Abstract
STUDY DESIGN:
Cross-sectional.
OBJECTIVES:
To quantify the magnitude and distribution of muscle fat infiltration (MFI) within the cervical multifidus and semispinalis cervicis muscles of participants with chronic whiplash-associated disorders (WAD) as compared to those who fully recover from a whiplash injury and healthy controls.
BACKGROUND:
Previous research has established the presence of increased MFI throughout the cervical extensor muscles of individuals with WAD when compared to healthy controls. These changes appear to be greater in deepest muscles (eg, multifidus and semispinalis cervicis) than the more superficial muscles. A detailed analysis of the distribution of MFI within these deep extensor muscles in chronic WAD, Recovered, and Control groups would provide foundation for further investigation of specific mechanisms, etiologies, and targets for treatments.
METHODS:
Fifteen participants: WAD (n=5), Recovered (n=5), and Controls (n=5) were studied using a 3-dimensional (3D) fat/water separation magnetic resonance imaging (MRI) sequence. Bilateral measures of cervical multifidus and semispinalis cervicis MFI in 4 quartiles (Q1 – Q4; medial to lateral) at cervical levels C3 through C7 were included in the analysis. Intrarater and interrater reliability were established. A mixed model analysis was performed to control for covariates, identify interaction effects, and compare MFI distribution between groups.
RESULTS:
The Limits of Agreement (LOA) confirmed strong intrarater and interrater agreement at all levels (C3-C7). Gender, age, and body mass index (BMI) were identified as significant covariates on MFI. Significant interactions were found between group and muscle quartile (P<.001) and between muscle quartile and cervical level (P<.001). Pairwise comparisons for intra-quartile MFI between groups revealed significantly greater MFI in the WAD group when compared to the Recovered group in Q1 (P<.001), Q2 (P<.001), and Q3 (P=.03). When compared to the Control group, the WAD group had significantly greater MFI in Q1 (P=.002) and Q2 (P=.045). The Control group had significantly higher MFI in comparison to the Recovered group in Q1 (P=.048).
CONCLUSION:
This study provides preliminary data mapping the spatial distribution of MFI in the cervical multifidus and semispinalis cervicis muscles in individuals with chronic WAD, those who have recovered from a whiplash injury, and healthy controls. MFI is more concentrated in the medial portion of the muscles in WAD, recovered, and control participants. However, the magnitude of MFI in the medial quartiles (Q1 and Q2) is greatest in the chronic WAD group.
Keywords: Imaging, MRI, neck, spine, whiplash, muscle
Introduction
Previous studies using magnetic resonance imaging (MRI) have revealed widespread fatty infiltrates in the neck extensor5 and flexor8 muscles of individuals with chronic whiplash associated disorders (WAD). These high levels of muscle fat infiltration (MFI) were not present in those with chronic non-traumatic neck pain6 or those without a history of neck disorders5. While widespread, the greatest magnitude of MFI was consistently observed in the deepest muscular layer of the extensors (eg, the multifidus and semispinalis cervicis) when compared to the more superficial musculature (eg, semispinalis capitis, splenius capitis, and upper trapezius).5, 9 The specific role of MFI in the development and maintenance of chronic WAD is not fully understood.7, 24 Improvements in our mechanistic understanding of the development of structural changes (eg, composition and morphology) in the cervical muscles of patients with chronic WAD may shed light on their potential contribution to poor functional recovery.
The muscles of the human neck are responsible for the majority of the postural stability of the cervical spinal column.18 The extensors are layered and can be divided into functional groups based on location and attachments. The most superficial extensor muscles attach the shoulder girdle to the cranium (eg. upper trapezius). The intermediate layer extensors (eg, portions of the splenius and semispinalis capitis), attach the shoulder girdle to individual vertebrae, or individual vertebrae to the cranium. The deep extensors, including the cervical multifidus and the semispinalis cervicis, attach vertebrae directly to other vertebrae. The deep extensors likely play a specific role in segmental support of the cervical spine and fine neck postural control.15 Degeneration of these complex muscles, as MFI might indicate, may have implications for altered biomechanics of the neck and postural stability in those with chronic WAD. Associations between MFI and commonly observed impairments (eg, proprioceptive deficits) and other functional characteristics, such as stiffness and altered motor control, are yet to be quantified.
Previous animal work suggests specific patterns of MFI are a consequential marker of induced experimental injuries involving the lumbar spine.13 Clinically, the magnitude and anatomical location of MFI alters plan of care with respect to surgical management of rotator cuff tears.11, 12, 16 Furthermore, measurement of and histological confirmation for MFI with a quantitative MRI approach has been produced in both an animal20 and human model.10
This preliminary study aims to establish the reliability of an MRI measure for mapping the magnitude and spatial distribution of MFI in the cervical multifidus and semispinalis cervicis muscles and to provide preliminary data for comparing MFI in individuals with chronic WAD, individuals who recovered from a whiplash injury, and healthy controls. Such data are necessary to inform future research on potential altered biomechanics (eg, changes in joint torques) and control patterns (eg, changes in task-dependent muscle activation) that may lead to or perpetuate persistent pain and disability in those with chronic WAD.
METHODS
Study Population
A total of 15 participants were included in this analysis. Five gender and age matched participants were selected for each group (WAD, Recovered, and Controls) from a concurrent prospective study. The MRI measures for MFI were performed by 2 raters who were blind to the clinical status of each participant. Each participant in the WAD group (n=5) had chronic neck pain (3 months – 5 years post motor vehicle collision [MVC]) and a neck disability index (NDI) greater than 30%. Those in the Recovered group (n=5) indicated full resolution of symptoms and were scanned at 3 months post MVC and had an NDI score of less than 10%. Those in the Control group (n=5) had no history of neck pain that required treatment in the past 10 years. Additional inclusion criteria consisted of no cervical spine fracture following MVC, no previous neck injury, and no previously diagnosed nervous system disorders. All participants provided informed consent before participating in the study. The protocol for this study was approved by the Institutional Review Board of Northwestern University.
MRI Measures and Analysis
A 3-dimensional multi-echo gradient echo acquisition was performed to collect the data required for the analysis of phase related to the precessional differences in muscle fat and water. A standard 12-channel head coil and 4-channel neck coil were used as receiver coils to improve the signal-to-noise ratio. The axial FLASH dual echo, gradient echo sequence was 4:23 minutes with an in-plane resolution of 0.7mm using a rectangular field of view of 75% and thickness of 3 mm and slab oversampling of 22% with 36 partitions to prevent aliasing, TR/TE1/TE2 6.59/2.45/3.68 ms with a FOV of 190 × 320 mm. This scan covered the cephalad portion of C3 through the caudal portion of the C7 vertebral end plate. By collecting data at an echo time when water and fat are in-phase and at an echo time when water and fat are out of phase, 2 images are produced. The ratio of the pixel intensities within a coregistered voxel in each image gives an index of MFI.
The MRI analysis consisted of manually tracing defined regions of interest (ROI) bilaterally over the cervical multifidus and semispinalis cervicis muscles on axial MR slices. A customized program was developed using MATLAB (MathWorks™ USA) to quantify the magnitude of MFI in each quartile of the ROI. Quartiles were defined by 2 dimensional coordinates containing an equal number of pixels running from medial (Quartile 1- Q1) to lateral (Quartile 4 – Q4) based on the orientation of the muscle as viewed in the transverse plane (FIGURE 1). MFI, a unitless measure of fat within the muscle, was calculated as the ratio of pixel intensities from the fat and water images:
where IF = Intensity of fat signal and IW = Intensity of water signal
FIGURE 1.
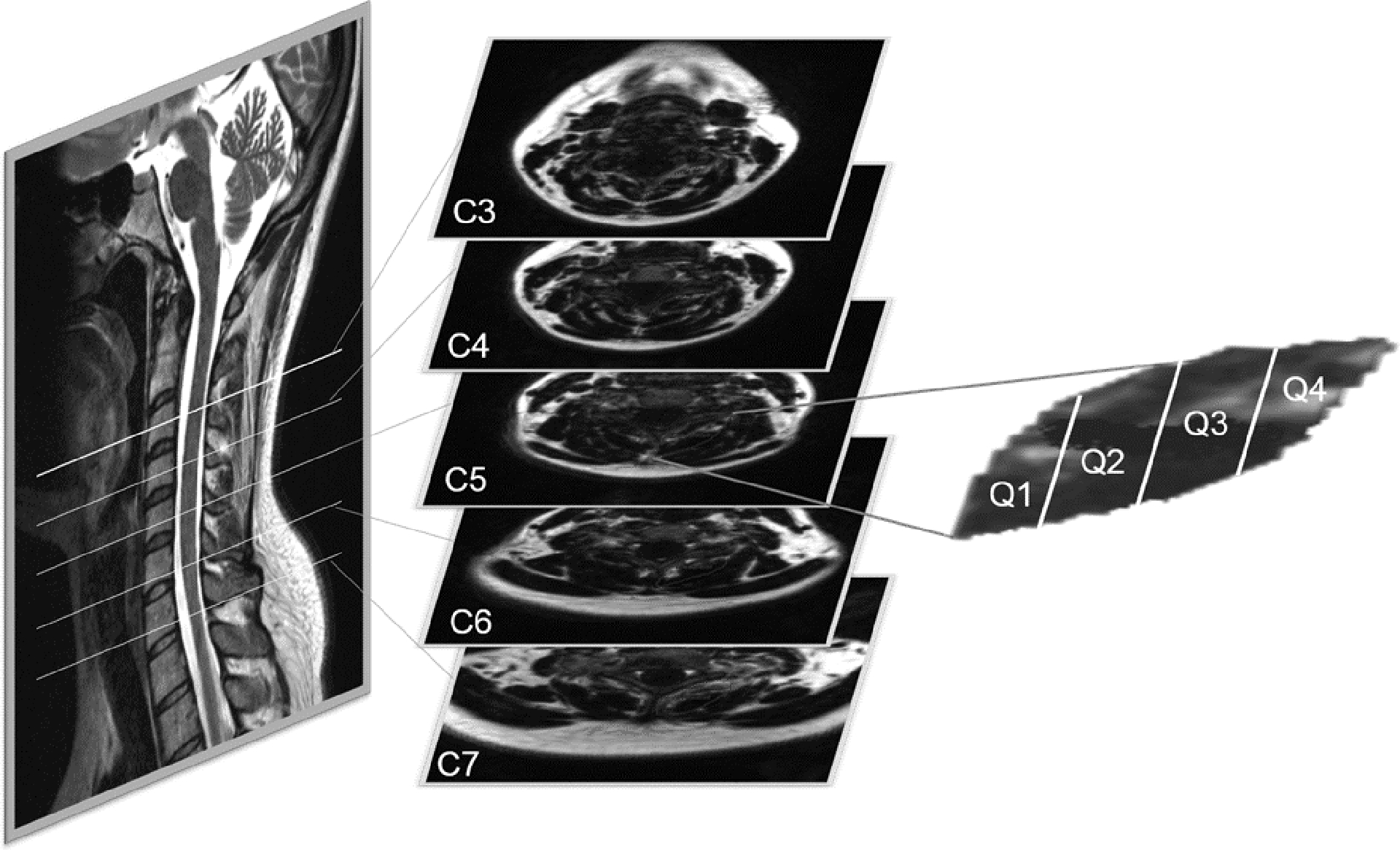
Regions of interest (ROIs) were drawn bilaterally around the Multifidus and Semispinalis Cervicis muscles (only one side shown above) at 5 cervical levels (C3-C7) for each participant. Each ROI was divided into quartiles (Q1 (medial) - Q4 (lateral)) for muscle fat infiltration (MFI) analysis.
The quartile and whole MFI values at each cervical level (C3-C7) are the average of measurements at 5 axial slices that span the length of the vertebral level. For example, the MFI value of Q2 at cervical level C4 of Subject A, is the average of 5 MFI measurements from sequential axial MR slices that span from the top to the bottom of the C4 vertebral body. An MFI measure for all 4 quartiles was created in this manner bilaterally at each level for each participant.
To test repeatability, 2 authors (RA and MH) experienced in this technique, independently traced the ROI once for each cervical level of 6 randomly selected participants (4 WADs and 2 Controls) while being blind to the other’s assessment.
Statistical Analysis
Intrarater and interrater reliability of intra-quartile MFI were examined through construction of Bland-Altman plots and calculation of the limits of agreement (LOA) using the method proposed by Bland & Altman.2 Intraclass correlation coefficients (ICCs) were also calculated for both intrarater and interrater reliability data using a 2-way random effects structure and absolute agreement (ICC[2,1]).
A repeated measures linear mixed model approach was used to investigate differences between groups in the magnitude and spatial distribution of MFI. A within-subject factor of level * quartile was used to account for the spatial repetition of measures of MFI across cervical level and muscle quartile. Preliminary investigation of MFI across all cervical levels revealed a non-linear quadratic-type relationship between MFI and cervical level. Thus, the quadratic effect of cervical level on MFI was entered into the model as a fixed effect. The main and interactive effects of muscle quartile and group (WAD, Recovered, Control) and the 3-way interactive effect of muscle quartile with group and cervical level were also included in the model. Inter-subject differences in the change in MFI across cervical level and across quartiles were included in the model as random effects. Age, gender, and BMI were entered as covariates given the previous evidence of their effect on MFI. Pairwise comparisons with Bonferroni corrections for multiple comparisons were used to investigate between group differences in intra-quartile MFI. All statistical procedures were performed using IBM SPSS 22.0 (IBM). For all analyses, the significance level was set to P ≤ .05.
RESULTS
Demographic data for each participant and their respective research groups are provided in TABLE 1. Raw data for MFI by group, quartile, and cervical level are provided in TABLE 2.
TABLE 1.
Demographic data for each participant and their respective groups. (Mean ± SD)
| Group | Gender | Age (years) | BMI (kg/m2) | NDI (x/100) |
|---|---|---|---|---|
| WAD-1 | F | 28 | 27 | 32 |
| WAD-2 | F | 40 | 36 | 38 |
| WAD-3 | M | 25 | 33 | 42 |
| WAD-4 | F | 20 | 26 | 30 |
| WAD-5 | M | 40 | 26 | 64 |
| WAD* | 30.6 ± 9.0 | 29.6 ± 4.6 | 41.2 ± 13.6 | |
| Rec-1 | F | 28 | 25 | 0 |
| Rec-2 | F | 40 | 26 | 6 |
| Rec-3 | M | 27 | 26 | 0 |
| Rec-4 | F | 27 | 27 | 2 |
| Rec-5 | M | 42 | 28 | 0 |
| Rec* | 32.8 ± 7.5 | 26.4 ± 1.1 | 1.6 ± 2.6 | |
| Ctrl-1 | F | 29 | 21 | - |
| Ctrl-2 | F | 47 | 33 | - |
| Ctrl-3 | M | 30 | 27 | - |
| Ctrl-4 | F | 27 | 21 | - |
| Ctrl-5 | M | 42 | 27 | - |
| Ctrl* | 35.0 ± 8.9 | 25.8 ± 5.0 | - |
Abbreviations: BMI, body mass index; Ctrl, Control; F, female; M, male; NDI, neck disability index; Rec, Recovered; WAD, whiplash associated disorder
Summary data are means and standard deviations except for gender which is made up of 3 females and 2 males in each group.
TABLE 2.
Raw data for muscle fat infiltration (MFI) by group, quartile, and cervical level for the multifidus and semispinalis cervicis muscles.*
| Cervical Level | Quartile | WAD | Recovered | Control |
|---|---|---|---|---|
| C3 | Q1 | 0.332 ± 0.090 | 0.231 ± 0.033 | 0.232 ± 0.064 |
| Q2 | 0.319 ± 0.096 | 0.220 ± 0.032 | 0.232 ± 0.083 | |
| Q3 | 0.304 ± 0.067 | 0.206 ± 0.046 | 0.218 ± 0.056 | |
| Q4 | 0.325 ± 0.052 | 0.239 ± 0.044 | 0.231 ± 0.056 | |
| Whole | 0.323 ± 0.065 | 0.226 ± 0.032 | 0.230 ± 0.061 | |
| C4 | Q1 | 0.305 ± 0.077 | 0.190 ± 0.061 | 0.206 ± 0.049 |
| Q2 | 0.259 ± 0.074 | 0.155 ± 0.044 | 0.179 ± 0.074 | |
| Q3 | 0.184 ± 0.062 | 0.110 ± 0.021 | 0.129 ± 0.052 | |
| Q4 | 0.214 ± 0.049 | 0.140 ± 0.027 | 0.147 ± 0.041 | |
| Whole | 0.244 ± 0.56 | 0.150 ± 0.031 | 0.167 ± 0.051 | |
| C5 | Q1 | 0.313 ± 0.075 | 0.177 ± 0.018 | 0.217 ± 0.073 |
| Q2 | 0.252 ± 0.057 | 0.141 ± 0.041 | 0.166 ± 0.060 | |
| Q3 | 0.178 ± 0.052 | 0.108 ± 0.016 | 0.113 ± 0.034 | |
| Q4 | 0.176 ± 0.060 | 0.134 ± 0.021 | 0.126 ± 0.031 | |
| Whole | 0.234 ± 0.048 | 0.141 ± 0.021 | 0.157 ± 0.048 | |
| C6 | Q1 | 0.363 ± 0.074 | 0.214 ± 0.056 | 0.267 ± 0.064 |
| Q2 | 0.227 ± 0.073 | 0.131 ± 0.041 | 0.165 ± 0.044 | |
| Q3 | 0.161 ± 0.064 | 0.097 ± 0.022 | 0.112 ± 0.040 | |
| Q4 | 0.158 ± 0.091 | 0.116 ± 0.021 | 0.122 ± 0.048 | |
| Whole | 0.234 ± 0.071 | 0.142 ± 0.034 | 0.170 ± 0.045 | |
| C7 | Q1 | 0.381 ± 0.080 | 0.252 ± 0.085 | 0.289 ± 0.079 |
| Q2 | 0.265 ± 0.098 | 0.146 ± 0.044 | 0.188 ± 0.057 | |
| Q3 | 0.195 ± 0.071 | 0.119 ± 0.027 | 0.148 ± 0.051 | |
| Q4 | 0.197 ± 0.110 | 0.130 ± 0.031 | 0.158 ± 0.060 | |
| Whole | 0.266 ± 0.080 | 0.164 ± 0.045 | 0.199 ± 0.060 |
Abbreviation: WAD, whiplash-associated disorder
Data are means and standard deviations for 5 participants in each group. “Whole” is for all 4 quartiles for each level.
A high level of agreement was confirmed for intrarater and interrater measures of quartile MFI with Bland & Altman (FIGURE 2 A, B) and ICC (FIGURE 2 C, D) analyses. Bland & Altman plots showed no systematic relationship between MFI values and absolute differences for either intrarater or interrater data. LOA showed slightly better agreement within (−0.04, 0.06) than between (−0.07, 0.07) raters. ICCs for both intrarater (ICC[2,1] = 0.98, 95% CI = 0.97, 0.98) and interrater (ICC[2,1] = 0.93, 95% CI = 0.90, 0.94) reliability showed excellent levels of agreement.
FIGURE 2.
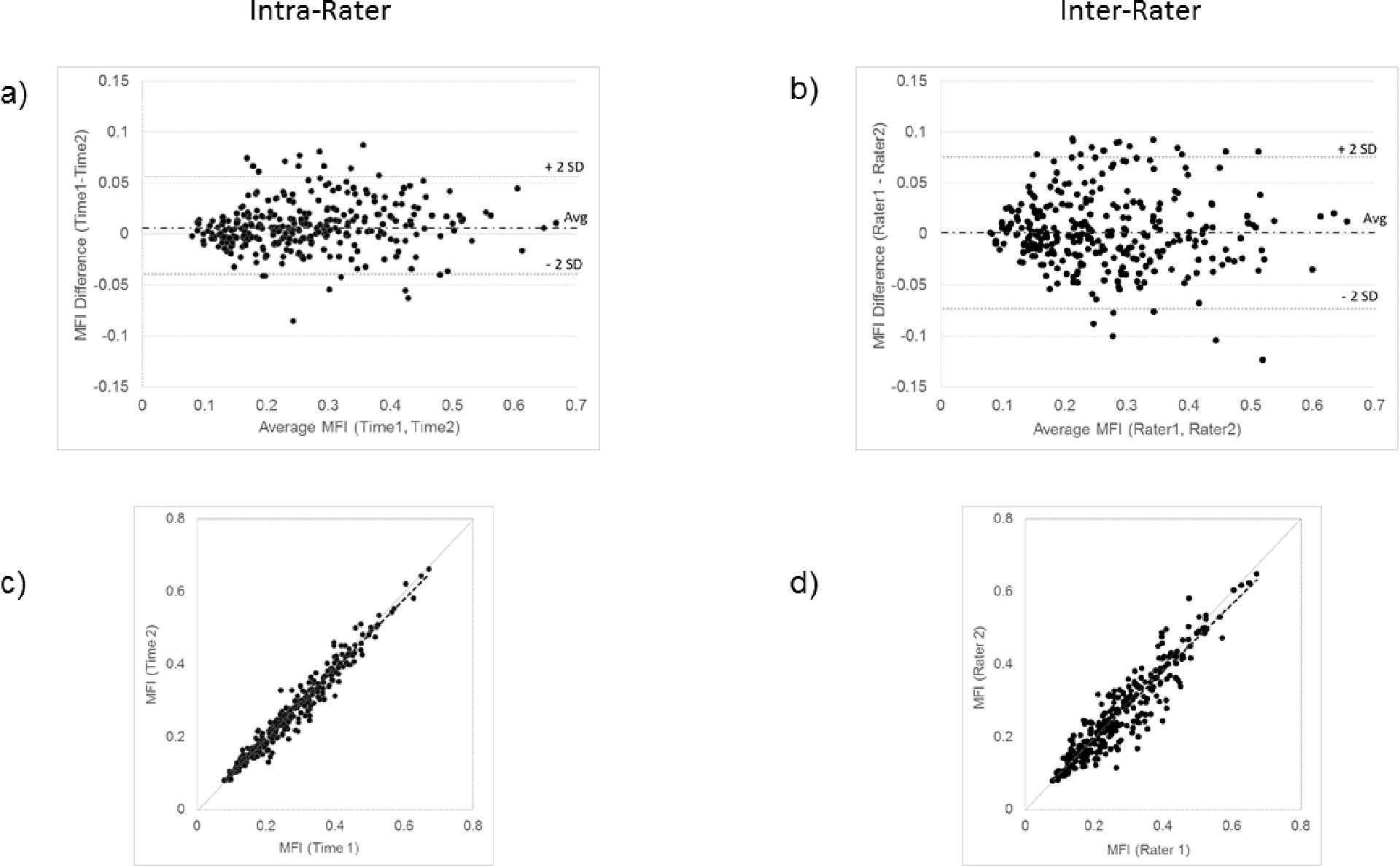
Intrarater and interrater reliability for the MFI quantification method. a) Bland-Altman Plot for intrarater reliability (Limits of Agreement (LOA)={−0.04, 0.06}), b) Bland-Altman Plot for interrater reliability (LOA={−0.07, 0.07}), c) Intrarater correlation (Intraclass Correlation Coefficient (ICC)[2,1] = 0.98, 95%CI = 0.97, 0.98), d) Interrater correlation (ICC[2,1] = 0.93, 95%CI = 0.90, 0.94).
Mixed model analysis revealed a significant effect of the covariates gender (F[1, 30.9] = 11.8, P=.002) and BMI (F[1, 30.9] = 28.0, P<.001) on MFI. There was no significant effect for age on MFI (F[1, 30.9] = 1.0, P=.316). There was a significant interaction between group and muscle quartile (F[6, 211.7] = 6.4, P<.001) and between muscle quartile and cervical level (F[3, 216.9] = 29.6, P<.001) but not between group and cervical level (F[2, 29.5] = 0.5, P=.61). The 3-way interaction between group, cervical level, and muscle quartile was not significant (F[6, 216.9] = 1.2, P=.32 indicating that between-group differences in intra-quartile MFI were consistent across cervical levels.
Pairwise comparisons for intra-quartile fat between groups are shown in FIGURE 3. The WAD group had significantly higher MFI values than the Recovered group in Q1 (df = 45.2, P<.001), Q2 (df = 46.17, P<.001), and Q3 (df = 47.6, P=.03). Significantly higher MFI was also observed in the WAD group in comparison to the Control group in Q1 (df = 43.0, P=.002) and Q2 (df = 43.8, P=.045). The Recovered group showed significantly lower MFI in comparison to the Control group in Q1 (df = 48.6, P=.048).
FIGURE 3.
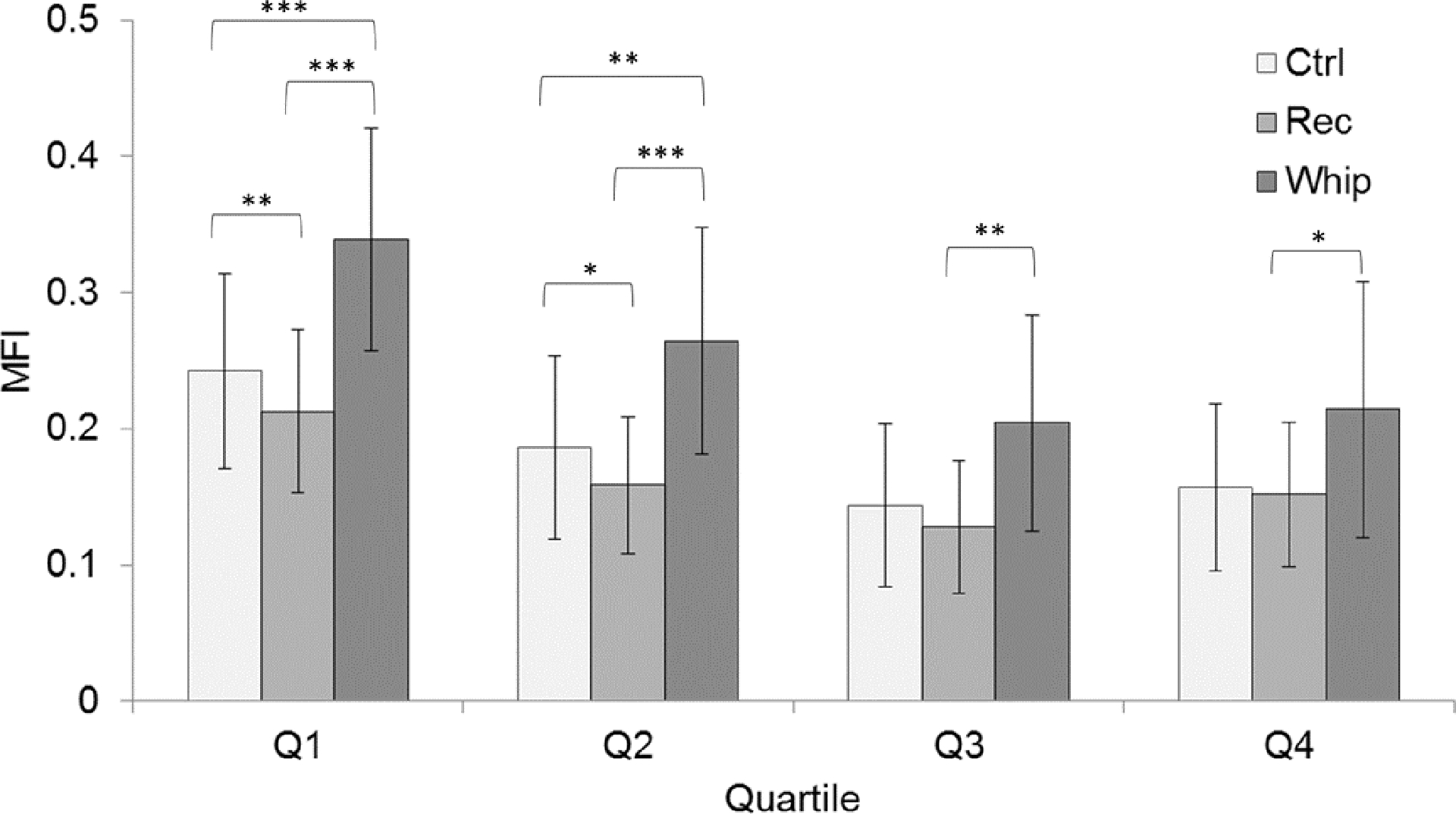
MFI means and standard deviations for between group comparisons at each quartile (Q1- medial, Q4- lateral) averaged over all cervical levels. The values are estimated marginal means and were based on the covariate values of age = 32.8 years, Body Mass Index (BMI) = 27.27 kg/m2, and cervical level = C4. Significance is denoted as * for P<.05, ** for P<.01, and *** for P<.001.
DISCUSSION
This study provides preliminary data mapping the magnitude and regional distribution of MFI in the deep extensor muscles in a small sample of individuals with chronic WAD, individuals who recovered from a whiplash injury, and healthy controls. Consistent with previous studies, the chronic WAD group presents with the greatest average MFI throughout the deep extensors when compared to controls5 and those who had recovered from a whiplash injury.7 Between group comparisons of intra-quartile MFI revealed a significantly greater MFI in the medial quartiles (Q1 and Q2) in the WAD group compared to both the Recovered and Control groups. Oddly, intra-quartile MFI was significantly greater in the WAD group compared to the recovered group, but not the Control group in Q3. Another unexpected result is that the Recovered group demonstrated significantly lower MFI in Q1 compared to the Control group. These 2 observations are difficult to explain, but may be the result of the low number of participants or factors related to trauma,4, 11 such as inflammation,13 that may drive (or inhibit) the development of MFI.23 Further mechanistic investigation of local and systemic serum quantities of inflammatory biomarkers (eg, TNF-α, C-Reactive Protein, and IL-1ß)23 is warranted as this could help explain the differential response (and presence) of MFI between the Recovered and Control group participants. Also, while we did not collect data regarding the various treatments being received by any of the participants with whiplash, it remains plausible that the presumed healthier muscle tissue (eg, less MFI) in recovered participants compared to the controls was, in part, influenced by an intervention effect. This is currently being documented and investigated in a larger prospective study.
Data from the plot of LOA suggest that the MFI quartile analysis is repeatable between and within raters experienced in this method. Despite the limited number of participants, this study was adequately powered to assess reliability for this measure because each ROI is considered an independent data point (5 cervical levels × 2 sides = 10 data points per participant) and the images were presented in a randomized fashion. Walter et al25 showed that with an assumed minimum agreement for ICC of 0.80 and a null hypothesis of 0.4, a minimum of 9 independent observations provides an acceptable 5% alpha error rate (P<.05) and 20% beta error rate. There can be a strong level of confidence in accepting the results of the measure as is demonstrated by the widths of the 95% CIs for the mean interrater differences in MFI across all cervical levels.
Also of interest are the interactions between quartile, cervical level, and group. Inter-quartile differences in MFI differed across cervical levels. MFI appears to be relatively equally distributed between quartiles at the highest analyzed cervical level (C3). But at lower cervical levels, Q1 is the highest of the quartiles while Q2, Q3, and Q4 decrease or stay the same (FIGURES 4a, 4b, 4c). This general pattern that is apparent in all groups may be related to the architecture of the deep paraspinal muscles rather than a true indicator of degeneration at these levels. The second significant interaction reveals that MFI within muscle quartiles differs between the study groups (FIGURE 3). These between-group differences are consistent across cervical levels. While preliminary, the findings support further prospective investigation with a larger cohort that may reveal the development of a characteristic MFI distribution pattern amongst participants with varying levels of pain-related disability. Such work is currently underway, aiming to identify specific injury mechanisms underlying MFI, biomechanical consequences of MFI, and potential treatment options.
FIGURE 4.
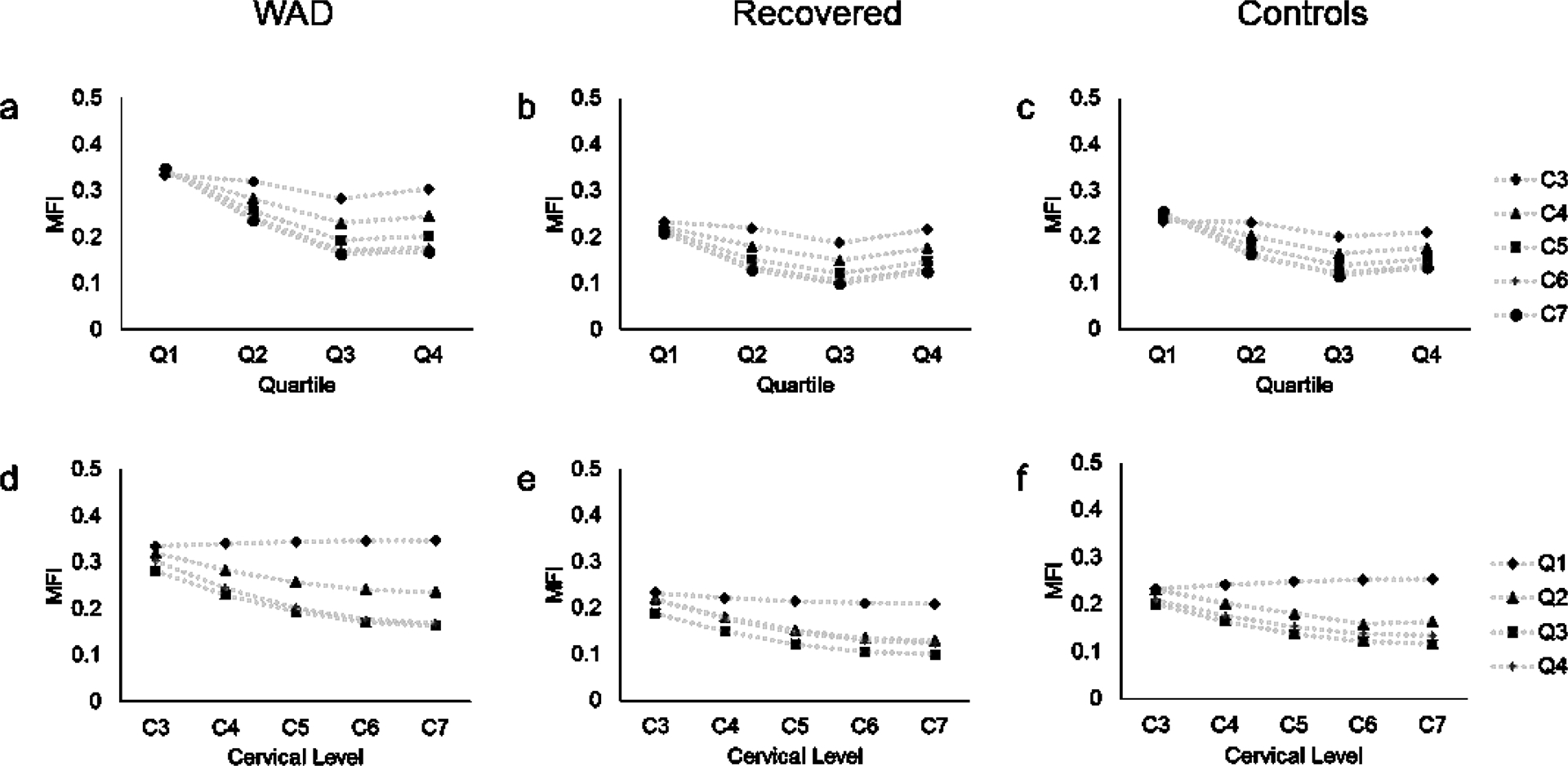
Mapping of MFI across quartiles of the Multifidus and Semispinalis Cervicis muscles for WAD (a), Recovered (b), and Control (c) groups and across cervical levels for WAD (d), Recovered (e), and Control (f) groups. There was a significant interaction between group and muscle quartile (P<.001) and between muscle quartile and cervical level (P<.001).
Although higher magnitudes of MFI in the multifidus muscles have been attributed to WAD, these current observations suggest that the intra-muscular changes may be more regionally situated than previously thought. The multifidus and semispinalis cervicis muscles have a complex architecture, with fascicles attaching to the spinous process (falling into Q1) of a superior vertebra and then traveling laterally to attach to the transverse process of an inferior vertebra (falling into Q4). While the precise biomechanical consequences of the spatial patterns of MFI within the deep extensors observed in this study are unknown, several hypotheses can be generated. If local MFI increases translate to deficits in motor function, the location of high MFI in specific neck muscles is relevant to the clinical presentation and the development of biomechanical models of the human head/neck. To our knowledge, the distribution and magnitude of MFI has not factored into the development of and use for current modeling efforts.
The magnitude and location of structural muscle changes may have implications for ongoing neck pain following a MVC, as they could alter the internal forces in joints and muscles. The multifidus and semispinalis cervicis both have attachments to the facet capsules, which have been consistently implicated in the generation of and maintenance for neck pain following whiplash.14, 17, 19, 26 Treatments targeting facetogenic pain are available. Radiofrequency neurotomy (RFN) interventions, which denervate the multifidus muscle, have been shown to attenuate the psychophysical signs/symptoms in whiplash (eg, reduced thermal/pressure pain thresholds)21 and low back pain3. However, considering the known resolution of facetogenic pain, the long-term consequences of RFN on the structure and function of the deep cervical muscles (and lumbar muscles3, 22) are largely unknown. It is noted that none of the participants in this study had undergone an RFN procedure. Further exploration with current quantitative imaging and emerging modeling methods are warranted to better understand the biomechanical and functional consequences of denervation on the spinal muscle system.1
CONCLUSION
The results of this study provide preliminary evidence of unique patterns of MFI distribution within the deep extensor muscles of individuals with chronic WAD, individuals who have recovered from a whiplash injury, and healthy controls. This provides foundation to explore whether the geography and magnitude of MFI contributes to persistent functional deficits common to some individuals with WAD. Further study to verify this pattern and to determine the potential influence of rehabilitative exercise amongst a larger cohort with varying amounts of MFI is warranted.
KEY POINTS.
Findings
The magnitude of MFI distributed across the deep cervical extensor muscles differs between groups in a small sample of individuals with chronic WAD, individuals who have recovered from a whiplash injury, and healthy controls.
Implications
Quantifying the magnitude and distribution of MFI in the cervical muscles may elucidate the mechanisms underlying disturbed neuromuscular control of posture and dynamic stability of the head and neck following whiplash injuries. Accordingly, such information may provide foundation for exploring and developing more informed treatment regimens.
Caution
It is necessary to establish a larger dataset of within muscle variation of MFI across all muscles in the cervical spine in individuals with WAD before more definitive biomechanical conclusions can be made. Furthermore, a scan-rescan reliability measure is also warranted to account for potential acquisition variability.
Acknowledgments
James Elliott is supported by NIH KL2 - KL2 RR025740
Rebecca Abbott and Mark Hoggarth are supported by NIH T32 training grant T32EB009406
Michelle Sterling is supported by a NHMRC Senior Research fellowship 1002489
Footnotes
The protocol for this study was approved by the Institutional Review Board of Northwestern University.
Elliott has an investment and ownership interest in a medical consulting start-up: Pain ID, LLC
References
- 1.Abbott RE, Parrish TB, Hoggarth MA, Smith AC, Elliott JM. Letter to the editor regarding Smuck M, Cristostomo RA, Demirjian R, et al. Morphologic change in the lumbar spine after lumbar medial branch radiofrequency neurotomy: a quantitative radiological study. Spine J. 2014;14:1088–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 3.Dreyfuss P, Stout A, Aprill C, Pollei S, Johnson B, Bogduk N. The significance of multifidus atrophy after successful radiofrequency neurotomy for low back pain. PM R. 2009;1:719–722. [DOI] [PubMed] [Google Scholar]
- 4.Dulor JP, Cambon B, Vigneron P, et al. Expression of specific white adipose tissue genes in denervation-induced skeletal muscle fatty degeneration. FEBS Lett. 1998;439:89–92. [DOI] [PubMed] [Google Scholar]
- 5.Elliott J, Jull G, Noteboom JT, Darnell R, Galloway G, Gibbon WW. Fatty infiltration in the cervical extensor muscles in persistent whiplash-associated disorders: a magnetic resonance imaging analysis. Spine (Phila Pa 1976). 2006;31:E847–855. [DOI] [PubMed] [Google Scholar]
- 6.Elliott J, Sterling M, Noteboom JT, Darnell R, Galloway G, Jull G. Fatty infiltrate in the cervical extensor muscles is not a feature of chronic, insidious-onset neck pain. Clinical Radiology. 2008;63:681–687. [DOI] [PubMed] [Google Scholar]
- 7.Elliott JM. Are there implications for morphological changes in neck muscles after whiplash injury? Spine. 2011;36:S205–210. [DOI] [PubMed] [Google Scholar]
- 8.Elliott JM, O’Leary S, Sterling M, Hendrikz J, Pedler A, Jull G. Magnetic resonance imaging findings of fatty infiltrate in the cervical flexors in chronic whiplash. Spine. 2010;35:948–954. [DOI] [PubMed] [Google Scholar]
- 9.Elliott JM, Pedler AR, Jull Ga, Van Wyk L, Galloway GG, OʼLeary SP. Differential changes in muscle composition exist in traumatic and nontraumatic neck pain. Spine. 2014;39:39–47. [DOI] [PubMed] [Google Scholar]
- 10.Gaeta M, Scribano E, Mileto A, et al. Muscle fat fraction in neuromuscular disorders: dual-echo dual-flip-angle spoiled gradient-recalled MR imaging technique for quantification–a feasibility study. Radiology. 2011;259:487–494. [DOI] [PubMed] [Google Scholar]
- 11.Gerber C, Meyer DC, Schneeberger AG, Hoppeler H, von Rechenberg B. Effect of tendon release and delayed repair on the structure of the muscles of the rotator cuff: an experimental study in sheep. J Bone Joint Surg Am. 2004;86-A:1973–1982. [DOI] [PubMed] [Google Scholar]
- 12.Hodges P, Holm AK, Hansson T, Holm S. Rapid atrophy of the lumbar multifidus follows experimental disc or nerve root injury. Spine (Phila Pa 1976). 2006;31:2926–2933. [DOI] [PubMed] [Google Scholar]
- 13.Lefaucheur JP, Gjata B, Lafont H, Sebille A. Angiogenic and inflammatory responses following skeletal muscle injury are altered by immune neutralization of endogenous basic fibroblast growth factor, insulin-like growth factor-1 and transforming growth factor-beta 1. Journal of neuroimmunology. 1996;70:37–44. [DOI] [PubMed] [Google Scholar]
- 14.Lord SM, Barnsley L, Wallis BJ, Bogduk N. Chronic cervical zygapophysial joint pain after whiplash. A placebo-controlled prevalence study. Spine (Phila Pa 1976). 1996;21:1737–1744; discussion 1744–1735. [DOI] [PubMed] [Google Scholar]
- 15.MacDonald DA, Moseley GL, Hodges PW. The lumbar multifidus: does the evidence support clinical beliefs? Man Ther. 2006;11:254–263. [DOI] [PubMed] [Google Scholar]
- 16.Meyer DC, Pirkl C, Pfirrmann CW, Zanetti M, Gerber C. Asymmetric atrophy of the supraspinatus muscle following tendon tear. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2005;23:254–258. [DOI] [PubMed] [Google Scholar]
- 17.Panjabi MM, Cholewicki J, Nibu K, Babat LB, Dvorak J. Simulation of whiplash trauma using whole cervical spine specimens. Spine (Phila Pa 1976). 1998;23:17–24. [DOI] [PubMed] [Google Scholar]
- 18.Panjabi MM, Cholewicki J, Nibu K, Grauer J, Babat LB, Dvorak J. Critical load of the human cervical spine: an in vitro experimental study. Clin Biomech (Bristol, Avon). 1998;13:11–17. [DOI] [PubMed] [Google Scholar]
- 19.Siegmund GP, Myers BS, Davis MB, Bohnet HF, Winkelstein BA. Mechanical evidence of cervical facet capsule injury during whiplash: a cadaveric study using combined shear, compression, and extension loading. Spine (Phila Pa 1976). 2001;26:2095–2101. [DOI] [PubMed] [Google Scholar]
- 20.Smith AC, Parrish TB, Abbott R, et al. Muscle-fat MRI: 1.5 Tesla and 3.0 Tesla versus histology. Muscle Nerve. 2014;50:170–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith AD, Jull G, Schneider G, Frizzell B, Hooper RA, Sterling M. Cervical radiofrequency neurotomy reduces central hyperexcitability and improves neck movement in individuals with chronic whiplash. Pain medicine. 2014;15:128–141. [DOI] [PubMed] [Google Scholar]
- 22.Smuck M, Crisostomo RA, Demirjian R, Fitch DS, Kennedy DJ, Geisser ME. Morphologic changes in the lumbar spine after lumbar medial branch radiofrequency neurotomy: a quantitative radiological study. Spine J. 2013; Nov 14. pii: S1529–9430(13)01218–7. doi: 10.1016/j.spinee.2013.06.096. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 23.Sterling M, Elliott JM, Cabot PJ. The course of serum inflammatory biomarkers following whiplash injury and their relationship to sensory and muscle measures: a longitudinal cohort study. PLoS One. 2013;8:e77903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sterling M, McLean SA, Sullivan MJL, Elliott JM, Buitenhuis J, Kamper SJ. Potential processes involved in the initiation and maintenance of whiplash-associated disorders: discussion paper 3. Spine. 2011;36:S322–329. [DOI] [PubMed] [Google Scholar]
- 25.Walter SD, Eliasziw M, Donner A. Sample size and optimal designs for reliability studies. Statistics in medicine. 1998;17:101–110. [DOI] [PubMed] [Google Scholar]
- 26.Winkelstein BA, Nightingale RW, Richardson WJ, Myers BS. The cervical facet capsule and its role in whiplash injury: a biomechanical investigation. Spine (Phila Pa 1976). 2000;25:1238–1246. [DOI] [PubMed] [Google Scholar]


