Abstract
Objective
This study compared the participation in four faecal immunochemical testing-based screening programmes for colorectal cancer in Flanders, France, Basque country and the Netherlands, to identify factors to further optimize faecal immunochemical testing programmes.
Method
Background information and data on performance indicators were collected and compared for the four programmes.
Results
Invitation method, reminders, funding, faecal immunochemical testing cut-off and follow-up after positive faecal immunochemical testing differed in the four programmes. In France, only an invitation letter is sent by mail, while the sample kit must be collected from the general practitioner. In the other programmes, an invitation letter including the sample kit is sent by mail. Participation rates vary substantially according to the method of invitation, with the highest participation rates in the Netherlands (73.0%) and Basque country (72.4%), followed by Flanders (54.5%) and France (28.6%). Basque country (92.8%) and France (88.4%), the two programmes with most active involvement of general practitioners in referral for colonoscopy, had the highest participation rates for colonoscopy.
Conclusions
Large differences in screening participation observed between programmes according to the invitation method used suggest that changes to the design of the programme, such as including the sample kit with the invitation, or active involvement of GPs, might increase participation.
Keywords: Colorectal cancer, screening, faecal immunochemical testing, invitation method, population-based, participation
Introduction
Many countries and regions have implemented colorectal cancer (CRC) screening by faecal occult blood testing (FOBT), in particular using faecal immunochemical testing (FIT).1 Screening using FOBT is recommended by European Union CRC screening guidelines.2 In France, a national population-based CRC screening programme was initiated in 2002 using guaiac FOBT, which was changed to FIT-based screening in April 2015. FIT-based screening was introduced in 2009 in the Basque country (Spain), in 2013 in Flanders (Belgium) and in 2014 in the Netherlands. As these four European programmes are geographically close and connected, and have recently been implemented, similar outcomes with respect to CRC screening may have been expected.
The effectiveness of population-based screening programmes is driven not only by the sensitivity of the screening method but also by the availability of resources, healthcare infrastructure and population preferences. Population preferences in particular will be reflected in participation rate. To determine the optimal screening method for the population, pilot studies were performed in the Basque country, Flanders and the Netherlands before the initiation of the screening programmes. The Basque Country pilot study in 2009 demonstrated a participation rate with FIT screening of 64.3%.3 In Flanders, the pilot study compared participation for two invitation strategies: FIT directly sent by mail (52.3%), or invitation only by mail and FIT to be collected from the general practitioner (GP) (24.6%).4 In the Dutch pilot studies comparing different screening methods, FIT screening resulted in the highest CRC detection per invitee compared with other screening methods, such as gFOBT, colonoscopy and sigmoidoscopy screening.5–7
Crucial to optimal screening performance is the organizational structure of the programme, including pre-invitation letters, reminders, and FIT mailing; however, almost all studies of these aspects have been carried out in trials.4,8,9 Actual performance in routine screening programmes may differ because many other factors are involved, such as government-endorsement, organization of healthcare systems and healthcare insurance. Participation in the trial preceding the implementation of the national Dutch programme, for example, was 60%, which was significantly lower than the 71% achieved in the first year of the national programme. It is unknown how the different organizational aspects will affect individuals residing in different countries, with different sociocultural backgrounds.
This study aimed to identify similarities and differences in the organizational structure of four large population-based CRC screening programmes using FIT in France, Basque country (Spain), Flanders (Belgium) and the Netherlands, and then compare these with important programme performance indicators of participation and detection rates to infer relationships between organizational structure and these programme outcomes.
Methods
Information on year of initiation, target population, eligible population, screening interval, methods of invitation to FIT screening and to colonoscopy following a positive FIT, funding and executive organization of the four screening programmes was collected. The target population was defined for each population-based CRC screening programme according to programme-specific policies. The eligible population is the target population minus those who are ineligible for screening based on exclusion criteria. The eligible population was all individuals who should have been invited in 2016. This number can deviate from the total target population, because of biennial screening or phased implementation of the national screening programme.
Data on performance indicators were extracted from each of the national or regional screening databases. In France, data were extracted from the database of French Public Health Agency (Santé Publique France) and Organized screening structure of the Big East region and the Pyrénées. Data from Flanders were extracted from the screening database, the Belgian Cancer Registry and reimbursement data from the Health insurance companies. All data from the Basque country were extracted from the programme (Programa de Cáncer ColoRectal) database, which is linked with medical records, population and hospital cancer registries. All data from the Netherlands were extracted from the national database for screening programmes (ScreenIT). Data on the invitees were collected in France starting in April 2015 until June 2017. Data on the invitees of 2016 were collected in the Basque country until December 2017, and in Flanders and the Netherlands until 30 June 2017.
Data were collected on the main performance indicators: participation rate, FIT positivity rate, participation rate in colonoscopy following positive FIT, detection rate of CRC or advanced neoplasia (AN) per participant and diagnostic yield. The definitions of performance indicators were those recommended in the European Union CRC screening guidelines.10
Participation rate was the number of persons sending back the FIT sample divided by the number receiving an invitation letter. For Flanders and France, persons were only considered participants if they returned the FIT sample within 12 months after the invitation. In the Basque country, participants were those who returned an assessable stool sample within six months following the invitation. In the Netherlands, individuals were considered participants until the date of the invitation of subsequent screening round. Positivity rate was the number of persons with a FIT result at or above the cut-off level, divided by the number of persons with an assessable stool sample. Participation rate colonoscopy was the number undergoing a colonoscopy divided by the number with a positive FIT result. Detection rate was defined as the number of persons with AN detected during colonoscopy per participant. AN was considered a relevant abnormality within a CRC screening programme, and was defined as CRC or any adenoma with histology showing ≥25% villous component or high-grade dysplasia or adenoma with size ≥10 mm. In Flanders, only adenomas with any villous component and/or high-grade dysplasia were counted as advanced adenoma, because no data were available on adenoma size or the amount of villous components. In the Basque country, in addition to histology, dysplasia and size, having ≥3 adenomas was also considered as advanced adenoma. Diagnostic yield of the programme was defined as the number of persons with AN detected during colonoscopy divided by all individuals who received an invitation. In Flanders, data of colonoscopy yield were not linked to the date of invitees of the programme. The denominator can contain individuals invited in previous year.
The organizational structures of the four programmes were compared using thematic analysis to identify similarities or differences. Outcomes of the performance indicators for each of the programmes were then compared. To rule out the possibility that the observed difference may be related to cultural differences between populations rather than organizational differences, the programme of Basque country in Spain was compared with the Basque country in France, as these two regions are very close with respect to geographical location and cultural background. The different subgroups were compared using chi-squared test. This test was performed using R version 3.5.0.
Results
The age range of the target populations differed, with France and the Basque country having the lowest starting age of 50 and the Basque country having the lowest stopping age of 69 (Table 1). All four programmes used a two-year screening interval. Exclusion criteria prior to invitation differed among the four programmes. In the Netherlands, persons are only excluded based on a positive FIT result of previous screening round (Table 1). France, Flanders and Basque country all excluded individuals with history of CRC, proctocolectomy and recently performed colonoscopy before invitation. France and Flanders also excluded individuals with a recently performed FIT test. Additionally, the Basque country excluded individuals with severe or terminal illness.
Table 1.
Background information on screening programmes.
| France | Flanders (Belgium) | Netherlands | Basque country (Spain) | |
|---|---|---|---|---|
| 1. General | ||||
| Year of introduction | Started in 2009 and switched nationally to the FIT in April 2015. | October 2013Phased implementation by age groups. In 2015, the programme was fully implemented. | 2014Phased implementation by age group. In 2019, the programme will be fully implemented. | 2009Phased implementation by provinces. In 2014, the programme was fully implemented. |
| Piloting | Yes | Yes | Yes | No |
| Public awareness campaigns | Yes. Through dedicated websites: National Cancer Institute (INCa), Health Insurance, some local initiatives. | Yes. Since 2015 short announcements on the Flemish television and since 2017 advertising in public transport. Both only during the month March (international CRC month). | Yes. Information on the website of the national institute of public health and the environment. Only at the start in 2014, there was extra media attention through television and radio. No advertising. | Yes. National yearly campaign by patient association and main results are presented in television, leaflets, radio and social network. |
| Population-based programme | National | Regional | National | Regional |
| Age group | 50–74 | 56–74Per 1 July 2017, it was extended to 55–74, and per July 2018, it was extended to 53–74 | 55–75 | 50–69 |
| Screening interval | Two years | Two years | Two years | Two years |
| Cut-off level (Hb/g faeces) | 30 µg | 15 µg | 47 µg | 20 µg |
| Brand name of the test | OC-Sensor, Eiken, Japan | OC-Sensor, Eiken, Japan | FOB-Gold, Sentinel, Italy | OC-Sensor, Eiken, Japan |
| 2. Methods of invitation | ||||
| Pre-invitation letter | No | No | Yes, three weeks in advance | Yes, four weeks in advance |
| Invitation by mail including FIT | No, test to be collected at GP’s office | Yes | Yes | Yes |
| Reminder letter | 12 Weeks and 24 weeks | 8 Weeks | 6 Weeks | 4 Weeks |
| Exclusion of individuals before invitation | Yes | Yes | No | Yes |
| Exclusion criteria mentioned in the invitation letter | No | Past or current treatment of colorectal cancer, occult blood in stool and unexplained and persistent change in the bowel movement patterns, colonoscopy in past 10 years, stool test in past two years, higher risk at CRC, having one or more first relatives who have/had CRC. | Past or current treatment of colorectal cancer, occult blood in stool and unexplained and persistent change in the bowel movement patterns. | Colonoscopy performed ≤5 years. |
| 3. Methods of invitation to diagnostic colonoscopy | ||||
| Invitation to follow-up colonoscopy | By letter. Results are sent to the participant, the general practitioner and the structure in charge of organized CRC screening. | By letter. Results are sent to the participant and the general practitioner. | By letter, including appointment for colonoscopy intake. Results are sent to the participant and if possible the general practitioner. | By letter. Results are sent to the participant. In that letter, participant is recommended to visit GP for colonoscopy referral. |
| Reminder following initial invitation colonoscopy | No | No, from 2019, persons receive a new invitation for colonoscopy intake two years after their positive FIT. When not following this second advise, two years later (four years after the positive FIT), they will receive a new FIT. | Yes, persons receive a no show letter. In case of no response, persons received a new invitation for colonoscopy intake after two years after positive FIT (until 2017). From 2018 onwards, persons will receive a new FIT after two years. | Yes, after 30 days, the participant is reminded to make an appointment with the general practitioner for colonoscopy referral. If participants reject to have colonoscopy follow-up, they will receive a new FIT after two years. |
| 4. Organization | ||||
| Funding of screen test | Free of charge, but not the GP’s encounter to collect the kit that is covered at 70% by national public health insurance. The remaining 30% are being covered by complementary insurance if the participant has one. | Free of charge | Free of charge | Free of charge |
| Funding of colonoscopy | Standard healthcare insurance covers 70%. The remaining 30% is covered by complementary insurance if any. | Standard healthcare insurance and partially payment of personal funds (out-of-pocket costs). | Standard healthcare insurance.Note: Not in all situations colonoscopy costs are fully covered by the healthcare insurance. Up to 350–850 euro are paid of personal funds (out-of-pocket costs, only once a year a deductible excess of all healthcare costs). | Free of charge |
| Responsible organization of the screening programme | INCa, National cancer institute, with dedicated screening structure in each of the departments. The latest are in charge of invitations. | Centre for Cancer Detection (CvKO). In Flanders, CvKO consists of five regional screening programmes, but the invitation letter, reminder letter and FIT results are organized by one central organization. The five regional screenings programmes offer free telephone advice and are responsible for administration. | The national institute of public health and the environment is the responsible organization of the programme. Five regional screening organizations are responsible for the execution of the programme. Those five regional screening organizations are brought together in one national cooperation. | The Basque Health Service is responsible for the execution of the programme, according with authorities planning, organizing and monitoring the quality of the process and results.The final responsibility of the programme is the Regional Ministry of Health in the Basque country. |
CRC: colorectal cancer; GP: general practitioner; FIT: faecal immunochemical testing.
Methods of invitation also differed among the four programmes. The eligible population in France received an invitation letter to collect the FIT sample kit at the GP. In Flanders, the invitation included the FIT sample kit. In the Basque country and the Netherlands, a pre-invitation letter was sent, followed by an invitation letter including the FIT sample kit. All four screening programmes used a reminder letter, but all at different time points, ranging from 30 days (Basque country) to six months (France). The programme in France sent two reminder letters. All four programmes used different cut-offs for a positive FIT and referral to colonoscopy: in Flanders, 15 µg Hb/g faeces; the Basque country, 20 µg Hb/g faeces; France, 30 µg Hb/g faeces and the Netherlands, 47 µg Hb/g faeces (Table 1).
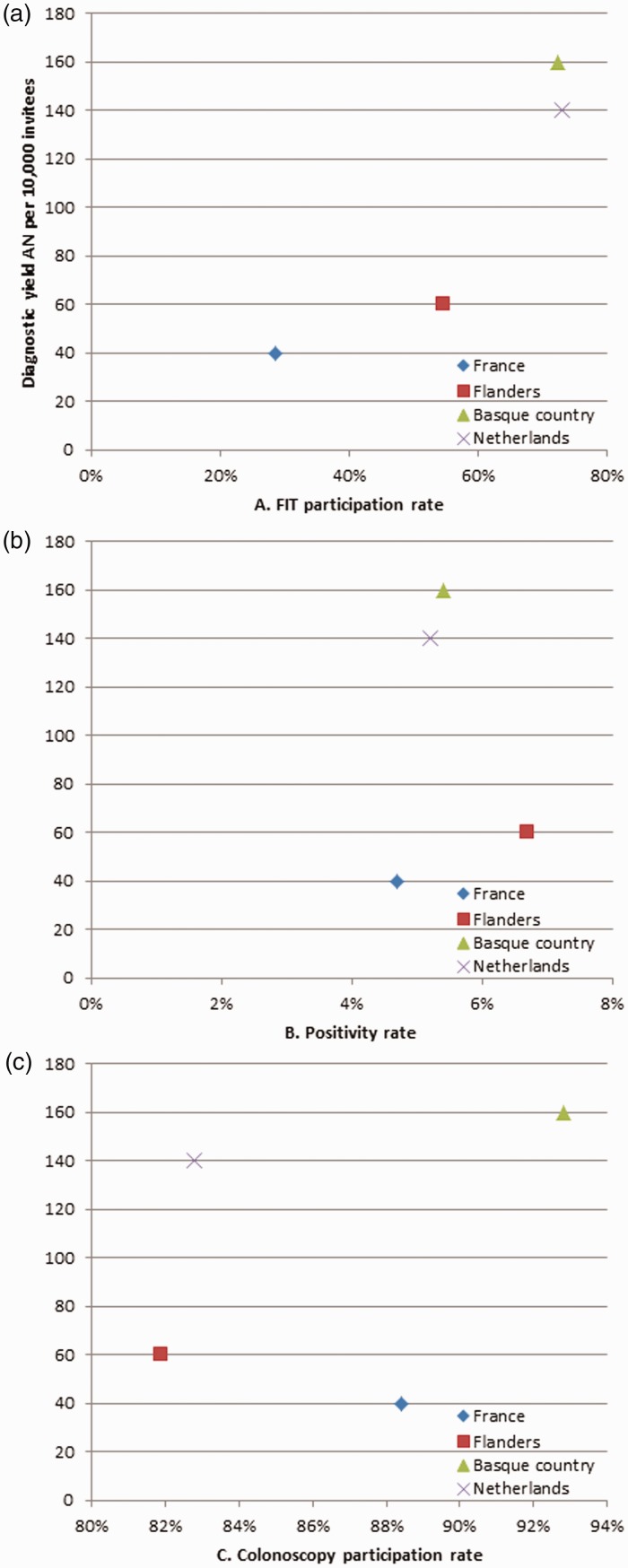
Among the four programmes, 18.9 million individuals were invited to participate in FIT screening. The highest participation rate was observed in the Netherlands (73.0%), followed by the Spanish Basque country (72.4%), Flanders (54.5%) and France (28.6%, p<0.001). Because of the different FIT cut-off levels used, the positivity rate differed between the four programmes, from 4.7% in France to 6.7% in Flanders (p<0.001). The participation rate for colonoscopy following a positive FIT result was 92.8% in the Basque country, 88.4% in France, 81.9% in Flanders and 82.8% in the Netherlands (p <0.001). Detection rate for AN per participant was highest in the Netherlands (2.3%) and lowest in Flanders (1.0%). Diagnostic yield for AN per invitee was highest in the Basque country (1.6%) and lowest in France (0.4%, p <0.001). Figure 1 shows the impact of FIT participation rate, positivity rate and colonoscopy participation rate on the diagnostic yield. This shows that programmes with the highest FIT participation rate have the highest diagnostic yield, but this is less visible for the positivity rate and colonoscopy participation rate.
Figure 1.
Diagnostic yield of advanced neoplasia by FIT participation rate.
AN: advanced neoplasia; FIT: faecal immunochemical testing.
In the comparison of the French with the Spanish Basque country, despite cultural similarities, differences in screening performance indicators were observed (Table 2). The participation rate in the Spanish part was 72.4%, 2.5 times as high as in the French part, with 24.6% (p <0.001) (Table 3). Participation rate to colonoscopy was high in both the Spanish and the French part (92.8% and 87.4%, respectively; p=0.37).
Table 2.
Performance indicators for France, Flanders, the Netherlands and Basque country.
| France | Flanders | Netherlands | Basque country | p | |
|---|---|---|---|---|---|
| Calendar year | 2015–2016 | 2016 | 2016 | 2016 | |
| Age (years) | 50–74 | 56–74 | 59–76 | 50–69 | |
| Target population | 19,043,771 | 1,447,434a | Unknown | 273,084 | |
| Eligible population | 16,701,387 | 830,665 | 1,543,223 | 239,601 | |
| Invited | 16,701,387 | 571,034 | 1,457,976 | 229,380 | |
| % | 100 | 68.7a | 94.5 | 87.7 | |
| Number of participants | 4,779,845 | 311,453 | 1,063,651 | 166,110 | <0.001 |
| Participation rate FIT % (95% CI) | 28.6 (28.6–28.6) | 54.5 (54.4–54.7)b | 73.0 (72.9–73.0) | 72.4 (72.2–72.6) | |
| Men | 27.8 | 53.1 | 71.1 | 70.0 | |
| Women | 30.8 | 56.0 | 74.8 | 74.6 | |
| Screen round | Any round | First and second | First and second | First to fourth round | |
| Cut-off level (Hb/g faeces) | 30 µg | 15 µg | 47 µg | 20 µg | |
| Positivity rate % (95% CI) | 4.7 (4.7–4.7) | 6.7 (6.7–6.8) | 5.4 (5.4–5.5) | 5.2 (5.1–5.3) | <0.001 |
| Participation rate colonoscopy % (95% CI) | 88.9 (88.8–89.0)c | 81.9 (81.4–82.4) | 82.8 (82.5–83.1) | 92.8 (92.3–93.4) | <0.001 |
| Detection rate | |||||
| AN% (95% CI) | 1.5 (1.5–1.5)c | 1.0 (1.0–1.1) | 2.3 (2.2–2.3) | 1.9 (1.9–2.0) | <0.001 |
| CRC% (95% CI) Diagnostic yield programme | 0.31 (0.30–0.32)c | 0.28 (0.26–0.30) | 0.35 (0.34–0.36) | 0.20 (0.18–0.22) | <0.001 |
| AN% (95% CI) | 0.4 (0.4–0.4)c | 0.6 (0.5–0.6) | 1.6 (1.6–1.7) | 1.4 (1.3–1.4) | <0.001 |
| CRC% (95% CI) | 0.09 (0.09–0.09)c | 0.15 (0.14–0.16) | 0.25 (0.25–0.26) | 0.15 (0.13–0.16) | <0.001 |
Note: Advanced neoplasia was defined as CRC or any adenoma with histology showing ≥25% villous component or high-grade dysplasia or adenoma with size ≥10 mm. In Basque country, also ≥3 adenomas were considered AN. In Flanders, only adenoma with a villous component and/or high-grade dysplasia was counted as advanced adenoma. There were no data available on the size or the amount of villous components in an adenoma. Detection rate: invitees with CRC or AN per participant. Diagnostic yield: individuals with CRC or AN per invitees.
AN: advanced neoplasia; NA: not available; FIT: faecal immunochemical testing.
aEligible population in Flanders is the total number aged 56–74 for two years minus those excluded for invitation. Eligible population for 2016 only could not be provided.
bCoverage by examination, also including opportunistic screening by FIT or colonoscopy, resulted in 65.5% of the target population to be screened.
cIn France, the participation rate of colonoscopy and number of colorectal cancers and advanced neoplasia was based on data from April 2015 until December 2015.
Table 3.
Outcome performance indicators Basque region.
| Basque country in France | Basque country in Spain | p | |
|---|---|---|---|
| Year | 2016 | 2016 | |
| Age | 50–74 | 50–69 | |
| Invited | 45,923 | 229,380 | |
| Number of participants | 11,293 | 166,110 | |
| Participation rate FIT % (95% CI) | 24.6 (24.2–25.0) | 72.4 (72.2–72.6) | <0.001 |
| Cut-off level (Hb/g faeces) | 30 µg | 20 µg | |
| Positivity rate % (95% CI) | 4.8 (4.4–5.2) | 5.2 (5.1–5.3) | 0.07 |
| Participation rate follow-up colonoscopy % (95% CI) | 87.4 (84.4–90.0) | 92.8 (92.3–93.4) | 0.37 |
| Detection rate | |||
| AN% (95% CI) | 1.4 (1.2–1.7) | 1.9 (1.9–2.0) | <0.001 |
| CRC% (95% CI) Diagnostic yield | 0.27 (0.19–0.39) | 0.20 (0.18–0.22) | |
| AN% (95% CI) | 0.4 (0.3–0.4) | 1.4 (1.3–1.4) | <0.001 |
| CRC% (95% CI) | 0.07 (0.05–0.10) | 0.15 (0.13–0.16) |
AN: advanced neoplasia; FIT: faecal immunochemical testing.
Note: Advanced neoplasia was defined as CRC or any adenoma with histology showing ≥25% villous component or high-grade dysplasia or adenoma with size ≥10 mm. In Basque country, also ≥3 adenomas were considered AN. Detection rate: invitees with CRC or AN per participant. Diagnostic yield: individuals with CRC or AN per invitees.
Discussion
Large differences in screening participation were observed between programmes, in line with the invitation method used. There are several possible explanations. First, sending the FIT home is more effective than having it collected at the GP. Almost all studies show a large increase in participation when including the FIT sample kit with the invitation.8,11–13 One Italian study showed only a modest increase in participation, but this study was performed in previously screened individuals (used to another screening strategy).14 One French study showed low uptake rates when directly mailing the FOBT.14 This inconsistency may be due to the test modality, gFOBT instead of FIT, resulting in lower participation rates.15
A second explanation for a higher FIT participation may be the advanced notification letter, as illustrated by the higher participation rate in the Spanish Basque country and the Netherlands. However, this will only explain a small proportion of the total difference, as studies have shown that sending a pre-invitation letter results in a three percentage point increase.9,16 Only one study from Australia showed a higher increase, nine percentage points, when a pre-invitation letter was sent.17 The higher participation with both direct mailing and the pre-invitation letter is in line with a recent systematic review.18 However, one large difference was that GP involvement improved participation. This has also been shown in France, with an increase of 4% if GPs were notified of the screening status of their patients so that they could actively promote CRC screening to non-participants.8 We showed the opposite in this study; in a country that sends out the FIT by mail with no involvement of GPs, such as the Netherlands, participation rates were very high, while in a country with active involvement of GPs, such as France, participation rates were substantially lower. We hypothesize that GP endorsement can have a positive impact on participation, as long this requires no effort for the participants. This is in line with findings of the CRC screening programme in England, which showed an increase in participation if the invitation letter was added with a GP endorsement banner.19
Our analysis of the two Basque regions in France and Spain showed that very similar cultures can have very different rates in screening participation, and that culture may not be the driving factor of performance differences between programmes, although we cannot rule out cultural differences completely. We know from the literature that cultural difference in screening attitude is also observed in the participation rates of other cancer screening programmes. In 2016, for example, participation in breast cancer screening was also lower in Flanders (51.9%) than in the Netherlands (77.6%) and the Basque country (80.1%), with France having the lowest participation rate (50.7%).20–22 The participation rate for breast cancer screening in France is similar to Flanders, while there is a much larger difference in participation rate for CRC screening. Gender cannot explain this difference, as both men and women show a similar pattern in participation. This again reflects the negative impact of using a different invitation method in France for FIT-based screening.
Participation rate to follow-up colonoscopy was high in all four screening programmes, although slightly below the recommended 85% in the Netherlands and Flanders. We hypothesize that higher participation to follow-up colonoscopy in Basque country and France may be the result of the active involvement of GPs during the screening process. In both countries, GPs play an active role in selecting the population eligible for FIT screening, at collection of the screening test, at follow-up after a negative FIT, or in defining the eligible population by excluding those with severe comorbidity from invitation. Consequently, those participating in FIT screening are all healthy enough to undergo follow-up colonoscopy. Conversely, it may also explain the lower participation to colonoscopy in the Netherlands, as there is no exclusion of individuals based on co-morbidities or medical history. Active involvement of GPs after positive FIT only for referral to colonoscopy, without involvement in the total screening process, will be less effective.8 Reimbursement differences for colonoscopy do not seem to explain participation differences. Although in the Basque country, the colonoscopy is free of charge, participation in France was only slightly lower, while French individuals may have significant expenses which have to be paid from personal funds. One explanation may be that in France, only the most motivated individuals collect the FIT sample kit at their GP practice, and they may thus also be more motivated to attend colonoscopy in case of a positive FIT.
Positivity rate differed for all four programmes, due to three important reasons: cut-off level of the FIT, target age group and screening round (first or subsequent round).23 Higher cut-off level and subsequent screening round will result in lower positivity rate, while higher age will result in higher positivity rate. The same explanations may partly hold for the difference in diagnostic yield of the programme. Previous research showed that using a higher FIT cut-off level will have a negative impact on detection rates.24 Nevertheless, the impact of positivity rate and colonoscopy participation rate on diagnostic yield was modest. Programmes with the highest FIT participation rate still have the highest diagnostic yield, regardless of their positivity rate or colonoscopy participation rate. This highlights the importance of high participation rates to primary screening, as this will result in the highest detection of AN. However, the difference in detection rates of AN is surprising, with the Netherlands having high detection rates while having the highest cut-off. This might be related to several factors: more incident screens in France and Basque country (lower detection), more prevalent screens in Flanders and the Netherlands, difference in age range (lower age, lower detection) and stricter definition of advanced adenomas in Flanders (lower detection).
Our study has three important strengths. It is the first study that gives detailed information on organizational structure of four large population-based programmes provided by representatives of each country. These details are generally unknown, as key elements of CRC screening programmes are only described in the local language (Flemish, French, Spanish/Basque, Dutch). These details can be used by other countries/regions considering CRC screening and are valuable for policymakers. In addition, our study contains the most recent outcomes of these large population-based programmes, all using the same test modality (FIT). Lastly, our study compared screening programmes of neighbouring countries with cultural similarities and differences, and can thus address the impact of cultural and organizational aspects on the uptake of CRC screening.
The study has also some limitations. Comparing quality indicators was challenging due to different definitions and differences in cut-off level and number of screening rounds. Unfortunately, we could not restrict the comparison to first screen round data only, as not all programmes have detailed information. In addition, data collection may be of concern as, for example, France does not yet have a centralized recording of quality indicators and does not centrally collect data on diagnostic yield.
Our findings suggest that organizational structure, for example, sending out the FIT, pre-invitation letter, involvement of the GP in the screening programme and colonoscopy referral, has an impact on the participation rate to FIT and follow-up colonoscopy. These results could be used to optimize each of the four screening programmes, or as an example for other organized FIT-based CRC screening programmes. Possibilities for optimization can differ for every organized programme, as healthcare systems, funding of the colonoscopy and available resources also differ. Interventions for optimization will have cost implications, and these results can therefore be used to explore the additional benefits and costs for each programme. France has already begun to optimize the screening programme, by deciding to mail the FIT with the first reminder, but only to those individuals who participated in previous rounds. The study by Giorgi-Rossi et al. suggests that these individuals may not be the best target.25
Although posting the FIT and actively approaching FIT positives for the colonoscopy seems to be most effective, this may be considered an infringement of free will.26 The goal of the screening programmes should not be high participation, but the level of informed choice, although infringement of free will should be considered in the light of the strength of the recommendation. For CRC screening, and in particular for colonoscopy in FIT positives, the evidence for net benefit is considered strong, and we can presume that the vast majority would weigh the balance of benefits and harm in the direction of having the intervention.27 Moreover, there is no indication that high participation in the Netherlands, for example, results in a lower level of informed choice.28,29 Besides these ethical considerations, there is also a remaining difference in participation that cannot be explained by the organizational structure and is difficult to unravel. This difference seems to be a difference in attitude towards screening in general between the different regions or countries, and it is unclear how this arises or how it can be solved.
Conclusion
This study shows that there is large variability in the design of these screening programmes, and that programmes with more evidence-based interventions in place (e.g. mailed-out FIT, pre-invitation and reminder letters) experience significantly higher screening participation than those without. Active involvement of GPs may result in slightly higher participation with colonoscopy after a positive FIT result, but should not come at the expense of extra barriers to collect the FIT, which can dramatically reduce primary screening participation.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Schreuders EH, Ruco A, Rabeneck L, et al. Colorectal cancer screening: a global overview of existing programmes. Gut 2015; 64: 1637–1649. [DOI] [PubMed] [Google Scholar]
- 2.Luxembourg: Council of the European Union. CotEUCrodocsEOJotEU-L, http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri-OJ:L:2003:327:0034:0038:EN:PDF (accessed 21 September 2017).
- 3.Portillo I, Idigoras I, Ojembarrena E, et al. [Main results of the colorectal cancer screening program in the Basque Country (Spain)] Principales resultados del programa de cribado de cancer colorrectal en el Pais Vasco. Gac Sanit 2013; 27: 358–361. [DOI] [PubMed] [Google Scholar]
- 4.Van Roosbroeck S, Hoeck S, Van Hal G. Population-based screening for colorectal cancer using an immunochemical faecal occult blood test: a comparison of two invitation strategies. Cancer Epidemiol 2012; 36: e317–e324. [DOI] [PubMed] [Google Scholar]
- 5.Hol L, van Leerdam ME, van Ballegooijen M, et al. Screening for colorectal cancer: randomised trial comparing guaiac-based and immunochemical faecal occult blood testing and flexible sigmoidoscopy. Gut 2010; 59: 62–68. [DOI] [PubMed] [Google Scholar]
- 6.Stoop EM, de Haan MC, de Wijkerslooth TR, et al. Participation and yield of colonoscopy versus non-cathartic CT colonography in population-based screening for colorectal cancer: a randomised controlled trial. Lancet Oncol 2012; 13: 55–64. [DOI] [PubMed] [Google Scholar]
- 7.van Rossum LG, van Rijn AF, Laheij RJ, et al. Random comparison of guaiac and immunochemical fecal occult blood tests for colorectal cancer in a screening population. Gastroenterology 2008; 135: 82–90. [DOI] [PubMed] [Google Scholar]
- 8.Rat C, Latour C, Rousseau R, et al. Interventions to increase uptake of faecal tests for colorectal cancer screening: a systematic review. Eur J Cancer Prev 2018; 27: 227–236. [DOI] [PubMed] [Google Scholar]
- 9.Senore C, Ederle A, DePretis G, et al. Invitation strategies for colorectal cancer screening programmes: the impact of an advance notification letter. Prev Med 2015; 73: 106–111. [DOI] [PubMed] [Google Scholar]
- 10.Moss S, Ancelle-Park R, Brenner H, International Agency for Research on Cancer. European guidelines for quality assurance in colorectal cancer screening and diagnosis. First Edition – evaluation and interpretation of screening outcomes. Endoscopy 2012; 44: SE49–SE64. [DOI] [PubMed] [Google Scholar]
- 11.Piette C, Durand G, Bretagne JF, et al. Additional mailing phase for FIT after a medical offer phase: the best way to improve compliance with colorectal cancer screening in France. Dig Liver Dis 2017; 49: 308–311. [DOI] [PubMed] [Google Scholar]
- 12.Ponti A, Anttila A, Ronco G, et al. Cancer screening in the European Union. Report on the implementation of Council Recommendation on Cancer Screening. Brussels: European Commission, https://ec.europa.eu/health/sites/health/files/major_chronic_diseases/docs/2017_cancerscreening_2ndreportimplementation_en.pdf (2017, accessed 24 October 2018).
- 13.Vanaclocha-Espi M, Ibanez J, Molina-Barcelo A, et al. Factors influencing participation in colorectal cancer screening programs in Spain. Prev Med 2017; 105: 190–196. [DOI] [PubMed] [Google Scholar]
- 14.Denis B, Gendre I, Perrin P. Participation in four rounds of a French colorectal cancer screening programme with guaiac faecal occult blood test: a population-based open cohort study. J Med Screen 2015; 22: 76–82. [DOI] [PubMed] [Google Scholar]
- 15.Moss S, Mathews C, Day TJ, et al. Increased uptake and improved outcomes of bowel cancer screening with a faecal immunochemical test: results from a pilot study within the national screening programme in England. Gut 2017; 66: 1631–1644. [DOI] [PubMed] [Google Scholar]
- 16.van Roon AH, Hol L, Wilschut JA, et al. Advance notification letters increase adherence in colorectal cancer screening: a population-based randomized trial. Prev Med 2011; 52: 448–451. [DOI] [PubMed] [Google Scholar]
- 17.Cole SR, Smith A, Wilson C, et al. An advance notification letter increases participation in colorectal cancer screening. J Med Screen 2007; 14: 73–75. [DOI] [PubMed] [Google Scholar]
- 18.Duffy SW, Myles JP, Maroni R, et al. Rapid review of evaluation of interventions to improve participation in cancer screening services. J Med Screen 2017; 24: 127–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wardle J, von Wagner C, Kralj-Hans I, et al. Effects of evidence-based strategies to reduce the socioeconomic gradient of uptake in the English NHS Bowel Cancer Screening Programme (ASCEND): four cluster-randomised controlled trials. Lancet 2016; 387: 751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The Spanish screening cancer network, www.cribado de cancer.es (accessed 13 January 2018).
- 21.Monitoring report of the Flemish Colorectal Cancer Screening Programme 2017, https://dikkedarmkanker.bevolkingsonderzoek.be/sites/default/files/atoms/files/Jaarrapport2017_DEF_0.pdf (accessed 13 January 2018).
- 22.Monitoring report of the French screening programme 2016–2017, https://bit.ly/2HIc0BP (accessed 13 April 2018).
- 23.Arana-Arri E, Idigoras I, Uranga B, et al. Population-based colorectal cancer screening programmes using a faecal immunochemical test: should faecal haemoglobin cut-offs differ by age and sex? BMC Cancer 2017; 17: 577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toes-Zoutendijk E, van Leerdam ME, Dekker E, et al. Real-time monitoring of results during first year of Dutch colorectal cancer screening program and optimization by altering fecal immunochemical test cut-off levels. Gastroenterology 2017; 152: 767–775. [DOI] [PubMed] [Google Scholar]
- 25.Giorgi Rossi P, Grazzini G, Anti M, et al. Direct mailing of faecal occult blood tests for colorectal cancer screening: a randomized population study from Central Italy. J Med Screen 2011; 18: 121–127. [DOI] [PubMed] [Google Scholar]
- 26.Hofmann B. Ethical issues with colorectal cancer screening – a systematic review. J Eval Clin Pract 2017; 23: 631–641. [DOI] [PubMed] [Google Scholar]
- 27.Lauby-Secretan B, Vilahur N, Bianchini F, et al. International Agency for Research on Cancer Handbook Working Group. The IARC perspective on colorectal cancer screening. N Engl J Med 2018; 378: 1734–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Haan MC, de Wijkerslooth TR, Stoop E, et al. Informed decision-making in colorectal cancer screening using colonoscopy or CT-colonography. Patient Educ Couns 2013; 91: 318–325. [DOI] [PubMed] [Google Scholar]
- 29.van Dam L, Korfage IJ, Kuipers EJ, et al. What influences the decision to participate in colorectal cancer screening with faecal occult blood testing and sigmoidoscopy? Eur J Cancer 2013; 49: 2321–2330. [DOI] [PubMed] [Google Scholar]