Abstract
Background and Objective
Understanding pharmacokinetic disposition of cefepime, a β-lactam antibiotic, is crucial for developing regimens to achieve optimal exposure and improved clinical outcomes. This study sought to develop and evaluate a unified population pharmacokinetic model in both pediatric and adult patients receiving cefepime treatment.
Methods
Multiple physiologically relevant models were fit to pediatric and adult subject data. To evaluate the final model performance, a withheld group of 12 pediatric patients and two separate adult populations were assessed.
Results
Seventy subjects with a total of 604 cefepime concentrations were included in this study. All adults (n = 34) on average weighed 82.7 kg and displayed a mean creatinine clearance of 106.7 mL/min. All pediatric subjects (n = 36) had mean weight and creatinine clearance of 16.0 kg and 195.6 mL/min, respectively. A covariate-adjusted two-compartment model described the observed concentrations well (population model R2, 87.0%; Bayesian model R2, 96.5%). In the evaluation subsets, the model performed similarly well (population R2, 84.0%; Bayesian R2, 90.2%).
Conclusion
The identified model serves well for population dosing and as a Bayesian prior for precision dosing.
Electronic supplementary material
The online version of this article (10.1007/s40262-020-00873-3) contains supplementary material, which is available to authorized users.
Key Points
| A unified cefepime population pharmacokinetic model has been developed from adult and pediatric patients and evaluates well in independent populations. |
| When paired with real-time β-lactam assays, a precision dosing approach will optimize drug exposure and improve clinical outcomes. |
Introduction
Cefepime is a commonly utilized antibiotic for nosocomial infections. Rising resistance, manifesting as increased cefepime minimum inhibitory concentrations (MICs), has led to more frequent clinical failures [1, 2]. To advise clinical outcomes according to MICs, the Clinical and Laboratory Standards Institute updated the susceptibility breakpoints and then created a category of susceptible-dose dependent for MICs of 4 and 8 mg/L for Enterobacteriaceae spp. [3]. Achieving goal pharmacokinetic exposures to effectively treat these higher MICs can require a precision dosing approach.
Cefepime, like other β-lactams, has pharmacodynamic activity governed by ‘time-dependent’ activity. The fraction of time that the unbound drug concentration exceeds the MIC (fT>MIC) for the dosing interval is the pharmacokinetic/pharmacodynamic (PK/PD) efficacy target for cefepime [4], and a target of 60–74% has been previously proposed [5–8]. For the currently approved cefepime product and combination agents in the pipeline [9, 10], understanding cefepime disposition and variability is crucial to for optimal treatment of patients. As inter- and intra-patient PK variability can impact the achievement of PD goals, understanding the precision of population dosing is important. Further, to fully realize precision dosing, individualized models (e.g., Bayesian models) are needed. Once developed, these models will form the basis for adaptive feedback and control strategies when paired with real-time drug assays. The purpose of this study was to: (1) develop and evaluate a unified cefepime population PK model for adult and pediatric patients, and (2) construct an individualized model that can be utilized to deliver precision cefepime dosing.
Materials and Methods
Study Populations
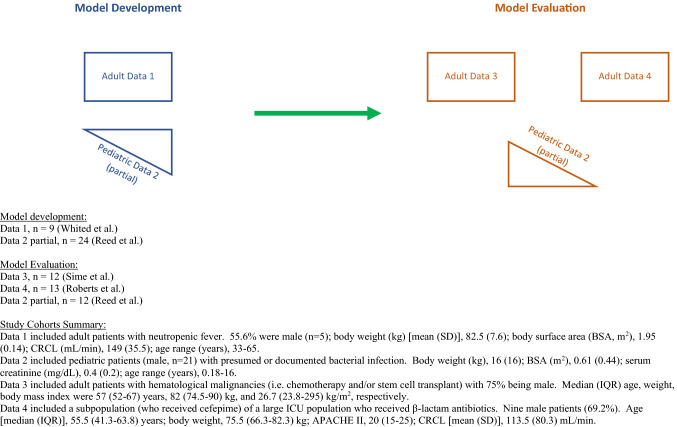
Data from four clinical cefepime PK studies representing unique groups of patients were compiled. Subject demographics and study methodologies have been previously described [11–14]. In brief, populations represented were febrile neutropenic adults with hematologic malignancies [13, 14], those with critical illness [12], and children with presumed or documented bacterial infections [11]. For the two studies that evaluated adults with neutropenic fever, Sime et al. prospectively enrolled 12 patients receiving chemotherapy and/or stem cell transplant who subsequently developed febrile neutropenia and were administered maximum doses of cefepime [13]. A total of 53 cefepime plasma concentrations in presumably steady-state dosing intervals (third, sixth, and ninth) were analyzed for PK target attainment. Whited et al. prospectively studied similar patients (n = 9) who were admitted to hematology-oncology services and were receiving cefepime at a maximum dosage for febrile neutropenia [14]. Cefepime PK samples were obtained during steady state and analyzed for population parameters. Critically ill adults were studied by Roberts et al. as a prospective multinational PK study and included 14 patients who received cefepime (only n = 13 were included for model evaluation) [12]. Last, Reed et al. characterized cefepime pharmacokinetics in hospitalized pediatric patients (above 2 months of age) who received cefepime as monotherapy for bacterial infections [11]. For our study, only those who received intravenous cefepime were included for model development.
Model Building Populations
Adult (n = 9) and partial pediatric (n = 24) datasets were utilized for PK model building (Fig. 1) [11, 14]. Model evaluation was performed with other datasets consisting of independent adult (n = 12, n = 13) and pediatric (n = 12) patients [11–13, 15]. Pediatric patients from Reed et al. [11] were randomized into the model building or the evaluation dataset. All clinical patient-level data included age, weight, and serum creatinine. An estimated creatinine clearance (CrCL) was calculated for each patient [16]. The Cockcroft–Gault formula (applied to all subjects) served as a standardized descriptor for the elimination rate constant. This study was exempted by the Institutional Review Board at Midwestern University Chicago College of Pharmacy.
Fig. 1.
Schematic for data sources in model development and evaluation
Pharmacokinetic Models
To construct the base PK models, the nonparametric adaptive grid (NPAG) algorithm [17, 18] within the Pmetrics (Version 1.5.2) package [18] for R [19] was utilized. Multiple physiologically relevant, one- and two-compartmental PK models were built and assessed. The one-compartment structural model included an intravenous cefepime dose into and parameterized total cefepime elimination rate constant (Ke) from the central compartment. The two-compartment model included additional parameterizations of intercompartmental transfer constants between central and peripheral compartments (KCP and KPC). In candidate models, total cefepime elimination was explored according to full renal and partial renal clearance (CL) models [i.e., non-renal elimination (KeIntcpt) and renal elimination descriptor (Ke0 vectorized as a function of glomerular filtration estimates)] [9, 20].
Assay error was included into the model using a polynomial equation in the form of standard deviation (SD) as a function of each observed concentration, Y (i.e., SD = C0 + C1 · Y). Observation weighting was performed using gamma (i.e., error = SD · gamma), a multiplicative variance model to account for extra process noise. Gamma was initially set at 4 with C0 and C1 equal to 0.5 and 0.15, respectively.
Covariate relationships were assessed using the ‘PMStep’ function in Pmetrics by applying stepwise linear regressions (forward selection and backwards elimination) of all covariates on PK parameters. Additionally, a priori analyses examined the effect of covariates on cefepime Ke, and both weight and CrCL were variables considered a priori to have a high potential likelihood to impact cefepime pharmacokinetics (Table 1 and Fig. S2 of the Electronic Supplementary Material [ESM]) [9, 21, 22]. Weight and CrCL were standardized to 70 kg and 120 mL/min, respectively. Further, an allometric scaler was applied to standardized weight (i.e., quotient of weight in kg divided by 70 kg raised to the negative 0.25th power) as a covariate adjustment to Ke (ESM). Ultimate model retention was governed according to criteria described below.
Table 1.
Population pharmacokinetic model builds and comparisons
| Models | Volume of distribution | Total elimination rate constant | -2LL | AIC | Change in OFV | Population | Bayesian | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bias | Imprecision | R2 | Bias | Imprecision | R2 | ||||||
|
Base 1-compt |
V0 | Ke | 3223 | 3229 | – | 0.82 | 21.3 | 0.23 | − 0.05 | 1.66 | 0.847 |
|
Base 2-compt |
V0 | Ke | 2994 | 3004 | 229 | 1.91 | 41.9 | 0.28 | 0.70 | 3.87 | 0.852 |
| 2-compt | V0 · (WT/70) | Ke · (WT/70) | 2990 | 3000 | 4 | 2.17 | 68.4 | 0.61 | − 0.18 | 1.12 | 0.965 |
|
Final 2-compt |
V0 · (WT/70) | [KeIntcpt + (CrCL/120) · Ke0] · (WT/70)− 0.25 | 2966 | 2978 | 34 | 0.53 | 7.75 | 0.87 | − 0.15 | 1.07 | 0.965 |
− 2LL − 2 log-likelihood, AIC Akaike’s information criterion, OFV objective function value, WT weight in kg
The best-fit PK and error model was identified by the change in objective function value (OFV) calculated as differences in − 2 log-likelihood, with a reduction of 3.84 in OFV corresponding to p < 0.05 based on Chi-square distribution and one degree of freedom. Further, the best-fit model was selected based on the rule of parsimony and the lowest Akaike’s information criterion scores. Goodness of fit of the competing models were evaluated by regression on observed vs. predicted plots, coefficients of determination, and visual predictive checks. Predictive performance was assessed using bias and imprecision in both population and individual prediction models. Bias was defined as mean weighted prediction error; imprecision was defined as bias-adjusted mean weighted squared prediction error. Posterior-predicted cefepime concentrations for each study subject were calculated using individual median Bayesian posterior parameter estimates.
Model Evaluation
To evaluate the final adjusted model, the NPAG algorithm [17, 18] was employed to assess the performance with separate data sets (Fig. 1). The population joint density from the best-fit covariate adjusted model was employed as a Bayesian prior for the randomly withheld pediatric data and separate adult data. In the evaluation process, structural model, model parameters, assay error, and observation weighting were unchanged. Goodness of fit of the competing models were determined as described above.
Simulations and Probability of Target Attainment
Simulation was performed to examine the exposures predicted by the final model, employing all support points from the population parameter joint density in the final NPAG analysis [18, 23]. Each support point was treated as a mean vector surrounded by the population variance–covariance matrix (i.e., covariance equal to the population covariance divided by the total number of support points). For each subject, 1000 simulated profiles were created with predicted outputs at 0.1-h intervals. Covariate values for each simulated subject were fixed based on arithmetic means of observed weight and CrCL for corresponding adult and pediatric populations. Semi-parametric Monte Carlo sampling was performed from the multimodal multivariate distribution of parameters with the parameter space concordant with the NPAG population analysis results (i.e., best-fit model) [Table S1 of the ESM] [23]. Maximum dosing regimens were simulated for adult and pediatric populations (total n = 33): 2 g every 8 h infused over 0.5 h and 50 mg/kg every 8 h infused over 0.5 h, respectively. Protein binding of 20% (i.e., 80% free fraction of total cefepime dose) was accounted for in predicting cefepime concentrations [9]. The PK/PD target of fT>MIC ≥ 68% was utilized across doubling MICs of 0.25–32 mg/L over the first 24 h of cefepime therapy [5]. Estimates are provided from the first 24 h of simulations as timely administration of effective antimicrobial agents is associated with increased survival [24].
Results
Demographics
A total of 70 clinically diverse subjects, contributing 683 cefepime concentrations, were included in this study (n = 33 subjects for model development; n = 37 subjects for evaluation) (Fig. 1). Adult subjects (n = 34), including those admitted to hematology and oncology services (n = 21) and intensive care units (n = 13), had a mean weight [SD] of 82.7 kg [21.5] and mean CrCL [SD] of 106.7 mL/min [58.4]. For the pediatric subjects (n = 36), means [SD] of weight and CrCL were 16.0 kg [16.1] and 195.6 mL/min [40.5], respectively. Adult subjects ranged in age from 22 to 82 years (mean, 55.4 years) while pediatric subjects ranged from approximately 2 months to 16 years of age (mean, 3.9 years).
Pharmacokinetic Model Selection, Parameters, and Evaluation
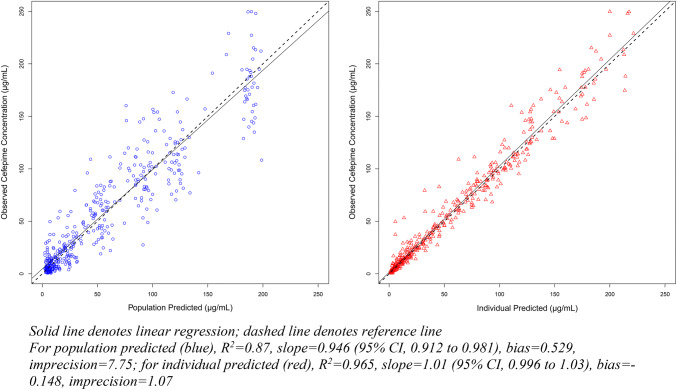
A total of 428 cefepime observations were available for model development. Cefepime concentrations ranged from 0.5 to 249.7 μg/mL. The base one- and two-compartment models (without covariate adjustment) produced reasonable fits for observed and Bayesian posterior-predicted cefepime concentrations (R2 = 84.7% and 85.2%, respectively), but population estimates were unsatisfactory (R2 = 22.7% and 27.8%, respectively) (Table 1). Weight and CrCL displayed relationships with the standard two-compartment model (i.e., base two-compartment model). Volume of distribution was associated with weight (p < 0.2) and Ke (total) was associated with CrCL (p < 0.2). After standardizing weight (to 70 kg) without an allometric scaler in the base two-compartment model, fits for both population and Bayesian posterior estimates against the observed data improved (R2 = 60.7% and 96.5%, respectively; OFV change, 4). Bias and imprecision for Bayesian posterior fits were − 0.18 and 1.12, respectively. When covariates (i.e., weight to volume of distribution and Ke; CrCL to Ke) and the allometric scaler were applied in the two-compartment model, Bayesian posteriors fit well (R2 = 96.5%; Fig. 2 right) with low bias and imprecision (− 0.15 and 1.07, respectively), and the population PK model produced good fits of the observed cefepime concentrations (R2 = 87.0%, bias = 0.53, imprecision = 7.75; Fig. 2 left). The OFV change from the weight-adjusted two-compartment model to the final model was significant at − 34 (p < 0.05) [Table 1].
Fig. 2.
Goodness-of-fit plots for best-fit population cefepime PK model (model development)
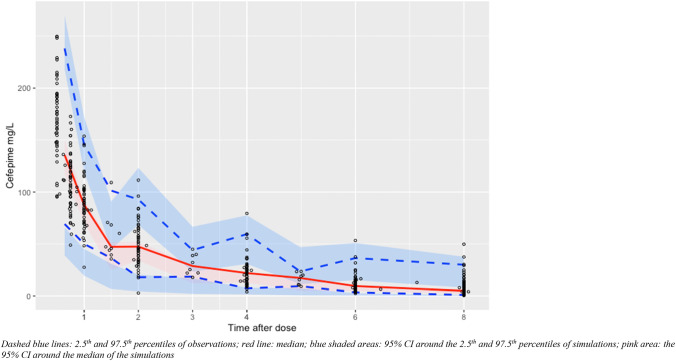
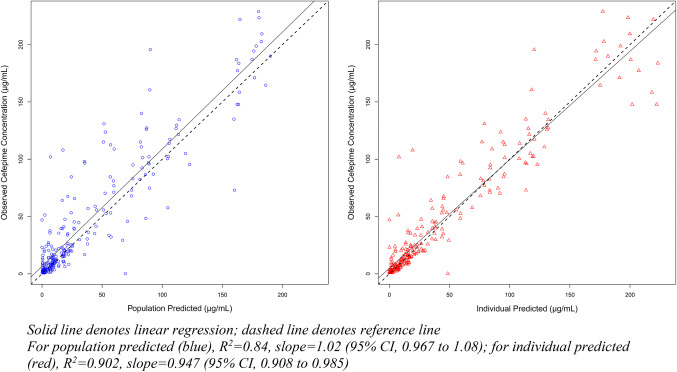
The final model also produced acceptable predicted checks (Fig. 3). Thus, a two-compartment model with weight and CrCL as covariate adjustments and allometric scaling was selected as the final PK model. The population parameter values from the final PK model are summarized in Table 2. Structural model and differential equations that define the population PK are listed in the ESM. The population parameter value covariance matrix can be found in Table 3. Additionally, weighted residual error plots for the best-fit model (Fig. S1) and scatter plots for covariates for the base structural model (Fig. S2) can be found included in the ESM. For the evaluation subset, Bayesian priors resulted in reasonably accurate and precise predictions (population R2 = 84.0%, Bayesian R2 = 90.2%; Fig. 4).
Fig. 3.
Visual Predictive Checks for the Best-fit PK Model. Dashed blue lines: 2.5th and 97.5th percentiles of observations; red line: median; blue shaded areas: 95% CI around the 2.5th and 97.5th percentiles of simulations; pink area: the 95% CI around the median of the simulations
Table 2.
Population pharmacokinetic parameter estimates from the final model
| Median (95% CI) | CV (%) | Shrinkage (%) | |
|---|---|---|---|
| V0 (L) | 11.172 (9.40, 12.50) | 22.66 | 14.7 |
| KeIntcpt (h−1) | 0.506 (0.33, 0.71) | 59.24 | 15.1 |
| Ke0 (h−1) | 0.236 (0.11, 0.40) | 133.95 | 6.8 |
| KCP (h−1) | 1.716 (1.02, 3.11) | 68.82 | 7.7 |
| KPC (h−1) | 1.502 (1.35, 1.90) | 60.63 | 8.8 |
V0 and KeIntcpt are standardized to weight (kg)/70 kg in the final structural model
Ke0 is standardized to weight (kg)/70 kg and creatinine clearance (mL/min)/120 mL/min in the final structural model
CI credibility interval, CV coefficient of variation
Table 3.
Population parameter value covariance matrix for the best-fit model
| V0 | KeIntcpt | Ke0 | KCP | KPC | |
|---|---|---|---|---|---|
| V0 | 6.366 | – | – | – | – |
| KeIntcpt | − 0.106 | 0.085 | – | – | – |
| Ke0 | − 0.238 | − 0.152 | 0.499 | – | – |
| KCP | − 1.576 | 0.005 | 0.145 | 2.354 | – |
| KPC | − 0.680 | 0.027 | − 0.188 | 1.441 | 1.414 |
Fig. 4.
Goodness-of-fit plots for evaluation of population cefepime PK model (model evaluation)
Simulation and Probability of Target Attainment
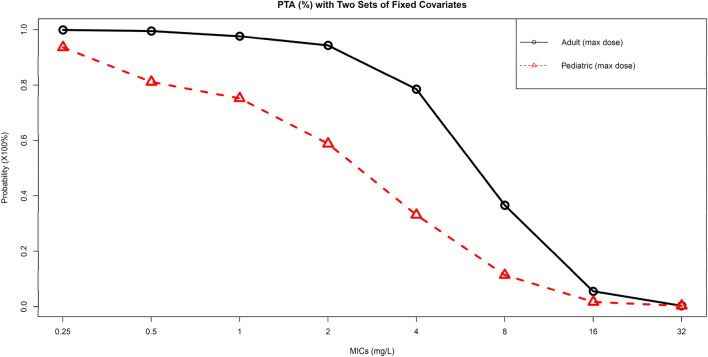
Results of the probability of target attainment (PTA) analysis are shown in Table 4 and Fig. 5 for the first 24 h of therapy. Two cefepime regimens were utilized to simulate PTA for adult and pediatric subjects. Cefepime dosage of 2 g every 8 h infused over 30 min produced PTAs of > 90% for MICs of 0.25–2 mg/L, while a more than two-fold drop of PTA was observed from MICs of 4 mg/L to 8 mg/L. The second cefepime regimen of 800 mg every 8 h (50 mg/kg based on a population mean weight of 16.0 kg) infused over 30 min achieved a PTA of > 90% only at an MIC of 0.25 mg/L, a PTA at 81.1% at an MIC of 0.5 mg/L and performs poorly across subsequent higher doubling MICs.
Table 4.
Probability of target attainment at different cefepime minimum inhibitory concentrations (MICs) for the first 24 h (h) of therapy (maximum recommended dosages for adult and pediatric patients)
| Cefepime regimen | Cefepime MICs (mg/L) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dose (mg) | Dosing interval (h) | Infusion time (h) | 0.25 (%) | 0.5 (%) | 1 (%) | 2 (%) | 4 (%) | 8 (%) | 16 (%) | 32 (%) |
| 2000 | 8 | 0.5 | 100 | 99.5 | 97.6 | 94.3 | 78.5 | 36.6 | 5.5 | 0.3 |
| 800 | 8 | 0.5 | 93.6 | 81.1 | 75.2 | 58.8 | 33.1 | 11.4 | 1.7 | 0.3 |
800 mg is based on 50 mg/kg
Fig. 5.
Probability of target attainment at different cefepime MICs
Discussion
This study created a population and individual PK model for adult and pediatric patients and can serve as a Bayesian prior for precision dosing. When paired with a real-time assay for cefepime, this model allows for precise and accurate predictions of cefepime disposition via adaptive feedback control. In the absence of real-time assays, these cefepime PK parameters facilitate more accurate population-based dosing strategies. Previous work by Rhodes et al. has shown an absolute difference of approximately 20% in survival probability across the continuum of achieving 0–100% fT>MIC in adult patients with Gram-negative bloodstream infections, thus understanding the dose and re-dosing interval necessary to achieve optimal PK exposures should greatly improve clinical outcomes for patients treated with cefepime [5].
Individualized dosing and therapeutic drug monitoring of β-lactam antibiotics (e.g., cefepime) are critically important to achieving optimal drug exposure (i.e., optimal fT>MIC as the PK/PD target) and improving clinical outcomes [4, 25, 26]. Precision medicine has been named as a major focus for the National Health Institute with $215 million invested [27], yet precision medicine has mostly focused on genomic differences [28, 29]. Precision dosing is an important facet of precision medicine, and renewed efforts in precision dosing in the real-world setting are being pursued [30]. Cefepime is a highly relevant example. While rigorous reviews and analyses are conducted during the development phase of an antibiotic, dose optimization is far less ideal for the types of patients who ultimately receive the drug. This is highlighted by the fact that although cefepime-associated neurotoxicity is rare, this serious and potentially life-threatening adverse event has been increasingly reported and few strategies exist for optimizing and delivering precision exposures [31, 32]. Lamoth et al. found that a cefepime trough concentration of ≥ 22 mg/L has a 50% probability of predicting neurotoxicity [33]. Huwyler et al. identified a similar predictive threshold of > 20 mg/dL (five-fold increased risk for neurologic events) [34]. In contrast, Rhodes et al. found the cut-off of 22 mg/L to be suboptimal [35]. Furthermore, Rhodes et al. performed simulations from literature cefepime data and observed a high intercorrelation amongst all PK parameters (i.e., area under the curve at steady state, maximum plasma concentration, and minimum plasma concentration), suggesting that more work is needed to establish the pharmacokinetic/toxicodynamic (PK/TD) profile for cefepime.
In addition to complications by these less-than-ideal PK/TD data, clinicians are left to treat patients with extreme age differences, organ dysfunction, and comorbid conditions affecting antibiotic pharmacokinetics/pharmacodynamics [26]. Further, a contemporary dose reduction strategy based on estimated renal function (e.g., estimated CrCL using the Cockcroft–Gault formula) is also likely to be confounded in these patients by intrinsic PK variability, such as changes in volume of distribution, and the challenges of accurately estimating the glomerular filtration rate at any point in time, leading to more ‘uncertainties’ in balancing dose optimization and adverse events [9, 36]. These ‘real-world’ patients are often under-represented, and thus not well understood, from a PK/PD and PK/TD standpoint during the drug approval process. Bridging to the more typical patients that are clinically treated is important and central to the mission of precision medicine. The findings of this study can be used to guide cefepime dosing in these ‘real-world’ patients.
Several other studies have reviewed population cefepime pharmacokinetics. Sime et al. observed that patients with neutropenic fever had a mean CL of 8.6 L/h and a mean elimination half-life of 2.7 h [13]. Nicasio et al. studied 32 critically ill patients receiving intravenous cefepime for ventilator-associated pneumonia and observed that means of total CL and elimination half-life (as calculated from mean total CL and mean volume of distribution) were 7.6 L/h and 2.0 h, respectively [37]. The noncompartmental analysis conducted (in the model development adult population) in our study produced similar results (CL, 7.59 L/h; elimination half-life, 2.98 h). Shoji et al. studied 91 pediatric patients and observed a mean of CL of approximately 1.86 L/h and a mean elimination half-life of 3.5 h [38]. In our pediatric population, means of CL and elimination half-life were 3.1 L/h and 3.0 h, respectively. Our simulation findings are similar to those of Shoji et al. that the maximum pediatric cefepime dosing did not adequately achieve optimal exposure to target higher MICs. While the cefepime package labeling recommends maximum dosages of 2 g every 8 h for adult patients with neutropenic fever and 50 mg/kg every 8 h for pediatric patients with pneumonia and/or neutropenic fever, there may be a need to extend these dosing regimens to other populations (in the absence of aforementioned indications) to achieve the best clinical outcomes by optimizing the PK/PD attainment goals [9].
Other studies also performed a simulation for PTA with different cefepime regimens and renal functions. Tam et al. found that with a PD target of 67% fT>MIC, 2 g every 8 h (30-minute infusion) achieved approximately 90% PTA for MIC of 8 mg/L in patients with CrCL of 120 mL/min while 2 g every 12 h achieved barely above 80% PTA for an MIC of 4 mg/L in the same population [39]. Nicasio et al. also conducted a simulation using a PD target of 50% fT>MIC in the critically ill with varying renal function. The maximum recommended dosage (2 g every 8 h) in patients with CrCL between 50 and 120 mL/min achieved a PTA of 78.1% at an MIC of 16 mg/L; however, when the same regimen was infused over 0.5 h, the PTA achieved was significantly lower [37]. Collectively, these findings suggest that cefepime exposure is highly variable and may be clinically suboptimal in a large number of patients commonly treated with cefepime. These findings support the need for precision dosing and therapeutic drug monitoring for β-lactam antibiotics to reach optimal PK/PD targets given the high variability in drug exposures.
Our study is not without limitations. Although a relatively large and diverse cohort was included in model development and evaluation, we did not specifically assess certain subgroups such as patients with morbid obesity and severe renal dysfunction. These conditions may require patient-specific models. Second, many studies to date included ‘real-world’ patients with various disease sates (e.g., neutropenic fever, renal failure, sepsis); however, all studies were conducted under the research protocol where doses, and administration times were all carefully confirmed. Additional efforts will be needed to evaluate model performance in clinical contexts.
Conclusions
A unified population model for cefepime in adult and pediatric populations was developed and demonstrated excellent performance on evaluation. Current cefepime dosages are often suboptimal, and population variability is high. Precision dosing approaches and real-time assays are needed for cefepime to optimize drug exposure and improve clinical outcomes.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
J.A. Roberts would like to acknowledge funding from the Australian National Health and Medical Research Council for a Centre of Research Excellence (APP1099452) and a Practitioner Fellowship (APP1117065).
Compliance with Ethical Standards
Funding
Study data are pre-existing. This study was conducted as a part of our normal work. No sources of funding were received for the conduct of this study or the preparation of this article.
Conflict of interest
Nathaniel J. Rhodes has received research funds from Hartford Hospital and honoraria from the American Society of Health-System Pharmacists and has no relevant conflict of interest regarding this work. Jeffrey Lipman has received honoraria (paid to the University of Queensland) from MSD and Pfizer. Jiajun Liu, Michael Neely, Fekade Sime, Jason A. Roberts, Patrick J. Kiel, Sean N. Avedissian, and Marc H. Scheetz have no conflicts of interest that are directly relevant to the content of this article.
References
- 1.Bhat SV, Peleg AY, Lodise TP, Jr, Shutt KA, Capitano B, Potoski BA, et al. Failure of current cefepime breakpoints to predict clinical outcomes of bacteremia caused by Gram-negative organisms. Antimicrob Agents Chemother. 2007;51(12):4390–4395. doi: 10.1128/AAC.01487-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rhodes NJ, Liu J, McLaughlin MM, Qi C, Scheetz MH. Evaluation of clinical outcomes in patients with Gram-negative bloodstream infections according to cefepime MIC. Diagn Microbiol Infect Dis. 2015;82(2):165–171. doi: 10.1016/j.diagmicrobio.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. 26th ed. CLSI supplement M100. Waye (PA): Clinical and Laboratory Standards Institute; 2016.
- 4.Craig WA. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis. 1998;26(1):1–10 (quiz 1–2). [DOI] [PubMed]
- 5.Rhodes NJ, Kuti JL, Nicolau DP, Van Wart S, Nicasio AM, Liu J, et al. Defining clinical exposures of cefepime for Gram-negative bloodstream infections that are associated with improved survival. Antimicrob Agents Chemother. 2015;60(3):1401–1410. doi: 10.1128/AAC.01956-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crandon JL, Bulik CC, Kuti JL, Nicolau DP. Clinical pharmacodynamics of cefepime in patients infected with Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2010;54(3):1111–1116. doi: 10.1128/AAC.01183-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamashita Y, Kamiyama H, Yamamoto A, Kanoh H, Yuhki Y, Ueda A, et al. Relationship between PK/PD of cefepime and clinical outcome in febrile neutropenic patients with normal renal function. Yakugaku Zasshi. 2016;136(12):1641–1649. doi: 10.1248/yakushi.16-00168. [DOI] [PubMed] [Google Scholar]
- 8.Craig WA. Interrelationship between pharmacokinetics and pharmacodynamics in determining dosage regimens for broad-spectrum cephalosporins. Diagn Microbiol Infect Dis. 1995;22(1–2):89–96. [DOI] [PubMed]
- 9.Hospira, Inc. Maxipine (cefepime hydrochloride) [package insert]. US FDA. Revised June 2012. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/050679s036lbl.pdf. Accessed 16 Aug 2019.
- 10.Daigle D, Hamrick J, Chatwin C, Kurepina N, Kreiswirth BN, Shields RK, et al. 1370. Cefepime/VNRX-5133 broad-spectrum activity is maintained against emerging KPC- and PDC-variants in multidrug-resistant K. pneumoniae and P. aeruginosa. Open Forum Infect Dis. 2018;5(Suppl. 1):S419–20.
- 11.Reed MD, Yamashita TS, Knupp CK, Veazey JM, Jr, Blumer JL. Pharmacokinetics of intravenously and intramuscularly administered cefepime in infants and children. Antimicrob Agents Chemother. 1997;41(8):1783–1787. doi: 10.1128/AAC.41.8.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberts JA, Paul SK, Akova M, Bassetti M, De Waele JJ, Dimopoulos G, et al. DALI: defining antibiotic levels in intensive care unit patients: are current beta-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis. 2014;58(8):1072–1083. doi: 10.1093/cid/ciu027. [DOI] [PubMed] [Google Scholar]
- 13.Sime FB, Roberts MS, Tiong IS, Gardner JH, Lehman S, Peake SL, et al. Adequacy of high-dose cefepime regimen in febrile neutropenic patients with hematological malignancies. Antimicrob Agents Chemother. 2015;59(9):5463–5469. doi: 10.1128/AAC.00389-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whited L, Grove M, Rose D, Rhodes NJ, Scheetz MH, O’Donnell JN, et al. Pharmacokinetics of cefepime in patients with cancer and febrile neutropenia in the setting of hematologic malignancies or hematopoeitic cell transplantation. Pharmacotherapy. 2016;36(9):1003–1010. doi: 10.1002/phar.1807. [DOI] [PubMed] [Google Scholar]
- 15.Food and Drug Administration. Guidance Document: Population Pharmacokinetics Guidance for Industry. 2019. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/population-pharmacokinetics. Published July, 2019. Accessed 15 Aug 2019.
- 16.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 17.Leary R, Jelliffe R, Schumitzky A, Van Guilder M. An adaptive grid non-parametric approach to pharmacokinetic and dynamic (PK/PD) population models. In: Proceedings 14th IEEE symposium on computer-based medical systems. CBMS 2001, Bethesda, MD, USA, 2001, pp. 389–394.
- 18.Neely MN, van Guilder MG, Yamada WM, Schumitzky A, Jelliffe RW. Accurate detection of outliers and subpopulations with Pmetrics, a nonparametric and parametric pharmacometric modeling and simulation package for R. Ther Drug Monit. 2012;34(4):467–476. doi: 10.1097/FTD.0b013e31825c4ba6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.R Core Team. R: a language and environment for statistical computing. 2019. R Foundation for Statistical Computing, Vienna, Austria. Available from: https://www.R-project.org/. Accessed 20 Feb 2020.
- 20.Okamoto MP, Nakahiro RK, Chin A, Bedikian A. Cefepime clinical pharmacokinetics. Clin Pharmacokinet. 1993;25(2):88–102. doi: 10.2165/00003088-199325020-00002. [DOI] [PubMed] [Google Scholar]
- 21.Kovarik JM, ter Maaten JC, Rademaker CM, Deenstra M, Hoepelman IM, Hart HC, et al. Pharmacokinetics of cefepime in patients with respiratory tract infections. Antimicrob Agents Chemother. 1990;34(10):1885–1888. doi: 10.1128/AAC.34.10.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Georges B, Conil JM, Seguin T, Dieye E, Cougot P, Decun JF, et al. Cefepime in intensive care unit patients: validation of a population pharmacokinetic approach and influence of covariables. Int J Clin Pharmacol Ther. 2008;46(4):157–164. doi: 10.5414/CPP46157. [DOI] [PubMed] [Google Scholar]
- 23.Goutelle S, Bourguignon L, Maire PH, Van Guilder M, Conte JE, Jr, Jelliffe RW. Population modeling and Monte Carlo simulation study of the pharmacokinetics and antituberculosis pharmacodynamics of rifampin in lungs. Antimicrob Agents Chemother. 2009;53(7):2974–2981. doi: 10.1128/AAC.01520-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(6):1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 25.Ambrose PG, Bhavnani SM, Rubino CM, Louie A, Gumbo T, Forrest A, et al. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: it’s not just for mice anymore. Clin Infect Dis. 2007;44(1):79–86. doi: 10.1086/510079. [DOI] [PubMed] [Google Scholar]
- 26.Huttner A, Harbarth S, Hope WW, Lipman J, Roberts JA. Therapeutic drug monitoring of the beta-lactam antibiotics: what is the evidence and which patients should we be using it for? J Antimicrob Chemother. 2015;70(12):3178–3183. doi: 10.1093/jac/dkv201. [DOI] [PubMed] [Google Scholar]
- 27.Office of the Press Secretary. The White House. FACT SHEET: President Obama’s Precision Medicine Initiative. 2015. https://obamawhitehouse.archives.gov/the-press-office/2015/01/30/fact-sheet-president-obama-s-precision-medicine-initiative. Published January 30, 2015. Accessed 15 Aug 2019.
- 28.Ginsburg GS, Phillips KA. Precision medicine: from science to value. Health Aff (Millwood). 2018;37(5):694–701. doi: 10.1377/hlthaff.2017.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gameiro GR, Sinkunas V, Liguori GR, Auler-Junior JOC. Precision medicine: changing the way we think about healthcare. Clinics (Sao Paulo). 2018;3(73):e723. doi: 10.6061/clinics/2017/e723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Food and Drug Administration UoNC. Precision dosing: defining the need and approaches to deliver individualized drug dosing in the real-world setting.; August 12, 2019, 2019; FDA White Oak Campus Federal Research Center 10903 New Hampshire Ave. Silver Spring, MD 20993.
- 31.Food and Drug Administration. Cefepime and risk of seizure in patients not receiving dosage adjustments for kidney impairment. 2016. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-cefepime-and-risk-seizure-patients-not-receiving-dosage-adjustments. Published January 19, 2016. Accessed 15 Aug 2019.
- 32.Appa AA, Jain R, Rakita RM, Hakimian S, Pottinger PS. Characterizing cefepime neurotoxicity: a systematic review. Open Forum Infect Dis. 2017;4(4):ofx170. [DOI] [PMC free article] [PubMed]
- 33.Lamoth F, Buclin T, Pascual A, Vora S, Bolay S, Decosterd LA, et al. High cefepime plasma concentrations and neurological toxicity in febrile neutropenic patients with mild impairment of renal function. Antimicrob Agents Chemother. 2010;54(10):4360–4367. doi: 10.1128/AAC.01595-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huwyler T, Lenggenhager L, Abbas M, Ing Lorenzini K, Hughes S, Huttner B, et al. Cefepime plasma concentrations and clinical toxicity: a retrospective cohort study. Clin Microbiol Infect. 2017;23(7):454–459. doi: 10.1016/j.cmi.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 35.Rhodes NJ, Kuti JL, Nicolau DP, Neely MN, Nicasio AM, Scheetz MH. An exploratory analysis of the ability of a cefepime trough concentration greater than 22 mg/L to predict neurotoxicity. J Infect Chemother. 2016;22(2):78–83. doi: 10.1016/j.jiac.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 36.Michels WM, Grootendorst DC, Verduijn M, Elliott EG, Dekker FW, Krediet RT. Performance of the Cockcroft-Gault, MDRD, and new CKD-EPI formulas in relation to GFR, age, and body size. Clin J Am Soc Nephrol. 2010;5(6):1003–1009. doi: 10.2215/CJN.06870909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nicasio AM, Ariano RE, Zelenitsky SA, Kim A, Crandon JL, Kuti JL, et al. Population pharmacokinetics of high-dose, prolonged-infusion cefepime in adult critically ill patients with ventilator-associated pneumonia. Antimicrob Agents Chemother. 2009;53(4):1476–1481. doi: 10.1128/AAC.01141-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shoji K, Bradley JS, Reed MD, van den Anker JN, Domonoske C, Capparelli EV. Population pharmacokinetic assessment and pharmacodynamic implications of pediatric cefepime dosing for susceptible-dose-dependent organisms. Antimicrob Agents Chemother. 2016;60(4):2150–2156. doi: 10.1128/AAC.02592-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tam VH, McKinnon PS, Akins RL, Drusano GL, Rybak MJ. Pharmacokinetics and pharmacodynamics of cefepime in patients with various degrees of renal function. Antimicrob Agents Chemother. 2003;47(6):1853–1861. doi: 10.1128/AAC.47.6.1853-1861.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.