Abstract
Purpose
Probiotics may prevent healthcare-associated infections, such as ventilator-associated pneumonia, Clostridioides difficile-associated diarrhea, and other adverse outcomes. Despite their potential benefits, there are no summative data examining the cost-effectiveness of probiotics in hospitalized patients. This systematic review summarized studies evaluating the economic impact of using probiotics in hospitalized adult patients.
Methods
We searched MEDLINE, EMBASE, CENTRAL, ACP Journal Club, and other EBM reviews (inception to January 31, 2019) for health economics evaluations examining the use of probiotics in hospitalized adults. Independently and in duplicate, we extracted data study characteristics, risk of bias, effectiveness and total costs (medications, diagnostics/procedures, devices, personnel, hospital) associated with healthcare-associated infections (ventilator-associated pneumonia, Clostridioides difficile-associated diarrhea and antibiotic-associated diarrhea). We used Grading of Recommendations Assessment, Development and Evaluation methods to assess certainty in the overall cost-effectiveness evidence.
Results
Of 721 citations identified, we included seven studies. For the clinical outcomes of interest, there was one randomized-controlled trial (RCT)-based health economic evaluation, and six model-based health economic evaluations. Probiotics showed favourable cost-effectiveness in six of seven (86%) economic evaluations. Three of the seven studies were manufacturer-supported, all which suggested cost-effectiveness. Certainty of cost-effectiveness evidence was very low because of risk of bias, imprecision, and inconsistency.
Conclusion
Probiotics may be an economically attractive intervention for preventing ventilator-associated pneumonia, Clostridioides difficile-associated diarrhea, and antibiotic-associated diarrhea in hospitalized adult patients. Nevertheless, certainty about their cost-effectiveness evidence is very low. Future RCTs examining probiotics should incorporate cost data to inform bedside practice, clinical guidelines, and healthcare policy.
Trial registration: PROSPERO CRD42019129929; Registered 25 April, 2019.
Electronic supplementary material
The online version of this article (10.1007/s12630-019-01525-2) contains supplementary material, which is available to authorized users.
Résumé
Objectif
Les probiotiques pourraient prévenir les infections nosocomiales, telles que la pneumonie acquise sous ventilation, la diarrhée associée au Clostridioides difficile, et d’autres atteintes néfastes. Malgré leurs bienfaits potentiels, il n’existe aucune donnée sommative examinant la rentabilité des probiotiques chez des patients hospitalisés. Cette revue systématique a résumé les études évaluant l’impact économique de l’utilisation de probiotiques chez des patients adultes hospitalisés.
Méthode
Nous avons effectué des recherches dans les bases de données MEDLINE, EMBASE, CENTRAL, ACP Journal Club et d’autres ressources médicales fondées sur des données probantes (de leur création jusqu’au 31 janvier 2019) pour en extraire les évaluations économiques examinant l’utilisation de probiotiques auprès d’adultes hospitalisés. Nous avons extrait, de façon indépendante et en double, les caractéristiques des données des études, le risque de biais, l’efficacité et les coûts totaux (médicaments, diagnostics et interventions, dispositifs, personnel et hôpitaux) associés aux infections nosocomiales (pneumonie acquise sous ventilation, diarrhée associée au Clostridioides difficile et diarrhée associée aux antibiotiques). Nous avons utilisé l’échelle GRADE (Grading of Recommendations Assessment, Development and Evaluation) afin d’évaluer le degré de certitude des données probantes globales de rentabilité.
Résultats
Parmi les 721 citations identifiées, sept études ont été incluses. En ce qui touchait aux critères d’évaluation présélectionnés, on comptait une évaluation économique de la santé basée sur une étude randomisée contrôlée (ERC) et six évaluations économiques de la santé fondées sur des modèles. Les probiotiques ont démontré une rentabilité favorable dans six des sept (86 %) évaluations économiques. Trois des sept études étaient financées par l’industrie, suggérant toutes la rentabilité des probiotiques. Le degré de certitude des données probantes de rentabilité était très faible en raison du risque de biais, d’imprécision et d’incohérence.
Conclusion
Les probiotiques pourraient constituer une intervention séduisante d’un point de vue économique pour prévenir la pneumonie acquise sous ventilation, la diarrhée associée au Clostridioides difficile et la diarrhée associée aux antibiotiques chez les patients adultes hospitalisés. Toutefois, le degré de certitude quant aux données probantes de rentabilité est très faible. Davantage d’ERC examinant les probiotiques devraient intégrer les données de coûts afin de guider la pratique au chevet, les recommandations cliniques et les politiques de soins de santé.
Enregistrement de l’étude : PROSPERO CRD42019129929; enregistrée le 25 avril 2019.
Probiotics are defined as “live microorganisms, which when administered in adequate amounts, confer a health benefit on the host.”1 Mechanisms by which probiotics offer potential health benefits are not yet fully elucidated. They may include enhanced gut barrier function, reduced gastrointestinal pathogenic bacterial load through competitive inhibition, modification of the gut microbiome, and modulation of the host immune system. These effects may reduce the incidence of healthcare-associated infections.2,3
Probiotics have been studied in randomized-controlled trials (RCT) in a variety of conditions in the hospital setting with evidence suggesting benefits, including the reduction of healthcare-associated infections.4,5 In the intensive care unit (ICU), probiotics have been studied for the prevention of ventilator-associated pneumonia (VAP).3,6 Multiple probiotic strains (i.e., Lactobacillus, Bifidobacterium, Saccharomyces) and doses have been systematically reviewed; a meta-analysis revealed a risk reduction of 0.74 for VAP (95% confidence interval [CI], 0.61 to 0.90; P = 0.002), showing a potential effect across species.6 As the most common healthcare-associated infection in ICU, VAP is associated with a two-fold attributable risk of death, and an attributable cost of 10,000–13,000 USD/patient.7
Further evidence suggests that probiotics can reduce the incidence of diarrhea, specifically Clostridioides difficile-associated diarrhea (CDAD), which can cause pseudomembranous colitis, toxic megacolon, and death.8 A Cochrane systematic review and meta-analysis of 31 RCTs including 8,672 patients receiving concurrent administration of probiotics (any dose, any strain) and antibiotics showed that probiotics prevented CDAD compared with placebo (based on moderate certainty evidence), with heterogeneous evidence for a specific species or dose effect.8 Treatment for CDAD is expensive (8,911–30,049 USD/patient).9
Among critically ill patients, the clinical effectiveness of probiotics in preventing VAP, CDAD, and other infectious outcomes was evaluated in a recently completed but as yet unpublished multicentre RCT (Probiotics: Prevention of Severe Pneumonia and Endotracheal Colonization Trial - PROSPECT; NCT01782755), with additional RCTs ongoing (PRINCESS: Probiotics to reduce infections in care home residents; ISRCTN16392920).
Health economic evaluations produce important evidence to inform clinical decisions and health policy creation. The objective of this systematic review is to summarize cost or cost-effectiveness evidence of a broad spectrum of strategies involving probiotics (different doses and strains) in hospitalized adult patients. The research question was: in hospitalized adult patients (population), do probiotics (intervention: any strain, any dose) vs placebo/no treatment (comparator: usual care) show cost-effectiveness in preventing healthcare-associated infections (VAP, CDAD, and antibiotic-associated diarrhea [AAD])?
Methods
Data sources and searches
Our search strategy is outlined in eAppendix 1 (available as Electronic Supplementary Material [ESM]). Searches were performed by a clinical librarian (A.I.) with experience in conducting electronic literature searches. Searches underwent peer-review of electronic search strategies by a professional librarian and our authors. No publication type or language restrictions were applied.
To identify additional potentially relevant studies, we also checked reference lists of identified articles within our systematic review search, to examine what source inputs were utilized in their economic evaluations.
Study selection and quality assessment
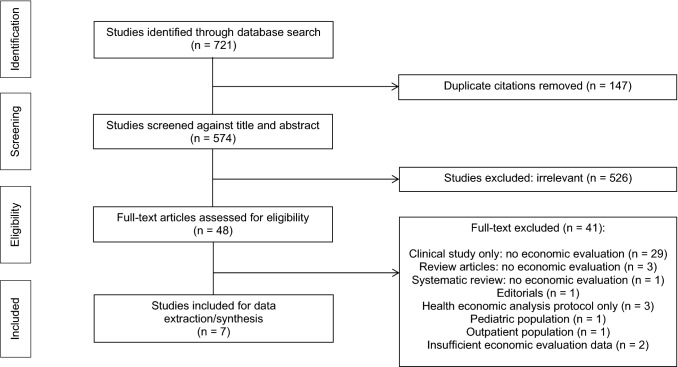
Two reviewers independently assessed each citation and applied inclusion/exclusion criteria (Figure). Two reviewers (V.L./J.B.) independently screened abstracts in the first stage, and the full-text in the second stage. Disagreements were resolved by a third reviewer (B.R./F.X.). We listed the characteristics of the included studies (Table 1). Quality of studies was critically appraised (Table 2) using the Joanna Briggs Institute for Critical Appraisal of Economic Evaluations tool10 and the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement.11 Our systematic review has been registered in PROSPERO (international prospective register of systematic reviews): CRD42019129929 (www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=129929). Our literature search commenced before registration, and data extraction was underway (but not completed) when registered (started January 31, 2019, registered April 25, 2019).
Figure.
E-PROSPECT PRISMA flow diagram. Selection criteria: (1) full economic evaluation (cost-minimization, cost-benefit, cost-effectiveness, cost-utility) conducted alongside clinical studies or via economic modeling, (2) the study described hospitalized patients, (3) the study included probiotics as a treatment, (4) the study described drug acquisition costs, the costs of providing prophylaxis, costs of complications, (5) the study described the effect of prophylaxis with respect to one of our clinical outcomes of interest including VAP, CDAD and antibiotic-associated diarrhea (AAD)
Table 1.
Summary of health economic studies of probiotics
| Study | Study design | Patient population | Economic perspective | Time horizon | Comparison | Cost (currency/year) | Primary clinical outcome | Primary economic outcome |
|---|---|---|---|---|---|---|---|---|
| Trial-based health economic analysis | ||||||||
| Allen et al. (2013) |
Cost-effectiveness Cost-utility (trial-based economic analysis) |
Elderly hospitalized adults >65 yr (medical, surgical) treated with antibiotics | Payer, societal | 12 weeks | Lactobacillus or Bifidobacterium vs placebo | GBP (2012) |
AAD CDAD |
Total healthcare costs ICER cost for AAD ICER per QALY |
| Model-based health economic analysis | ||||||||
| Branch-Elliman et al. (2015) |
Cost-benefit analysis Cost-effectiveness analysis (model-based decision tree analysis) |
Adult medical-surgical patients (mechanical ventilation >12 hr) | Payer, societal | 4 weeks | Probiotics, subglottic endotracheal tubes, VAP prevention bundles, chlorhexidine oral care, selective oral decontamination, selective gut decontamination, silver endotracheal tubes | USD (2013) | VAP | Cost-benefit ratio per VAP prevented |
| Fansiet al.(2012)*** |
Cost-effectiveness (model-based decision tree analysis) |
Adult hospitalized patients (50–70 yr), hospitalization of 5 or more days, and antibiotic therapy of at least 3 days but no more than 14 days | Payer | 3 weeks | Lactobacillus acidophilus/casei vs placebo | USD (2009) | CDAD | Cost savings per dose |
| Leal et al. (2016) | Cost-effectiveness analysis (model-based decision tree analysis) | Adult (>18 yr) hospitalized patients treated with antibiotics | Payer | 4 weeks | Lactobacillus acidophilus/casei vs no treatment | CAD (2015) | CDAD | Cost savings per CDAD avoided |
| Lenoir-Wijnkoop et al. (2014) *** | Cost-effectiveness (model-based decision tree analysis) | Elderly hospitalized patients (>65 yr) treated with antibiotics | Payer | Until recovery/death | Fermented milk (FM) with Lactobacillus paracasei vs placebo | GBP (2010) |
AAD CDAD |
Cost savings per AAD avoided |
| Shen et al. (2017) |
Cost-effectiveness Cost-utility (model-based decision tree analysis) |
Hospitalized adults (mean age: 68 yr) | Payer | 52 weeks | Lactobacillus acidophilus/casei/Saccharomyces boulardii vs no treatment | USD (2013) | CDAD |
ICER cost for CDAD ICER per QALY |
| Vermeersch et al. *** (2018) | Cost-effectiveness (model-based decision tree analysis) | Hospitalized adults (mean age: 68 yr) | Payer, societal | Until hospital discharge/death | Saccharomyces boulardii vs no treatment | € (2017) |
AAD (non-complicated) CDAD (complicated) |
Cost savings per patient for AAD and CDAD |
AAD = antibiotic-associated diarrhea; CAD = Canadian; CDAD = Clostridium Difficile-associated diarrhea; CFU = colony-forming units; FM = fermented milk; GBP = Great Britain pound; ICER = incremental cost-effectiveness ratio; ICU = intensive care unit; NR = not reported; QALY = quality-adjusted life year; RCT = randomized-controlled trial; USD = United States dollar; VAP = ventilator-associated pneumonia.
*** Industry-sponsored study.
Table 2.
Critical appraisal of study articles
| Paper | Were the outcomes accurately measured? | Were the costs accurately measured? | Do incremental costs and outcomes differ between subgroups? | Are prophylaxis benefits worth the harm and costs? | Generalizability: could other patient populations expect similar outcomes? | Generalizability: could other patient populations expect to experience similar costs? |
|---|---|---|---|---|---|---|
| Allen et al. (2013) | Yes | Yes—data from literature, databases, reference costs | Yes | Equivocal (no benefit and no difference in cost) | Yes | Yes |
| Branch-Elliman et al. (2015) | Yes | Yes—data from literature, databases, reference costs | Yes | Yes | Yes | Yes |
| Kamdeu Fansi et al.*** (2012) | Yes | Yes—data from a hospital, consumer price index, pharmacy Red Book | Yes | Yes | Yes | Yes |
| Leal et al. (2016) | Yes | Yes—data from literature, Alberta pharmacy and infection control, laboratory services, consumer price index | Yes | Yes | Yes | Yes |
| Lenoir-Wijnkoop et al.*** (2014) | Yes | Yes—data from literature and local price lists | Yes | Yes | Yes | Yes |
| Shen et al. (2017) | Yes | Yes—data from literature, databases, consumer price index | Yes | Yes | Yes | Yes |
| Vermeersch et al.*** (2018) | Yes | Yes—data from literature, databases, consumer price index | Yes | Yes | Yes | Yes |
NR = not reported.
*** Industry-sponsored study
Modified from the Joanna Briggs Institute Critical Appraisal Tool for Economic Evaluations (Gomersall et al.)10
Data extraction
Independently and in duplicate, our reviewers (V.L./J.B.) extracted data using pre-developed abstraction forms (eAppendix 2 available as ESM). We attempted to contact study authors for all study-related data, if not previously published. All currencies were converted to Canadian dollars (CAD) for the year 2018 utilizing the World Bank Official Exchange Rate.12 Incremental costs, effectiveness outcomes, or cost-effectiveness ratios are presented in Table 3.
Table 3.
Incremental costs, effects, and cost efficacy ratios for the probiotics vs comparator (placebo/no treatment/usual care)
| Reference | Costs inputs | Clinical effects inputs (healthcare-associated infections avoided, life-years or QALYS gained) | Incremental outputs (incremental costs, incremental cost benefit or cost effectiveness ratios - cost per healthcare associated-infection avoided or life-years or QALYS gained) | Subgroup analysis | Sensitivity analysis | Most economically attractive drug |
|---|---|---|---|---|---|---|
| Allen et al. (2013) |
Total healthcare costs per patient did not differ significantly between the probiotic (£8020; 95% CI, 7620 to 8420) and placebo arms (£8010; 95% CI, 7600 to 8420) Probiotics: (15,629 CAD; 95% CI, 14,850 to 16,409) Placebo: (15,601 CAD; 95% CI, 14,811 to 16,409) |
Probiotics and occurrence of AAD/CDAD: No difference with probiotics usage and placebo for AAD: 10.8 vs 10.4%, RR, 1.04; 95% CI, 0.84 to 1.28; P = 0.71 or CDAD: probiotics (12/1470, 0.8%), vs placebo (17/1491, 1.2%); RR, 0.71; 95% CI, 0.34 to 1.47; P = 0.35 |
Incremental Cost (AAD): 8.74 GBP; 95% CI, 4.32 to 21.78 17.03 CAD; 95% CI, -8.42 to 42.44 ICER: base case analysis: 22,701 GBP per QALY (44,239.07 CAD per QALY) |
Yes | Yes | No difference (base case) |
| Branch-Elliman et al. (2015) |
VAP: 15,975 USD [7,000–35,000] per case (22,623 CAD [9,913–49,566]) Probiotics cost: 2.18 USD; range, 1–10 3.09 CAD; range, 1.42–14.16 |
Primary outcome: VAP risk reduction (RR): 0.48 (range, 0.1–0.9) (Model effects inputs: 83.8% ICU survivors, 20% VAP, 15.4% mortality, 1% remained in ICU) |
Incremental cost benefit ratio: low estimate for VAP: 7,000–14,000 USD (9,913–19,826 CAD) vs willingness to pay threshold of 50,000–100,000 (70,809–141,617 CAD) per VAP case Prophylactic probiotics and subglottic endotracheal tube are cost-effective for preventing VAP |
Yes | Yes | Probiotics, suction ETT, VAP bundle (base case) |
| Kamdeu Fansi et al. (2012)*** |
Hospital care for CDAD patient (per day hospitalized): 1,424.16 USD (2,016.85 CAD) 2.50 USD (3.55 CAD) (Lactobacillus acidophilus/casei, per dose-unit) |
Probiotic-double dose (Pro-2) (15.5%) lower AAD vs probiotic-single dose (Pro-1) (28.2%) with each probiotic lower AAD incidence vs placebo (44.1%). In patients with AAD, Pro-2 (2.8 days) and Pro-1 (4.1 days) had shorter symptom duration vs placebo (6.4 days). Pro-2 (1.2%) had lower CDAD incidence vs Pro-1 (9.4%). Each treatment group had a lower CDAD incidence vs placebo (23.8%). Gastrointestinal symptoms were less common in the treatment groups vs placebo and in Pro-2 vs Pro-1. |
Estimated mean per patient’s savings (incremental cost): 1,968 USD (2,152 CAD) - single dose 2,661 USD (2,910 CAD) - double dose Compared with the placebo option (if used an average of 13 days by all patients at risk of developing AAD and CDAD) |
Yes | Yes | Probiotics (base case) |
| Leal et al. (2016) |
Cost of probiotics: 24 CAD/treatment (2018): 24.94 CAD Costs of CDAD: 11,862 CAD (12,326.60 CAD 2018) |
Risk of CDAD vs cost of probiotics Lower risk of CDI: 5.5 vs 2.0% |
Incremental cost: cost-savings: 518 CAD (539 CAD 2018)/patient Patients treated with oral probiotics lower overall cost compared with usual care (CAD 327 [340 CAD 2018] vs 845 [878 CAD 2018]) |
Yes | Yes | Probiotics (base case) |
| Lenoir-Wijnkoop et al. (2014)*** |
Non-severe CDAD patient (1st, 2nd, 3rd line): 2502, 3104, 2808 GBP (4,745, 5,587, 5,226 CAD) Severe CDAD patient (1st, 2nd, 3rd line): 6292, 6236, 5110 GBP (11,933, 11,827, 9,691 CAD) |
Probiotic group, 12% (7/57) developed AAD compared with 34% (19/56) in the placebo group (P = 0.007). None of the patients randomized to the FM with probiotic developed CDAD, while 17% (9/53) in the placebo group developed CDAD (P = 0.001). Risk ratio (RR) for the total population from Hickson’s study was 0.35 (12/34) |
Incremental cost: Probiotic intervention to prevent AAD generated estimated mean cost savings of £339 (643 CAD) per hospitalized patient over the age of 65 years and treated with antibiotics, compared to no preventive probiotic. Incremental cost-savings: 243 GBP (461 CAD)/case treated with antibiotics by preventing non-CDAD 96 GBP (182)/case treated with antibiotics through preventing CDAD |
Yes | Yes | Probiotics (base case) |
| Shen et al. (2017) |
CDAD (inpatient cost per case): 7,670 USD [3,830–11,500] CDAD (outpatient cost per case): 440 USD [210–620] CDAD (inpatient cost per case): 10,502.98 CAD [5,244.65-15,747.62] CDAD (outpatient cost per case): 602.52 CAD [287.57–849.00] |
Probiotic efficacy vs no treatment: <0.73 RR, baseline risk CDAD >1.6%, risk of probiotic-associated bacteremia/fungemia (<0.26%) |
Incremental cost: cost-savings of 840 USD (1,150 CAD)/case of CDAD averted Base case (age, 65–84; CDI risk, 2.9%); probiotics dominant (-13 USD incremental cost [18 CAD], +0.00005 QALYs); probiotics dominated no probiotics (less costly, greater QALYs) ICERs (scenarios): Probiotics RR 0.51 (WTP: 100,000 USD (135,348 CAD)) Age 18-44, CDI risk 0.6%: ICER 884,100 USD/QALY (1,196,609 CAD/QALY) - not cost effective Age, 45–64; CDI risk, 1.5%; ICER, 156,100 USD/QALY (211,278 CAD/QALY) - not cost effective Age, 65–84; CDI risk, 1.2%; ICER, 1,257,100 USD/QALY (1,701,456 CAD/QALY) - not cost effective Age >85; CDI risk, 3.8%; probiotics dominant (-31 USD incremental cost [42 CAD], +0.00014 QALYs) ICER, 19,200 USD (26,292 CAD)/QALY if baseline CDAD risk was low <1.2% |
Yes | Yes | Probiotics (in certain scenarios: base case - age 65–84 & CDI risk 2.9%, age >85, CDI risk 3.8%) |
| Vermeersch et al.*** (2018) |
AAD – non-complicated (cost per case): €277 or 418 CAD (hospital): €2150.30 or 3237.78 CAD (societal) CDAD - complicated (inpatient cost per case): €588.80 or 886.58 CAD (hospital): €2239.10 or 3,371.49 CAD (societal) |
Base case: AAD: 9.6% (71/743 patients), CDAD 5.6% (4/71 AAD patients) AAD RRR 48% S. boulardii vs no treatment CDAD RRR 47% S. boulardii vs no treatment |
Incremental cost: cost savings of €50.03 or 75.74 CAD (bottom-up) and €28.10 or 42.31 CAD (top-down) per AAD patient treated with antibiotics (healthcare provider) Incremental cost: cost savings of €95.20 or 143.35 CAD (bottom-up) and €14.70 or 22.13 CAD (top-down) per AAD patient treated with antibiotics (hospital/societal) |
Yes | Yes |
Probiotics (base case) |
AAD = antibiotic-associated diarrhea; CAD = Canadian dollar; CDAD = Clostridium Difficile-associated diarrhea; CDI = Clostridium Difficile infection; CI = confidence interval; ETT = endotracheal tube; GBP = Great Britain pound; ICER = incremental cost-effectiveness ratio; RR = risk reduction; RRR = relative risk reduction; USD = United States dollar; VAP = ventilator associated pneumonia; WTP = willingness-to-pay threshold.
*** Industry-sponsored study.
Adjusted to Canadian dollar (CAD) – 2018.
Risk of bias assessment
Randomized-controlled trials used as data sources for the health economic evaluation were assessed using the Cochrane Collaboration Risk of Bias (ROB) tool.13 Non-randomized trials were assessed using the Newcastle-Ottawa Scale.14 Surveys were assessed using the ROB tool from the McMaster University Clinical Advances Through Research and Information Translation (CLARITY).15 The assessment schemas are found in eAppendix 3 (available as ESM) or in the footnotes of eAppendix 4A–D (available as ESM).
For model-based economic designs, we assessed ROB in the contributing inputs from multiple source studies for the models. We decided a priori that, if each source input in a particular economic model had low ROB, the overall model would likely have a low ROB (even for varied types of studies—from RCTs to surveys). If any source study had an unknown/high ROB (identified as the weakest link), the entire economic evaluation would be assessed an unknown/high ROB. For source articles drawn from systematic reviews, guidelines documents, or economic evaluations, we did not assess ROB unless that source was not previously assessed in eAppendix 4A–D (available as ESM). We did not assess ROB when data were derived from an externally established public database (i.e., Consumer Price Index).
Data synthesis and analysis
We summarized the economic evaluation data (e.g., resource utilization, costs, cost-effectiveness ratios) in terms of point estimates and 95% CIs or ranges, if available. Categorical variables were reported as counts/proportions. Given the heterogeneity among the included studies, we could not conduct a meta-analysis. This review is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta Analyses (PRISMA) guidelines.16
Grading of recommendations assessment, development, and evaluation (GRADE)
We used the GRADE approach (Table 4) to assess the following domains: ROB, indirectness, imprecision, inconsistency, and other considerations. Certainty in evidence from RCTs started as high, while observational studies started as low. Final quality was rated high, moderate, low, or very low.17
Table 4.
Grading of Recommendations Assessment, Development and Evaluation (GRADE) of Probiotics Systematic Review Outcomes: VAP, CDAD, AAD
| Certainty assessment | Impact | Certainty | Importance | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No of studies | Study design (sources) | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | |||
| Prevention of ventilator associated pneumonia (VAP)18 | |||||||||
| 1 | 1 model-based health economic evaluation (observational studies)a | Seriousb | Not serious | Not serious | Erious | None | Branch-Elliman et al.18 constructed a cost-benefit decision model with a Markov model based on multicenter observational data. One hundred and twenty unique combinations of VAP prevention strategies were examined. Probiotics, along with subglottic suction ET tubes, and the Institute for Healthcare Improvement VAP Prevention Bundle was the preferred strategy for best cost-benefit ratio. |
⊕◯◯◯ Very low |
CRITICAL |
| Prevention of Clostridium Difficile associated diarrhea (CDAD)19–24 | |||||||||
| 6 | 6 model-based health economic evaluations (randomized and observational trials)b, 1 RCT-based health economic evaluation | Seriousb | Seriousc | Not serious | Seriousd | None |
Allen et al.19 concluded no difference in total healthcare costs per patient the probiotic and placebo arms. All other studies concluded that a probiotic was a cost-effective intervention to prevent CDAD. On this basis, there were serious concerns about inconsistency. Allen et al. suggested that a probiotic reduces and increases risk of CDAD (RR, 0.71; 95% CI, 0.34 to 1.47; P = 0.35). This, in addition to the weight of the study based on the sample size, raised serious concerns about imprecision. |
⊕◯◯◯ Very low |
CRITICAL |
| Prevention of antibiotic-associated diarrhea (AAD)19,22,24 | |||||||||
| 3 | 3 model-based health economic evaluations (randomized and observational trials)e, 1 RCT-based health economic evaluation | Seriousb | Seriousc | Not serious | Seriousd | None |
In the PLACIDE study, Allen et al19 concluded no difference in total healthcare costs per patient in the probiotic and placebo arms. All other studies concluded that a probiotic was a cost-effective intervention to prevent AAD. On this basis, there were serious concerns about inconsistency. The PLACIDE study suggested that a probiotic reduces and increases the risk of AAD (RR, 1.04; 95% CI, 0.84 to 1.28; P =0.71). This, in addition to the weight of the study based on the sample size, raised serious concerns about imprecision. |
⊕◯◯◯ Very low |
CRITICAL |
AAD = antibiotic-associated diarrhea; CDAD = Clostridium Difficile-associated diarrhea; CI = confidence interval; ET = endotracheal tube; RCT = randomized-controlled trial; RR = relative risk; VAP = ventilator-associated pneumonia.
aDecision tree analysis with observational studies as input (no RCTs)
bMultiple source data, observational cohort/case-control studies, and surveys had high risk of bias, which downgraded this category
cInconsistency came from one study (Allen et al.)19 that found no benefit in the use of probiotics to prevent CDAD, and concluded that they were not cost effective, while all other studies concluded that probiotics had a benefit for AAD/CDAD. There was no pooled estimate with a 95% CI, as the outcomes for some of the studies were not available or were too heterogeneous to pool (i.e., cost per treatment [with multiple dose regimens of probiotics] vs incremental cost-effectiveness ratios vs cost-utility vs cost-savings).
dConfidence interval crosses 0 for Allen et al19. study, and many of the included studies were small
eIncluded RCT, decision tree analysis, and systematic reviews/meta-analyses were used for source data (6 RCTs)
Results
Study comparisons, populations, and format
Out of 721 records identified through database searches, 147 duplicates were removed and 526 excluded based on an irrelevant title/abstract. The full-text of 48 papers was retrieved for comprehensive evaluation, of which 41 were excluded (Figure).
Of seven studies included in this systematic review (Table 1), one study was a RCT-based cost-effectiveness analysis (CEA).18 Six studies were model-based economic evaluations using CEA or incremental cost.19–24 Two also reported cost-utility analysis.19,23 One evaluation investigated VAP,18 six investigated CDAD,19–24 and three investigated AAD.19,22,24
Study perspectives, time horizon, and funding
Three studies were conducted in the United States,18,20,23 two in the United Kingdom,19,22 one in Canada,25 and one in Belgium.26 Four studies were conducted from the societal perspective18,19,24 (aggregation of all perspectives, taking into account time costs, opportunity costs, and community preferences, i.e., patient, payer, hospital)27 and seven from the perspective of a specific payer (four public and three private payers).18–24 The time horizon (duration of time for follow-up, over which health outcomes and costs are calculated) ranged from three to 52 weeks. A probiotic manufacturer supported three of seven (43%) studies.20,22,24
Study quality and risk of bias
Study quality is summarized in Table 2. Two studies obtained effectiveness data from meta-analysis,21,23 while seven studies obtained data from RCTs or observational trials.18–24 All performed sensitivity analyses.18–24
For assessing ROB in RCTs (eAppendix 4A [available as ESM]), three studies19–21 had a low ROB, while four studies28–31 had unclear/high ROB. Common ROB issues were selection, performance, detection, attrition, and reporting bias. For observational study ROB (eAppendix 4B [available as ESM]), there were six high-quality cohort observational studies,25,28,29,32–34 and ten low-quality cohort studies.26,30,31,35–40 Common ROB were selection (only selected group of patients representing the intervention cohort, no description of non-exposed non-intervention cohort, and no demonstration that outcome of interest was absent at the start of the study), comparability (study did not control for age, antibiotic/probiotic exposure, or additional factors), and outcome (short follow-up).
For ROB in case-control studies (eAppendix 4C [available as ESM]), there was one high quality study41 and one low quality study.42 Common ROB were selection (no description of case definition, representativeness shows potential for selection bias, no description of control—case-study only, no description of source), comparability (study did not control for age, antibiotic/probiotic exposure, or additional factors), and outcome (no method of ascertainment for controls, non-response rate, and different with no designation).
For ROB in surveys (eAppendix 4D [available as ESM]), there were two high-quality studies,43,44 and two studies with a mix of low/high ROB.18,45 Common ROB issues were low response rates (< 50%), missing data (> 15% within questionnaires), and no evidence of reliability/validity of the survey instrument.
Cost and effect estimates
The cost and effect estimates are shown in Table 3. Individual natural units and unit cost per resource are presented in eAppendix 5 (available as ESM).
Ventilator-associated pneumonia
One evaluation investigated VAP (Table 3). Using a Markov model for a cost-benefit analysis, prophylactic probiotics (with subglottic endotracheal tubes) showed cost benefit for preventing VAP, with a willingness-to-pay (WTP) of 50,000–100,000 USD (70,807–141,614 CAD) per case of VAP averted (median [range] cost estimate of 15,958 USD [7,000–35,000] or 22,623 CAD [9,913–49,566] per VAP case). The incremental cost-effectiveness ratio (ICER) between probiotics and no probiotics showed dominance of probiotics over placebo (with usual care). Sensitivity analysis showed continued dominance in a multiple scenarios (reducing cost of VAP, and increasing hourly nursing wages). There was a substantial increase in cost-savings with probiotics when VAP risk reduction was increased vs placebo.18
Clostridioides difficile-associated diarrhea
Among six studies examining the cost-effectiveness of probiotics in CDAD (Table 3), four studies found probiotics to be cost-effective/incremental cost-saving,20,22–24 one study showed no difference,19 and one study showed cost-effectiveness in certain scenarios.23
Kamdeu Fansi et al. found a cost-savings dose response for probiotics vs placebo. There was a cost-saving of 1,968 USD (2,152 CAD) for a single dose of probiotics (per CDAD case prevented) compared with placebo. For a double dose of probiotics per day, there was a cost-saving of 2,661 USD (2,910 CAD) compared with placebo.20 Leal et al. showed cost-savings of 538 CAD per patient (340 CAD for probiotics vs 878 CAD for usual care) for CDAD.21
Shen et al. showed a cost-saving of 840 USD (1,150 CAD) per case of CDAD averted, with dominance of probiotics (lower cost and higher effectiveness) in the base case. Nevertheless, there were scenarios (i.e., young patients) in which the ICER was not cost-effective (age, 18–44 yr; CDAD risk, 0.6%: ICER, 884,100 USD/quality-adjusted life-year [QALY] and 1,196,609 CAD/QALY).23 Furthermore, Allen et al. showed there was no difference in total healthcare costs between probiotics (£8020; 95% CI, 7620 to 8420 and (15,629 CAD; 95% CI, 14,850 to 16,409) and placebo (£8010; 95% CI, 7600 to 8420 and 15,601 CAD; 95% CI, 14,811 to 16,409).19
Antibiotic-associated diarrhea
Among three studies examining the cost-effectiveness of probiotics for AAD (Table 3), two studies found probiotics to be cost-effective,22,24 with one study showing no difference between probiotics and placebo.19
Lenoir-Wijnkoop et al. showed a mean cost-saving of £339 (642.94 CAD) per hospitalized patient for probiotics vs no treatment for prevention of AAD.22 Vermeersch et al. found cost-savings of €50.30 (75.74 CAD) using a bottom-up approach and €28.10 (42.31 CAD) using a top-down approach per AAD patient treated with antibiotics from a payer’s perspective. From a hospital/societal perspective, there was a cost-saving of €95.20 (143.35 CAD) (bottom-up) and €14.70 (22.13 CAD) (top-down) per AAD patient treated with probiotics.19
Conversely, Allen et al. found that probiotics were not cost-effective, with an ICER for AAD prevention of £4531.36 (£3,439.80–£5,622.92; 8,830.58 CAD [6,703.39–10,957.79]), and a base-case cost-utility of £189,662 (369,608 CAD) per QALY, for a WTP threshold of < £20,000 (38,975 CAD)/QALY.19
Sponsorship, economic perspective, trial vs. model-based, and placebo vs. no probiotic subgroup comparisons
Overall, of the seven studies included, six (86%) economic evaluations favoured probiotics as cost-effective/cost-saving in the base case. Three studies (43%) were sponsored by the manufacturer (Lactobacillus acidophilus/casei/paracasei). All three reported favourable findings towards probiotics. Three of four studies without manufacturer sponsorship favoured probiotics. Publication bias cannot be excluded.
The one trial-based economic evaluation did not show cost-effectiveness for its outcome,19 while all six model-based evaluations showed cost-effectiveness in their base cases and certain sensitivity analyses.18,20–24 For economic perspective subgroups, six of seven (86%) payer perspectives were cost-effective, while two of three (66%) of societal perspectives were cost-effective. For comparators, control arms (placebo vs no probiotic subgroups), two of three (66%) with placebo control arms were cost-effective, while four of four (100%) with no treatment/usual care control arms were cost-effective.
Grading of recommendations assessment, development, and evaluation assessment
The GRADE assessment46 (Table 4) found very low certainty of evidence for probiotic use for VAP, CDAD, and AAD.
The outcome of VAP included one model-based economic evaluation. We downgraded for ROB (serious ROB from multiple model inputs with unclear/high ROB) and imprecision (serious for only one study in analysis).18
The outcome of CDAD included six health economic evaluations (one RCT-based and five model-based). We downgraded for ROB (serious: multiple model inputs with unclear/high ROB), inconsistency (serious: one not cost-effective and five cost-effective) and imprecision (CI crosses zero for one RCT economic evaluation, with many small studies included).19–24
The outcome of AAD included thee health economic evaluations (one RCT-based and two model-based). We downgraded for ROB (serious: multiple model inputs with unclear/high ROB), inconsistency (serious: one study not cost-effective, and two studies cost-effective) and imprecision (serious: CIs crossing zero in the largest RCT to date, with many small studies included).19,22,24
Discussion
In this systematic review of economic evaluations of probiotics in hospitalized adult patients, we found that most of the studies suggest probiotics are cost-saving/cost-effective in preventing VAP, CDAD, or AAD.18–24 Nevertheless, the largest trial-based RCT paired with a health economic evaluation to date found no difference in clinical outcomes, and no cost-effectiveness/cost-utility.19 The conclusions drawn from the collective studies in this systematic review are based on very low certainty evidence from the ROB and GRADE assessments, precluding strong inferences or definitive recommendations regarding probiotics.
We found no prior systematic reviews that focused on economic evaluations of probiotic prophylaxis in hospitalized patients, hence we conducted our own. Among economic evaluations included in this review, incremental costs/ICERs were expressed in costs per healthcare-associated infection event prevented, but heterogeneity in reporting prevented meta-analysis conduction. Further, variable time horizons make comparisons of economic evaluations problematic (specifically ICERs) as costs and resource utilization may change over different time horizons. Changes in time horizons or perspectives can lead to differing parameters (costs [direct vs indirect], or outcomes [patient vs payer]). Many studies only reported incremental costs rather than true ICERs. Results from different perspectives, time horizons, and variable incremental cost reporting all represent disparate cost outcomes, which need to be interpreted carefully within context.
Moreover, there are large ranges in WTP, which are difficult to interpret with no conventional WTP benchmarks for prevention of VAP, CDAD, and AAD. Different countries may differ on values quality of life and WTP, making benchmarks difficult to establish across jurisdictions. Cost-utility parameters (like cost per life-year or QALY gained) were less commonly reported. If cost per QALYs were available, it would help to inform economic comparisons with other healthcare interventions.
Compared with other infection-prevention strategies, probiotics appear to be similarly cost-effective. A study examining concomitantly administered central-line associated bloodstream infection (CLABSIs) and VAP programs combined documented ICERs of 14,250.74 USD (20,533.24 CAD)/life-year gained and 23,277.86 USD (33,540.02 CAD)/QALY.47 Multifaceted quality improvement programs for reducing CLABSIs in ICUs have shown dominance (lower cost and higher effectiveness) in 80% of model scenarios using probabilistic sensitivity analysis.48 A proactive model infection-control program for multi-drug resistant (MDR) organisms in general-surgical ICUs showed an ICER of 3,804 USD (5,320.01 CAD) per case averted of transmission of MDR organisms in one year compared with standard infection control. For a WTP threshold of 14,000 USD (19,579.43 CAD) per transmission averted, there is a 42% probability of being cost-effective, and 100% probability when WTP thresholds were 22,000 USD (30,767.68 CAD).49 These similarities suggest that adoption of probiotics for prevention of healthcare-associated infections could be cost-effective.
New interventions studied in economic evaluations are occasionally sponsored by drug manufacturers. This potentially introduces bias in model construction and interpretation of results. In a retrospective analysis of 107 studies in five leading medical journals with regard to outcome and sources of funding, trials sponsored by pharmaceutical companies were more likely to favour the new drug over traditional therapy.50,51
In our systematic review, three studies were funded by manufacturers and all found the sponsored intervention to be more economically attractive, which could suggest potential publication bias (although this was not proven). This is tempered by three of four peer-review funded studies that also showed cost-effectiveness. Hence, methodologically rigorous trials with concomitant economic evaluations from peer-review funded studies are needed to ensure proper interpretation of results.
Strengths of our review include adherence to rigorous methodology, consisting of a comprehensive search strategy, broad eligibility criteria, and study selection by two independent adjudicators to minimize selection bias.17 We conducted data abstraction and appraisal in duplicate, using established criteria for assessing economic evaluations.11 We performed assessments of study quality employing ROB assessments, including assessment of source studies utilized in model-based economic evaluations.13–15 We performed assessment of level of certainty using GRADE.17 We also addressed the relationship of for-profit industry sponsorship potentially influencing the reporting of economic evaluations.
This review also has limitations. The inclusion of only seven studies influences precision. Rare product-specific complications such as probiotic-induced complications (i.e., bacteremia) are unclear, underscoring the need for additional safety data. Overall GRADE certainty of evidence was very low for all outcomes, rendering conclusions non-definitive. Our review included only adult patients and may not be applicable to pediatric populations. Evaluated reports varied widely with respect to patient population, time horizon of therapy, and payer perspective, which challenges the generalizability and interpretation of these findings.
Conclusion
This systematic review found that probiotics may be an economically attractive strategy for the prevention of healthcare-associated infections in most studies. Nevertheless, our GRADE summary indicates a very low quality/certainty of evidence, such that inferences are weak regarding the health economic evaluation of probiotics in adult hospitalized patients. Future RCTs should include concomitant economic evaluations, including clinical outcomes and costs associated with probiotics, to inform bedside practice, clinical guidelines, and healthcare policy. To this end, an economic evaluation of PROSPECT (E-PROSPECT) is planned.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (PDF 105 kb) eAppendix 1 Search strategy
Supplementary material 2 (PDF 85 kb) eAppendix 2 Data extraction form (change to a form)
Supplementary material 3 (PDF 78 kb) eAppendix 3 Risk of bias (ROB) assessment methods
Supplementary material 4 (PDF 145 kb) eAppendix 4A-B Risk of bias table 4A) Risk of bias assessment for source clinical studies utilized in health economic analysis of probiotics (randomized-controlled trials); 4B) Risk of Bias Assessment for Source Clinical Studies Utilized in Health Economic Analysis of Probiotics (Observational Studies – Utilizing the Newcastle-Ottawa Quality Assessment Scale for Cohort Studies); 4C) Risk of Bias Assessment for Source Clinical Studies Utilized in Health Economic Analysis of Probiotics (Observational Studies – Utilizing the Newcastle-Ottawa Quality Assessment Scale for Case-Control Studies); 4D) Risk of Bias Assessment for Source Clinical Studies Utilized in Health Economic Analysis of Probiotics (Evidence Partners and CLARITY for Risk of Bias of Surveys)
Supplementary material 5 (PDF 175 kb) eAppendix 5 Costing data (natural units, unit costs, and/or total costs)
Acknowledgments
Author contributions
Vincent Lau, Bram Rochwerg, Feng Xie, John Basmaji, Jana Balakumaran, Alla Iansavichene, Jennie Johnstone, and Deborah Cook made substantial contributions to study conception and design, and acquisition, analysis and interpretation of data; and drafted the submitted article and revised it critically for important intellectual content. Conception: Lau, Rochwerg, Xie, Cook. Background: Vincent Lau, Feng Xie, Bram Rochwerg, Alla Iansavichene, Jennie Johnstone, and Deborah Cook. Design: Vincent Lau, Bram Rochwerg, Feng Xie, and Deborah Cook. Acquisition of data: Vincent Lau, Jana Balakumaran, John Basmaji, Bram Rochwerg, Feng Xie, Alla Iansavichene, Jennie Johnstone, and Deborah Cook. Drafting the manuscript: Vincent Lau, Bram Rochwerg, Feng Xie, John Basmaji, Jana Balakumaran, Alla Iansavichene, Jennie Johnstone, and Deborah Cook. Revising the manuscript: Vincent Lau, Bram Rochwerg, Feng Xie, John Basmaji, Jana Balakumaran, Alla Iansavichene, Jennie Johnstone, and Deborah Cook.
Acknowledgements
We are grateful to the Canadian Critical Care Trials Group and the Canadian Institute of Health Research who have supported PROSPECT. We also thank Juanita Meyer (Library Services, London Health Sciences Center) for her assistance with the full-text acquisition and search strategy.
Competing interests
Several co-authors (Drs. Deborah Cook, Jennie Johnstone, Bram Rochwerg) are co-investigators in PROSPECT (Probiotics: Prevention of Severe Pneumonia and Endotracheal Colonization Trial) NCT01782755.
Funding statement
D Cook holds a Canada Research Chair in Knowledge Translation in Intensive Care Medicine from the Canadian Institutes of Health Research.
Editorial responsibility
This submission was handled by Dr. Sangeeta Mehta, Associate Editor, Canadian Journal of Anesthesia.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. Diarrhoea. Available from URL: http://www.who.int/topics/diarrhoea/en/ (accessed September 2019).
- 2.Marshall JC. Gastrointestinal flora and its alterations in critical illness. Curr Opin Clin Nutr Metab Care. 1999;2:405–411. doi: 10.1097/00075197-199909000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Brenchley JM, Douek DC. Microbial translocation across the GI tract. Annu Rev Immunol. 2012;30:149–173. doi: 10.1146/annurev-immunol-020711-075001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hao Q, Lu Z, Dong BR, Huang CD, Wu T. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst Rev 2011; 9: CD006895. [DOI] [PubMed]
- 5.Hempel S, Newberry SJ, Maher AR, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhea: a systematic review and meta-analysis. JAMA. 2012;307:1959–1969. doi: 10.1001/jama.2012.3507. [DOI] [PubMed] [Google Scholar]
- 6.Manzanares W, Lemieux M, Langlois PL, Wischmeyer PE. Probiotic and synbiotic therapy in critical illness: a systematic review and meta-analysis. Crit Care. 2016 doi: 10.1186/s13054-016-1434-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Safdar N, Dezfulian C, Collard HR, Saint S. Clinical and economic consequences of ventilator-associated pneumonia: a systematic review. Crit Care Med. 2005;33:2184–2193. doi: 10.1097/01.CCM.0000181731.53912.D9. [DOI] [PubMed] [Google Scholar]
- 8.Goldenberg JZ, Yap C, Lytvyn L, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev 2017; 12: CD006095. [DOI] [PMC free article] [PubMed]
- 9.Nanwa N, Kendzerska T, Krahn M, et al. The economic impact of Clostridium difficile infection: a systematic review. Am J Gastroenterol. 2015;110:511–519. doi: 10.1038/ajg.2015.48. [DOI] [PubMed] [Google Scholar]
- 10.Gomersall JS, Jadotte YT, Xue Y, Lockwood S, Riddle D, Preda A. Conducting systematic reviews of economic evaluations. Int J Evid Based Healthc. 2015;13:170–178. doi: 10.1097/XEB.0000000000000063. [DOI] [PubMed] [Google Scholar]
- 11.Husereau D, Drummond M, Petrou S, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMJ. 2013 doi: 10.1136/bmj.f1049. [DOI] [PubMed] [Google Scholar]
- 12.The World Bank. Official exchange rate (LCU per US$, period average). Available from URL: https://data.worldbank.org/indicator/PA.NUS.FCRF (accessed September 2019).
- 13.Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011 doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wells GA, Shea B, O’Connell D, et al. The Ottawa Hospital Research Institute. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available from URL: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed September 2019).
- 15.Agarwal A, Guyatt G, Busse J. Evidence Partners. Methods commentary: risk of bias in cross-sectional surveys of attitudes and practices. Available from URL: https://www.evidencepartners.com/resources/methodological-resources/risk-of-bias-cross-sectional-surveys-of-attitudes-and-practices/ (accessed September 2019).
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009; 62: 1006-12. [DOI] [PubMed]
- 17.Schünemann H, Brożek J, Guyatt G, Oxman A. GRADE Handbook; 2013. Available from URL: https://gdt.gradepro.org/app/handbook/handbook.html (accessed September 2019).
- 18.Branch-Elliman Westyn, Wright Sharon B., Howell Michael D. Determining the Ideal Strategy for Ventilator-associated Pneumonia Prevention. Cost–Benefit Analysis. American Journal of Respiratory and Critical Care Medicine. 2015;192(1):57–63. doi: 10.1164/rccm.201412-2316OC. [DOI] [PubMed] [Google Scholar]
- 19.Allen SJ, Wareham K, Wang D, et al. A high-dose preparation of lactobacilli and bifidobacteria in the prevention of antibiotic-associated and Clostridium difficile diarrhoea in older people admitted to hospital: a multicentre, randomised, double-blind, placebo-controlled, parallel arm trial (PLACIDE) Health Technol Assess. 2013;17:1–140. doi: 10.3310/hta17570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamdeu Fansi AA, Guertin JR, LeLorier J. Savings from the use of a probiotic formula in the prophylaxis of antibiotic-associated diarrhea. J Med Econ. 2012;15:53–60. doi: 10.3111/13696998.2011.629015. [DOI] [PubMed] [Google Scholar]
- 21.Leal JR, Heitman SJ, Conly JM, Henderson EA, Manns BJ. Cost-effectiveness analysis of the use of probiotics for the prevention of Clostridium difficile–associated diarrhea in a provincial healthcare system. Infect Control Hosp Epidemiol. 2016;37:1079–1086. doi: 10.1017/ice.2016.134. [DOI] [PubMed] [Google Scholar]
- 22.Lenoir-Wijnkoop I, Nuijten MJ, Craig J, Butler CC. Nutrition economic evaluation of a probiotic in the prevention of antibiotic-associated diarrhea. Front Pharmacol. 2014 doi: 10.3389/fphar.2014.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen NT, Leff JA, Schneider Y, et al. Cost-effectiveness analysis of probiotic use to prevent Clostridium difficile infection in hospitalized adults receiving antibiotics. Open Forum Infect Dis. 2017 doi: 10.1093/ofid/ofx148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vermeersch SJ, Vandenplas Y, Tanghe A, Elseviers M, Annemans L. Economic evaluation of S. boulardii CNCM I-745 for prevention of antibioticassociated diarrhoea in hospitalized patients. Acta Gastroenterol Belg 2018; 81: 269-76. [PubMed]
- 25.Kuntz JL, Polgreen PM. The importance of considering different healthcare settings when estimating the burden of Clostridium difficile. Clin Infect Dis. 2015;60:831–836. doi: 10.1093/cid/ciu955. [DOI] [PubMed] [Google Scholar]
- 26.Elseviers MM, Van Camp Y, Nayaert S, et al.) Prevalence and management of antibiotic associated diarrhea in general hospitals. BMC Infect Dis 2015; DOI: 10.1186/s12879-015-0869-0. [DOI] [PMC free article] [PubMed]
- 27.ISPOR. Good Research Practices for Measuring Drug Costs in Cost-Effectiveness Analyses: A Societal Perspective; 2010. Available from URL: https://www.ispor.org/heor-resources/good-practices-for-outcomes-research/article/good-research-practices-for-measuring-drug-costs-in-cost-effectiveness-analyses-a-societal-perspective (accessed September 2019). [DOI] [PubMed]
- 28.Salminen MK, Tynkkynen S, Rautelin H, et al. Lactobacillus bacteremia during a rapid increase in probiotic use of Lactobacillus rhamnosus GG in Finland. Clin Infect Dis. 2002;35:1155–1160. doi: 10.1086/342912. [DOI] [PubMed] [Google Scholar]
- 29.Song X, Bartlett JG, Speck K, Naegeli A, Carroll K, Perl TM. Rising economic impact of clostridium difficile-associated disease in adult hospitalized patient population. Infect Control Hosp Epidemiol. 2008;29:823–828. doi: 10.1086/588756. [DOI] [PubMed] [Google Scholar]
- 30.Miller MA, Hyland M, Ofner-Agostini M, et al. Morbidity, mortality, and healthcare burden of nosocomial Clostridium difficile-associated diarrhea in Canadian hospitals. Infect Control Hosp Epidemiol. 2002;23:137–140. doi: 10.1086/502023. [DOI] [PubMed] [Google Scholar]
- 31.Kyne L, Hamel MB, Polavaram R, Kelly CP. Health care costs and mortality associated with nosocomial diarrhea due to Clostridium difficile. Clin Infect Dis. 2002;34:346–353. doi: 10.1086/338260. [DOI] [PubMed] [Google Scholar]
- 32.Pepin J, Alary ME, Valiquette L, et al. Increasing risk of relapse after treatment of Clostridium difficile colitis in Quebec. Canada. Clin Infect Dis. 2005;40:1591–1597. doi: 10.1086/430315. [DOI] [PubMed] [Google Scholar]
- 33.van Walraven C. The Hospital-patient One-year Mortality Risk score accurately predicted long-term death risk in hospitalized patients. J Clin Epidemiol. 2014;67:1025–1034. doi: 10.1016/j.jclinepi.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 34.Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372:825–834. doi: 10.1056/NEJMoa1408913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dubberke ER, Reske KA, Olsen MA, McDonald LC, Fraser VJ. Short- and long-term attributable costs of Clostridium difficile-associated disease in nonsurgical inpatients. Clin Infect Dis. 2008;46:497–504. doi: 10.1086/526530. [DOI] [PubMed] [Google Scholar]
- 36.Lawrence SJ, Puzniak LA, Shadel BN, Gillespie KN, Kollef MH, Mundy LM. Clostridium difficile in the intensive care unit: epidemiology, costs, and colonization pressure. Infect Control Hosp Epidemiol. 2007;28:123–130. doi: 10.1086/511793. [DOI] [PubMed] [Google Scholar]
- 37.Riley TV, Codde JP, Rouse IL. Increased length of hospital stay due to Clostridium difficile associated diarrhoea. Lancet. 1995;345:455–456. doi: 10.1016/S0140-6736(95)90439-5. [DOI] [PubMed] [Google Scholar]
- 38.Ananthakrishnan AN, McGinley EL, Binion DG. Excess hospitalisation burden associated with Clostridium difficile in patients with inflammatory bowel disease. Gut. 2008;57:205–210. doi: 10.1136/gut.2007.128231. [DOI] [PubMed] [Google Scholar]
- 39.Kofsky P, Rosen L, Reed J, Tolmie M, Ufberg D. Clostridium difficile–a common and costly colitis. Dis Colon Rectum. 1991;34:244–248. doi: 10.1007/BF02090164. [DOI] [PubMed] [Google Scholar]
- 40.Wassenberg MW, Kluytmans JA, Box AT, et al. Rapid screening of methicillin-resistant Staphylococcus aureus using PCR and chromogenic agar: a prospective study to evaluate costs and effects. Clin Microbiol Infect. 2010;16:1754–1761. doi: 10.1111/j.1469-0691.2010.03210.x. [DOI] [PubMed] [Google Scholar]
- 41.Henrich TJ, Krakower D, Bitton A, Yokoe DS. Clinical risk factors for severe Clostridium difficile-associated disease. Emerg Infect Dis. 2009;15:415–422. doi: 10.3201/eid1503.080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sunenshine RH, McDonald LC. Clostridium difficile-associated disease: new challenges from an established pathogen. Cleve Clin J Med. 2006;73:187–197. doi: 10.3949/ccjm.73.2.187. [DOI] [PubMed] [Google Scholar]
- 43.Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014;370:1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sullivan PW, Ghushchyan V. Preference-based EQ-5D index scores for chronic conditions in the United States. Med Decis Making. 2006;26:410–420. doi: 10.1177/0272989X06290495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bauer MP, Notermans DW, van Benthem BH, et al. Clostridium difficile infection in Europe: a hospital-based survey. Lancet. 2011;77:63–73. doi: 10.1016/S0140-6736(10)61266-4. [DOI] [PubMed] [Google Scholar]
- 46.Brunetti Massimo, Shemilt Ian, Pregno Silvia, Vale Luke, Oxman Andrew D., Lord Joanne, Sisk Jane, Ruiz Francis, Hill Suzanne, Guyatt Gordon H., Jaeschke Roman, Helfand Mark, Harbour Robin, Davoli Marina, Amato Laura, Liberati Alessandro, Schünemann Holger J. GRADE guidelines: 10. Considering resource use and rating the quality of economic evidence. Journal of Clinical Epidemiology. 2013;66(2):140–150. doi: 10.1016/j.jclinepi.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 47.Dick AW, Perencevich EN, Pogorzelska-Maziarz M, Zwanziger J, Larson EL, Stone PW. A decade of investment in infection prevention: a cost effectiveness analysis. Am J Infect Control. 2015;43:4–9. doi: 10.1016/j.ajic.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Herzer KR, Niessen L, Constenla DO, Ward WJ Jr, Pronovost PJ. Cost-effectiveness of a quality improvement programme to reduce central line-associated bloodstream infections in intensive care units in the USA. BMJ Open 2014; DOI: 0.1136/bmjopen-2014-006065. [DOI] [PMC free article] [PubMed]
- 49.Jayaraman SP, Jiang Y, Resch S, Askari R, Klompas M. Cost-effectiveness of a model infection control program for preventing multi-drug-resistant organism infections in critically ill surgical patients. Surg Infect (Larchmt) 2016;17:589–595. doi: 10.1089/sur.2015.222. [DOI] [PubMed] [Google Scholar]
- 50.Kelly RE, Cohen LJ, Semple RJ, et al. Relationship between drug company funding and outcomes of clinical psychiatric research. Psychol Med. 2006;36:1647–1656. doi: 10.1017/S0033291706008567. [DOI] [PubMed] [Google Scholar]
- 51.Yaphe J, Edman R, Knishkowy B, Herman J. The association between funding by commercial interests and study outcome in randomized controlled drug trials. Fam Pract. 2001;18:565–568. doi: 10.1093/fampra/18.6.565. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1 (PDF 105 kb) eAppendix 1 Search strategy
Supplementary material 2 (PDF 85 kb) eAppendix 2 Data extraction form (change to a form)
Supplementary material 3 (PDF 78 kb) eAppendix 3 Risk of bias (ROB) assessment methods
Supplementary material 4 (PDF 145 kb) eAppendix 4A-B Risk of bias table 4A) Risk of bias assessment for source clinical studies utilized in health economic analysis of probiotics (randomized-controlled trials); 4B) Risk of Bias Assessment for Source Clinical Studies Utilized in Health Economic Analysis of Probiotics (Observational Studies – Utilizing the Newcastle-Ottawa Quality Assessment Scale for Cohort Studies); 4C) Risk of Bias Assessment for Source Clinical Studies Utilized in Health Economic Analysis of Probiotics (Observational Studies – Utilizing the Newcastle-Ottawa Quality Assessment Scale for Case-Control Studies); 4D) Risk of Bias Assessment for Source Clinical Studies Utilized in Health Economic Analysis of Probiotics (Evidence Partners and CLARITY for Risk of Bias of Surveys)
Supplementary material 5 (PDF 175 kb) eAppendix 5 Costing data (natural units, unit costs, and/or total costs)