levels at 12, 24, 48 and 72 h after surgical incision |
| Chest X-ray (evaluated by an independent trained radiologist who was not be involved in the study) on POD1, POD2 and POD3 |
| Monitoring of patients' clinical progress and secondary endpoints by daily SOFA scores, laboratory and physical examinations |
| Follow-up period (POD4-28) |
| Evaluation of secondary endpoints, in-hospital stay, 28-days and in-hospital mortality |
PCT procalcitonin; FiO2 fractional inspired oxygen; ABG arterial blood gas sample; CVBG central venous blood gas sample; PRBC packed red blood cells; MAP mean arterial pressure; POD postoperative day; PaO2/FiO2 ratio of arterial oxygen partial pressure to fractional inspired oxygen; dCO2 central venous-to-arterial carbon dioxide difference; PPC postoperative pulmonary complications; SOFA sequential organ failure assessment
Outcomes
The primary outcome variables were intraoperative respiratory mechanics and gas exchange parameters, as indicated by Cstat and PaO2/FiO2 determined at the end of surgery.
Secondary outcomes were early PCT kinetics, hypoxaemia (PaO2/FiO2 < 300 mmHg) within the first 3 postoperative days (POD) and postoperative organ dysfunctions: incidence of circulatory failure, gastrointestinal and renal dysfunction, hematologic and coagulation disorders and infections within POD1-28 (Table 2). Blood samples were collected at 0, 2, 6, 12, 24, 48 and 72 h after surgical incision, in order to evaluate PCT kinetics and the changes of absolute values between T0-T24-T48. Tertiary endpoints were ICU days, in-hospital stay, in-hospital and 28-days mortality.
Table 2.
Secondary endpoints
| Endpoint | Time frame | Detailed description |
|---|---|---|
| Hypoxaemia | 3 days | PaO2/FiO2 < 300 mmHg |
| Circulatory failure | 28 days |
Hypotension—MAP < 65 mmHg Severe cardiac arrhythmia—40/min < HR > 150/min ScvO2 < 70% dCO2 > 7 mmHg Serum lactate > 2 mmol/L Severe metabolic acidosis (actual bicarbonate < 18 mmol/L) Acute coronary syndrome Acute left ventricular failure Pulmonary embolism Cardiac arrest |
| Gastrointestinal dysfunction | 28 days |
Constipation Ileus Anastomotic leakage Reoperation Disorders of liver function |
| Renal dysfunction | 28 days | RIFLE criteria |
| Hematologic and coagulation disorders | 28 days |
Severe bleeding Coagulopathy—INR > 1.5 |
| Infection | 28 days | Any infection except from pneumonia |
PaO2/FiO2 ratio of arterial oxygen partial pressure to fraction of inspired oxygen; MAP mean arterial pressure; HR heart rate; ScvO2 central venous oxygen saturation; dCO2 arterial to central venous carbon dioxide difference; INR international normalized ratio
Statistical analysis
Primary endpoints of the study were the difference in the intraoperative Cstat values and PaO2/FiO2 ratios. Based on preliminary results of two recent clinical studies in which the effects of intraoperative recruiting manoeuvres on compliance and the PaO2/FiO2 ratio were investigated [22, 25], their sample size calculation was 13 patients per group. We estimated that to show a similar clinically significant effect (i.e.: 25% improvement in compliance with a SD of 8.9 and improvement of PaO2/FiO2 by 115 mmHg with a SD of 125) for a study to have 80% power to show a significant difference in the primary endpoints, a minimum of 30 patients in total (15 per group) were required. To allow for dropout, we decided to randomize 20 patients in each group.
Statistical analysis was conducted on an intention-to-treat basis. Data distribution was tested by the Kolmogorov–Smirnov analysis. Normally distributed data are presented as mean and SD and skewed data as median (interquartile range, IQR). Comparing related samples, the paired and unpaired t test were used for normally distributed data and the Wilcoxon signed rank test and Mann–Whitney U test for skewed data. Differences in proportions were evaluated using the Fisher’s exact test, and risk ratio with associated 95% CI. Analysis of the primary endpoint (PPC) was carried out by the unpaired Student t test. Two-way repeated-measures analysis of variance (2-way RM ANOVA) was used to compare the groups serum PCT levels. Relationship between PCT levels and organ dysfunctions was evaluated using the Pearson’s correlation. Statistical analysis of SOFA scores, ICU days, in-hospital stay, in-hospital and 28-days mortality data of groups were implemented by the χ2 test. P value of less than 0.05 was considered statistically significant. MedCalc Statistical Software v14.8.1 (MedCalc Software bvba, Ostend, Belgium) was used for statistical analysis.
Results
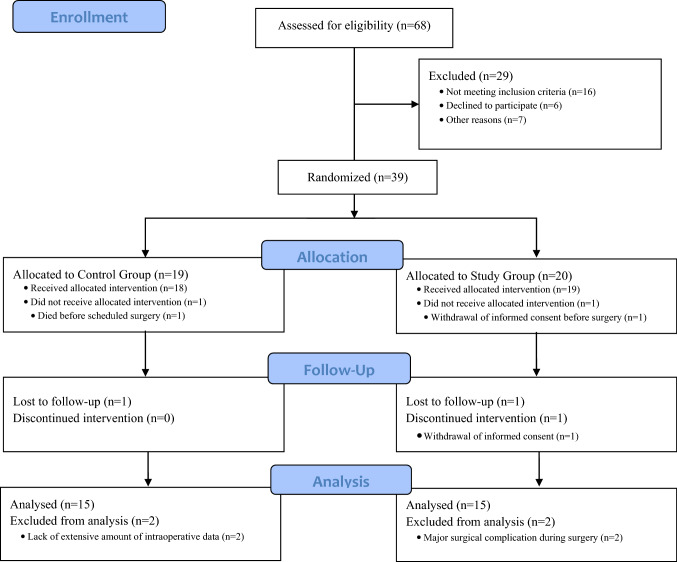
Of 68 patients who were assessed for eligibility, 39 patients were randomized, and 30 patients completed the study (Fig. 1). The baseline clinical characteristics and demographic data of the groups were comparable (Table 3). Participants’ ARISCAT Scores for PPC were calculated retrospectively.
Fig. 1.
CONSORT (Consolidated Standards of Reporting Trials) flow diagram showing the progress of participants during the trial
Table 3.
Demographic data and clinical characteristics
| CG (n = 15) | SG (n = 15) | P value | |
|---|---|---|---|
| Male sex (n) | 13 (86.7) | 13 (86.7) | 1.000 |
| Age (years) | 61.47 (7.37) | 64.27 (7.03) | 0.245 |
| ASA physical status | |||
| 1 | 1 (6.7) | 1 (6.7) | |
| 2 | 12 (80.0) | 12 (80.0) | |
| 3 | 2 (13.3) | 2 (13.3) | |
| RFRI (%) | 2.57 [2.05–3.57] | 2.78 [2.09–3.78] | 0.479 |
| ARISCAT score | 45.67 [42.47–50.46] | 44.4 [41.88–47.51] | 0.644 |
| BMI (kg m−2) | 27.42 (4.00) | 27.66 (2.58) | 0.829 |
| IBW (kg) | 67.33 (8.79) | 67.44 (9.52) | 0.971 |
| Duration of anaesthesia (min) | 384.00 (107.01) | 418.2 (70.49) | 0.342 |
| Duration of surgery (min) | 352.47 (103.58) | 378.00 (63.52) | 0.442 |
| Type of surgery | |||
| Ileal conduit | 13 (86.7) | 10 (66.7) | 0.208 |
| Orthotopic bladder substitute | 0 (0) | 4 (26.7) | 0.105 |
| Intraoperative inoperablea | 2 (13.3) | 1 (6.6) | 0.551 |
| PEEP during surgery (cmH2O) | |||
| 6 | 15 (100.0) | 0 (0.0) | |
| 8 | 7 (46.7) | ||
| 10 | 6 (40.0) | ||
| 12 | 1 (6.65) | ||
| 14 | 1 (6.65) |
Data are expressed as number n (%), mean (SD) or median [IQR]
ASA American Society of Anesthesiologists physical status classification; RFRI Respiratory Failure Risk Index (Gupta); ARISCAT Score Assess Respiratory Risk in Surgical Patients in Catalonia; BMI body mass index, IBW ideal body weight (calculation was based on the ARMA Trial of the ARDS Network Investigators); PEEP positive end-expiratory pressure; SD standard deviation; IQR interquartile range
aDue to intraoperatively observed intraabdominal status or excessive propagation of bladder tumor, only radical cystectomy and ureterocutaneostomy was performed without ileal conduit
Italics value indicates number of subjects or number of events
PEEPopt levels were higher in SG than in CG (Table 3). The PaO2/FiO2, Cstat, together with all other intraoperative respiratory mechanics parameters were significantly better in SG (Table 4).
Table 4.
Intraoperative respiratory mechanics and oxygenation
| CG (n = 15) | SG (n = 15) | P value | |
|---|---|---|---|
| PaO2/FiO2 (mmHg) | 404.15 (115.87) | 451.24 (121.78) | 0.005 |
| Cstat (ml cmH2O−1) | 45.22 (9.13) | 52.54 (13.59) | < 0.0001 |
| Vds/Vt (%) | 23.05 [20.05–25.50] | 21.14 [17.94–24.93] | 0.001 |
| Raw (cmH2O L−1 s−1) | 6.84 (2.39) | 5.86 (1.31) | < 0.0001 |
| P (cmH2O) | 9.73 (4.02) | 8.26 (1.74) | < 0.0001 |
| Respiratory rate (min−1) | 16.04 [14.04–16.75] | 17.07 [15.01–18.87] | 0.0001 |
| EtCO2 (mmHg) | 37.63 [36.23–38.16] | 38.00 [36.96–39.52] | 0.017 |
| (a-Et)PCO2 (mmHg) | 7.25 (0.92) | 5.76 (1.39) | 0.007 |
Data are expressed as mean (SD) or median [IQR]
Cstat static pulmonary compliance; Vds/Vt dead space fraction; Raw airway resistance; △P driving pressure; EtCO2 end-tidal carbon dioxide tension; (a-Et)PCO2 arterial to end-tidal carbon dioxide difference; PaO2/FiO2 ratio of arterial oxygen partial pressure to fraction of inspired oxygen; SD standard deviation; IQR interquartile range
We found no significant differences between intraoperative haemodynamic parameters, fluid administration and transfused units of PRBC of groups, however norepinephrine requirements in SG were significantly higher (Table 5).
Table 5.
Intraoperative haemodynamic parameters and management
| CG (n = 15) | SG (n = 15) | P value | |
|---|---|---|---|
| MAP (mmHg) | 79 [72–84] | 76 [71–83.25] | 0.040 |
| HR (min−1) | 74 [67–82] | 72 [61–85] | 0.062 |
| ScvO2 (%) | 86.8 [82.95–89.98] | 85.9 [81.90–89.30] | 0.248 |
| dCO2 (mmHg) | 6.3 [4.75–7.98] | 6.65 [4.90–8.05] | 0.724 |
| Lactate (mmol l−1) | 1.1 [0.83–1.50] | 1.2 [0.98–1.40] | 0.277 |
| pH | 7.33 (0.04) | 7.32 (0.04) | 0.307 |
| stHCO3− (mmol l−1) | 22.70 (1.42) | 21.83 (1.52) | 0.0002 |
| Fluid management | |||
| Crystalloids (ml) | 2212.53 (1102.16) | 2331.53 (889.49) | 0.775 |
| Colloids (ml) | 433.33 (225.72) | 573.33 (194.45) | 0.078 |
| Fluids (ml kg−1 h−1) | 3.99 [3.08–4.63] | 4.41 [3.37–5.06] | 0.646 |
| ∑ Fluids (ml) | 3765.87 (1218.72) | 3931.53 (1006.09) | 0.745 |
| Urine output (ml) | 1051.33 (423.39) | 1023.33 (606.47) | 0.741 |
| Blood loss (ml) | 1000.0 (622.5) | 1250.0 (882.5) | 0.125 |
| Fluid balance (ml) | 1702.4 (1054.42) | 1566.73 (1071.56) | 0.761 |
| PRBC units transfused (U) | 2 [0–2] | 2 [0–2] | 0.859 |
| 0 U | 7 (46.7) | 7 (46.7) | 1.000 |
| 1–3 U | 6 (40.0) | 5 (33.3) | 0.705 |
| > 3 U | 2 (13.3) | 3 (20.0) | 0.626 |
| Norepinephrine (mcg min−1) | 3 [0–5] | 7 [3–14] | < 0.0001 |
| ∑ Norepinephrine (mg) | 1.29 [0.40–2.85] | 2.8 [1.99–5.01] | 0.006 |
Data are expressed as number n (%), mean (SD) or median [IQR]
MAP mean arterial pressure; HR heart rate; ScvO2 central venous oxygen saturation; dCO2 arterial to central venous carbon dioxide difference; stHCO3− arterial standard bicarbonate; PRBC packed red blood cells; U unit; SD standard deviation; IQR interquartile range
Italics value indicates number of subjects or number of events
For secondary outcomes, postoperative PaO2/FiO2 values from the end of surgery (POD0) within the first three POD were higher in SG, however these differences were not significant (298.67 ± 44.48 mmHg vs. 307.60 ± 48.22 mmHg, OR:0.63, 95% CI 0.25 to 1.63, P = 0.342). There were no significant intergroup differences neither in haemodynamic and metabolic results, nor in IAP values, fluid balance and transfusion requirements, however serum blood urea nitrogen and creatinine levels were significantly lower and daily urine output was significantly higher in CG indicating a higher incidence of postoperative renal dysfunction in SG (Table 6). In contrast, intergroup comparison of renal complications based on RIFLE Criteria proved no significant difference (34 vs. 41, OR: 1.31, 95% CI 0.81–2.10, P = 0.277).
Table 6.
Postoperative results on POD1 to POD3
| POD1 | POD2 | POD3 | Composite results | P value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CG (n = 15) | SG (n = 15) | CG (n = 15) | SG (n = 15) | CG (n = 15) | SG (n = 15) | CG (n = 15) | SG (n = 15) | OR (95% CI) | ||
| Oxygenation | ||||||||||
| PaO2/FiO2 (mmHg) | 277.75 (72.79) | 299.85 (79.61) | 279.77 (81.43) | 270.02 (70.63) | 310.31 (87.98) | 311.83 (70.10) | 298.67 (44.68) | 307.60 (48.22) | 0.63 (0.25–1.63) | 0.342 |
| Haemodynamic data | ||||||||||
| MAP (mmHg) | 80.27 (14.06) | 79.97 (15.14) | 88.50 (13.84) | 83.07 (16.79) | 89.67 (10.57) | 84.13 (14.45) | 75.20 (10.97) | 72.39 (11.74) | 1.19 (0.52–2.73) | 0.673 |
| HR (min−1) | 84.13 (15.83) | 82.07 (18.91) | 80.00 (21.0) | 83.50 (20.0) | 80.67 (10.39) | 81.97 (14.24) | 82.23 (9.21) | 82.82 (11.57) | 1.12 (0.44–2.90) | 0.809 |
| ScvO2 (%) | 71.54 (7.56) | 70.67 (7.72) | 70.45 (5.89) | 70,78 (5.73) | 71.31 (6.11) | 70.49 (6.89) | 71.64 (4.47) | 70.94 (5.15) | 0.89 (0.36–2.25) | 0.814 |
| dCO2 (mmHg) | 6.58 (2.92) | 7.04 (2.58) | 6.68 (2.75) | 6.02 (2.41) | 6.25 (2.06) | 5.73 (1.96) | 5.96 (2.59) | 5.76 (2.38) | 0.43 (0.17–1.08) | 0.072 |
| Lactate (mmol l−1) | 1.28 (0.45) | 1.58 (0.74) | 1.09 (0.38) | 1.25 (0.65) | 1.22 (0.48) | 1.02 (0.33) | 1.20 (0.44) | 1.28 (0.63) | 3.72 (1.09–12.64) | 0.057 |
| pH | 7.43 (0.04) | 7.42 (0.05) | 7.43 (0.03) | 7.44 (0.05) | 7.42 (0.02) | 7.43 (0.03) | 7.43 (0.03) | 7.43 (0.04) | 0.33 (0.01–8.22) | 0.496 |
| stHCO3− (mmol l−1) | 25.19 (2.17) | 24.48 (2.77) | 25.14 (2.56) | 25.84 (2.72) | 24.71 (2.45) | 25.07 (1.88) | 25.02 (2.38) | 25.13 (2.53) | 1.05 (0.47–2.95) | 0.705 |
| IAP (mmHg) | 12.99 (6.19) | 11.69 (5.63) | 13.86 (7.93) | 12.08 (5.12) | 12.16 (6.68) | 11.75 (3.97) | 13.03 (6.92) | 11.84 (4.92) | 0.45 (0.23–0.87) | 0.062 |
| Fluid management | ||||||||||
| Crystalloids (ml) | 3000 [2500–3587] | 3000 [2700–3000] | 2700 [2000–3262] | 2500 [1650–3325] | 2500 [1500–2975] | 1600 [1500–2075] | 2800 [2000–3187] | 2300 [1600–3000] | 0.314 | |
| Colloids (ml) | 200 [0–200] | 400 [300–450] | 0 [0–0] | 0 [0–100] | 0 [0–0] | 0 [0–0] | 0 [0–0] | 0 [0–25] | 0.083 | |
| Oral intake (ml) | 1200 [1100–1925] | 800 [525–1100] | 1300 [1050–2000] | 1400 [875–1738] | 2000 [1325–2425] | 1500 [1225–2000] | 1400 [1100–2000] | 1230 [750–1850] | 0.089 | |
| Urine output (ml) | 3050 [2125–4150] | 2460 [2125–2900] | 3800 [2745–4538] | 2800 [2713–3425] | 3700 [3050–4185] | 2600 [2150–3175] | 3600 [2835–4300] | 2750 [2275–3212] | 0.001 | |
| Blood loss (ml) | 50 [0–200] | 100 [0–200] | 0 [0–0] | 0 [0–0] | 0 [0–0] | 0[0–0] | 0 [0–0] | 0 [0–12.5] | 0.685 | |
| Fluid balance (ml) | 800 [490–2185] | 1700 [660–1890] | 200 [-640 to 1580] | 830 [-62 to 2125] | 300 [-12 to 950] | 800 [-25 to 1575] | 460 [-100 to 1532] | 1200 [162–1938] | 0.114 | |
| PRBC units transfused | ||||||||||
| 0 U | 9 (60) | 9 (60) | 10 (67) | 10 (67) | 15 (100) | 11 (73) | 34 (76) | 30 (67) | 1.55 (0.62–3.88) | 0.354 |
| 1–3 U | 6 (40) | 6 (40) | 4 (27) | 5 (33) | 0 | 4 (27) | 10 (22) | 15 (33) | 0.57 (0.22–1.46) | 0.242 |
| > 3 U | 0 | 0 | 1 (6) | 0 | 0 | 0 | 1 (2) | 0 (0) | 0.33 (0.01–8.22) | 0.496 |
| Laboratory results | ||||||||||
| Platelet count (G l−1) | 197 (57.57) | 191 (40.92) | 183 (56.47) | 163 (38.47) | 186 (63.93) | 162 (45.99) | 189 (58.41) | 172 (42.97) | 1.12 (0.52–3.25) | 0.814 |
| Bilirubin (μmol l−1) | 10.4 (3.09) | 16.6 (13.08) | 7.9 (2.36) | 12.3 (9.90) | 8.5 (2.33) | 10.7 (6.54) | 8.9 (2.77) | 13.2 (10.28) | 5.50 (0.62–19.11) | 0.127 |
| Creatinine (μmol l−1) | 102 [83.50–132.75] | 131 [94.00–180.75] | 94 [80.00–123.75] | 136 [88.50–163.00] | 92.0 [79.25–128.50] | 124.0 [73.25–157.25] | 94 [80.00–128.25] | 131 [88.75–166.50] | 2.05 (0.89–4.75) | 0.022 |
| BUN (mmol l−1) | 4.9 [3.9–5.9] | 5.1 [4.3–8.8] | 4.4 [3.4–5.4] | 5.3 [3.5–7.6] | 4.8 [3.9–5.5] | 5.1 [4.5–7.9] | 4.6 [3.8–5.3] | 5.1 [4.3–7.9] | 3.25 (0.61–6.52) | 0.044 |
Data are expressed as number n (%), mean (SD) or median [IQR]
POD postoperative day; CG control group; SG study group; OR odds ratio; PaO2/FiO2 ratio of arterial oxygen partial pressure to fractional inspired oxygen; MAP mean arterial pressure; HR heart rate; ScvO2 central venous oxygen saturation; dCO2 arterial to central venous carbon dioxide difference; stHCO3− arterial standard bicarbonate; IAP intraabdominal pressure; PRBC packed red blood cells; U unit; BUN blood urea nitrogen; SD standard deviation; IQR interquartile range
Italics value indicates number of subjects or number of events
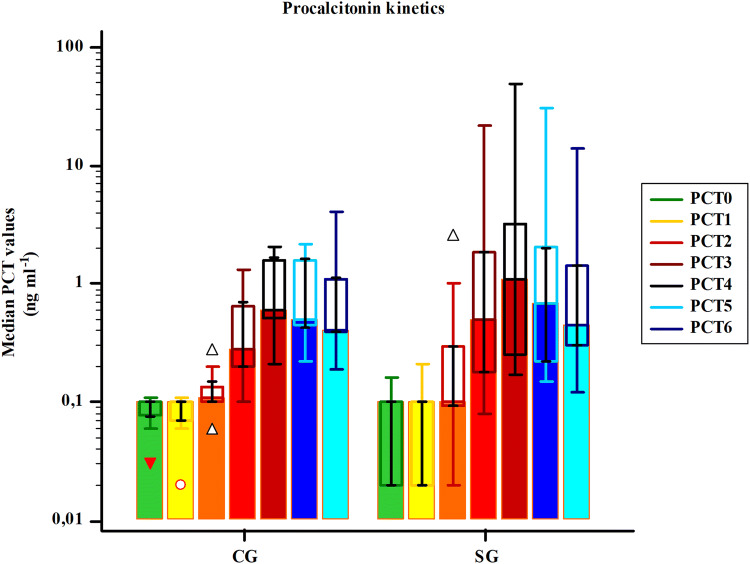
A six-fold increase in CG and a 6.7-fold increase in SG from baseline PCT levels were observed at the end of the first 24 h (POD0), followed by a 16.7% decrease on POD1 and a further 14% decrease on POD2 in CG. Decrease in PCT values in SG on POD1 was 19.5%, followed by a 26.3% decrease on POD2 (Fig. 2). However, no significant differences were found in PCT kinetics in the early postoperative period between groups (F = 2.82, P = 0.076). In contrast, the absolute PCT values of subjects were significantly different (F = 107.5, P < 0.001).
Fig. 2.
Median procalcitonin values indicating procalcitonin kinetics of groups. PCT procalcitonin; PCT0 baseline; PCT1 2 h after surgical incision; PCT2 6 h; PCT3 12 h; PCT4 24 h; PCT5 48 h; PCT6 72 h
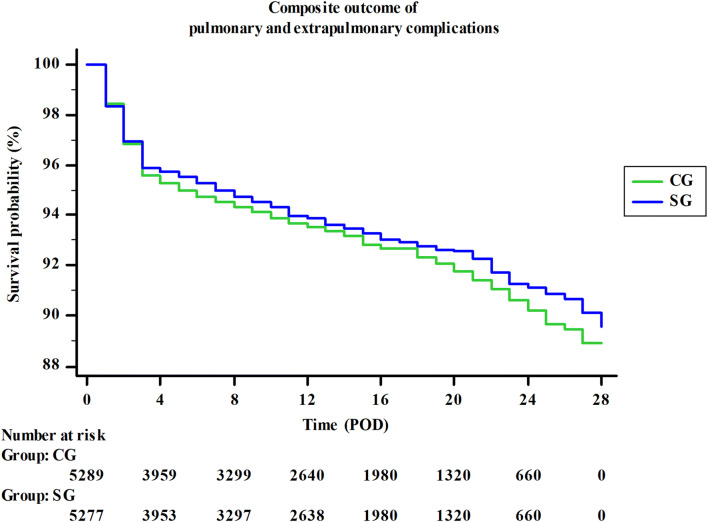
Except from gastrointestinal disorders and infections, there were no significant differences in secondary outcomes between groups (Table 7). Composite outcome results indicated a slight (0.5%), but not significant reduction of postoperative complications in SG (OR: 0.93, 95% CI 0.79–1.07, P = 0.295, Fig. 3). There were no significant differences in ICU and in-hospital length of stay between the groups. One patient in SG died on POD5 due to massive gastrointestinal bleeding originated from gastric stress ulcer, but it was considered not to be a result of group’s assigned intervention, and mortality data analysis proved also no significant difference (Table 7).
Table 7.
Outcome results
| CG (n = 15) | SG (n = 15) | OR (95% CI) | P value | |
|---|---|---|---|---|
| Secondary outcome | ||||
| PaO2/FiO2 (mmHg) | 298.67 (44.68) | 307.60 (48.22) | 0.63 (0.25–1.63) | 0.342 |
| Circulatory | 126 (3.0) | 141 (3.4) | 1.15 (0.91–1.48) | 0.249 |
| Gastrointestinal | 128 (7.6) | 90 (5.7) | 0.73 (0.56–0.97) | 0.026 |
| Renal | 34 (8.1) | 41 (10.3) | 1.31 (0.83–2.16) | 0.270 |
| Haematologic | 20 (2.4) | 17 (2.1) | 0.89 (0.45–1.68) | 0.745 |
| Infection | 7 (0.5) | 18 (1.5) | 3.03 (1.26–7.28) | 0.013 |
| Tertiary outcome | ||||
| ICU length of stay (days) | 4 [3, 4] | 3 [2–4] | 0.33 (0.08–1.48) | 0.108 |
| In-hospital stay (days) | 20.20 (13.08) | 18.23 (11.45) | 0.94 (0.21–4.29) | 0.678 |
| Mortality | 0 (0.0) | 1 (6.7) | 3.21 (0.12–85.20) | 0.486 |
| Composite outcome | 372 (7.1) | 350 (6.6) | 0.94 (0.81–1.09) | 0.396 |
Data are expressed as number n (%), mean (SD) or median [IQR]
CG control group; SG study group; OR odds ratio; PaO2/FiO2 ratio of arterial oxygen partial pressure to fraction of inspired oxygen; ICU intensive care unit
Italics value indicates number of subjects or number of events
Fig. 3.
Composite outcome for postoperative complications. Composite outcome results indicated a slight, but not significant decrease in postoperative complications in SG as compared to CG. POD postoperative day; CG control group; SG study group
Discussion
Despite many efforts and promising results of recent research, postoperative complications remained a worldwide healthcare problem after major abdominal surgery [30–34]. Open radical cystectomy with urinary diversion (ileal conduit or orthotopic bladder substitute) is considered major abdominal surgery and associated with high rates of postoperative complications: at least 50–72% of patients develop complications [26–29], of which approximately 6% are PPC [27, 35]. As inappropriate mechanical ventilation may lead to VILI resulting tissue oxygenation disorders leading pulmonary and extrapulmonary organ dysfunctions, it has some rationale that improved intraoperative respiratory mechanics and gas exchange may reduce the incidence of postoperative complications.
The purpose of our investigator-initiated, interventional, prospective, RCT was to assess the effects of an individualized intraoperative LPV on intraoperative respiratory mechanics, oxygenation and their potential correlation with the inflammatory response following open radical cystectomy and urinary diversion. Regarding the primary outcomes of respiratory mechanics and gas exchange we found significant differences in favour of the SG as compared to the CG.
In 1963, Bendixen et al. found that higher TV during anaesthesia resulted in less atelectasis and acidosis with improved oxygenation compared to lower TV [36]. Based on their results, 10–15 ml kg−1 TV during mechanical ventilation was recommended almost for 50 years. Ashbaugh et colleagues described acute respiratory distress syndrome (ARDS) in 1967, however potential harms of high TV were only recognized in the 1970s and 1980s [37]. Amato et al. suggested the use of low TV ventilation in ARDS patients in 1998, but protective ventilatory management as the standard of care was only recommended after the ARMA Trial conducted by the ARDS Network Investigators in 2000 [38, 39].
A meta-analysis of 20 studies carried out by Serpa Neto et al. in 2012 indicated decreased risk of lung injury and mortality with the use of LPV in patients without ARDS [40]. Since Futier and colleagues published the results of IMPROVE Trial in 2013, intraoperative LPV has gained increasing interest and importance during general anaesthesia in routine anaesthetic care [5, 17, 41, 42]. The use of low TV (6 ml kg−1 of IBW) became common in intraoperative settings, however the so called intraoperative open lung approach (OLA) applying ARM and appropriate levels of PEEP remained controversial [5, 43–45]. Although Zaky et al. proved that applying PEEP and regular ARM during general anaesthesia improved aeration of the lungs, results of the PROVHILO Trial suggested that OLA strategy with a high level of PEEP and regular ARM during open abdominal surgery does not protect against PPC, or even may worsen outcomes due to an increased risk of intraoperative hypotension and higher vasopressor requirements [46, 47]. Additionally, Ferrando et al. compared three types of individualized OLA strategies to standard LPV in a multicentre RCT in Spain. They have not found any difference on outcomes between the OLA strategies, however PEEP had to be increased in 14% of patients in the standard LPV group due to intraoperative hypoxaemia [48].
Research about the effects of individual LPV applying PEEPopt levels has provided a new direction over the past decade [49–51]. Titrating PEEP to achieve individual optimal levels has a strong pathophysiological rationale with potential benefits. Spadaro et al. found that the increased pulmonary shunt induced by general anaesthesia may be reduced only with the use of higher PEEP levels during laparoscopic surgery as compared to open abdominal surgery [23]. Liu and colleagues found significantly improved oxygenation, pulmonary function and reduced incidence of PPC after laparoscopic radical gastrectomy with the use of intraoperative decremental titrated individual PEEP [52]. However, it should not be forgotten that PEEPopt is rather a compromise than a realistic goal due to the heterogenous regional distribution of ventilation and compliance of the lungs. A PEEP that is appropriate in one region may be harmful in another one: in non-dependent lung parts overinflation can occur, in dependent parts atelectasis may develop [53, 54]. Maisch et al. defined PEEPopt as the PEEP that prevents atelectasis after ARM and minimizes alveolar dead space ventilation without over-distension [55].
There are several types of PEEP titration methods in order to determine the individual PEEPopt. Static or dynamic pulmonary compliance directed methods, Vds/Vt guided technique based on volumetric capnography or electrical impedance tomography (EIT), and transpulmonary pressure directed PEEP titration procedures are worth to mention [56–59]. Most authors agree that decremental titration should be performed, however, there is no recommendation about best practice. Pereira et colleagues found that EIT guided PEEP individualization could reduce PPC while improving intraoperative oxygenation and reducing ΔP as well, causing minimal side effects [51]. Another Spanish RCT by Ferrando et al. suggested that individualized PEEP settings with the use of ARM may confer an enhanced lung protection in patients undergoing major abdominal surgery [60]. Additionally, in two Italian physiological studies conducted by D’Antini and Rauseo in 2018, OLA applying titrated optimal PEEP levels resulted in improved respiratory mechanics, better gas exchange, decreased transpulmonary pressures and ΔP without significant haemodynamic effects [24, 25]. Reducing ΔP as a goal of ventilatory settings has some rationale: decreased lung stress and strain may attenuate intrapulmonary inflammatory response [61, 62].
On the one hand, surgery, especially major abdominal surgery, alone induces host inflammatory response via damage associated molecular patterns (DAMPs) pathway that is necessary for postoperative recovery, however an overwhelming inflammatory response may lead to multi-organ dysfunction in the postoperative period [10, 11, 14, 16]. On the other hand, injurious intraoperative ventilatory management may cause further complications by exacerbating the local intrapulmonary inflammation and amplifying the surgery induced inflammatory response [8]. Potential advantages and some disadvantages of intraoperative LPV during abdominal surgery are well-known, however, the exact role and impact of inappropriate mechanical ventilation caused inflammatory response, on systemic and local intrapulmonary complications remained uncertain.
As radical cystectomy and urinary diversion is considered a high-risk, major abdominal surgery with an operating time lasting for several hours, we hypothesized that it has some rationale that optimizing intraoperative mechanical ventilation applying individually appropriate PEEP levels may improve respiratory mechanics, oxygenation, attenuate the inflammatory response and decrease the incidence of complications in the postoperative period.
Results of our current trial are similar to those reported in earlier RCTs. Intraoperative oxygenation and respiratory mechanics improved significantly with the use of an individual PEEPopt. Additionally, dead space ventilation and ΔP were significantly lower in the SG. We could not prove any significant intergroup differences in host inflammatory response, however the daily decrease in PCT levels was more pronounced in SG. Composite outcomes were also better in SG, but results were not significant statistically. Moreover, higher PEEP values in SG resulted in higher incidence of intraoperative hypotension, significantly higher vasopressor requirements and more kidney injury in the postoperative period. A significant correlation was found between PCT values and SOFA scores. Moreover, SOFA Scores had a significant impact on postoperative ICU length of stay but not on in-hospital days.
Although, sample size was suitable for the analysis of the physiological primary endpoints our study has several limitations. Firstly, available resources restricted our possibility to recruit enough patients to investigate robust clinical outcomes such as PPCs. Therefore, multicentre studies are needed to elaborate this further. Second, we could not perform detailed haemodynamic monitoring during surgery, hence rescue fluid boluses and norepinephrine therapy were based on mean arterial pressure, central venous oxygen saturation and central venous-to-arterial carbon dioxide difference as surrogates for more appropriate measures. Finally, during the out-of-hospital follow-up period outcomes (e.g. constipation or infection) were only assessed by phone call visits.
In conclusion our study confirmed the results of previous physiological trials on individualized LPV during major abdominal surgery. Although, we found significant advantages on gas exchange and pulmonary mechanics in the SG and our results have some promising details and may further improve our knowledge on the effects of optimal intraoperative ventilatory strategies applied in patients undergoing major abdominal surgery, whether these have any effect on short and long term outcomes require further investigations.
Acknowledgements
The authors extend thanks to the nursing staff of the study centres, especially Gabriella Gombor and Katalin Gornicsár for their assistance with the study. Preliminary data for this study were presented as a poster presentation at the Euroanaesthesia meeting, 1–3 June 2019, Vienna. This trial was supported by departmental funding.
Author contributions
All authors contributed to the study conception and design. Statistical analysis was designed by Ildikó László, Zoltán Ruszkai and Zsolt Molnár. Material preparation and data collection were performed by Zoltán Ruszkai, Erika Kiss, Gergely Péter Bokrétás, Dóra Vizserálek, Ildikó Vámossy and Erika Surány. Statistical analysis was performed by Zoltán Ruszkai, Ildikó László and Zsolt Molnár. The first draft of the manuscript was written by Zoltán Ruszkai and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by departmental funding.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This investigator-initiated, double-centre, single-blinded (subject), interventional, prospective, randomized controlled trial (RCT) was approved by the Hungarian Scientific and Medical Research Council Ethics Committee (21586–4/2016/EKU, on 17 June 2016), the Local Ethics Committee of Péterfy Sándor Hospital Budapest (CO-338–045, on 12 September 2016) and the Regional Ethics Committee of the University of Szeged (149/2016-SZTE, on 19 September 2016). This study was conducted in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Zoltán Ruszkai, Email: z.ruszkai.md@gmail.com.
Erika Kiss, Email: kiss.erika.zentayne@med.u-szeged.hu.
Ildikó László, Email: laszlo.ildiko@med.u-szeged.hu.
Gergely Péter Bokrétás, Email: bokretas.gergely@peterfykh.hu.
Dóra Vizserálek, Email: vizseralek.dora@gmail.com.
Ildikó Vámossy, Email: vamossy.ildiko@gmail.com.
Erika Surány, suranye@freemail.hu.
István Buzogány, Email: buzogany.istvan@peterfykh.hu.
Zoltán Bajory, Email: bajory.zoltan@med.u-szeged.hu.
Zsolt Molnár, Email: zsolt.lajos.molnar@aok.pte.hu.
References
- 1.Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Eng J Med. 2013;369:2126–2136. doi: 10.1056/NEJMra1208707. [DOI] [PubMed] [Google Scholar]
- 2.Ricard J-D, Dreyfuss D, Saumon G. Ventilator-induced lung injury. Eur Respir J. 2003;22(Suppl 42):2–9. doi: 10.1183/09031936.03.00420103. [DOI] [PubMed] [Google Scholar]
- 3.Futier E, Marret E, Jaber S. Perioperative positive pressure ventilation: an integrated approach to improve pulmonary care. Anesthesiology. 2014;121:400–408. doi: 10.1097/ALN.0000000000000335. [DOI] [PubMed] [Google Scholar]
- 4.Sutherasan Y, Vargas M, Pelosi P. Protective mechanical ventilation in the non-injured lung: review and meta-analysis. Crit Care. 2014;18:211. doi: 10.1186/cc13778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The LAS VEGAS Investigators Epidemiology, practice of ventilation and outcome for patients at increased risk of postoperative pulmonary complications: LAS VEGAS—an observational study in 29 countries. Eur J Anaesthesiol. 2017;34:492–507. doi: 10.1097/EJA.0000000000000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rotta AT, Steinhorn DM. Conventional mechanical ventilation in pediatrics. J Pediatr (Rio J) 2007;83(Suppl 2):S100–108. doi: 10.2223/JPED.1617. [DOI] [PubMed] [Google Scholar]
- 7.Picca A, Lezza A, Leeuwenburgh C, et al. Fueling inflamm-aging through mitochondrial dysfunction: mechanisms and molecular targets. Int J Mol Sci. 2017;18:933. doi: 10.3390/ijms18050933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuipers MT, van der Poll T, Schultz MJ, et al. Bench-to-bedside review: damage-associated molecular patterns in the onset of ventilator-induced lung injury. Crit Care. 2011;15:235. doi: 10.1186/cc10437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunction to immunotherapy. Nat Rev Immunol. 2013;13:862–874. doi: 10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lindberg M, Hole A, Johnsen H, et al. Reference intervals for procalcitonin and C-reactive protein after major abdominal surgery. Scand J Clin Lab Invest. 2002;62:189–194. doi: 10.1080/003655102317475443. [DOI] [PubMed] [Google Scholar]
- 11.Sarbinowski R, Arvidsson S, Tylman M, et al. Plasma concentration of procalcitonin and systemic inflammatory response syndrome after colorectal surgery. Acta Anaesth Scand. 2005;49:191–196. doi: 10.1111/j.1399-6576.2004.00565.x. [DOI] [PubMed] [Google Scholar]
- 12.Mokart D, Merlin M, Sannini A, et al. Procalcitonin, interleukin 6 and systemic inflammatory response syndrome (SIRS): early markers of postoperative sepsis after major surgery. Br J Anaesth. 2005;94:767–773. doi: 10.1093/bja/aei143. [DOI] [PubMed] [Google Scholar]
- 13.Bogár L, Molnár Z, Tarsoly P, et al. Serum procalcitonin level and leukocyte antisedimentation rate as early predictors of respiratory dysfunction after oesophageal tumour resection. Crit Care. 2006;10:R110. doi: 10.1186/cc4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minami E, Ito S, Sugiura T, et al. Markedly elevated procalcitonin in early postoperative period in pediatric open heart surgery: a prospective cohort study. J Intensive Care. 2014;2:38. doi: 10.1186/2052-0492-2-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trasy D, Tánczos K, Németh M, et al. Early procalcitonin kinetics and appropriateness of empirical antimicrobial therapy in critically ill patients*** A prospective observational study. J Crit Care. 2016;34:50–55. doi: 10.1016/j.jcrc.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 16.Paruk F, Chausse J. Monitoring the post surgery inflammatory host response. J Emerg Crit Care Med 2019; 3:5356. https://jeccm.amegroups.com/article/view/5356. Accessed 02 Oct, 2019.
- 17.Futier E, Constantin J-M, Paugam-Burtz C, et al. A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N Engl J Med. 2013;369:428–437. doi: 10.1056/NEJMoa1301082. [DOI] [PubMed] [Google Scholar]
- 18.Futier E, Constantin J-M, Pelosi P, et al. Intraoperative recruitment maneuver reverses detrimental pneumoperitoneum-induced respiratory effects in healthy weight and obese patients undergoing laparoscopy. Anesthesiology. 2010;113:1310–1319. doi: 10.1097/ALN.0b013e3181fc640a. [DOI] [PubMed] [Google Scholar]
- 19.Whalen FX, Gajic O, Thompson GB, et al. The effects of the alveolar recruitment maneuver and positive end-expiratory pressure on arterial oxygenation during laparoscopic bariatric surgery. Anesth Analg. 2006;102:298–305. doi: 10.1213/01.ane.0000183655.57275.7a. [DOI] [PubMed] [Google Scholar]
- 20.Talley HC, Bentz N, Georgievski J, et al. Anesthesia providers’ knowledge and use of alveolar recruitment maneuvers. J Anesth Clin Res. 2012;3:325. [Google Scholar]
- 21.Hess DR. Recruitment maneuvers and PEEP titration. Respir Care. 2015;60(11):1688–1704. doi: 10.4187/respcare.04409. [DOI] [PubMed] [Google Scholar]
- 22.Cinnella G, Grasso S, Natale C, et al. Physiological effects of a lung-recruiting strategy applied during one-lung ventilation. Acta Anaesth Scand. 2008;52:766–775. doi: 10.1111/j.1399-6576.2008.01652.x. [DOI] [PubMed] [Google Scholar]
- 23.Spadaro S, Karbing DS, Mauri T, et al. Effect of positive end-expiratory pressure on pulmonary shunt and dynamic compliance during abdominal surgery. Br J Anaesth. 2016;116(6):855–861. doi: 10.1093/bja/aew123. [DOI] [PubMed] [Google Scholar]
- 24.D’Antini D, Rauseo M, Grasso S, et al. Physiological effects of the open lung approach during laparoscopic cholecystectomy: focus on driving pressure. Minerva Anestesiol. 2018;84(2):159–167. doi: 10.23736/S0375-9393.17.12042-0. [DOI] [PubMed] [Google Scholar]
- 25.Rauseo M, Mirabella L, Grasso S, et al. Peep titration based on the open lung approach during one lung ventilation in thoracic surgery: a physiological study. BMC Anesthesiol. 2018;18(1):156. doi: 10.1186/s12871-018-0624-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liedberg F. Early complications and morbidity of radical cystectomy. Eur Urol Suppl. 2010;9:25–30. [Google Scholar]
- 27.Tsaturyan A, Petrosyan V, Crape B, et al. Risk factors of postoperative complications after radical cystectomy with continent or conduit urinary diversion in Armenia. Springerplus. 2016;5:134. doi: 10.1186/s40064-016-1757-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pavone C, Candela L, Fontana D, et al. Postoperative complications and 90-day mortality in radical cystectomy in high-risk patients: a monocentric retrospective observational study. Urol J. 2018;85:111–117. doi: 10.1177/0391560317751600. [DOI] [PubMed] [Google Scholar]
- 29.Schulz GB, Grimm T, Buchner A, et al. Surgical high-risk patients with ASA ≥ 3 undergoing radical cystectomy: morbidity, mortality, and predictors for major complications in a high-volume tertiary center. Clin Genitourin Cancer. 2018;16:e1141–e1149. doi: 10.1016/j.clgc.2018.07.022. [DOI] [PubMed] [Google Scholar]
- 30.Jakobson T, Karjagin J, Vipp L, et al. Postoperative complications and mortality after major gastrointestinal surgery. Medicina (Kaunas) 2014;50(2):111–117. doi: 10.1016/j.medici.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 31.Kelkar KV. Post-operative pulmonary complications after non-cardiothoracic surgery. Indian J Anaesth. 2015;59:599–605. doi: 10.4103/0019-5049.165857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Straatman J, Cuesta MA, de Lange-de Klerk ESM, et al. Long-term survival after complications following major abdominal surgery. J Gastrointest Surg. 2016;20:1034–1041. doi: 10.1007/s11605-016-3084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel K, Hadian F, Ali A, et al. Postoperative pulmonary complications following major elective abdominal surgery: a cohort study. Perioper Med. 2016;5:10. doi: 10.1186/s13741-016-0037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simões CM, Carmona MJC, Hajjar LA, et al. Predictors of major complications after elective abdominal surgery in cancer patients. BMC Anesthesiol. 2018;18:49. doi: 10.1186/s12871-018-0516-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gakis G (2018, March). Open RC remains most effective treatment despite complications risks. Paper presented at the meeting of the European Association of Urology, Copenhagen, Denmark. Retrieved from https://eau18.uroweb.org/open-rc-remains-most-effective-treatment-despite-complications-risks
- 36.Bendixen HH, Hedley-Whyte J, Laver MB. Impaired oxygenation in surgical patients during general anesthesia with controlled ventilation—a concept of atelectasis. N Engl J Med. 1963;269:991–996. doi: 10.1056/NEJM196311072691901. [DOI] [PubMed] [Google Scholar]
- 37.Ashbaugh DG, Bigelow DB, Petty TL, et al. Acute respiratory distress in adults. The Lancet. 1967;2:319–323. doi: 10.1016/s0140-6736(67)90168-7. [DOI] [PubMed] [Google Scholar]
- 38.Amato MB, Barbas CS, Medeiros DM, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338:347–354. doi: 10.1056/NEJM199802053380602. [DOI] [PubMed] [Google Scholar]
- 39.Acute Respiratory Distress Syndrome Network Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Eng J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 40.Serpa Neto A, Cardoso SO, Manetta JA, et al. Association between use of lung-protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndrome: a meta-analysis. JAMA. 2012;308:1651–1659. doi: 10.1001/jama.2012.13730. [DOI] [PubMed] [Google Scholar]
- 41.Lipes J, Bojmehrani A, Lellouche F. Low tidal volume ventilation in patients without acute respiratory distress syndrome: a paradigm shift in mechanical ventilation. Crit Care Res Pract. 2012;2012:416862. doi: 10.1155/2012/416862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alencar R, D'Angelo V, Carmona R, et al. Patients with uninjured lungs may also benefit from lung-protective ventilator settings. F1000Res. 2017;6:2040. doi: 10.12688/f1000research.12225.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fischer MO, Courteille B, Guinot PG, et al. Perioperative ventilatory management in cardiac surgery: a french nationwide survey. Medicine (Baltimore) 2016;95:e2655. doi: 10.1097/MD.0000000000002655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Colinet B, Van der Linden P, Bissot M, et al Mechanical ventilation practices in the operating room. Survey of the Anesthesiology Society of Charleroi “VENTISAC”. Acta Anaesth Belg. 2017;68:81–86. [Google Scholar]
- 45.Ruszkai Z, Kiss E, Molnár Z. Perioperative lung protective ventilatory management during major abdominal surgery: a hungarian nationwide survey. J Crit Care Med. 2019;5:19–27. doi: 10.2478/jccm-2019-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zaky A, Lang JD. The use of intraoperative positive end expiratory pressure. J Anesthe Clinic Res. 2011;4:4. [Google Scholar]
- 47.Hemmes SN, Gama De Abreu M, Pelosi P, et al. High versus low positive end-expiratory pressure during general anaesthesia for open abdominal surgery (PROVHILO trial): a multicentre randomised controlled trial. The Lancet. 2014;384:495–503. doi: 10.1016/S0140-6736(14)60416-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferrando C, Soro M, Unzueta C, et al. Individualised perioperative open-lung approach versus standard protective ventilation in abdominal surgery (iPROVE): a randomised controlled trial. Lancet Respir Med. 2018;6:193–203. doi: 10.1016/S2213-2600(18)30024-9. [DOI] [PubMed] [Google Scholar]
- 49.Goldenberg NM, Steinberg BE, Lee WL, et al. Lung-protective ventilation in the operating room: time to implement? Anesthesiology. 2014;121:184–188. doi: 10.1097/ALN.0000000000000274. [DOI] [PubMed] [Google Scholar]
- 50.Kacmarek RM, Villar J. Lung-protective ventilation in the operating room: individualized positive end-expiratory pressure is needed! Anesthesiology. 2018;129:1057–1059. doi: 10.1097/ALN.0000000000002476. [DOI] [PubMed] [Google Scholar]
- 51.Pereira SM, Tucci MR, Morais CCA, et al. Individual positive end-expiratory pressure settings optimize intraoperative mechanical ventilation and reduce postoperative atelectasis. Anesthesiology. 2018;129:1070–1081. doi: 10.1097/ALN.0000000000002435. [DOI] [PubMed] [Google Scholar]
- 52.Liu J, Meng Z, Iv R, et al. Effect of intraoperative lung-protective mechanical ventilation on pulmonary oxygenation function and postoperative pulmonary complications after laparoscopic radical gastrectomy. Braz J Med Biol Res. 2019;52(6):e8523. doi: 10.1590/1414-431X20198523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gattinoni L, Pelosi P, Crotti S, et al. Effects of positive end-expiratory pressure on regional distribution of tidal volume and recruitment in adult respiratory distress syndrome. Am J Respir Crit Care Med. 1995;151:1807–1814. doi: 10.1164/ajrccm.151.6.7767524. [DOI] [PubMed] [Google Scholar]
- 54.Hedenstierna G. Optimum PEEP during anesthesia and in intensive care is a compromise but is better than nothing. Turk J Anaesthesiol Reanim. 2016;44:161–162. doi: 10.5152/TJAR.2016.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maisch S, Reissmann H, Fuellekrug B, et al. Compliance and dead space fraction indicate an optimal level of positive end-expiratory pressure after recruitment in anesthetized patients. Anesth Analg. 2008;106:175–181. doi: 10.1213/01.ane.0000287684.74505.49. [DOI] [PubMed] [Google Scholar]
- 56.Pelosi P, de Abreu MG, Rocco PRM. New and conventional strategies for lung recruitment in acute respiratory distress syndrome. Crit Care. 2010;14:210. doi: 10.1186/cc8851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Long Y, Liu DW, He HW, et al. Positive end-expiratory pressure titration after alveolar recruitment directed by electrical impedance tomography. Chin Med J (Engl) 2015;128:1421–1427. doi: 10.4103/0366-6999.157626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao Z, Chang M-Y, Chang M-Y, et al. Positive end-expiratory pressure titration with electrical impedance tomography and pressure–volume curve in severe acute respiratory distress syndrome. Ann Intensive Care. 2019;17:7. doi: 10.1186/s13613-019-0484-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baedorf Kassis E, Loring SH, Talmor D. Should we titrate peep based on end-expiratory transpulmonary pressure? - yes. Ann Transl Med. 2018;6:390. doi: 10.21037/atm.2018.06.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ferrando C, Suarez-Sipmann F, Tusman G, et al. Open lung approach versus standard protective strategies: effects on driving pressure and ventilatory efficiency during anesthesia—A pilot, randomized controlled trial. PLoS ONE. 2017;12:e0177399. doi: 10.1371/journal.pone.0177399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aoyama H, Yamada Y, Fan E. The future of driving pressure: a primary goal for mechanical ventilation? J Intensive Care. 2018;6:64. doi: 10.1186/s40560-018-0334-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pelosi P, Ball L. Should we titrate ventilation based on driving pressure? Maybe not in the way we would expect. Ann Transl Med. 2018;6:389. doi: 10.21037/atm.2018.09.48. [DOI] [PMC free article] [PubMed] [Google Scholar]