Abstract
Objective
We assessed oxidant–antioxidant status and evaluated the role of lipid peroxidation, oxidative DNA damage, and protein oxidation in the development and severity of neonatal respiratory distress syndrome (RDS).
Methods
Forty preterm neonates with RDS were compared with another 40 preterm neonates without RDS enrolled as controls. Total antioxidant capacity (TAC), malondialdehyde (MDA), advanced oxidation protein products (AOPPs), 8-hydroxy-2-deoxyguanosine (8-OHdG), and trace elements (copper and zinc) were measured in cord blood (day 0) for all neonates and repeated on day 3 for the RDS group.
Results
Day 0 serum levels of MDA, AOPPs, and 8-OHdG were significantly higher in neonates with RDS than controls with a further increase on day 3. Days 0 and 3 levels of TAC, copper, and zinc were significantly lower in the RDS group compared with controls. Elevated serum levels of 8-OHdG and AOPPs were associated with severe RDS, invasive mechanical ventilation, and high mortality rate. 8-OHdG and AOPPs were positively correlated with MDA, oxygenation index, duration of ventilation, and duration of hospitalization.
Conclusions
Increased lipid, protein, and DNA oxidation is accompanied by alterations in the antioxidant defense status, which may play a role in the pathogenesis and severity of RDS.
Introduction
Respiratory distress syndrome (RDS) is a major cause of morbidity and mortality in premature infants.1 Newborns and particularly preterm infants are at high risk of oxidative stress and are susceptible to free radical oxidative damage.2,3 The pathogenesis of oxidative stress in RDS is based on the rapid formation of the reactive oxygen species (ROS), which overcome the capacity of the antioxidative defense system.4 ROS can cause damage to a variety of cellular molecules, including lipids, proteins, and DNA, which results in cell injury and may induce respiratory cell death.5,6 ROS also interact with pulmonary surfactant, thus delaying the normal functioning of the lung.5 This process can contribute to the pathogenesis of RDS and very likely leads to severe RDS.7
ROS have been implicated in the molecular damage seen in the bronchoalveolar lavage (BAL) fluid of patients with RDS. BAL fluid normally contains a large amount of the antioxidant glutathione; but in patients with RDS, it is mostly in the oxidized form. Hydrogen peroxide was detected in the expired air of RDS patients.4 Moreover, in neonates with RDS and bronchopulmonary dysplasia (BPD), lipid hydroperoxide is increased and glutathione concentrations are decreased in BAL fluid.8
Malondialdehyde (MDA) is one of the most frequently used indicators of the overall lipid peroxidation.9 Advanced oxidation protein products (AOPPs) is a protein oxidation biomarker, which originates under oxidative and carbonyl stress and increases global inflammatory activity.10,11 8-Hydroxy-2-deoxyguanosine (8-OHdG) is a commonly used marker of oxidative stress-derived DNA damage.12,13
Total antioxidant capacity (TAC) evaluates the overall antioxidant status resulting from antioxidant production and consumption in response to oxidative stress.14 Thus, TAC provides an integrated parameter rather than the simple sum of measurable antioxidants.15 Trace elements such as copper, manganese, selenium, and zinc act as cofactors of antioxidant enzymes to protect the body from oxygen free radicals that are produced during oxidative stress. It is necessary to maintain a balance between the harmful pro-oxidant components produced and the antioxidant compounds that counter these effects.16
Identification of the potential biomarkers of oxidative stress in RDS that could monitor disease progression represents a promising challenge. To test the hypothesis that the presence of oxidant–antioxidant imbalance in preterm neonates contributes to the development and severity of RDS, this study assessed lipid peroxidation, protein oxidation, and oxidative DNA damage in relation to neonatal RDS severity.
Patients and methods
Study population
This prospective study was carried out in the neonatal intensive care unit (NICU), Ain Shams University Hospitals. Informed consent was taken from the parents or caregivers of each participant before enrollment. This study was approved by the ethical committee of Ain Shams University and is in accordance with the Declaration of Helsinki of 1975.
This study included 80 preterm neonates <34 weeks gestational age. Neonates with respiratory distress due to causes other than RDS, major malformations and genetic disorders, fetal distress, difficult delivery, infant of diabetic or hypertensive mother, neonatal sepsis, hemolytic diseases, and hyperbilirubinemia were excluded from the study.
Cord blood samples were withdrawn from all neonates at birth. Samples were centrifuged and then stored at −80 °C. Out of 162 neonates assessed for eligibility, 38 did not meet the inclusion criteria, 49 were excluded, and 11 guardians declined to participate. Thus, 113 preterm neonates were followed up for the first 12 h of life and 40 neonates of them had established the diagnosis of RDS (RDS group). Neonates were considered to have RDS if they had marked respiratory distress with tachypnea, nasal flaring, grunting, and subcostal, intercostal, and/or suprasternal retractions at birth combined with radiological findings on chest X-ray.17 They were compared with 40 consequently born healthy preterm neonates (control group). Neonates were considered to be healthy controls if they had no signs of respiratory distress or any other medical problem and did not need any respiratory support.
Perinatal history and clinical examination
Detailed perinatal history was obtained for all neonates, followed by a thorough clinical examination focusing on maternal diseases, premature rupture of membranes, maternal medication during this pregnancy, antenatal steroids, surfactant administration, and the need for mechanical ventilation. Gestational age was determined using Ballard scores.18 Apgar score at 1 and 5 min was assessed.19
RDS grading and respiratory management
Grading of RDS was done according to chest X-ray findings: grade I—fine, diffuse reticulogranular pattern over the lung fields, grade II—a denser lung with the presence of air bronchogram, which overlaps the heart, grade III—increased density and the presence of air bronchograms beyond the heart border, and grade IV—white lung.20
Our NICU protocol is to initiate continuous positive airway pressure (CPAP) from birth with early selective surfactant administration for babies showing signs of RDS and needed more than 30% inspired oxygen to maintain saturation in the normal range using the INSURE (intubate–surfactant–extubate to CPAP) technique.21 Age of start of mechanical ventilation, maximum positive inspiratory pressure (PIP), and maximum positive end expiratory pressure (PEEP) were recorded and oxygenation index was calculated.22 The ventilator parameters were adjusted according to the clinical condition, arterial blood gases, and chest X-ray.
Laboratory analysis
For laboratory analysis, cord blood samples were collected on day 0 of life from preterm neonates with RDS and controls, then peripheral blood samples were obtained on day 3 from neonates with RDS. Serum levels of AOPPs and 8-OHdG were assessed by enzyme-linked immunosorbent assay using kit supplied by SunLong (SunLong Biotech Co., Ltd, Zhejiang, China). Serum TAC was assessed using colorimetric kit (Bio-Diagnostics, Egypt, Cat. No., MD 2513). Serum levels of MDA were determined by measuring the production of thiobarbituric acid reactive substances (TBARS) using colorimetric kit (Bio-Diagnostics, Egypt, Cat. No., MD 2529). Quantitative determination of serum copper and zinc was done using SOLAR system Unicam 939 atomic absorption spectrometer (Unicam, Cambridge, UK).
Follow-up
All included neonates with RDS were followed up to assess disease progression and mortality, as well as duration of mechanical ventilation and duration of hospitalization. Patients were also evaluated for other comorbidities: intraventricular hemorrhage, patent ductus arteriosus, pulmonary hypertension, pneumothorax, pulmonary hemorrhage, BPD, necrotizing enterocolitis, and retinopathy of prematurity.
Primary and secondary outcomes
The primary outcome was to assess the levels of AOPPs and 8-OHdG along with MDA, TAC, and trace elements in neonates with RDS and healthy controls. Secondary outcomes included duration of mechanical ventilation, duration of hospitalization, and mortality, as well as examining the relation between oxidative stress markers (AOPPs and 8-OHdG) and the degree of RDS.
Sample size calculation
Using STATA program, setting α-error at 5% and power at 90%, results of a previous study5 showed that the mean 8-OHdG in cases was 39.6 ± 11.5 compared with 23.35 ± 7.6 in controls. Based on these values, a sample of at least 15 preterm neonates per group was calculated; however, we included 40 cases and 40 controls to achieve a better statistical power.
Statistical analysis
Analysis of data was done using Statistical Program for Social Science version 21 (SPSS Inc., Chicago, IL, USA). Qualitative data were presented as number and percentages, while quantitative data were presented as ranges, mean and standard deviations, or median and interquartile range. In order to compare quantitative parametric variables between three groups, analysis of variance (ANOVA) test with post hoc test was used, while for comparing two groups, Student’s t test was applied. Comparison of nonparametric variables was carried out using Mann–Whitney tests. Qualitative variables were compared using X2 test or Fischer’s exact test when frequencies were below five. Pearson’s or Spearman’s correlation coefficients were used as appropriate to assess the correlation between two quantitative parameters in the same group. Multivariable linear regression analysis was employed to determine the relation between oxidative stress markers and clinical, laboratory, and radiological variables. Logistic regression analysis was performed with estimating the odds ratio (OR) and 95% confidence interval (CI) to define predictors of RDS severity. Receiver operating characteristic (ROC) curve was used to determine the best cutoff values of AOPPs and 8-OHdG that could predict RDS severity and mortality with the highest balanced sensitivity and specificity. The area under the curve (AUC) was calculated for each plot. A p value <0.05 was considered significant in all analyses.
Results
The clinical characteristics of neonates with RDS were shown in Table 1. Gestational age and birth weight were significantly lower among neonates with RDS than controls (30.9 ± 1.9 vs. 33.6 ± 0.5 weeks and 1.58 ± 0.43 vs. 2.29 ± 0.06 kg, respectively; p < 0.001). RDS group included 25 males and 15 females with a male to female ratio of 1.7:1, while the control group included 26 males and 14 females with a male to female ratio of 1.8:1 with no significant difference in sex distribution between both groups (p > 0.05). No significant difference was found between mothers of neonates with RDS and control group as regards maternal age, mode of delivery, and antenatal steroid intake (p > 0.05).
Table 1.
Clinical characteristics of neonates with RDS
| Variables | RDS group (n = 40) |
|---|---|
| Gestational age (weeks), mean ± SD | 30.9 ± 1.9 |
| Males, n (%) | 25 (62.5) |
| Birth weight (kg), mean ± SD | 1.58 ± 0.43 |
| Apgar score, median (IQR) | |
| 1 min | 6 (5–7) |
| 5 min | 7 (7–8) |
| Age of start of mechanical ventilation (h), median (IQR) | 6 (3–8) |
| Duration of mechanical ventilation (days), median (IQR) | 7 (5–10) |
| Type of respiratory support, n (%) | |
| Non-invasive mechanical ventilation | |
| NCPAP | 9 (22.5) |
| NIPPV | 5 (12.5) |
| Invasive mechanical ventilation | |
| CMV | 18 (45) |
| HFV | 8 (20) |
| Maximum PIP (CmHO2), mean ± SD | 16.4 ± 1.01 |
| Maximum PEEP (CmHO2), mean ± SD | 4.4 ± 0.5 |
| Oxygenation index, mean ± SD | |
| Before surfactant treatment | 30.1 ± 6.3 |
| After surfactant treatmenta | 12.8 ± 3.7 |
| RDS radiological grade, n (%) | |
| I | 0 (0) |
| II | 12 (30) |
| III | 12 (30) |
| IV | 16 (40) |
| Bronchopulmonary dysplasia, n (%) | 1 (2.5) |
| Retinopathy of prematurity, n (%) | 1 (2.5) |
| Necrotizing enterocolitis, n (%) | 0 (0) |
| Pulmonary hypertension, n (%) | 0 (0) |
| Pneumothorax, n (%) | 0 (0) |
| Pulmonary hemorrhage, n (%) | 0 (0) |
| Duration of hospitalization (days), median (IQR) | 13.5 (5.5–18.5) |
RDS respiratory distress syndrome, IQR interquartile range, NCPAP nasal continuous positive airway pressure, NIPPV nasal intermittent positive pressure ventilation, CMV conventional mechanical ventilation, HFV high-frequency ventilation, PIP positive inspiratory pressure, PEEP positive end expiratory pressure
aOxygenation index was assessed in 16 patients treated by surfactant
Comparison of hematological data on day 0 between neonates with RDS and the control group revealed no significant difference (p > 0.05). As regards oxidative stress markers, we found that day 0 and day 3 levels of MDA, AOPPs, and 8-OHdG were higher in neonates with RDS compared with control group, while TAC, zinc, and copper were lower on day 0 and day 3 (p < 0.05 for all) (Table 2). Moreover, upon comparing the oxidative stress markers among neonates with RDS on day 0 and day 3, MDA, AOPPs, and 8-OHdG were increased on day 3, while TAC and zinc were decreased on day 3 (p < 0.001 for all) (Table 2).
Table 2.
Oxidative stress markers among neonates with RDS and control groups
| Variables | Control group (n = 40) | RDS group (n = 40) | P value | |||
|---|---|---|---|---|---|---|
| Day 0 | Day 3 | RDS Day 0 vs. control | RDS Day 3 vs. control | RDS Day 0 vs. RDS Day 3 | ||
| TAC (mmol/L) | 3.3 ± 1.1 | 2.58 ± 0.81 | 1.1 ± 0.43 | <0.001 | <0.001 | <0.001 |
| MDA (nmol/mL) | 8.8 ± 2.5 | 12.3 ± 4.3 | 20.4 ± 5.1 | 0.031 | <0.001 | <0.001 |
| AOPPs (ng/mL) | 27.0 ± 15.5 | 82.6 ± 48.0 | 196.3 ± 63.1 | <0.001 | <0.001 | <0.001 |
| 8-OHdG (pg/ml) | 1200.0 ± 823.3 | 2562.5 ± 1166.8 | 6200.0 ± 2420.0 | <0.001 | <0.001 | <0.001 |
| Copper (mg/L) | 0.71 ± 0.47 | 0.51 ± 0.19 | 0.35 ± 0.1 | 0.006 | <0.001 | 0.262 |
| Zinc (mg/dL) | 14.2 ± 6.1 | 9.1 ± 4.5 | 5.1 ± 2.2 | 0.003 | <0.001 | <0.001 |
Data were expressed as mean plus or minus SD, where ANOVA with post hoc test was used for comparison between three groups
RDS respiratory distress syndrome, TAC total antioxidant capacity, MDA malondialdehyde, AOPPs advanced oxidation protein products, 8-OHdG 8-hydroxy-desoxyguanosine, ANOVA analysis of variance
Analysis of the levels of AOPPs and 8-OHdG in relation to maternal clinical characteristics of RDS group showed lower day 3 AOPPs levels among neonates born to mothers with antenatal steroid intake than those without (p = 0.003). As regards neonatal data, day 0 and day 3 AOPPs and 8-OHdG levels were significantly higher in neonates in RDS grade III and IV compared with those in grade II (p < 0.05). Serum AOPPs levels on day 3 were also increased in neonates who received surfactant (p = 0.004). Moreover, levels of both markers were increased among patients on invasive mechanical ventilation and among those who died (p < 0.05) (Table 3).
Table 3.
Levels of AOPPs and 8-OHdG in relation to neonatal clinical characteristics among the RDS group
| Variables | Number (%) | AOPPs (ng/mL) Day 0 | P value | AOPPs (ng/mL) Day 3 | P value | 8-OHdG (pg/mL) Day 0 | P value | 8-OHdG (pg/mL) Day 3 | P value |
|---|---|---|---|---|---|---|---|---|---|
| Antenatal steroids intake | |||||||||
| Positive | 24 (60) | 80.6 ± 48.4 | 0.794 | 173.1 ± 57.8 | 0.003 | 2458.3 ± 1250.4 | 0.496 | 6000 ± 2595.9 | 0.529 |
| Negative | 16 (40) | 85.6 ± 48.9 | 230.9 ± 55.5 | 2718.8 ± 1048.3 | 6500 ± 2175.6 | ||||
| PROM | |||||||||
| Positive | 12 (30) | 80.14 ± 49.67 | 0.627 | 204.58 ± 72.35 | 0.591 | 2589.3 ± 1225.1 | 0.828 | 6089.3 ± 2490.9 | 0.664 |
| Negative | 28 (70) | 88.33 ± 45.39 | 192.68 ± 59.71 | 2500 ± 1066 | 6458.3 ± 2330.2 | ||||
| Sex | |||||||||
| Male | 25 (62.5) | 82.4 ± 49.3 | 0.968 | 192 ± 68.98 | 0.589 | 2420 ± 1057.5 | 0.325 | 6060 ± 2310.8 | 0.643 |
| Female | 15 (37.5) | 83 ± 47.4 | 203.3 ± 53.2 | 2800 ± 1333.6 | 6433.3 ± 2658.3 | ||||
| RDS radiological grade | |||||||||
| Grade II | 12 (30) | 40.8 ± 25.5 | <0.001 | 151.7 ± 50.7 | 0.002 | 1625 ± 644 | <0.001 | 4416.7 ± 1459 | 0.001 |
| Grade III and IV | 28 (70) | 100.5 ± 44.2 | 215.4 ± 58.6 | 2964.3 ± 1113.3 | 6964.3 ± 2360.7 | ||||
| Surfactant | |||||||||
| Positive | 26 (65) | 80.9 ± 49.2 | 0.861 | 230.6 ± 62.4 | 0.004 | 2468.8 ± 1056.2 | 0.684 | 5604.2 ± 2090.2 | 0.055 |
| Negative | 14 (35) | 83.7 ± 48.2 | 173.3 ± 53.2 | 2625 ± 1253.3 | 7093.8 ± 2665.9 | ||||
| IVH | |||||||||
| Positive | 8 (20) | 82.3 ± 46.3 | 0.97 | 213.1 ± 61.5 | 0.10 | 2881.8 ± 1728.1 | 0.347 | 6177.4 ± 2599.8 | 0.91 |
| Negative | 32 (80) | 82.8 ± 50.5 | 180.9 ± 61.8 | 2467.7 ± 965.5 | 6277.7 ± 1787.2 | ||||
| PDA | |||||||||
| Positive | 17 (42.5) | 78.5 ± 41.3 | 0.44 | 186.6 ± 57.1 | 0.61 | 2500 ± 1322.8 | 0.752 | 6473.6 ± 2573.7 | 0.50 |
| Negative | 23 (57.5) | 85.8 ± 51.8 | 199 ± 65.2 | 2619.1 ± 1035.6 | 5952.3 ± 2307.1 | ||||
| Mechanical ventilation | |||||||||
| Invasive | 26 (65) | 95 ± 42.4 | 0.024 | 219.2 ± 58.9 | 0.001 | 2942.3 ± 1098.4 | 0.004 | 6865.4 ± 2463.9 | 0.016 |
| Non-invasive | 14 (35) | 59.6 ± 3 0.7 | 153.6 ± 47.3 | 1857.1 ± 969.3 | 4964.3 ± 1834.1 | ||||
| Mortality | |||||||||
| Alive | 34 (85) | 75.9 ± 44.9 | 0.032 | 181.9 ± 55.3 | <0.001 | 2485.3 ± 1202.8 | 0.325 | 5676.5 ± 2018.4 | <0.001 |
| Died | 6 (15) | 120.8 ± 51.1 | 277.5 ± 38.9 | 3000 ± 894.4 | 9166.7 ± 2523.2 | ||||
Data were expressed as mean plus or minus SD, where Student’s t test was used for comparisons
RDS respiratory distress syndrome, AOPPs advanced oxidation protein products, 8-OHdG 8-hydroxy-desoxyguanosine, PROM premature rupture of membranes, IVH intraventricular hemorrhage, PDA patent ductus arteriosus, ANOVA analysis of variance
Logistic regression analysis included all the significant variables between RDS grade II vs. grades III and IV revealed that maternal disease (rheumatic heart disease or bronchial asthma), gestational age, and day 0 levels of AOPPs and 8-OHdG were the significant independent variables related to RDS severity (Table 4).
Table 4.
Logistic regression analysis for predictors of RDS severity
| Independent variables | OR | 95% CI for OR | P value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Maternal disease | 7.727 | 1.416 | 42.175 | 0.018 |
| Gestational age (weeks) | 0.201 | 0.074 | 0.546 | 0.002 |
| Apgar score 1 min | 0.343 | 0.095 | 1.348 | 0.103 |
| Apgar score 5 min | 1.091 | 0.120 | 1.415 | 0.202 |
| Day 0 MDA (nmol/mL) | 4.633 | 0.928 | 23.137 | 0.062 |
| Day 0 AOPPs (ng/mL) | 1.056 | 1.016 | 2.098 | 0.006 |
| Day 0 8-OHdG (pg/mL) | 1.002 | 1.001 | 3.003 | 0.008 |
Dependent variable: RDS grade severity.
OR odds ratio, CI confidence interval, MDA malondialdehyde, AOPPs advanced oxidation protein products, 8-OHdG 8-hydroxy-desoxyguanosine, RDS respiratory distress syndrome
ROC curve analysis revealed that AOPPs cutoff value of 47 ng/mL at day 0 could predict RDS grade severity with a sensitivity of 95.4% and specificity of 80.1% and AUC 0.896, while 8-OHdG cutoff value of 2418 pg/mL at day 0 could predict RDS grade severity with a sensitivity of 84.3% and specificity of 94.7% and AUC 0.831. Furthermore, AOPPs cutoff value of 95 ng/mL at day 0 could predict mortality with a sensitivity of 86.1% and specificity of 89.8% and AUC 0.826. while 8-OHdG cutoff value of 7250 pg/mL at day 3 could predict mortality with a sensitivity of 92.3% and specificity of 93.4% and AUC 0.897.
Both AOPPs and 8-OHdG were positively correlated with each other (r = 0.503, p < 0.001 and r = 0.622, p < 0.001 on day 0 and day 3, respectively). Day 0 MDA was positively correlated to day 0 and day 3 levels of AOPPs (day 0: r = 0.553, p = 0.011 and day 3: r = 0.518, p = 0.033) and 8-OHdG (day 0: r = 0.721 and day 3: r = 0.78, p < 0.001 for both). None of the studied biomarkers of oxidative stress was correlated to gestational age or birth weight (p > 0.05).
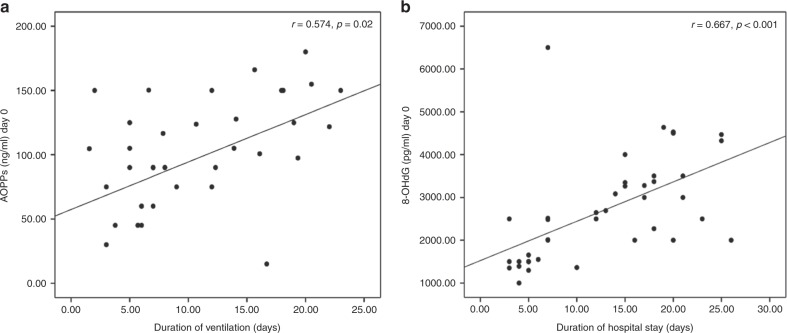
AOPPs was positively correlated to oxygenation index (day 0: r = 0.512, p = 0.013 and day 3: r = 0.559, p = 0.002), duration of ventilation (day 0: r = 0.574, p = 0.02 and day 3: r = 0.692, p < 0.001), and duration of hospitalization (day 0: r = 0.348, p = 0.028 and day 3: r = 0.387, p = 0.014). Similarly, 8-OHdG showed positive correlation with oxygenation index (day 0: r = 0.403, p = 0.03 and day 3: r = 0.354, p = 0.041), duration of ventilation (day 0; r = 0.436, p = 0.036 and day 3: r = 0.499, p = 0.008) and duration of hospitalization (day 0: r = 0.667, p < 0.001 and day 3: r = 0.634, p < 0.001) (Fig. 1). Multivariable regression analysis showed that oxygenation index, duration of ventilation, and day 0 levels of MDA and 8-OHdG were the significant independent variables related to day 0 levels of AOPPs among the RDS group (Table 5).
Fig. 1.
Positive correlations between day 0 levels of advanced oxidation protein products and duration of ventilation (a) and day 0 levels of 8-hydroxy-desoxyguanosine and duration of hospital stay (b) among neonates with respiratory distress syndrome (RDS)
Table 5.
Multivariable regression analysis of the factors affecting day 0 AOPPs levels among the RDS group
| Independent variables | Unstandardized coefficients | Standardized coefficients | P value | |
|---|---|---|---|---|
| B | Standard error | β | ||
| Oxygenation index | 4.156 | 1.218 | 0.521 | 0.003 |
| Duration of ventilation | 2.817 | 0.672 | 0.419 | 0.023 |
| Duration of hospitalization | 7.051 | 3.816 | 0.287 | 0.072 |
| Day 0 MDA (nmol/mL) | 7.855 | 2.004 | 0.679 | 0.001 |
| Day 0 8-OHdG (pg/ml) | 1.873 | 0.034 | 0.482 | 0.008 |
Dependent variable: AOPPs
RDS respiratory distress syndrome, MDA malondialdehyde, AOPPs advanced oxidation protein products, 8-OHdG 8-hydroxy-desoxyguanosine
Discussion
In preterm babies, the perinatal transition is accompanied by the immaturity of the antioxidant systems and the reduced ability to induce efficient homeostatic mechanisms designed to control overproduction of cell-damaging free radicals.23
The mean gestational age and birth weight was lower among our RDS patients than controls; yet, all the studied patients and controls were preterm neonates <34 weeks gestational age. This difference could be explained by the fact that the risk of RDS is inversely proportional to gestational age and birth weight; RDS occurs in approximately 5% of near-term infants, 30% of infants <30 weeks gestational age, and 60% of premature infants <28 weeks gestational age.24 Gestational age is considered as an independent predictor of RDS.25 Moreover, in a report from the National Institute of Child Health and Human Development Neonatal Research Network, the incidence of RDS was 44% in infants weighing 501–1500 g, with 71% reported in infants weighing 501–750 g, 54% reported in infants weighing 751–1000 g, 36% reported in infants weighing 1001–1250 g, and 22% reported in infants weighing 1251–1500 g.26 In the study by Negi et al.,5 gestational age and birth weight were higher in control group than neonates with RDS.
Several reports discussed oxidative stress in preterm infants;7,14,27 however, few studies have addressed the role of oxidative stress in pathogenesis of RDS.5,28 In this context, Negi et al.5 found convincing evidence of oxidative damage and diminished antioxidant defense in newborns with RDS. Results of logistic regression analysis showed a significant association of oxidative damage markers and total antioxidant status with the incidence of RDS in newborns.
In the current study, we found higher day 0 MDA levels in neonates with RDS compared with the control group that further increased on day 3 compared with day 0. This denotes increased lipid peroxidation in RDS, which comes in parallel with the disease progression. In agreement with our findings, Negi et al.5 examined the product of lipid peroxidation, MDA, using TBARS assay in neonates with RDS and it was significantly increased in the RDS group than controls. Similar results were obtained by Matyas et al.29 In another study, Elshemy et al.28 assessed lipid peroxidation among RDS neonates using serum peroxide and found that it was significantly increased in RDS neonates.
In the view of protein and DNA oxidation process, our results showed a significant increase in day 0 levels of AOPPs and 8-OHdG in the RDS group compared with controls. These levels showed further increase at day 3 compared to day 0. Few studies reported that 8-OHdG5 and protein carbonyl,5,30,31 another marker for protein oxidation, were elevated in neonates with RDS compared to controls. This oxidative protein and DNA damage has been suggested to be one of the contributing factors to the pathogenesis of neonatal RDS.5,32
Our results suggest that the severity of oxidative stress correlates with the severity of RDS; we observed that AOPPs and 8-OHdG levels on day 0 and day 3 were significantly higher in neonates with RDS grades III and IV compared with those having RDS grade II. Logistic regression analysis showed that these biomarkers were significant independent variables related to RDS severity and the cutoff for prediction of RDS severity was determined. In our study, day 3 AOPP levels were higher among patients who received surfactant. Furthermore, levels of AOPPs and 8-OHdG were increased among RDS patients who needed invasive mechanical ventilation support and were also higher in those who died. We found significant relations between the two markers and oxygenation index, duration of ventilation, and duration of hospitalization. The increase in levels of oxidative stress biomarkers on day 3 with increasing the severity of RDS and the use of invasive mechanical ventilation could be related to the use of exogenous oxygen. This points out to the deleterious effects of hyperoxia.
There exist, however, several potential causes of intra- and extracellular oxidant stress in the preterm newborns with RDS. The high inspiratory concentrations of oxygen required to achieve adequate arterial oxygenation and pro-oxidant drugs (e.g., non-steroidal anti-inflammatory drugs) can all promote ROS accumulation and the utilization and depletion of antioxidative factors.4 Joung et al.33 reported that 8-OHdG measured on day 3 was correlated with the duration of mechanical ventilation. In this context, Dursun et al.34 stated that mechanical ventilation and CPAP can induce oxidative stress. These findings support those reported by Falk et al.,35 who found that mechanical ventilation can induce oxidative stress, which plays a critical role in the occurrence of pulmonary injury in intubated neonates. Moreover, Gladstone and Levine36 found that the amount of oxidatively modified protein may provide a quantitative assessment of oxygen toxicity. Gitto et al.4 suggested that hyperoxic exposure itself, although essential for the survival of RDS infants, probably induces excessive production of ROS in the respiratory system.
We also found that day 3 levels of AOPPs were significantly lower in neonates born to mothers who received antenatal steroids, which is most likely because antenatal steroid administration reduces the incidence and severity of RDS.37 Moreover, antenatal steroid intensifies that activity of the antioxidant enzymes: glutathione, superoxide dismutases, and catalases.38
In the present work, there were significant positive correlations between AOPP levels at days 0 and 3 with MDA. Buonocore et al.32 observed a positive correlation between AOPPs and total hydroperoxide (a derivative of lipid peroxidation). This implies that the oxidative stress damage is a parallel process involving both lipids and proteins proportionately.
We found that increased oxidative stress is accompanied by reduced antioxidant defense since day 0 and day 3 levels of TAC were decreased in patients with RDS compared with the control group, which may be due to its consumption. Furthermore, copper and zinc levels were significantly lower among the studied neonates with RDS than controls. An experimental model by Sarricolea et al.39 has been developed to investigate the effect of copper deficiency on lung maturity in the newborn rat. A greater thickness of the air–blood barrier was observed in the lungs of the copper-deficient group, which might explain the RDS observed in this group.
The limitation of this study is that MDA was assessed by a colorimetric kit for TBARS. Although the rapidity, ease of use, and cost of this assay made it the most common method, it may limit the likelihood of detecting true differences in the level of lipid peroxidation. Therefore, it is important to use high-performance liquid chromatography assay for MDA as a reliable method to measure lipid peroxidation.40 Investigation of other effective markers of lipid peroxidation (such as isoprostanes) or oxidative stress-induced changes in the antioxidant system (e.g., reduced glutathione/glutathione disulfide ratio) would provide additional information.
In conclusion, the increased lipid, protein, and DNA oxidation is accompanied by alterations in the antioxidant defense status, which may play a role in the pathogenesis and severity of RDS. AOPPs and 8-OHdG could be used as serum biomarkers for oxidative stress among neonates with RDS to monitor disease progression. Maternal antenatal steroid intake and limiting the exposure of the preterm infants to oxygen at high concentration and high positive pressure during ventilation might decrease the risk of oxidative stress among neonates with RDS. Investigating the relation between the studied markers and proinflammatory cytokines, such as interleukin-6 (IL-6) and IL-8, as well as other markers of oxidative stress, are warranted.
Author contributions
All authors were involved in the concept, design, data collection, analysis, and drafting of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dizdar EA, et al. Total antioxidant capacity and total oxidant status after surfactant treatment in preterm infants with respiratory distress syndrome. Ann. Clin. Biochem. 2011;48:462–467. doi: 10.1258/acb.2011.010285. [DOI] [PubMed] [Google Scholar]
- 2.Davis JM, Auten RL. Maturation of the antioxidant system and the effects on preterm birth. Semin. Fetal Neonatal Med. 2010;15:191–195. doi: 10.1016/j.siny.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Yzydorczyk C, et al. Oxidative stress after preterm birth: origins, biomarkers, and possible therapeutic approaches. Arch. Pediatr. 2015;22:1047–1055. doi: 10.1016/j.arcped.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 4.Gitto E, Pellegrino S, D’Arrigo S, Barberi I, Reiter RJ. Oxidative stress in resuscitation and in ventilation of newborns. Eur. Respir. J. 2009;34:1461–1469. doi: 10.1183/09031936.00032809. [DOI] [PubMed] [Google Scholar]
- 5.Negi R, et al. A novel approach to study oxidative stress in neonatal respiratory distress syndrome. BBA Clin. 2015;3:65–69. doi: 10.1016/j.bbacli.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ikegami M, Kallapur S, Michna J, Jobe AH. Lung injury and surfactant metabolism after hyperventilation of premature lambs. Pediatr. Res. 2000;47:398–404. doi: 10.1203/00006450-200003000-00019. [DOI] [PubMed] [Google Scholar]
- 7.Ozsurekci Y, Aykac K. Oxidative stress related diseases in newborns. Oxid. Med. Cell Longev. 2016;2016:2768365. doi: 10.1155/2016/2768365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fabiano A, et al. The development of lung biochemical monitoring can play a key role in the early prediction of bronchopulmonary dysplasia. Acta Paediatr. 2016;105:535–541. doi: 10.1111/apa.13233. [DOI] [PubMed] [Google Scholar]
- 9.Giera M, Lingeman H, Niessen WM. Recent advancements in the LC- and GC-based analysis of malondialdehyde (MDA): a brief overview. Chromatographia. 2012;75:433–440. doi: 10.1007/s10337-012-2237-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gangemi S, et al. Increase of novel biomarkers for oxidative stress in patients with plasma cell disorders and in multiple myeloma patients with bone lesions. Inflamm. Res. 2012;61:1063–1067. doi: 10.1007/s00011-012-0498-7. [DOI] [PubMed] [Google Scholar]
- 11.Yunanto A, Firdaus RT, Triawanti, Suhartono E. Advance oxidation protein products (AOPPs) of newborn at risk of sepsis as novel parameter for early-onset neonatal sepsis. Int. J. Biosci. Biochem. Bioinforma. 2014;4:112–133. [Google Scholar]
- 12.Kroese LJ, Scheffer PG. 8-Hydroxy-2′’-deoxyguanosine and cardiovascular disease: a systematic review. Curr. Atheroscler. Rep. 2010;16:452. doi: 10.1007/s11883-014-0452-y. [DOI] [PubMed] [Google Scholar]
- 13.Sova H, Jukkola-Vuorinen A, Puistola U, Kauppila S, Karihtala P. 8-Hydroxydeoxyguanosine: a new potential independent prognostic factor in breast cancer. Br. J. Cancer. 2010;102:1018–1023. doi: 10.1038/sj.bjc.6605565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nemec A, et al. Total antioxidant capacity (TAC) values and their correlation with individual antioxidants in serum of healthy beagles. Acta Vet. Brno. 2000;69:297–303. doi: 10.2754/avb200069040297. [DOI] [Google Scholar]
- 15.Cadenas E, Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic. Biol. Med. 2000;29:222–230. doi: 10.1016/S0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- 16.Leung FY. Trace elements that act as antioxidants in parenteral micronutrition. J. Nutr. Biochem. 1998;9:304–307. doi: 10.1016/S0955-2863(98)00018-7. [DOI] [Google Scholar]
- 17.Reuter S, Moser C, Baack M. Respiratory distress in the newborn. Pediatr. Rev. 2014;35:417–428. doi: 10.1542/pir.35-10-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ballard JL, et al. New Ballard Score, expanded to include extremely premature infants. J. Pediatr. 1991;119:417–423. doi: 10.1016/S0022-3476(05)82056-6. [DOI] [PubMed] [Google Scholar]
- 19.Apgar V. A proposal for a new method of evaluation of the newborn infant. Originally published in July 1953, volume 32, pages 250–259. Anesth. Analg. 2015;120:1056–1059. doi: 10.1213/ANE.0b013e31829bdc5c. [DOI] [PubMed] [Google Scholar]
- 20.Greenough A, et al. A simple chest radiograph score to predict chronic lung disease in prematurely born infants. Br. J. Radiol. 1999;72:530–533. doi: 10.1259/bjr.72.858.10560333. [DOI] [PubMed] [Google Scholar]
- 21.Sweet DG, et al. European Consensus Guidelines on the Management of Respiratory Distress Syndrome—2016 Update. Neonatology. 2017;111:107–125. doi: 10.1159/000448985. [DOI] [PubMed] [Google Scholar]
- 22.Ortiz RM, Cilley RE, Bartlett RH. Extracorporeal membrane oxygenation in pediatric respiratory failure. Pediatr. Clin. N. Am. 1987;34:39–46. doi: 10.1016/S0031-3955(16)36179-X. [DOI] [PubMed] [Google Scholar]
- 23.Perrone S, et al. Whole body hypothermia and oxidative stress in babies with hypoxic–ischemic brain injury. Pediatr. Neurol. 2010;43:236–240. doi: 10.1016/j.pediatrneurol.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 24.Sweet LR, et al. Respiratory distress in the neonate: case definition & guidelines for data collection, analysis, and presentation of maternal immunization safety data. Vaccine. 2017;35:6506–6517. doi: 10.1016/j.vaccine.2017.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.St Clair C, et al. The probability of neonatal respiratory distress syndrome as a function of gestational age and lecithin/sphingomyelin ratio. Am. J. Perinatol. 2008;25:473–480. doi: 10.1055/s-0028-1085066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fanaroff AA, et al. Trends in neonatal morbidity and mortality for very low birthweight infants. Am. J. Obstet. Gynecol. 2007;196:147 e141–147 e148. doi: 10.1016/j.ajog.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 27.Gitto E, Reiter RJ, Karbownik M, Xian-Tan D, Barberi I. Respiratory distress syndrome in the newborn: role of oxidative stress. Intens. Care Med. 2001;27:1116–1123. doi: 10.1007/s001340100977. [DOI] [PubMed] [Google Scholar]
- 28.Elshemy AS, Mohamed IL, Bakheet MS. Serum lipid peroxide and antioxidant vitamins (E, C) in neonates with respiratory distress syndrome. Int. J. Nutr. Metab. 2014;6:37–42. doi: 10.5897/IJNAM2014.0169. [DOI] [Google Scholar]
- 29.Matyas M, Blaga L, Hasmasanu M, Zaharie G. PO-0747 the study of oxidative stress at preterm newborns with respiratory distress syndrome. Arch. Dis. Child. 2014;99:A499–A500. doi: 10.1136/archdischild-2014-307384.1386. [DOI] [Google Scholar]
- 30.Buss IH, Darlow BA, Winterbourn CC. Elevated protein carbonyls and lipid peroxidation products correlating with myeloperoxidase in tracheal aspirates from premature infants. Pediatr. Res. 2000;47:640–645. doi: 10.1203/00006450-200005000-00014. [DOI] [PubMed] [Google Scholar]
- 31.Schock BC, Sweet DG, Halliday HL, Young IS, Ennis M. Oxidative stress in lavage fluid of preterm infants at risk of chronic lung disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001;281:L1386–L1391. doi: 10.1152/ajplung.2001.281.6.L1386. [DOI] [PubMed] [Google Scholar]
- 32.Buonocore G, Perrone S, Longini M, Terzuoli L, Bracci R. Total hydroperoxide and advanced oxidation protein products in preterm hypoxic babies. Pediatr. Res. 2000;47:221–224. doi: 10.1203/00006450-200002000-00012. [DOI] [PubMed] [Google Scholar]
- 33.Joung KE, et al. Correlation of urinary inflammatory and oxidative stress markers in very low birth weight infants with subsequent development of bronchopulmonary dysplasia. Free Radic. Res. 2011;45:1024–1032. doi: 10.3109/10715762.2011.588229. [DOI] [PubMed] [Google Scholar]
- 34.Dursun A, Okumus N, Erol S, Bayrak T, Zenciroglu A. Effect of ventilation support on oxidative stress and ischemia-modified albumin in neonates. Am. J. Perinatol. 2016;33:136–142. doi: 10.1055/s-0035-1560044. [DOI] [PubMed] [Google Scholar]
- 35.Falk DJ, et al. Mechanical ventilation promotes redox status alterations in the diaphragm. J. Appl. Physiol. (1985) 2006;101:1017–1024. doi: 10.1152/japplphysiol.00104.2006. [DOI] [PubMed] [Google Scholar]
- 36.Gladstone IM, Jr., Levine RL. Oxidation of proteins in neonatal lungs. Pediatrics. 1994;93:764–768. [PubMed] [Google Scholar]
- 37.Heljic S, Maksic H, Kalkan I, Krdalic B. The effects of antenatal corticosteroids and surfactant replacement on neonatal respiratory distress syndrome. Bosn J. Basic Med. Sci. 2009;9:225–228. doi: 10.17305/bjbms.2009.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilinska M, Borszewska-Kornacka MK, Niemiec T, Jakiel G. Oxidative stress and total antioxidant status in term newborns and their mothers. Ann. Agric. Environ. Med. 2015;22:736–740. doi: 10.5604/12321966.1185786. [DOI] [PubMed] [Google Scholar]
- 39.Sarricolea ML, Villa-Elizaga I, Lopez J. Respiratory distress syndrome in copper deficiency: an experimental model developed in rats. Biol. Neonate. 1993;63:14–25. doi: 10.1159/000243903. [DOI] [PubMed] [Google Scholar]
- 40.Khoubnasabjafari M, Ansarin K, Jouyban A. Reliability of malondialdehyde as a biomarker of oxidative stress in psychological disorders. Bioimpacts. 2015;5:123–127. doi: 10.15171/bi.2015.20. [DOI] [PMC free article] [PubMed] [Google Scholar]