Abstract
Background:
Non-motor symptoms (NMS) are common in Parkinson’s disease (PD), but their relationships to nigrostriatal degeneration remain largely unexplored.
Methods:
We evaluated 18 NMS scores covering 5 major domains in relation to concurrent and future dopamine transporter (DAT) imaging in 344 PD patients from the Parkinson’s Progression and Markers Initiative (PPMI). We standardized NMS assessments into z-scores for side-by-side comparisons. Patients underwent sequential DaTSCAN imaging at enrollment and at months 12, 24, and 48. Specific binding ratios (SBR) were calculated using the occipital lobe reference region. We evaluated the association of striatal DAT binding at the four time points with each baseline NMS using mixed-effects regression models.
Results:
Multiple baseline NMS were significantly associated with DAT binding at baseline and at follow-up scans. REM sleep behavior disorder (RBD) symptoms showed the strongest association – mean striatal SBR declined with increasing RBD symptom z-score (average of time-point-specific slopes per unit change in z-score: βAVG = −0.083, SE=0.017; p<0.0001). In addition, striatal DAT binding was linearly associated with increasing baseline z-scores: positively for the memory (βAVG =0.055, SE=0.022; p=0.01) and visuospatial (βAVG =0.044, SE=0.020; p=0.03) cognitive domains, and negatively for total anxiety (βAVG = −0.059, SE=0.018; p=0.001). Striatal DAT binding showed curvilinear associations with odor identification, verbal discrimination recognition, and autonomic dysfunction z-scores (p=0.001, p=0.0009, and p=0.0002, respectively). Other NMS were not associated with DAT binding.
Conclusions:
Multiple NMS, RBD symptoms in particular, are associated with nigrostriatal dopaminergic changes in early PD.
Keywords: Non-motor symptoms, Parkinson’s disease, Dopamine transporter binding
Parkinson’s disease (PD) is characterized by progressive loss of dopaminergic neurons in the substantia nigra pars compacta. The clinical motor presentation at the time of PD diagnosis reflects an extensive loss of nigrostriatal neurons that has occurred during a prolonged prodromal period.1 Advancements in neuroimaging techniques have allowed in vivo visualization of the integrity of the nigrostriatal dopaminergic system in PD. Dopamine transporter (DAT) imaging with single photon emission computed tomography (SPECT) using radioligands such as [123I]FP-CIT or [123I]β-CIT shows promise for monitoring Parkinson’s progression and severity.2,3 However, due to concerns about high cost, radiation exposures, accessibility, and the potential of being influenced by drugs, the neuroimaging has been mostly used in research settings, and clinically for differential parkinsonian syndromes from benign tremor phenomena.
Certain non-motor symptoms (NMS), such as olfactory impairment and rapid eye movement sleep behavior disorder (RBD), may reflect early neuropathological changes in extranigral nerve structures1; some NMS may progress in parallel with nigrostriatal dopaminergic pathology during PD progression. Importantly, a number of these NMS may occur years if not decades before PD diagnosis, and thus have been investigated as markers for prodromal PD.4 As these NMS can be readily evaluated with simple screeners or tests, it is of great interest to know whether they are also correlated with nigrostriatal degeneration in PD, particularly in prodromal PD. However, current evidence is limited to a small number of studies that focused exclusively on one or a few NMS.5–7 Using data from sequential DAT-SPECT imaging in the Parkinson’s Progression and Markers Initiative (PPMI), we evaluated whether the presence of selected NMS from five major NMS domains are associated with concurrent and future nigrostriatal dopaminergic function in newly diagnosed PD patients.
METHODS
Study Participants
PPMI is a longitudinal, international multicenter study designed to identify biomarkers of PD progression. Details of the study’s aims and methodology have been published8 and are on the PPMI website (http://www.ppmi-info.org/study-design). A total of 423 newly diagnosed, drug naïve PD patients met stringent enrollment criteria8 and underwent a comprehensive series of clinical and neuroimaging assessments (see supplementary material). Sixty-four patients withdrew their consent during the course of the study, and we chose to exclude them from the analyses. After further excluding 15 cases without baseline DAT imaging, our final analytical sample included 344 patients with early PD.
NMS Assessments
The NMS assessments at baseline included an extensive set of validated tests and questionnaires that examined five major areas of non-motor functions: including sleep, olfaction, neurobehavioral, autonomic function, and cognition (Supplemental Table S1). Sleep disturbance was evaluated by the Epworth Sleepiness Scale (ESS) and RBD Screening Questionnaire (RBDSQ). Olfaction was assessed by the University of Pennsylvania Smell Identification Test (UPSIT). Neurobehavioral assessments included the State-Trait Anxiety Inventory (STAI), Questionnaire for Impulsive-Compulsive Disorder in Parkinson’s Disease (QUIP), and the 15-item version of the Geriatric Depression Scale (GDS-15). Autonomic dysfunction was evaluated using the Scales for Outcomes in Parkinson’s disease – Autonomic (SCOPA-AUT). Neuropsychological tests included the Montreal Cognitive Assessment (MoCA) as a global cognitive screening test and a battery of tests evaluating cognitive domains: memory, visuospatial functions, working memory-executive functions, and attention-processing speed.
DAT Imaging Acquisition and Processing
All PD participants underwent [123I]FP-CIT SPECT imaging at enrollment and months 12, 24, and 48. SPECT imaging acquisition was performed 4±0.5 hours after an intravenous injection of 111 to 185 MBq (3–5 mCi) of 123I-ioflupane (DaTscan), a time point at which striatal specific binding ratios (SBRs) are stable.9 Raw projection data were acquired into a 128 x 128 matrix stepping each 3 or 4 degrees for a total of 120 or 90 projections, respectively, in a window centered on 159±10% KeV. The recommended image acquisition was performed in step and shoot mode, with each head rotating 360 degrees using a parallel hole collimator. SPECT raw projection data was then imported into a HERMES (Hermes Medical Solutions, Stockholm, Sweden) system for iterative reconstruction. Reconstructions for all imaging centers were done centrally at the Institute for Neurodegenerative Disorders, New Haven, Connecticut to ensure consistency of the reconstructions. Following reconstruction, data were transferred to the PMOD (PMOD Technologies, Zurich, Switzerland) brain software for subsequent processing. Attenuation correction ellipses were drawn on the images and a Chang zero attenuation correction was applied. The site-specific attenuation coefficient, µ, was empirically derived from phantom data acquired during a preceding technical site visit. Spatial normalization was performed to standard Montreal Neurologic Institute space so that all scans were in the same anatomical alignment; trained analysts assessed the quality of normalization visually. Count densities were extracted from four striatal regions of interest (left and right caudate nucleus and left and right putamen) with the occipital cortex as the reference region. SBRs were calculated separately for each striatal regions according to the formula: SBR = [(striatal region count density) / (occipital region count density)] −1. We used the mean total SBR (the average of the four SBRs from the aforementioned striatal regions) as the primary outcome measure.
Statistical Analysis
We presented means and standard deviations for continuous variables and proportions for categorical variables. We compared baseline characteristics between eligible PD cases who completed all four SPECT imaging assessments (termed “completers”) and those who did not (termed “non-completers”) using Mann-Whitney-Wilcoxon rank-sum tests or χ2 tests as appropriate. To allow comparability across different NMS measures, original test scores on global cognition and all NMS with the exception of neuropsychological assessments were converted to z-scores (mean=0, standard deviation=1). For neuropsychological measures, raw scores were standardized to z-scores according to published normative data that are corrected for age, education, and/or sex as appropriate. We then calculated a composite score for each cognitive domain by averaging the z-scores of the neuropsychological tests contributing to that domain. For UPSIT and cognitive domains assessments, lower baseline z-scores represent worsening of the respective functions, whereas for all remaining NMS, higher baseline z-scores represent worsening of the respective functions.
We calculated a mean SBR separately for caudate nucleus, putamen, and the overall striatum for each of the four neuroimaging assessment periods. We used mean total SBR as the primary outcome and mean caudate and putamen SBR as secondary. We used mixed-effects regression models to assess whether striatal DAT binding at baseline and the three subsequent visits was associated with an individual NMS at baseline – a separate model for each of the NMS. We treated time of DAT imaging as a categorical variable, thus allowing a possible nonlinear decline in SBR through time. Though we anticipated a linear relationship between SBR and NMS z-score, we adopted a modeling strategy that checked for curvature. We modeled the relationship between SBR and an NMS as quadratic in the NMS z-score with a separate intercept, separate slope, and separate quadratic term for each categorical imaging time. If a simultaneous test of the four quadratic terms showed no evidence of curvature, we removed those terms from the model. In models retaining quadratic terms, we tested whether the four terms differed; similarly, in models with only linear terms, we tested whether slopes differed. We report regression parameters for individual imaging times as well as their averages across the four imaging times. For example, these time-point-specific slopes estimate the change in SBR at each time-point per unit change in the NMS z-score. Each model also included terms to adjust for relevant covariates: dichotomous (0–1) terms for sex and race; linear continuous terms for age and education, and separate linear continuous terms for duration of PD at each time of DAT imaging. To account for correlations among repeated SBR measurements for each subject, we modeled within-subject variation using an unstructured covariance matrix.
In all tables and figures, we present the estimated SBR at each time point for a specific NMS for a hypothetical white male with average values of all other covariates at baseline of the PPMI participants (61 years old, 16-years of education, and disease duration of 6 months from PD diagnosis). Lastly, for NMS that were significantly associated with change in mean total SBR across the 48 months, we conducted a post-hoc analysis by creating a summary NMS z-score and examining whether the combined score would strengthen the association. We performed all analyses using SAS, version 9.4 (SAS Institute Inc., Cary, NC). All statistical tests were 2-sided with α=0.05.
Standard protocol approvals, registrations, and patient consents
Each participating PPMI site obtained written informed consent from all participants and received approval from an ethical standards committee on human experimentation.
RESULTS
Of the 344 eligible PD cases who provided baseline clinical and imaging data (Table 1), 298 (87%) completed SPECT imaging at month 12, 295 (86%) at month 24, 258 (75%) at month 48, and 225 (65 %) completed all four. We saw no statistically significant differences in baseline demographic or clinical characteristics between completers and non-completers, although non-completers seem to have more severe disease (HY stage 2 in non-completers vs. HY stage 1 in completers).
Table 1.
Demographic and clinical characteristics of PD patients at enrollment
| Characteristics | Enrolled (n=344) |
Completersa (N=225) |
Non-completersb (N=119) |
|---|---|---|---|
| Age at enrollment, y | 61.3 (9.7) | 60.9 (9.6) | 61.9 (9.9) |
| Race (% white) | 93.6 | 95.6 | 89.9 |
| Sex (% male) | 66.3 | 67.1 | 64.7 |
| Education, y | 15.6 (3.0) | 15.5 (2.9) | 15.7 (3.1) |
| Disease duration, mo | 6.4 (6.4) | 6.6 (6.7) | 6.1 (5.8) |
| Duration since symptom onset, mo | 23.4 (21.6) | 23.1 (17.4) | 24.1 (28.0) |
| Time from symptom onset to diagnosis, mo | 17.0 (20.4) | 16.5 (16.2) | 18.1 (26.6) |
| Tremor at diagnosis, (%) | 77.0 | 75.6 | 79.8 |
| Rigidity at diagnosis, (%) | 76.2 | 78.2 | 72.3 |
| Bradykinesia at diagnosis, (%) | 82.3 | 83.6 | 79.8 |
| Postural instability at diagnosis, (%) | 7.3 | 7.6 | 6.7 |
| Family history of PD, (%) | 14.4 | 13.1 | 16.8 |
| UPDRS Part III score | 20.5 (8.7) | 20.3 (8.4) | 21.0 (9.4) |
| Hoehn and Yahr stage, median | 2.0 | 1.0 | 2.0 |
Abbreviations: PD = Parkinson disease; UPDRS = Unified Parkinson’s Disease Rating Scale. Data are given as mean (SD) for continuous variables, and percentage (%) for categorical variables.
Completers are those among the enrolled cases who completed all four SPECT imaging assessments.
Non-completers are those among the enrolled cases who completed fewer than four SPECT imaging assessments.
We observed markedly reduced DAT binding in both striatal regions over the study period (Table 2). Sequential DAT imaging showed a 26.6% [17.0%] reduction in mean total striatal DAT availability from baseline to month 48, with an annualized decline of 9.7% [14.6%] during the initial 12 months of the study, 6.1% [15.5%] during the second year, and 12.2% [15.4%] during the subsequent two years (Table 2). The mean percent loss of DAT binding from baseline to month 48 was greater in the putamen (30.3% [21.1%]) than in the caudate (24.7% [17.8%]).
Table 2.
DAT striatal specific binding ratios (SBR) in PD patients across the 48-month follow-upa
| Mean (SD) DAT SBR |
|||
|---|---|---|---|
| Time of Scan | Striatum | Caudate | Putamen |
| Baseline (n=344) | 1.41 (0.37) | 1.98 (0.53) | 0.81 (0.27) |
| Month 12 (n=298) | 1.25 (0.35) | 1.79 (0.50) | 0.70 (0.24) |
| Month 24 (n=295) | 1.16 (0.37) | 1.67 (0.52) | 0.66 (0.25) |
| Month 48 (n=258) | 1.01 (0.33) | 1.48 (0.50) | 0.55 (0.20) |
| Mean (SD) Percent Change in DAT SBRb |
|||
| Time of Scan | Striatum | Caudate | Putamen |
| 12 months since baseline | −9.7 (14.6) | −8.5 (16.4) | −11.4 (19.7) |
| 24 months since baseline | −15.8 (16.6) | −14.7 (16.6) | −17.4 (21.1) |
| 48 months since baseline | −26.6 (17.0) | −24.7 (17.8) | −30.3 (21.1) |
Abbreviations: DAT = dopamine transporter; PD = Parkinson disease.
Mean and percent change in DAT SBR are unadjusted.
Among completers of all four DAT imaging assessments (N=225).
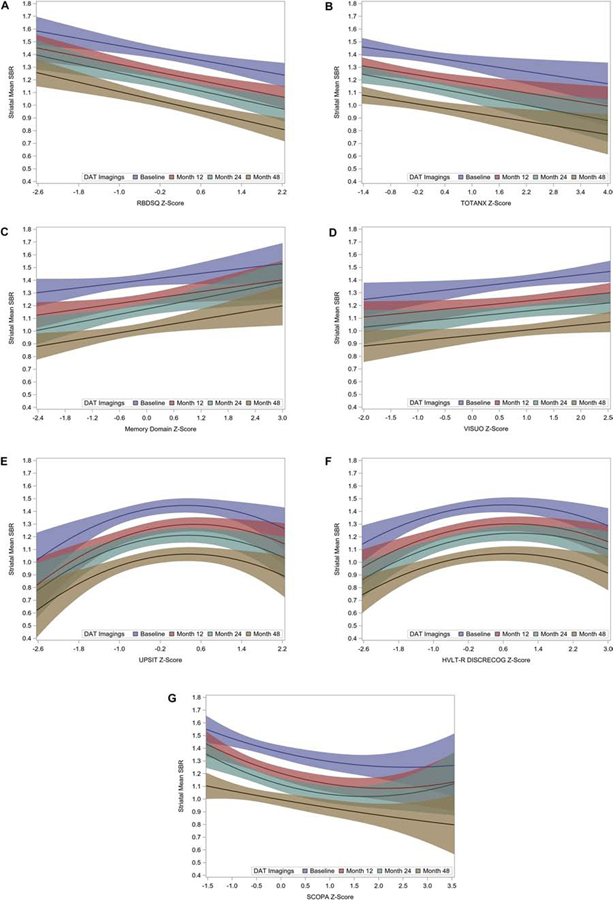
Several NMS measured at baseline were significantly associated with mean striatal SBR at baseline and subsequent follow-up visits (Table 3). With the exception of three NMS, we found no evidence of curvilinear associations with mean striatal SBR (Fig 1A–D), and we reported the mean slope across all four time points because they did not differ significantly (p >0.1). Among all measured NMS, baseline RBDSQ z-score showed the strongest relationship with DAT mean striatal SBR (Table 3). For each one-unit increase in baseline RBDSQ z-score, mean striatal SBR declined by 0.083 (SE, 0.017; p<0.0001) (Fig. 1A). Similar associations between mean striatal SBR and NMS severity were noted for total anxiety, memory and visuospatial cognitive domains (Table 3 & Fig. 1B, C, D).
Table 3.
Nonmotor symptoms in relation to DAT mean striatal specific binding ratios (SBR) over time in PD
| Nonmotor Symptomsb | Change in DAT Mean Striatal SBR per unit change in NMS z-scorea |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Month 12 | Month 24 | Month 48 | Overall 4 Year Period | |||||||
| β (SE) | P Value | β (SE) | P Value | β (SE) | P Value | β (SE) | P Value | Average βc (SE) | P Valueh | ||
| Sleep Disturbances | |||||||||||
| Epworth sleepiness scale | −0.038 (0.020) | 0.05 | −0.027 (0.018) | 0.14 | −0.039 (0.020) | 0.05 | −0.028 (0.019) | 0.13 | −0.033 (0.018) | 0.06 | |
| RBDSQ | −0.071 (0.019) | 0.0003 | −0.080 (0.018) | <.0001 | −0.088 (0.020) | <.0001 | −0.092 (0.019) | <.0001 | −0.083 (0.017) | <.0001 | |
| Neurobehavioral | |||||||||||
| Total anxiety | −0.053 (0.020) | 0.01 | −0.059 (0.019) | 0.002 | −0.067 (0.020) | 0.001 | −0.057 (0.019) | 0.003 | −0.059 (0.018) | 0.001 | |
| State anxiety | −0.043 (0.020) | 0.03 | −0.055 (0.019) | 0.003 | −0.054 (0.020) | 0.01 | −0.061 (0.019) | 0.001 | −0.053 (0.018) | 0.003 | |
| Trait anxiety | −0.056 (0.020) | 0.005 | −0.054 (0.019) | 0.004 | −0.072 (0.020) | 0.001 | −0.045 (0.019) | 0.02 | −0.057 (0.018) | 0.002 | |
| Geriatric depression | −0.020 (0.020) | 0.32 | −0.037 (0.018) | 0.05 | −0.032 (0.020) | 0.12 | −0.024 (0.019) | 0.22 | −0.028 (0.018) | 0.12 | |
| Cognitive Domainsd | |||||||||||
| Global | 0.122 (0.020) | 0.55 | −0.005 (0.019) | 0.79 | 0.001 (0.021) | 0.96 | −0.003 (0.019) | 0.89 | 0.001 (0.019) | 0.94 | |
| Executive–Working Memory | 0.016 (0.029) | 0.60 | 0.014 (0.027) | 0.62 | 0.021 (0.030) | 0.48 | 0.028 (0.028) | 0.31 | 0.020 (0.026) | 0.45 | |
| Letter number sequencing | 0.017 (0.024) | 0.46 | 0.011 (0.022) | 0.61 | 0.0008 (0.024) | 0.75 | −0.003 (0.023) | 0.90 | 0.008 (0.022) | 0.70 | |
| Semantic fluency | 0.0002 (0.020) | 0.90 | 0.005 (0.019) | 0.80 | 0.015 (0.021) | 0.46 | 0.031 (0.019) | 0.11 | 0.013 (0.018) | 0.47 | |
| Processing Speed– Attention | 0.041 (0.023) | 0.08 | 0.044 (0.022) | 0.06 | 0.028 (0.024) | 0.25 | 0.038 (0.022) | 0.10 | 0.038 (0.022) | 0.08 | |
| Visuospatial | 0.049 (0.022) | 0.03 | 0.042 (0.020) | 0.04 | 0.042 (0.022) | 0.06 | 0.042 (0.021) | 0.04 | 0.044 (0.020) | 0.03 | |
| Memory | 0.042 (0.023) | 0.08 | 0.051 (0.022) | 0.02 | 0.070 (0.024) | 0.004 | 0.059 (0.022) | 0.01 | 0.055 (0.022) | 0.01 | |
| HVLT-R immediate recall | 0.042 (0.018) | 0.02 | 0.037 (0.017) | 0.03 | 0.056 (0.018) | 0.002 | 0.045 (0.017) | 0.009 | 0.045 (0.016) | 0.006 | |
| HVLT-R delayed recall | 0.009 (0.017) | 0.60 | 0.018 (0.016) | 0.27 | 0.022 (0.018) | 0.22 | 0.028 (0.016) | 0.09 | 0.019 (0.016) | 0.22 | |
| HVLT-R discrimination recognitione, f | (slope) | 0.035 (0.017) | 0.05 | 0.047 (0.016) | 0.004 | 0.051 (0.018) | 0.004 | 0.041 (0.017) | 0.02 | 0.044 (0.016) | 0.006 |
| (curvature) | −0.027 (0.009) | 0.004 | −0.030 (0.008) | 0.0005 | −0.026 (0.009) | 0.005 | −0.029 (0.009) | 0.001 | −0.028 (0.008) | 0.0008 | |
| Olfaction | |||||||||||
| UPSITe, f | (slope) | 0.032 (0.021) | 0.14 | 0.048 (0.020) | 0.02 | 0.034 (0.022) | 0.12 | 0.036 (0.021) | 0.08 | 0.037 (0.020) | 0.06 |
| (curvature) | −0.051 (0.018) | 0.005 | −0.041 (0.017) | 0.01 | −0.054 (0.018) | 0.003 | −0.058 (0.017) | 0.0006 | −0.051 (0.016) | 0.002 | |
| Autonomic | |||||||||||
| SCOPA-AUTe, g | (slope) | −0.092 (0.025) | 0.0002 | −0.112 (0.023) | <.0001 | −0.105 (0.025) | <.0001 | −0.062 (0.024) | 0.010 | −0.093 (0.023) | <.0001 |
| (curvature) | 0.015 (0.013) | 0.24 | 0.024 (0.012) | 0.04 | 0.035 (0.013) | 0.008 | 0.0006 (0.012) | 0.62 | 0.020 (0.012) | 0.09 | |
Abbreviations: DAT = dopamine transporter; NMS = Non-motor symptoms; PD = Parkinson disease; RBDSQ = REM Sleep Behavior Disorder Screening Questionnaire; SCOPA-AUT = Scales for Outcomes in Parkinson’s disease – Autonomic; UPSIT = University of Pennsylvania Smell Identification Test.
Unless otherwise noted, parameters (β) are slope estimates from a model with separate slopes for each imaging time.
With the exception of UPSIT, cognitive domains, and SCOPA-AUT where lower baseline z-scores represents worsening of the respective functions, for all remaining NMS, higher baseline z-scores represent worsening of the respective functions.
We saw no evidence of differences among time-point-specific slopes for any individual NMS (p>0.1), thereby justifying the reporting of an average slope for each NMS.
The global domain included the Montreal Cognitive Assessment (MoCA) test. The memory domain included the immediate recall, delayed recall, and discrimination recognition of the Hopkins Verbal Learning Test-Revised (HVLT-R). The visuospatial domain included the Benton Judgment of Line Orientation (JOLO). The working memory-executive domain included the Letter Number Sequencing (LNS) and the Semantic Fluency-Animal tests. The attention-processing speed included the Symbol Digit Modalities Test (SDMT).
Coefficients reported are from a quadratic model with separate slope and curvature parameters for each imaging time. Two-degree-of-freedom test that average slope and average curvature are both zero: p=0.0009 for HVLT-R; p=0.001 for UPSIT; and p=0.0002 for SCOPA_AUT.
HVLT-R discrimination recognition and UPSIT showed evidence of a common curvature at all timepoints but no evidence of differential curvature.
SCOPA-AUT showed evidence of differential curvature among different time points.
The p-values are not adjusted for multiple comparisons; if the Bonferroni correction is applied at experiment-wise error rate of 0.05 for 21 tests (the number of NMS considered), the significance cut-off would be set at 0.05/21= 0.0024.
Figure 1.
Relationships among mean striatal specific binding ratio (SBR) at baseline and follow-ups and NMS z-scores. Color coding indicates the time of DAT imaging; the four time-point-specific slopes for each NMS were not significantly different statistically (p>0.1). Shading represents 95% confidence bands for each time-point-specific regression. A) REM Sleep Behavior Disorder Screening Questionnaire (RBDSQ), B) Total Anxiety (State-Trait Anxiety Inventory for Adults (STAI)), C) Memory Cognitive Domain, D) Visuospatial Cognitive Domain, E) University of Pennsylvania Smell Identification Test (UPSIT), F) Hopkins Verbal Learning Test – Discrimination Recognition, G) Scales for Outcomes in Parkinson’s Disease-Autonomic Questionnaire (SCOPA-AUT). In A) through D), fitted models have separate intercepts and slopes for each imaging time but no curvature. In E) and F), fitted models have separate intercepts and slopes for each imaging time but a common curvature parameter; in G), fitted model has separate intercepts, slopes, and curvature parameters for each imaging time.
Three NMS showed curvilinear associations with mean striatal SBR. Olfactory identification (UPSIT) and HVLT-R discrimination recognition each exhibited common curvature across different imaging times (overall association test covering both average slope and average quadratic terms, p= 0.001 and p= 0.0009, respectively). For both these NMS, mean striatal SBR increased with z-score for low z-scores, then leveled off, and decreased (especially for olfaction) at the highest z-scores (Fig. 1E & Fig. 1F). Autonomic dysfunction (SCOPA-AUT) exhibited different curvature parameters at different imaging times (overall association, p= 0.0002); mean striatal SBR decreased at low z-scores then leveled off (Fig. 1G).
To put these NMS z-score slope estimates in the context of age-related changes, we calculated the change in striatal mean SBR associated with a unit increase in baseline NMS z-score and used the estimated age coefficient from our fitted models to estimate the number years of aging that would be required to achieve the same estimated change in SBR. These estimates ranged from 6 years for visuospatial function to 11 years for RBDSQ. Results were similar in separate analyses for caudate (Supplemental Table S2) and putamen (Supplemental Table S3). We did not detect significant associations for other NMS symptoms.
Finally, we refit the model using a summary z-score of the six NMS that were individually associated with SBR, and this only modestly improved the overall association. For each one-unit change in the summary NMS z-score, the average change in mean total SBR was −0.11 (SE, 0.017, p<0.0001) compared to the slope with the strongest estimate of −0.083 among the four individual NMS.
DISCUSSION
NMS encompasses a wide array of neuropsychological, sensory, autonomic, and sleep symptoms that are common among PD patients.10 The Braak hypothesis suggests that PD pathology may begin in extranigral structures, such as the olfactory bulb and medulla, before spreading to the nigrostriatal system.11,12 Speculatively, non-cognitive and behavioral NMS presentation in prodromal PD may reflect these extranigral pathologies13 and may progress in parallel with nigrostriatal dopaminergic pathology during PD. A number of large prospective studies identified some of these symptoms, including RBD and olfactory impairment, as highly predictive of future risk of PD14. However, the potential connections between these extranigral NMS symptoms and nigrostriatal pathology are poorly understood. A few studies investigated striatal DAT binding among special cohorts of individuals with idiopathic RBD (iRBD) or hyposmia and found significant reductions in tracer uptake. Moreover, both conditions predicted conversion to clinical PD.11,12,15–18
In this study, we aimed to identify current NMS that might reflect current and future DAT binding. To our knowledge, this study is the first to compare multiple NMS side-by-side in relation to DAT binding, albeit we were only able to these comparisons among newly diagnosed PD patients. We found that several baseline NMS symptoms, including RBDSQ, olfactory identification, anxiety, autonomic function, and memory, particularly verbal discrimination recognition, and visuospatial cognitive functions were significantly associated with both current and future DAT levels. These findings support the exciting possibility of using NMS as indirect markers for nigrostriatal changes in PD.
Of these NMS, RBD symptoms showed the strongest association with concurrent and future DAT binding. RBD affects roughly 40% of PD patients,10 half of them may have developed RBD before PD diagnosis. In longitudinal studies of polysomnography (PSG) confirmed iRBD patients, up to 80% eventually developed PD or a related synucleinopathy in the next decade or so.11,16,19 Clinical studies also showed that iRBD patients had reduced striatal DAT binding or abnormal midbrain hyperechogenicity compared to controls.15 Moreover, reduced DAT binding predicted phenotypical conversion of iRBD to clinical neurodegenerative diseases.11,15,16 Our data further support these clinical observations. RBD symptoms, even assessed with a simple screener, is strongly associated with current and future nigrostriatal deficits in newly diagnosed PD.
Compared to RBD, olfactory impairment or hyposmia is much more prevalent in older adults with and without PD.20 At the population level, it is among the most sensitive predictor for future PD risk21; however, it is also much less specific to PD than RBD.10 Among studies of first-degree relatives of PD patients,7,18 hyposmia was associated with DAT deficits in both cross-sectional and longitudinal analyses. When combined with DAT imaging, it was highly predictive of conversion to clinical PD in prodromal PD.17,18 Further, several previous studies showed significant correlations between olfactory impairment and striatal DAT binding in early PD.5,6,22 However, these studies are all cross-sectional and only had DaTSCAN imaging at a single time point and in a small sample of patients. Our study is much larger and included DAT scans at four time points. In our analyses, we found the relationship between UPSIT z-score and striatal SBR was not linear, increasing first and then leveling off and decreasing. We also found curvilinear associations for z-scores of verbal discrimination recognition and autonomic dysfunction. They, however, appear more consistent with a flattening of an initially steeper trend rather than with a frank change in direction.
In the current study, memory, and more specifically immediate recall, was also significantly associated with DAT progression. Previous smaller studies of newly diagnosed PD patients showed significant correlations between dopaminergic tracer uptake in specific regions and executive functions.23,24 Further, in first-degree relatives of PD patients, cognitive performance on global cognition and executive function/working memory measures strengthened the association between hyposmia and DAT reduction.7 Conversely, previous studies showed that lower baseline striatal DAT binding was a significant predictor of global cognitive decline over a 22-month period among de novo PD patients.25 In our study, a moderate association was also observed for the visuospatial cognitive domain. This observation is consistent with reports that visuospatial impairment is an early feature of cognitive deterioration in PD.26 In one report from the PARS study it was observed that 38 subjects with hyposmia and DAT scan abnormalities had significantly lower mean scores on measures of global cognition, executive function/working memory, and episodic memory than scores of subjects with either hyposmia only or DAT abnormality only.7 Further, hyposmic subjects with mild (1 SD below the mean) cognitive problems were at fourfold risk for DAT abnormality (on the basis of hyposmia alone) compared to normosmic subjects without cognitive impairment. Taken together, these reports suggest that cognitive impairment occurs early in the disease course and may be partially mediated/or contributed to nigrostriatal disease pathology.7 Indeed, it has been estimated that, already at PD diagnosis, up to one third of PD patients have mild cognitive impairment (MCI).27,28 If this proportion is correct, it stands to reason that subclinical cognitive changes should be observable in a sizeable minority of PD patients before diagnosis.
There is a lack of data on autonomic dysfunction in relation to DAT binding. One study29 linked occurrences of autonomic dysfunction to RBD status and showed that they are more pronounced in Parkinson’s patients with RBD than those without. Our finding of a significant association between autonomic function and nigrostriatal deficits at baseline and month 12 provides further support for the evidence that autonomic dysfunction may develop early and are an integral part of PD pathogenesis.
The current study builds on the strength of the PPMI cohort, a landmark international effort to systematically search for clinical, biochemical and neuroimaging biomarkers for the progression of PD. The large sample size of newly diagnosed PD patients with serial DAT imaging allowed us to examine nigrostriatal deficits at various time points. The assessment at the study baseline of a wide range of NMS and their severity with well-validated instruments offers a unique opportunity to examine multiple NMS simultaneously in relation to objective changes in nigrostriatal dopaminergic function. We chose not to dichotomize NMS variables in data analyses to take advantage of the full spectrum of symptom severity which was often ignored in previous studies on NMS and PD.
Our analysis also has several notable limitations. First, investigating nigrostriatal pathology in relation to these NMS would hold the most interest in prodromal PD when motor signs are not yet clinically evident; however, our subjects were not prodromal but were recently diagnosed PD patients. Nevertheless, for statistically significant NMS, the relationship between SBR and NMS z-score at baseline did not change markedly whether SBR was measured at baseline or years later. Whether this finding could be extrapolated into the prodromal stage of PD remains to be investigated. Second, while nigrostriatal degeneration is central to PD, its NMS presentation is heterogenous. Therefore, NMS severity may simply correlate with disease progression, rather than directly relate to the progressive loss of dopaminergic neurons in PD. Third, DAT availability may be affected by dopamine replacement therapy (DRT) as the disease progresses. However, PPMI patients are drug naïve at enrollment when NMS were assessed. Further, an early PPMI publication showed that change in DAT availability over the first year, during which over half of participants began DRT, was not significantly different between those who did and did not initiate DRT.30 Fourth, the cognitive battery is limited and did not cover certain domains such as the language domain. Fifth, the curvilinear associations we observed for three of the NMS with mean striatal SBR are puzzling. Future studies are needed to confirm or refute these observations. Finally, the PPMI cohort comprises predominately white volunteers who were committed to multiple comprehensive examinations and longitudinal follow-ups. Therefore, our findings may not be readily generalizable to general PD patients.
In summary, our findings suggest that in newly diagnosed PD patients, certain non-motor features are associated with nigrostriatal dopamine binding and supports the exciting possibility of using NMS to assist with monitoring clinical PD and patient stratification for clinical research. If these findings could be extrapolated to the prodromal stage of PD, assessment of NMS may also hold great potential in characterizing high-risk populations and in measuring prodromal disease progression.
Supplementary Material
Highlights.
Multiple non-motor symptoms were associated with dopamine transporter (DAT) binding.
REM sleep behavior disorder (RBD) symptoms showed the strongest association.
Striatal DAT binding was positively associated with memory and visuospatial cognitive domains.
Striatal DAT binding was negatively associated with total anxiety.
Striatal DAT binding showed curvilinear associations with odor identification, verbal discrimination recognition, and autonomic dysfunction.
Acknowledgements:
We thank PPMI participants for their commitments to scientific research. Data used in the preparation of this article are from the Parkinson’s Progression Markers Initiative (PPMI) database (www.ppmi-info.org/data). For up-to-date information on the study, visit www.ppmi-info.org.
Funding: Parkinson’s Progression Markers Initiative, a public-private partnership, is funded by the Michael J. Fox Foundation for Parkinson’s Research and funding partners, including Abbvie, Avid Radiopharmaceuticals, Biogen Idec, Bristol-Myers Squibb, Covance, Élan, GE Healthcare, Genentech, GSK-GlaxoSmithKline, Lilly, Merck, MSD-Meso Scale Discovery, Piramal, Pfizer, Roche, and UCB (www.ppmi-info.org/fundingpartners). This work was supported by the Intramural Research Program of the National Institutes of Health, the National Institute of Environmental Health Sciences (Z01-ES-101986). The study is also in part supported by National Institute of Neurological Disorders and Stroke (NS082151 to XH), from the National Institute of Environmental Health Sciences (ES019672 to XH) and Penn State College of Medicine Translational Brain Research Center. HC is supported by a start-up fund from Michigan State University (GE100455), the Parkinson’s Foundation (Grant No. PF-IMP-1825), and the Office of the Assistant Secretary of Defense for Health Affairs, through the Parkinson’s Research Program (Award No. W81XWH-17–1-0536), and the National Institute of Environmental Health Sciences (R01ES029227). Opinions, interpretations, conclusions and recommendations are those of the author and are not necessarily endorsed by the Department of Defense and the National Institutes of Health.
Financial Disclosure: Dr. Tröster has received royalties from Oxford University Press, grant funding from Barrow Neurological Foundation and served on scientific advisory boards of Medtronic and Takeda.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None for all authors
REFERENCES
- 1.Berg D, Marek K, Ross GW, Poewe W. Defining at-risk populations for Parkinson’s disease: lessons from ongoing studies. Mov Disord 2012;27(5):656–665. [DOI] [PubMed] [Google Scholar]
- 2.Politis M Neuroimaging in Parkinson disease: from research setting to clinical practice. Nat Rev Neurol 2014;10(12):708–722. [DOI] [PubMed] [Google Scholar]
- 3.Benamer HT, Patterson J, Wyper DJ, Hadley DM, Macphee GJ, Grosset DG. Correlation of Parkinson’s disease severity and duration with 123I-FP-CIT SPECT striatal uptake. Mov Disord 2000;15(4):692–698. [DOI] [PubMed] [Google Scholar]
- 4.Berg D, Postuma RB, Adler CH, et al. MDS research criteria for prodromal Parkinson’s disease. Mov Disord 2015;30:1600–1611. [DOI] [PubMed] [Google Scholar]
- 5.Siderowf A, Newberg A, Chou KL, et al. [99mTc]TRODAT-1 SPECT imaging correlates with odor identification in early Parkinson disease. Neurology 2005;64:1716–1720. [DOI] [PubMed] [Google Scholar]
- 6.Berendse HW, Roos DS, Raijmakers P, Doty RL. Motor and non-motor correlates of olfactory dysfunction in Parkinson’s disease. J Neurol Sci 2011;310:21–24. [DOI] [PubMed] [Google Scholar]
- 7.Chahine LM, Weintraub D, Hawkins KA, et al. Cognition in individuals at risk for Parkinson’s: Parkinson associated risk syndrome (PARS) study findings. Mov Disord 2016;31:86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marek k, Jennings D, Lasch S, et al. The Parkinson Progression Marker Initiative (PPMI). Prog Neurobiol 2011;95:629–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Booij J, Hemelaar TG, Speelman JD, de Bruin K, Janssen AG, van Royen EA. One-day protocol for imaging of the nigrostriatal dopaminergic pathway in Parkinson’s disease by [123I]FPCIT SPECT. J Nucl Med 1999;40:753–761. [PubMed] [Google Scholar]
- 10.Chen H, Zhao EJ, Zhang W, et al. Meta-analyses on prevalence of selected Parkinson’s nonmotor symptoms before and after diagnosis. Transl Neurodegener 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iranzo A, Valldeoriola F, Lomena F, et al. Serial dopamine transporter imaging of nigrostriatal function in patients with idiopathic rapid-eye-movement sleep behaviour disorder: a prospective study. Lancet Neurol 2011;10:797–805. [DOI] [PubMed] [Google Scholar]
- 12.Stiasny-Kolster K, Doerr Y, Moller JC, et al. Combination of ‘idiopathic’ REM sleep behaviour disorder and olfactory dysfunction as possible indicator for alpha-synucleinopathy demonstrated by dopamine transporter FP-CIT-SPECT. Brain 2005;128:126–137. [DOI] [PubMed] [Google Scholar]
- 13.Adler CH, Beach TG. Neuropathological basis of nonmotor manifestations of Parkinson’s disease. Mov Disord 2016. [DOI] [PMC free article] [PubMed]
- 14.Postuma RB, Aarsland D, Barone P, et al. Identifying prodromal Parkinson’s disease: pre-motor disorders in Parkinson’s disease. Mov Disord 2012;27:617–626. [DOI] [PubMed] [Google Scholar]
- 15.Iranzo A, Lomena F, Stockner H, et al. Decreased striatal dopamine transporter uptake and substantia nigra hyperechogenicity as risk markers of synucleinopathy in patients with idiopathic rapid-eye-movement sleep behaviour disorder: a prospective study [corrected]. Lancet Neurol 2010;9:1070–1077. [DOI] [PubMed] [Google Scholar]
- 16.Iranzo A, Tolosa E, Gelpi E, et al. Neurodegenerative disease status and post-mortem pathology in idiopathic rapid-eye-movement sleep behaviour disorder: an observational cohort study. Lancet Neurol 2013;12:443–453. [DOI] [PubMed] [Google Scholar]
- 17.Ponsen MM, Stoffers D, Wolters E, Booij J, Berendse HW. Olfactory testing combined with dopamine transporter imaging as a method to detect prodromal Parkinson’s disease. J Neurol Neurosurg Psychiatry 2010;81:396–399. [DOI] [PubMed] [Google Scholar]
- 18.Jennings D, Siderowf A, Stern M, et al. Conversion to Parkinson Disease in the PARS Hyposmic and Dopamine Transporter-Deficit Prodromal Cohort. JAMA Neurol 2017;74:933–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Postuma RB, Gagnon JF, Bertrand JA, Genier Marchand D, Montplaisir JY. Parkinson risk in idiopathic REM sleep behavior disorder: preparing for neuroprotective trials. Neurology 2015;84:1104–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dong J, Pinto JM, Guo X, et al. The Prevalence of Anosmia and Associated Factors Among U.S. Black and White Older Adults. J Gerontol A Biol Sci Med Sci 2017;72:1080–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nalls MA, McLean CY, Rick J, et al. Diagnosis of Parkinson’s disease on the basis of clinical and genetic classification: a population-based modelling study. Lancet Neurol 2015;14:1002–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deeb J, Shah M, Muhammed N, et al. A basic smell test is as sensitive as a dopamine transporter scan: comparison of olfaction, taste and DaTSCAN in the diagnosis of Parkinson’s disease. QJM 2010;103:941–952. [DOI] [PubMed] [Google Scholar]
- 23.Nobili F, Campus C, Arnaldi D, et al. Cognitive-nigrostriatal relationships in de novo, drug-naive Parkinson’s disease patients: a [I-123]FP-CIT SPECT study. Mov Disord 2010;25:35–43. [DOI] [PubMed] [Google Scholar]
- 24.Polito C, Berti V, Ramat S, et al. Interaction of caudate dopamine depletion and brain metabolic changes with cognitive dysfunction in early Parkinson’s disease. Neurobiol Aging 2012;33:206 e229–239. [DOI] [PubMed] [Google Scholar]
- 25.Ravina B, Marek K, Eberly S, et al. Dopamine transporter imaging is associated with long-term outcomes in Parkinson’s disease. Mov Disord 2012;27:1392–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aarsland D, Bronnick K, Larsen JP, Tysnes OB, Alves G. Cognitive impairment in incident, untreated Parkinson disease: the Norwegian ParkWest study. Neurology 2009;72:1121–1126. [DOI] [PubMed] [Google Scholar]
- 27.Pedersen KF, Larsen JP, Tysnes OB, Alves G. Prognosis of mild cognitive impairment in early Parkinson disease: the Norwegian ParkWest study. JAMA Neurol 2013;70:580–586. [DOI] [PubMed] [Google Scholar]
- 28.Broeders M, Velseboer DC, de Bie R, et al. Cognitive change in newly-diagnosed patients with Parkinson’s disease: a 5-year follow-up study. J Int Neuropsychol Soc 2013;19:695–708. [DOI] [PubMed] [Google Scholar]
- 29.Postuma RB, Gagnon JF, Vendette M, Montplaisir JY. Markers of neurodegeneration in idiopathic rapid eye movement sleep behaviour disorder and Parkinson’s disease. Brain 2009;132:3298–3307. [DOI] [PubMed] [Google Scholar]
- 30.Smith KM, Xie SX, Weintraub D. Incident impulse control disorder symptoms and dopamine transporter imaging in Parkinson disease. J Neurol Neurosurg Psychiatry 2016;87:864–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.