Abstract
Background
Coeliac disease (CD) results from an immune-mediated reaction to gluten in genetically predisposed individuals. In rare cases CD may occur with acute features deferring the diagnosis and exposing these patients to possible life-threatening complications. Herein we present the case of a young woman with a coeliac crisis, that is, a sudden clinical onset characterised by severe electrolyte imbalance due to an unknown (previously unrecognised) CD.
Methods
This is a case report and literature review revealing that coeliac crisis is under-reported, with a total of 48 adult cases so far published. The diagnosis in our case was established by histopathological analysis of multiple duodenal biopsies. The patient’s serum was tested by enzyme-linked immunoassay to detect antitransglutaminase IgA antibodies.
Results
In contrast to cases reported in the literature, with male gender predominance and a mean age of 50±17 years, our patient was a young female case of coeliac crisis. However, like in our patient, a higher incidence of coeliac crisis was associated with the human leucocyte antigen (HLA)-DQ2 haplotype, versus HLA-DQ8, and a severe (Marsh-Oberhüber 3c) duodenal mucosa atrophy. Notably, there is no clear correlation between the antitissue transglutaminase 2 IgA antibody titre and coeliac crisis onset/severity, as confirmed by our case report.
Conclusions
The present case highlights that CD may manifest quite abruptly with a severe malabsorption syndrome, that is, electrolyte abnormalities and hypoproteinaemia. Our case should alert physicians, in particular those in the emergency setting, that even a typically chronic disorder, such as CD, may show life-threatening complications requiring urgent management.
Keywords: diarrhoea, gluten-free diet, malabsorption, intestinal failure, coeliac disease
Introduction
Coeliac disease (CD) is a multisystemic, immune-mediated illness evoked by gluten ingestion in genetically susceptible individuals.1 The main target organ of the autoimmune reaction against the enzyme tissue transglutaminase (TG2) is the small bowel, where the gluten-related inflammatory cascade causes a progressive mucosal damage leading to severe villous atrophy.1 2 From a clinical standpoint, CD is a multifaceted chronic condition displaying a broad spectrum of intestinal (ranging from mild irritable bowel syndrome-like to severe malabsorption symptoms) and extraintestinal manifestations targeting several tissues and organs (eg, skin, endocrine/exocrine glands, nervous system, joint/muscles). As a result, CD remains a challenging condition to be diagnosed, thus causing a significant delay in establishing the appropriate therapy and increasing related morbidity.3–5
A potentially life-threatening and neglected clinical manifestation of CD is the so-called ‘coeliac crisis’, characterised by acute, massive watery diarrhoea, severe dehydration and metabolic disturbances, leading to neuromuscular weakness, tetanic seizures, cardiac arrhythmias and even sudden death in extreme cases.6–8 This condition is largely under-reported and under-recognised both in children and adults, with a total of 48 adult cases published so far.6–46 In most cases, coeliac crisis develops due to voluntary or inadvertent gluten ingestion in patients with or without an established diagnosis of CD. Only rarely a coeliac crisis heralds the clinical onset of CD, requiring hospitalisation and rapid therapeutic management due to possible occurrence of severe complications with high morbidity and mortality.9–13
Herein we describe the case of a patient admitted to our emergency department for a severe life-threatening coeliac crisis as the first manifestation of a previously unknown CD.
Case report
A 34-year-old woman was admitted to the emergency unit complaining of limb numbness and watery diarrhoea (8–10 bowel movements/day) which started 2 weeks earlier. The patient reported a weight loss of about 10 kg in the last 2 months in the absence of hyporexia. Her clinical history unravelled microcytic anaemia treated with oral iron replacement. Physical examination showed severe weakness of the limbs with a bilaterally positive Trousseau’s sign without cardiorespiratory abnormalities. Vital parameters were within the normal range. The abdomen was flat, without tenderness, while auscultation disclosed increased intestinal sounds. Her ECG showed a sinus rhythm with type 1 atrioventricular block, flat T waves associated with U waves and an elongated QTc interval (570 ms). Laboratory tests revealed severe electrolyte imbalance, with hyponatraemia (133 mmol/L), hypokalaemia (1.6 mmol/L), hypocalcaemia (ionised calcium of 0.9 mmol/L), hypophosphataemia (1.6 mg/dL) and hypomagnesaemia (1.4 mmol/L). Furthermore, the patient had hypochromic microcytic anaemia (haemoglobin of 85 g/L, with a mean cell volume of 68 fL and a mean cell haemoglobin of 20.6 pg), normal platelet count (297×10ˆ9/L), iron (serum iron 18 µg/dL; ferritin 2 ng/mL) and folate deficiency (2 ng/mL), as well as hypoproteinaemia and hypoalbuminaemia (total serum protein 4.4 g/dL; albumin 2.6 g/dL). Due to severe electrolyte imbalance, a conspicuous electrolyte replacement was rapidly administered, leading to a slight improvement in electrocardiographic abnormalities. The patient was then admitted to the internal medicine ward for adequate investigation and treatment. During the hospitalisation, the common causes of infectious diarrhoea were excluded by stool cultures, and the faecal occult blood test resulted negative. Both ultrasound and abdominal X-ray examinations were unremarkable. Liver function tests revealed a slight increase of transaminases, with alanine transaminase and aspartate transaminase values of 47 U/L and 60 U/L (n.v. 5-35 for both parameters), respectively. Based on the lack of fever, normal C reactive protein and the presence of non-bloody, watery diarrhoea, the patient was further evaluated with an upper endoscopy. The examination revealed stigmata of villous atrophy at the duodenal level (figure 1), where biopsies were taken from the bulb and the second portion. Histopathological analysis showed the presence of a severe villous atrophy (Marsh-Oberhüber grade 3c) (figure 2) without any evidence of aberrant intraepithelial lymphocytes.14 Based on the histopathological result, we used enzyme-linked immunoassay to test IgA anti-TG2, which turned to be positive at low titre (23 U/mL, n.v. <10 U/mL). This result was associated with the positivity of IgA antiendomysial antibodies (1:80) revealed by indirect immunofluorescence.47 48 The genetic test showed human leucocyte antigen (HLA)-DQ2 positivity. Therefore, a firm diagnosis of CD was established and the patient started a gluten-free diet (GFD). Due to rapid improvement after gluten withdrawal, a course with steroid treatment was deemed not necessary. Since diarrhoea and paraesthesia showed significant improvement with complete regression in about a week, the patient was discharged in good health.
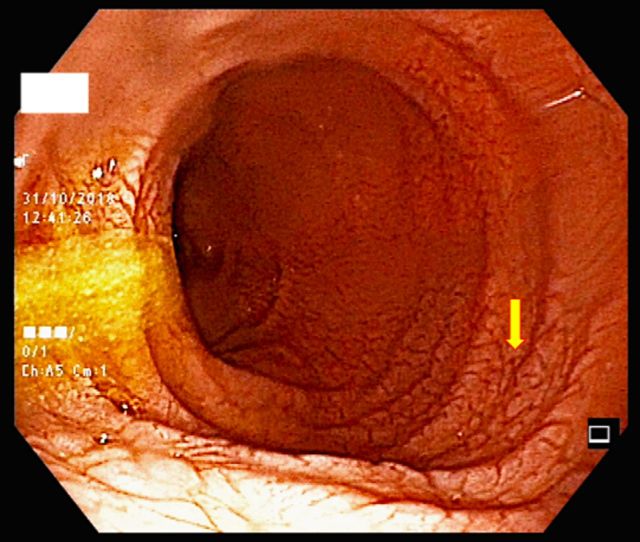
Figure 1.
Representative duodenal endoscopic picture showing decreased mucosal folds carrying a scalloping profile, together with a mosaic pattern and an increased vascular network (arrow), all suggestive of villous atrophy.
Figure 2.
Representative microphotograph of the duodenal mucosa showing villous atrophy and crypt hyperplasia with dense inflammatory infiltrate of the lamina propria (Marsh-Oberhüber lesion grade 3c). H&E staining, original magnification 200×.
Discussion and review of the literature
In the vast majority of cases, the natural history of CD is characterised by chronic evolution without acute exacerbations. Conversely, coeliac crisis is burdened by severe acute symptoms such as abdominal pain and distension, massive diarrhoea and weight loss, causing a life-threatening malabsorption syndrome. In most cases, gluten is introduced inadvertently, whereas in some patients with poor compliance to GFD a voluntary ingestion may occur.3 6 7 Only seldom a coeliac crisis can herald the onset of CD,10 as it was in the herein reported case. Among the reported cases described in table 1, there was a greater prevalence of female than male gender (28 vs 20), with a mean age of 50±17 years, which is higher than the age of the present case.9–46 Like in the present case, a higher incidence of coeliac crisis has been reported in patients carrying the HLA-DQ2 haplotype of genetic susceptibility to the disease as compared with those with HLA-DQ8, whereas the biopsy specimens showed signs characteristic of Marsh-Oberhüber 3 (from ‘a’ to ‘c’) mucosal lesions.1 Other common clinical features included weight loss, hypoproteinaemia and electrolyte abnormalities, whereas coagulation abnormalities (ie, prothrombin time elongation) along with markedly reduced platelet count were uncommon. No clear correlation was found between the anti-TG2 IgA antibody titre and coeliac crisis onset/severity, as also supported by our case showing only a twofold increase above the upper normal limit.
Table 1.
Synopsis highlighting the main features of adult cases (n=48) with coeliac crisis published so far
| Reference | Age | Sex | Weight loss | Favorable evolution with GFD | Diagnosis | HLA | Hb (g/L) | PLT (x109/L) | INR | Hypo-proteinaemia | Electrolyte Abnormalities | LOS (days) | Death | ||
| Biopsy (Marsh grade) | EMA | TG2 (UI/mL) | |||||||||||||
| Ozaslan et al9 | 75 | M | Yes | Yes | N/A | Positive | N/A | N/A | 59 | 97 | N/A | Yes | Yes | 10 | No |
| 55 | F | N/A | Yes | N/A | Positive | N/A | N/A | 79 | 43 | N/A | Yes | Yes | 8 | No | |
| Poudyal et al10 | 20 | M | No | Yes | N/A | N/A | 100 | DQ8 | 86 | N/A | N/A | No | Yes | N/A | No |
| Gutiérrez et al11 | 26 | F | N/A | Yes | 3c | N/A | 23.4 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Wolf et al12 | 36 | F | Yes | Yes | 3c | N/A | N/A | N/A | <110 | N/A | N/A | N/A | Yes | N/A | No |
| Forrest et al15 | 43 | F | Yes | Yes | 3c | N/A | 608 | N/A | 78 | <100 | 2.1 | Yes | Yes | 107 | No |
| Akbal et al16 | 52 | F | Yes | Yes | N/A | Positive | Positive | N/A | <100 | N/A | N/A | Yes | Yes | N/A | No |
| Al Shammeri et al17 | 50 | F | N/A | Yes | N/A | 1/160 | 15 | N/A | N/A | N/A | N/A | N/A | Yes | N/A | No |
| Atikou et al19 | 26 | F | N/A | Yes | 3 | Positive | N/A | N/A | N/A | N/A | N/A | No | Yes | N/A | No |
| Parry and Acharya20 | 83 | M | N/A | Yes | N/A | Positive | 6 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | No |
| Magro and Pullicino21 | 38 | M | Yes | Yes | 3c | N/A | 35.9 | N/A | 152 | N/A | 1.6 | Yes | Yes | N/A | No |
| Lindo Ricce et al22 | 63 | F | N/A | No | 3c | N/A | 45 | DQ2 | N/A | N/A | N/A | Yes | Yes | 30 | No |
| de Almeida Menezes et al25 | 31 | M | Yes | Yes | N/A | N/A | N/A | N/A | N/A | N/A | 3.48 | Yes | Yes | 6 | No |
| Gonzalez et al26 | 76 | M | Yes | Yes | N/A | N/A | N/A | N/A | 102 | N/A | >10 | N/A | Yes | 10 | No |
| Da Costa Becker et al27 | 49 | F | Yes | Yes | 3c | Positive | Positive | N/A | 72 | 250 | >1.5 | Yes | Yes | N/A | No |
| Ferreira et al28 | 56 | M | Yes | Yes | N/A | N/A | N/A | DQ2 | <110 | N/A | >1.2 | No | Yes | 7 | No |
| Helvaci et al29 | 25 | F | Yes | Yes | 3c | N/A | 100 | N/A | N/A | N/A | N/A | Yes | Yes | 14 | No |
| Chen et al30 | 24 | F | Yes | Yes | 3c | N/A | 99 | N/A | 93 | N/A | 3.2 | No | Yes | 8 | No |
| Sbai et al31 | 43 | M | Yes | Yes | 3c | N/A | 19 | N/A | N/A | N/A | N/A | No | Yes | 5 | No |
| Bul et al32 | 46 | M | Yes | Yes | N/A | N/A | 48 | N/A | 110 | N/A | N/A | Yes | No | N/A | No |
| Tiwari et al33 | 26 | F | N/A | Yes | N/A | N/A | 100 | N/A | 158 | N/A | N/A | Yes | Yes | N/A | No |
| Bou-Abboud et al34 | 69 | M | N/A | Yes | 3b | N/A | 132 | N/A | 48 | N/A | 1.2 | Yes | Yes | N/A | No |
| Yilmaz et al35 | 75 | M | No | Yes | N/A | 3+ | Positive | N/A | 81 | 180 | N/A | Yes | Yes | 6 | No |
| 82 | F | Yes | N/A | N/A | 1+ | Positive | N/A | 76 | 156 | N/A | Yes | Yes | 8 | No | |
| Ribeiro do Vale et al36 | 58 | M | Yes | Yes | 3c | 1/640 | 142 | N/A | 116 | N/A | 1.6 | No | Yes | 11 | No |
| Mrad et al37 | 64 | F | Yes | Yes | 3c | Positive | 200 | N/A | 87 | N/A | >1.2 | Yes | Yes | N/A | No |
| Jamma et al38 | 34 | F | Yes | Yes | 3b | N/A | 113 | DQ8 | N/A | N/A | N/A | N/A | Yes | 7 | No |
| 51 | M | Yes | Yes | 3c | N/A | 200 | DQ2 | N/A | N/A | N/A | N/A | Yes | 11 | No | |
| 48 | F | Yes | Yes | 3b | N/A | 0 | DQ2 | N/A | N/A | N/A | N/A | Yes | 4 | No | |
| 70 | M | Yes | Yes | 3 | N/A | 100 | N/A | N/A | N/A | N/A | N/A | Yes | N/A | No | |
| 48 | F | Yes | N/A | 3 | N/A | N/A | DQ2 | N/A | N/A | N/A | N/A | Yes | 7 | No | |
| 68 | F | Yes | Yes | 3 | N/A | 6 | DQ2 | N/A | N/A | N/A | N/A | Yes | 5 | No | |
| 67 | F | Yes | Yes | 3c | N/A | 250 | DQ2 | N/A | N/A | N/A | N/A | Yes | 8 | No | |
| 74 | F | Yes | Yes | 3c | N/A | 24.5 | N/A | N/A | N/A | N/A | N/A | No | 7 | No | |
| 65 | M | Yes | Yes | 3 | N/A | 21.3 | DQ2 | N/A | N/A | N/A | N/A | Yes | 10 | No | |
| 68 | M | Yes | Yes | 3b | N/A | 117 | DQ2 | N/A | N/A | N/A | N/A | Yes | 11 | No | |
| 65 | F | Yes | Yes | 3c | N/A | 22 | DQ2 | N/A | N/A | N/A | N/A | No | 13 | No | |
| 49 | F | Yes | Yes | 3 | N/A | 83.7 | DQ2 | N/A | N/A | N/A | N/A | Yes | 4 | No | |
| Hammami et al39 | 26 | F | Yes | No | N/A | N/A | N/A | N/A | N/A | N/A | N/A | Yes | Yes | 7 | Yes |
| Toyoshima et al 40 | 30 | F | Yes | Yes | N/A | 1/1280 | 5 | N/A | 114 | N/A | N/A | Yes | Yes | N/A | No |
| Kelly et al41 | 23 | F | N/A | Yes | N/A | N/A | 33.9 | N/A | 120 | 94 | N/A | N/A | Yes | 21 | No |
| Krishna et al42 | 67 | M | Yes | Yes | 3 | Negative | 4 | DQ8 | 124 | 160 | N/A | Yes | Yes | 15 | No |
| Chandan et al43 | 58 | M | Yes | Yes | 3c | N/A | 200 | N/A | 47 | N/A | N/A | Yes | Yes | N/A | No |
| Kizilgul et al44 | 50 | M | Yes | Yes | 3 | 158.7 | 200 | N/A | 94 | N/A | N/A | N/A | Yes | N/A | No |
| Selen et al45 | 37 | F | Yes | Yes | 3 | Positive | Positive | N/A | 80 | N/A | N/A | Yes | Yes | N/A | No |
| Gupta et al46 | 30 | F | Yes | Yes | 3b | N/A | N/A | N/A | <110 | N/A | N/A | Yes | Yes | 7 | No |
| Present Case | 34 | F | Yes | Yes | 3c | 1/80 | 23 | DQ2 | 85 | 297 | 2,52 | Yes | Yes | 9 | No |
EMA, antiendomysial antibodies; F, female; GFD, gluten-free diet; Hb, haemoglobin; HLA, human leucocyte antigen; INR, international normalised ratio; LOS, length of stay; M, male; N/A, not applicable; PLT, platelets; TG2, tissue transglutaminase.
Among the major features of this case were the abrupt onset of symptoms, in particular of those related to electrolyte imbalance, and the severity of the clinical picture prompting admission to the emergency department. Further to severe dehydration, the patient presented with neuromuscular weakness, a finding detected in other reports,7 9 13–21 and electrocardiographic abnormalities due to the key role exerted by potassium in regulating cell excitability. Likely, hypocalcaemia and hypomagnesaemia also contributed to worsening cellular excitability. According to previous evidence,6 9–13 hypoproteinaemia with hypalbuminaemia and metabolic acidosis were found in our patient as hallmarks of malabsorption, whereas paraesthesia was likely related to electrolyte imbalance. However, the variety of possible clinical pictures in coeliac crisis should be underlined, ranging from central nervous system involvement with tetraplegia/paraplegia and ataxia,17–19 psychosis22 as well as seizures,23 24 to coagulopathy11 25–27 and acute kidney injury.28 29 In all cases described so far, coeliac crisis required urgent hospitalisation. The milestone treatment is fluid resuscitation with correction of the electrolyte imbalance, which can lead to life-threatening cardiac arrhythmias. Nutritional support is also of paramount importance, and clinicians should take coeliac crisis in mind during differential diagnosis of severe acute diarrhoea with weight loss, as patients’ prognosis can dramatically improve with a simple dietary intervention.
In our case, like in almost all cases described, GFD led to a dramatic improvement in clinical picture. Nutritional management should take into consideration the possible occurrence of a refeeding syndrome, which can be fatal if not recognised and treated properly, as described in one patient with coeliac crisis.39 In less than 20% of cases (8 of 48 cases, 16%), corticosteroids were administered during management.49 However, we decided not to use steroids since their usefulness was recently questioned.38 50 It was reported previously that immunosuppression with corticosteroids and azathioprine for autoimmune hepatitis or prednisone for Bell’s palsy did not prevent the occurrence of coeliac crisis in patients.17 51 Moreover, steroid therapy may increase electrolyte depletion, facilitating the occurrence of refeeding syndrome.22 Finally, despite the acute onset of malabsorption syndrome in adulthood, our case did not show features of complicated CD52 53 (ie, refractory CD), and the clinical picture dramatically improved in a few days with GFD, still the only effective treatment available.54
Conclusion
The present case highlights the possibility that CD may manifest quite abruptly with a severe malabsorption syndrome and related electrolyte abnormalities and hypoproteinaemia. This would imply that even a typically chronic disorder, such as CD, may have an acute onset in a small proportion of patients, which emergency physicians should be aware of. Although rarely encountered in clinical practice, this acute onset of CD requires hospitalisation and immediate treatment (ie, electrolyte replacement and protein correction) in order to avoid life-threatening complications.
Footnotes
Contributors: MG, RDG and GC: design and conceptualisation. MG, EG and FA reviewed the literature and wrote the first draft of the manuscript. AS, RC, LL, GZ, UV, RDG and GC participated in the clinical assessment of the patient and critically reviewed the paper.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data sharing not applicable as no data sets generated and/or analysed for this study.
References
- 1.Caio G, Volta U, Sapone A, et al. Celiac disease: a comprehensive current review. BMC Med 2019;17:142. 10.1186/s12916-019-1380-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valitutti F, Fasano A. Breaking down barriers: how understanding celiac disease pathogenesis informed the development of novel treatments. Dig Dis Sci 2019;64:1748–58. 10.1007/s10620-019-05646-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trovato CM, Raucci U, Valitutti F, et al. Neuropsychiatric manifestations in celiac disease. Epilepsy Behav 2019;99:106393. 10.1016/j.yebeh.2019.06.036 [DOI] [PubMed] [Google Scholar]
- 4.Volta U, Caio G, Stanghellini V, et al. The changing clinical profile of celiac disease: a 15-year experience (1998-2012) in an Italian referral center. BMC Gastroenterol 2014;14:194. 10.1186/s12876-014-0194-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caio G, Volta U. Coeliac disease: changing diagnostic criteria? Gastroenterol Hepatol Bed Bench 2012;5:119–22. [PMC free article] [PubMed] [Google Scholar]
- 6.Waheed N, Cheema HA, Suleman H, et al. Celiac crisis: a rare or rarely recognized disease. J Ayub Med Coll Abbottabad 2016;28:672–5. [PubMed] [Google Scholar]
- 7.Mones RL, Atienza KV, Youssef NN, et al. Celiac crisis in the modern era. J Pediatr Gastroenterol Nutr 2007;45:480–3. 10.1097/MPG.0b013e318032c8e7 [DOI] [PubMed] [Google Scholar]
- 8.Rostami-Nejad M, Villanacci V, Hogg-Kollars S, et al. Endoscopic and histological pitfalls in the diagnosis of celiac disease: a multicentre study assessing the current practice. Rev Esp Enferm Dig 2013;105:326–33. 10.4321/S1130-01082013000600003 [DOI] [PubMed] [Google Scholar]
- 9.Ozaslan E, Köseoğlu T, Kayhan B. Coeliac crisis in adults: report of two cases. Eur J Emerg Med 2004;11:363–5. 10.1097/00063110-200412000-00015 [DOI] [PubMed] [Google Scholar]
- 10.Poudyal R, Lohani S, Kimmel WB. A case of celiac disease presenting with celiac crisis: rare and life threatening presentation. J Community Hosp Intern Med Perspect 2019;9:22–4. 10.1080/20009666.2019.1571883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gutiérrez S, Toro M, Cassar A, et al. [Celiac crisis: presentation as bleeding diathesis]. Acta Gastroenterol Latinoam 2009;39:53–4. [PubMed] [Google Scholar]
- 12.Wolf I, Mouallem M, Farfel Z. Adult celiac disease presented with celiac crisis: severe diarrhea, hypokalemia, and acidosis. J Clin Gastroenterol 2000;30:324–6. 10.1097/00004836-200004000-00026 [DOI] [PubMed] [Google Scholar]
- 13.Altamimi E. Celiac crisis with severe hypokalemia and paraplegia as a first presentation of celiac disease in a child. Jordan Med J 2012;46:61–3. [Google Scholar]
- 14.Oberhuber G, Granditsch G, Vogelsang H. The histopathology of coeliac disease: time for a standardized report scheme for pathologists. Eur J Gastroenterol Hepatol 1999;11:1185. 10.1097/00042737-199910000-00019 [DOI] [PubMed] [Google Scholar]
- 15.Forrest EA, Wong M, Nama S, et al. Celiac crisis, a rare and profound presentation of celiac disease: a case report. BMC Gastroenterol 2018;18:59. 10.1186/s12876-018-0784-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akbal E, Erbağ G, Binnetoğlu E, et al. An unusual gastric ulcer cause: celiac crisis. Wien Klin Wochenschr 2014;126:661–2. 10.1007/s00508-014-0588-3 [DOI] [PubMed] [Google Scholar]
- 17.Al Shammeri O, Duerksen DR. Celiac crisis in an adult on immunosuppressive therapy. Can J Gastroenterol 2008;22:574–6. 10.1155/2008/453520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oba J, Escobar AM, Schvartsman BGS, et al. Celiac crisis with ataxia in a child. Clinics 2011;66:173–5. 10.1590/S1807-59322011000100031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atikou A, Rabhi M, Hidani H, et al. [Celiac crisis with quadriplegia due to potassium depletion as presenting feature of celiac disease]. Rev Med Interne 2009;30:516–8. 10.1016/j.revmed.2008.11.012 [DOI] [PubMed] [Google Scholar]
- 20.Parry J, Acharya C. Celiac crisis in an older man. celiac crisis in an older man. Am Geriatr Soc 2010;58:1818–9. [DOI] [PubMed] [Google Scholar]
- 21.Magro R, Pullicino E. Coeliac crisis with severe hypokalaemia in an adult. Malta Medical Journal 2012;24:36–9. [Google Scholar]
- 22.Lindo Ricce M, Rodriguez-Batllori Arán B, Jiménez Gómez M, et al. Enfermedad celíaca no respondedora: crisis celíaca vs. enfermedad celíaca refractaria Con respuesta a corticoides. Gastroenterología y Hepatología 2017;40:529–30. 10.1016/j.gastrohep.2016.07.003 [DOI] [PubMed] [Google Scholar]
- 23.Hijaz NM, Bracken JM, Chandratre SR. Celiac crisis presenting with status epilepticus and encephalopathy. Eur J Pediatr 2014;173:1561–4. 10.1007/s00431-013-2097-1 [DOI] [PubMed] [Google Scholar]
- 24.Caio G, De Giorgio R, Venturi A, et al. Clinical and immunological relevance of anti-neuronal antibodies in celiac disease with neurological manifestations. Gastroenterol Hepatol Bed Bench 2015;8:146–52. [PMC free article] [PubMed] [Google Scholar]
- 25.de Almeida Menezes M, Cabral V, Silva Lorena SL. Celiac crisis in adults: a case report and review of the literature focusing in the prevention of refeeding syndrome. Rev Esp Enferm Dig 2017;109:67–8. 10.17235/reed.2016.4073/2015 [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez JJ, Elgamal M, Mishra S, et al. Severe coagulopathy as a rare feature of celiac crisis in a patient previously diagnosed with celiac disease. Am J Case Rep 2019;20:290–3. 10.12659/AJCR.913731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Da Costa Becker SM, Appel-da-Silva MC, D’Incao RB, et al. Celiac crisis and hemorrhagic diathesis in an adult. Scientia Medica 2014;24:284–7. [Google Scholar]
- 28.Ferreira R, Pina R, Cunha N, et al. A celiac crisis in an adult: raising awareness of a life-threatening condition. Eur J Case Rep Intern Med 2016;3:000384. 10.12890/2016_000384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Helvacı Özant, Yıldız S, Korucu B, et al. Coeliac crisis mimicking nephrotic syndrome in a post-partum patient. Scott Med J 2019;64:116–8. 10.1177/0036933019853170 [DOI] [PubMed] [Google Scholar]
- 30.Chen A, Linz CM, Tsay JL, et al. Celiac crisis associated with herpes simplex virus esophagitis. ACG Case Rep J 2016;3:e159. 10.14309/crj.2016.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sbai W, Bourgain G, Luciano L, et al. Celiac crisis in a multi-trauma adult patient. Clin Res Hepatol Gastroenterol 2016;40:e31–2. 10.1016/j.clinre.2015.10.003 [DOI] [PubMed] [Google Scholar]
- 32.Bul V, Sleesman B, Boulay B. Celiac Disease Presenting as Profound Diarrhea and Weight Loss - A Celiac Crisis. Am J Case Rep 2016;17:559–61. 10.12659/AJCR.898004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tiwari A, Qamar K, Sharma H, et al. Urinary tract infection associated with a celiac crisis: a preceding or precipitating event? Case Rep Gastroenterol 2017;11:364–8. 10.1159/000475921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bou-Abboud C, Katz J, Liu W. Firing of an implantable cardiac defibrillator: an unusual presentation of celiac crisis. ACG Case Rep J 2016;3:e137. 10.14309/crj.2016.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yilmaz B, Aksoy EK, Kahraman R, et al. Atypical presentation of celiac disease in an elderly adult: celiac crisis. J Am Geriatr Soc 2015;63:1712–4. 10.1111/jgs.13583 [DOI] [PubMed] [Google Scholar]
- 36.Ribeiro do Vale R, da Silva Conci N, Santana AP, et al. Ls Laborda and Felipe dA Silva as. celiac crisis: an unusual presentation of gluten-sensitive enteropathy. Autopsy Case Rep 2018;8:e2018027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mrad RA, Ghaddara HA, Green PH, et al. Celiac crisis in a 64-year-old woman: an unusual cause of severe diarrhea, acidosis, and malabsorption. ACG Case Reports Journal 2015;2:95–7. 10.14309/crj.2015.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jamma S, Rubio-Tapia A, Kelly CP, et al. Celiac crisis is a rare but serious complication of celiac disease in adults. Clin Gastroenterol Hepatol 2010;8:587–90. 10.1016/j.cgh.2010.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hammami S, Aref HL, Khalfa M, et al. Refeeding syndrome in adults with celiac crisis: a case report. J Med Case Rep 2018;12:22 10.1186/s13256-018-1566-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toyoshima MTK, Queiroz MS, Silva MER, et al. Celiac crisis in an adult type 1 diabetes mellitus patient: a rare manifestation of celiac disease. Arq Bras Endocrinol Metab 2013;57:650–2. 10.1590/S0004-27302013000800011 [DOI] [PubMed] [Google Scholar]
- 41.Kelly E, Cullen G, Aftab AR, et al. Coeliac crisis presenting with cytomegalovirus hepatitis. Eur J Gastroenterol Hepatol 2006;18:793–5. 10.1097/01.meg.0000224471.28626.a6 [DOI] [PubMed] [Google Scholar]
- 42.Krishna K, Krishna SG, Coviello-malle JM, et al. Celiac crisis in a patient with chronic lymphocytic leukemia and hypogammaglobulinemia. Clin Res Hepatol Gastroenterol 2011;35:70–3. 10.1016/j.gcb.2010.08.002 [DOI] [PubMed] [Google Scholar]
- 43.Chandan S, Ahmad D, Hewlett A. Celiac crisis: a rare presentation of celiac disease in adults. 2016.. ACG 2016 conference paper. [Google Scholar]
- 44.Kizilgul M, Kan S, Celik S, et al. Celiac crisis in an adult type 1 diabetes mellitus patient presented with diarrhea, weight loss and hypoglycemic attacks. Int J Diabetes Dev Ctries 2017;37:85–7. 10.1007/s13410-016-0477-6 [DOI] [Google Scholar]
- 45.Selen T, Ince M, Çelik S, et al. Gluten sensitive enteropathy presenting with celiac crisis as the initial presentation: case report. Turkiye Klinikleri Journal of Gastroenterohepatology 2014;21:60–2. 10.5336/gastro.2014-42592 [DOI] [Google Scholar]
- 46.Gupta T, Mandot A, Desai D, et al. Celiac crisis with hypokalemic paralysis in a young lady. Indian J Gastroenterol 2006;25:259–60. [PubMed] [Google Scholar]
- 47.Volta U, Fabbri A, Parisi C, et al. Old and new serological tests for celiac disease screening. Expert Rev Gastroenterol Hepatol 2010;4:31–5. 10.1586/egh.09.66 [DOI] [PubMed] [Google Scholar]
- 48.Amarri S, Alvisi P, De Giorgio R, et al. Antibodies to deamidated gliadin peptides: an accurate predictor of coeliac disease in infancy. J Clin Immunol 2013;33:1027–30. 10.1007/s10875-013-9888-z [DOI] [PubMed] [Google Scholar]
- 49.Balaban DV, Dima A, Jurcut C, et al. Celiac crisis, a rare occurrence in adult celiac disease: a systematic review. WJCC 2019;7:311–9. 10.12998/wjcc.v7.i3.311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gupta S, Kapoor K. Steroids in celiac crisis: doubtful role! Indian Pediatr 2014;51:756–7. [PubMed] [Google Scholar]
- 51.Volta U, Caio G, Tovoli F, et al. Gut–liver axis: an immune link between celiac disease and primary biliary cirrhosis. Expert Rev Gastroenterol Hepatol 2013;7:253–61. 10.1586/egh.13.5 [DOI] [PubMed] [Google Scholar]
- 52.Caio G, Volta U, Ursini F, et al. Small bowel adenocarcinoma as a complication of celiac disease: clinical and diagnostic features. BMC Gastroenterol 2019;19:45 10.1186/s12876-019-0964-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vanoli A, Di Sabatino A, Furlan D, et al. Small Bowel Carcinomas in Coeliac or Crohn’s Disease: Clinico-pathological, Molecular, and Prognostic Features. A Study From the Small Bowel Cancer Italian Consortium. J Crohns Colitis 2017;11:942–53. 10.1093/ecco-jcc/jjx031 [DOI] [PubMed] [Google Scholar]
- 54.Caio G, Ciccocioppo R, Zoli G, et al. Therapeutic options for coeliac disease: what else beyond gluten-free diet? Digestive and Liver Disease 2020;52:130–7. 10.1016/j.dld.2019.11.010 [DOI] [PubMed] [Google Scholar]