Abstract
We present a comprehensive survey of the scientific literature pertaining to non-indigenous and invasive zooplankton published across the first decades of the twenty-first century (i.e., 2000–2018). We provide a concise summary of the manner in which the scientific community has allocated its efforts to this issue in recent decades, and to illuminate trends that emerge from the literature. Our search yielded 620 publications encompassing 139 invasive zooplankton species, with invasive zooplankton reported from every region of the planet—including the Arctic and Antarctic. Most taxa were reported in a small number of publications, with the majority being mentioned in only a single paper. In contrast, approximately half of the surveyed publications concerned just four species: Bythotrephes longimanus, Mnemioposis leidyi, Cercopagis pengoi, and Daphnia lumholtzi. Our survey reveals strong geographic patterns among the literature, with most publications arising from economically developed western nations. We found that the majority of publications pertained to holoplanktonic organisms from freshwater habitats, especially from the North American Great Lakes. Based on these results, we present several recommendations for future research topics that may hold considerable opportunity for growth in our understanding of the invasion process.
Electronic supplementary material
The online version of this article (10.1007/s10750-019-04096-x) contains supplementary material, which is available to authorized users.
Keywords: Non-indigenous, Aquatic, Bythotrephes longimanus, Mnemioposis leidyi, Cercopagis pengoi, Daphnia lumholtzi
Introduction
Zooplankton constitute a diverse assemblage of microscopic organisms that occupy a crucial intermediate position in the food webs of freshwater, estuarine, and marine ecosystems. In transferring energy from primary producers (photosynthetic protists, bacteria, and single-celled plants) to macroscopic invertebrates and fishes, zooplankton have the capacity to shape the dynamics of entire ecosystems. Zooplankton tend to be well adapted for long-range dispersal via a wide range of natural vectors (e.g., airborne resting eggs, aquatic birds, coastal currents—Cáceres & Soluk, 2002; Frisch et al., 2007), but anthropogenic activities such as aquaculture, commercial shipping, and recreational boating now provide an unprecedented capacity for dispersal across large geographic distances (Carlton & Geller, 1993; Ruiz et al., 2000; Minchin, 2007). Accordingly, non-indigenous zooplankton are now reported from bodies of water across nearly every region of the globe, and in many cases have been associated with far ranging ecological and economic impacts (Pimentel et al., 2005; Gollasch 2006; Connelly et al., 2007; Cuhel & Aguilar, 2013).
Nearly two decades have elapsed since the last comprehensive review of the literature pertaining to non-indigenous and invasive zooplankton (Bollens et al., 2002), and in the years that have passed since this publication, a number of important developments have occurred within the field. This includes a large number of new invasions like the introduction of the mysid Hemimysis anomala G.O. Sars, 1907 (Pothoven et al., 2007), the copepod Thermocyclops crassus (Fischer, 1853) (Connolly et al., 2017), and the rotifer Brachionus leydigii Cohn, 1862 (Connolly et al., 2018) to the North American Great Lakes. Furthermore, some high-profile invaders have greatly expanded their range in recent decades. This includes the western expansion of zebra and quagga mussels (which exhibit planktonic larvae) across the continental United States (Nalepa & Schloesser, 2013) and the spread of the Ctenophore Mnemiospis leidyi A. Agassiz, 1865, to the Baltic Sea and adjacent waters (Hansson, 2006; Javidpour, 2006). Finally, a number of theoretical and technical developments have also shifted research interests and paradigms in recent decades. This includes a growing interest in the ecological consequences of climate change (Hoegh-Guldberg & Bruno, 2010; Li et al., 2011; Chapman, 2014), a decrease in the financial and technological barriers to genetic sequencing (Glenn, 2011; Peterson et al., 2012; Schlötterer et al., 2014), and an increased awareness of the role that disease may play in shaping zooplankton communities (Ebert, 2005; Decaestecker et al., 2007; Sommer et al., 2012; Cáceres et al., 2014).
We therefore present here a comprehensive survey of the scientific literature pertaining to non-indigenous and invasive zooplankton published across the first decades of the twenty-first century (i.e., 2000–2018). This survey updates and expands upon the prior work of Bollens et al. (2002), with an increased focus on identifying taxonomic and geographic trends across the literature. This survey is intended to provide a concise summary of the manner in which the scientific community has allocated its efforts to this issue in recent decades and to illuminate trends that emerge from the literature. We do not here present a detailed synthesis of the findings contained in these articles, nor do we undertake a meta-analysis of the data contained therein. Instead, our survey has been organized around five fundamental questions: Who, what, where, when, and why? Who are the non-indigenous zooplankton species represented in the literature? What aspects of introduction and invasion are being studied? Where are introductions being reported? When are these patterns different? Why do we observe these patterns? Finally, we conclude our review with a set of recommendations for future research based upon the answers to these questions.
Methods
We conducted an exhaustive search of the full set of databases available on the Web of Science platform for publications pertaining to non-indigenous and invasive zooplankton. This database encompasses more than 150 million records distributed across more than 30,000 journals, books, and proceedings. Our search encompassed 11 individual subcollections: Web of Science Core Collection, BIOSIS Citation Index, BIOSIS Previews, Current Contents Connect, Data Citation Index, Derwent Innovations Index, KCI—Korean Journal Database, Medline, Russian Science Citation Index, SciELO Citation Index, and Zoological Record.
We searched for all records in the period spanning the years 2000 to 2018 which contained the following search terms in the title, abstract, or associated keywords: Zooplankton AND (Invasion OR invasive OR exotic OR non-native OR non-indigenous OR introduced). We limited our search to only those items published in peer-reviewed journals in English. We then read the abstract of each item and removed those which were false positives for our search terms (e.g., interactions between invasive fish and native zooplankton, or mathematical models of zooplankton dynamics with additional variables introduced). We then collected the following data from each abstract: title, authors, publication year, journal, studied taxa, life history (holoplankton vs. meroplankton), habitat (freshwater, estuarine, brackish sea, marine), research topic, geographic region, and geographic subregion (Supplemental materials S1). In cases of ambiguity, we examined the full text of the associated article. We delineated geographic regions and subregions according to the United Nations Geoscheme (U.N. Statistics Division 2019). Our survey methodology closely follows Bollens et al. (2002) so that results may be comparable. Table 1 provides a listing of the terms and definitions that we employed to classify the research topics of the articles.
Table 1.
Glossary of research topics and definitions employed in our classification of publications
| Topic | Definition |
|---|---|
| Ballast water contents | Surveys of zooplankton found in ballast water |
| Ballast water treatment | Evaluation of ballast water treatment methods including ballast water exchange |
| Behavior | Predator escape responses, diel vertical migration, etc |
| Climate change | Studies which explicitly test climate change related hypotheses or demonstrate a climate–invader relationship |
| Community impacts | Impacts of invader on native members of community |
| Detection | Methods or statistical probabilities of invader detection |
| Disease | Agents of disease that infect or are transported by the invader, including parasites |
| Dispersal | Transport vectors, biological adaptations, and routes of dispersal. Does not include papers on general aspects of dispersal |
| Distribution and range | Reports on the geographic extent of invasion or detection in new locales |
| Ecosystem impacts | Changes to nutrient cycling, clarity, etc |
| Feeding | Estimates of feeding rates, prey selection experiments and/or grazing by the invader |
| General/review | Reviews or summaries of the extant literature |
| Genetics | Any study employing genetic methodologies, ranging from community metabarcoding to whole genome sequencing |
| Invasion theory | Traits associated with invasion success, conditions promoting invasion, or requirements for population establishment |
| Physiology/biology | Biological functions of the organism (e.g. growth, reproduction) and physiological tolerances to the environment |
| Population dynamics | Population phenology, demography, or dynamics |
| Predation | Predation upon the invader by other native or invasive taxa |
We excluded technical reports, grey literature, regional species checklists, and other non-peer-reviewed literature from our survey. In order to avoid biasing our survey results towards systems and species most familiar to the authors, we included only those publications which were returned in our search results. Therefore, readers should view the survey which we present here as a rigorous sampling of the literature but not an exhaustive list. The complete database of publications that we surveyed (including our classification notes) is provided as a supplemental item to this manuscript (Supplemental Materials S2). Note that throughout the text of this manuscript, we employ the general term “non-indigenous” in reference to any non-native species, but apply the narrower term “invasive” to only those species associated with negative ecological impacts, rapid geographic expansion, or large population sizes (see Davis & Thompson, 2000 for a discussion of this terminology).
Results
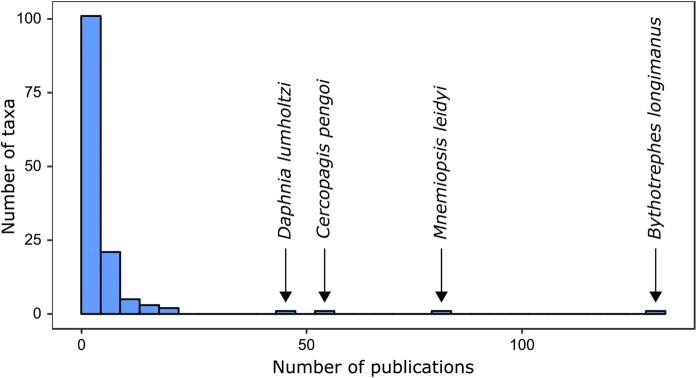
Our initial search returned a total of 1824 articles, which were individually verified to ensure peer-reviewed status and intended scope. After removing off-topic articles (the majority of which pertained to invasive fish), our final data set consisted of 620 articles. These articles encompassed a total of 139 zooplankton taxa which were listed as invasive, exotic, non-indigenous, or introduced. Taxa were identified to the species level in 93% of instances and to the genus level in the remainder. The vast majority of these taxa (90%) exhibited a holoplanktonic lifestyle, with meroplanktonic organisms primarily represented by larval bivalves and jellyfish medusae. Most taxa were documented in a small number of publications, with 63% of taxa mentioned in only a single paper (Fig. 1). Approximately half of the surveyed publications concerned just four species: Bythotrephes longimanus (Leydig, 1860) (n = 132), Mnemioposis leidyi (n = 82), Cercopagis pengoi (Ostroumov, 1891) (n = 52), and Daphnia lumholtzi G.O. Sars, 1885 (n = 47).
Fig. 1.
The total number of taxa across the entire survey, binned by the number of publications concerning each taxon. For example, more than 100 taxa were mentioned in only a single publication, but a single taxon (Bythotrephes longimanus) was mentioned in more than 100 publications
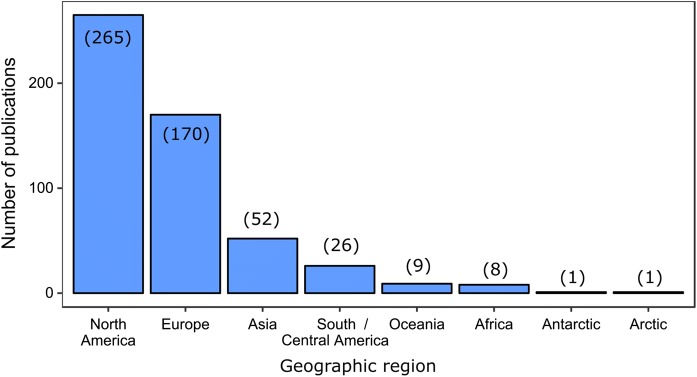
Our survey found reports of non-indigenous zooplankton across every region of the planet—including the Arctic and Antarctic (Fig. 2). The majority of publications that we surveyed (50%) concerned bodies of water in North America, with most records originating in US and Canadian waters. Records of non-indigenous zooplankton among Asian waters almost entirely pertained to the Black, Azov, and Caspian seas, with only 4 publications originating from Southeast Asian nations. All records from the Oceania region originated from New Zealand, without a single publication representing Australia or the South Pacific islands. Of all the surveyed articles which contained a field-based component, 61% (328 of 536) pertained to freshwater systems, with a large fraction of these studies occurring in the North American Great Lakes. The remainder of the studies were evenly divided among estuaries (16%), brackish seas (12%), and marine systems (10%). Brackish seas were represented by the Baltic, Black, Azov, and Caspian seas, with the majority of publications pertaining to the Black and Baltic seas.
Fig. 2.
The number of surveyed publications from 2000 to 2018 concerning invasive zooplankton species, shown by geographic region. The total number of publications from each region is listed in parentheses on each bar
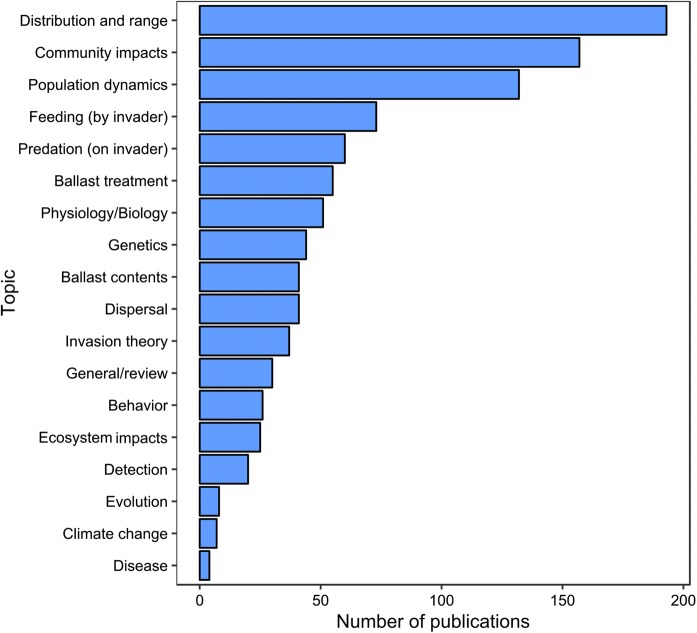
In order to estimate how well these results captured the extant literature, we compiled a complete list of publications from 10 randomly selected first authors represented in our database. We then calculated the number of publications from each author falling within the scope of our manuscript, but not captured by our search methodology. We found that across this subset of authors, 65% (SD = 24%) of the papers pertaining to zooplankton invasions were represented in our search. The complete set of publications that we surveyed encompassed a wide variety of topics, although some were encountered only rarely (Fig. 3). Three topics frequently arose in the publications that we surveyed: distribution and range, community impacts, and populations dynamics. Of the articles that we surveyed, 193 (31%) concerned the distribution and/or range of non-indigenous taxa, 157 (25%) reported on the community impacts of non-indigenous taxa, and 132 (21%) reported on population dynamics of non-indigenous taxa. The topics which appeared least frequently in our survey were evolution (n = 8), climate change (n = 7), and disease (n = 4).
Fig. 3.
The total number of surveyed publications concerning each of the selected research topics among the literature on invasive zooplankton across the period spanning 2000–2018
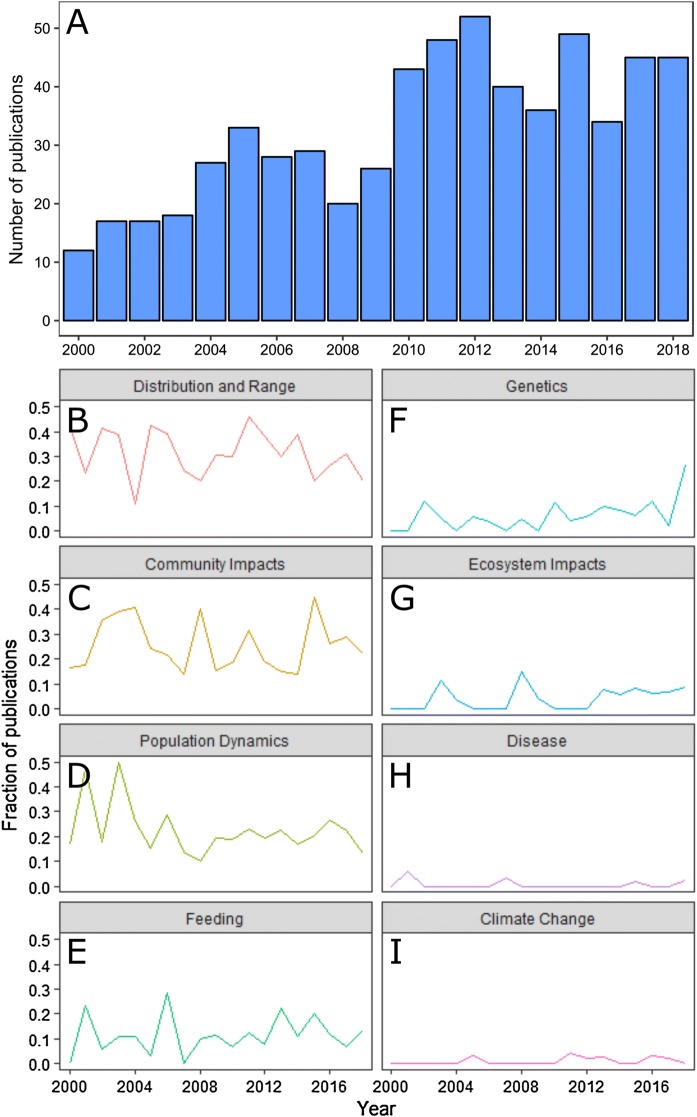
Our survey shows a large increase in the number of studies concerning non-indigenous and invasive zooplankton in recent decades (Fig. 4). This pattern has been highly variable from year to year, but a large and relatively consistent increase is clear. This increasing trend is most apparent when the data are divided into two equal periods: An earlier period spanning 2000–2009, and a later period spanning 2009–2018. We identified 214 publications from this earlier period, compared to 405 publications in the later period—which accounts for an increase of almost 90%. Only once in the earlier period were more than 30 publications produced in a single year, but in the latter period, more than 30 publications were produced in each year.
Fig. 4.
The temporal distribution of articles covered in our literature survey in terms of A the total number of articles published each year, and B–I the annual proportion of articles concerning selected subtopics
We observed clear geographic trends among the literature at multiple spatial scales. Of the 291 studies originating from the Americas, 90% pertained to North American waters, compared to 7.6% for South America, and 1.4% for Central America and the Caribbean. Among studies which originated from North American waters, almost all publications pertained to the continental United States and Canada. We observed similar geographic trends at finer geographic scales. For instance, among those studies conducted in North America, approximately half (117 of 263) of publications concerned the Great Lakes, and among South American publications, nearly all studies concerned the waters of Brazil.
Discussion
This survey has illuminated some unexpected trends across the invasive zooplankton literature (e.g., a very few species dominate the literature) as well as some expected, but previously unquantified trends (e.g., few publications pertain to tropical waters). In general, we observed a steady increase in the number of non-indigenous zooplankton reported during the 18-year period of study, along with a corresponding increase in the total number of publications. Our survey reveals strong geographic and taxonomic patterns among the literature, with most publications arising from economically developed western nations. The majority of publications that we reviewed pertained to holoplanktonic organisms from freshwater habitats, especially from the North American Great Lakes region. Most non-indigenous species were mentioned in only a single publication, with the bulk of the surveyed literature pertaining to a relatively small number of taxa.
We were surprised to discover that approximately half of the publications that we surveyed concerned just four species: Bythotrephes longimanus, Mnemioposis leidyi, Cercopagis pengoi, and Daphnia lumholtzi. This strong taxonomic bias among the literature likely exists for several reasons. Foremost is the fact that each of these taxa is closely associated with major ecological impacts across large, highly urbanized, and commercially important bodies of water (e.g., The Great Lakes, The Black Sea, and the Baltic Sea). These bodies of water tend to be associated with a large number of scientific institutions and thus receive an overall higher level of scientific scrutiny. Furthermore, the ecosystem impacts of these four invaders have been so pervasive that researchers may find it difficult to avoid considering these invasive species in some manner—regardless of the primary objectives of their studies.
Our survey updates and expands upon Bollens et al. (2002), which employed a similar methodology to review the non-indigenous zooplankton literature spanning from ~ 1970 to 2000. Comparing our results to this prior survey of the literature, we find a number of notable similarities and differences. Using identical search criteria to those employed in this review, Bollens et al. (2002) identified 252 publications concerning a total of 63 non-indigenous zooplankton taxa. We report a considerable expansion in the total number of reports across a similar span of time (620 articles—a 250% increase) and in the total number of non-indigenous taxa reported (139 taxa—a 220% increase). In the prior review, more than half of the publications (57% of 252) pertained to freshwater environments, which is similar to the proportion that we observed (61% of 620). Bollens et al. (2002) drew particular attention to a relative paucity of studies concerning meroplanktonic organisms (6.0% of 252), which appears to have slightly increased proportionally in recent decades (10% of 620). This earlier survey noted that a relatively low number and proportion of publications concerned community level (17% of 252) and ecosystem impacts (4.0% of 252). We observed a slight increase in the proportion of publications reporting community level impacts in recent decades (25% of 620), but no change in the proportion of publications reporting ecosystem impacts (4.0% of 620).
We note that most of the zooplankton invasions that we surveyed were reported from economically developed nations of the global West. One possible interpretation of this geographic trend is that it broadly mirrors the global distribution of wealth, a reasonable proxy for the global distribution of scientific funding (May, 1997; Davies et al., 2009). Alternatively, this wealth disparity might be a proxy for rates of commercial shipping, recreational boating, and other human associated vectors of introduction. This geographic trend may also to some degree reflect the fact that we surveyed only English language publications.
English is the most common language among the global scientific community, but its usage is far from universal (Hamel, 2007). This fact may account for the lack of records pertaining to Chinese waters in our survey and may also be a factor in the small number of publications that we surveyed from Latin American nations. Although given the increasing availability of professional services for English translation among the global scientific community, this language barrier may play a lesser role in recent years. While we do not have a quantitative measure of this linguistic bias, there are some anecdotal signs that the geographic trends that we observed are not entirely a matter of language. For example, our search yielded a small number of records from several nations where English is the primary language, such as Australia, Ireland, and the United Kingdom, but a relatively large number of records from France, Brazil, and Japan.
Of particular note is the difference in topics addressed in publications from economically developed versus developing regions. Among developing nations, publications tended to focus on the natural history of a given area with an emphasis on faunal surveys (e.g., Nandini et al., 2017). In some cases, authors reported a degree of uncertainty as to the origin of putatively non-indigenous species due to incomplete or ambiguous historical records (e.g., Segers, 2001). In contrast, publications from economically developed nations could often draw upon historical faunal surveys to assess the provenance of newly discovered taxa, and in many cases, to estimate a date of introduction (e.g., Ferrari & Orsi, 1984; Cordell et al., 2008; Dexter et al., 2015). Similarly, publications on specialized or resource intensive topics such as feeding rates, reproduction, and the genetics of non-indigenous taxa were almost exclusively the domain of economically developed nations.
At smaller spatial scales, we see that the research interests of individual scientists can play a significant part in the geographic patterns that we observe. Nearly all of the publications that we reviewed from New Zealand were affiliated with a single researcher (e.g., Duggan et al., 2006, 2012, 2014), and a single researcher was an author on all publications that we reviewed from the Philippines (e.g., Papa et al., 2012a, b; de Leon et al., 2016). Similarly, a high proportion of Black Sea records arose from a small number of authors working consistently on the issue of invasive ctenophores.
Recommendations for future research
Based upon the results of our survey and our own research experience, we have identified four topics which may present considerable opportunity for further growth in the understanding of zooplankton invasions: genetics, disease, ecosystem impacts, and climate change. In their earlier review, Bollens et al. (2002) reported a striking underutilization of genetic tools in the study of zooplankton invasions. Across the subsequent 18-year period that we reviewed, we report a slight increase in the number of publications with a genetic component, but far below what might be expected given the dramatic reduction in cost and the increased accessibility of genetic sequencing platforms in recent years (Glenn, 2011; Peterson et al., 2012; Goodwin et al., 2016). We found that only 7% of surveyed articles included a genetic component, with 8 publications on the metabarcoding of organisms in ballast water, 21 publications on invasion history, and 11 publications concerning species identification and/or taxonomy. Note that the apparent rapid increase in the number of publications with a genetic component (Fig. 4F) is driven by an unusually large value in 2018 alone. This may represent the beginning of an upward trend, or simply an outlying value. Nearly all reviewed genetic studies employed a small set of genetic markers such as cytochrome oxidase 1 (COI) or internal transcribed spacer 1 (ITS1), with only a few publications employing next-generation sequencing methods (Ghabooli et al., 2016; Dexter et al., 2018; Scott et al., 2018). Given the power of these tools to reconstruct the demographic history of populations (Estoup & Guillemaud, 2010), the maturation of eDNA technologies and protocols (Taberlet et al., 2012), and the constantly decreasing cost of implementation (Peterson et al., 2012), genetic and genomic tools represent a particularly promising area for growth within the field of invasion biology.
Another research topic which was largely absent from our survey of the invasive zooplankton literature was that of disease. Parasites and other pathogenic organisms have the capacity to act as major drivers of zooplankton population dynamics (Ebert, 2005; Cáceres et al., 2014). In recent years, there has been a growing appreciation for the role that disease may play in shaping seasonal dynamics of plankton communities (Sommer et al., 2012) and the evolution of local populations (Decaestecker et al., 2007; Ebert, 2008). It has been suggested that escape from pathogenic organisms may play a role in the invasion process (Mack et al., 2000), and in some cases, the rapid decline of an invader has been attributed to the subsequent arrival of pathogens and parasites from its native range (Simberloff & Gibbons, 2004). Invaders may also serve as vectors for the introduction of new parasites to native fauna (Mack et al., 2000; Grigorovich et al., 2001). Despite this wide-ranging set of consequences, our survey contained only 4 publications that examined some aspect of disease among non-indigenous zooplankton. This is clearly a facet of zooplankton invasions which merits greater investigation.
Some non-indigenous species of zooplankton have shown a tremendous capacity to alter not only the biological characteristics of the waterbodies to which they are introduced, but also the chemical and physical characteristics as well. In the most well-known example, zebra and quagga mussels (which disperse via planktonic larvae) have affected dramatic changes to water clarify and nutrient cycling, and markedly transformed benthic substrate across the invaded North American Great Lakes (Macisaac, 1996; Cuhel & Aguilar, 2013). While this may represent an extreme case, ecosystem impacts of similar scale have been reported across other species and waterbodies (Kideys et al., 2008; Penk et al., 2015; Walsh et al., 2016) and may be far more common than presently known (Simberloff, 2011). However, we observed that only a small percentage of the publications that we surveyed (4.0%) explicitly examined ecosystem-level impacts of non-indigenous zooplankton. As surveys of public attitudes regarding non-indigenous species have shown that general ecosystem impacts are a primary concern of public stakeholders (García-Llorente et al., 2008), we strongly recommend that researchers consider ecosystem-scale processes and impacts in future work.
The final research topic that we wish to highlight is that of climate, as the earth’s climate is undoubtably warming at a rapid pace due to the activities of human society (IPCC, 2014). A number of observational studies, laboratory experiments, and modeling efforts indicate that climate change may promote the global spread of invasive species and increased severity of invasion impacts (e.g., Lennon et al., 2001; Stachowicz et al., 2002; Winder et al., 2011; Chapman et al., 2016). Although many of the publications that we reviewed referenced climate change in some manner, we identified only 7 publications that explicitly tested hypotheses pertaining to climate–invasion linkages. This may be a consequence of the long periods of observation needed to detect climate signals in field-based studies, or the complex relationship between temperature and plankton community dynamics. Nonetheless, we highlight this climate–invasion linkage as an understudied and increasingly relevant facet of zooplankton ecology.
Conclusion
The increasing number of publications that pertain to non-indigenous zooplankton likely reflects both a growing awareness of this issue among the scientific community and a growing number of invaders across the globe. Our review highlights that fact that much of the current scientific understanding of zooplankton invasions is based upon a relatively small number of taxa and waterbodies. Our survey shows a potential need for greater examination of the waters associated with less economically developed nations, especially at tropical latitudes. Furthermore, we highlight considerable opportunity for growth in the areas of genetics, disease, ecosystem impacts, and climate–invasion linkages. We hope that this review will serve as a point of reference for the research community and a roadmap for the planning of future investigations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Funding for this research was provided by a US Environmental Protection Agency STAR grant (#FP91780901-0) awarded to E. Dexter and S. Bollens. Additional comments on this manuscript were provided by Stephen Katz, Stephanie Hampton, Gretchen Rollwagen-Bollens, and Séverine Vuilleumier as members of E. Dexter’s Ph.D. committee.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Bollens SM, Cordell JR, Avent S, Hooff R. Zooplankton invasions: a brief review, plus two case studies from the northeast Pacific Ocean. Hydrobiologia. 2002;480:87–110. doi: 10.1023/A:1021233018533. [DOI] [Google Scholar]
- Cáceres C, Soluk D. Blowing in the wind: a field test of overland dispersal and colonization by aquatic invertebrates. Oecologia. 2002;131:402–408. doi: 10.1007/s00442-002-0897-5. [DOI] [PubMed] [Google Scholar]
- Cáceres CE, Tessier AJ, Duffy MA, Hall SR. Disease in freshwater zooplankton: what have we learned and where are we going? Journal of Plankton Research. 2014;36:326–333. doi: 10.1093/plankt/fbt136. [DOI] [Google Scholar]
- Carlton JT, Geller JB. Ecological roulette: the global transport of nonindigenous marine organisms. Science. 1993;261:78–82. doi: 10.1126/science.261.5117.78. [DOI] [PubMed] [Google Scholar]
- Chapman S. Publishing trends on climate change vulnerability in the conservation literature reveal a predominant focus on direct impacts and long time-scales. Diversity and Distributions. 2014;20:1221–1228. doi: 10.1111/ddi.12234. [DOI] [Google Scholar]
- Chapman DS, Makra L, Albertini R, et al. Modelling the introduction and spread of non-native species: international trade and climate change drive ragweed invasion. Global Change Biology. 2016;22:3067–3079. doi: 10.1111/gcb.13220. [DOI] [PubMed] [Google Scholar]
- Connelly NA, O’Neill CR, Knuth BA, Brown TL. Economic impacts of zebra mussels on drinking water treatment and electric power generation facilities. Environmental Management. 2007;40:105–112. doi: 10.1007/s00267-006-0296-5. [DOI] [PubMed] [Google Scholar]
- Connolly JK, Watkins JM, Hinchey EK, Rudstam LG, Reid JW. New cyclopoid copepod (Thermocyclops crassus) reported in the Laurentian Great Lakes. Journal of Great Lakes Research. 2017;43:198–203. doi: 10.1016/j.jglr.2017.03.020. [DOI] [Google Scholar]
- Connolly JK, Watkins JM, Marshall CC, Adams JM, Rudstam LG, Błędzki LA. Brachionus leydigii (Monogononta: Ploima) reported from the western basin of Lake Erie. Journal of Great Lakes Research. 2018;44:1123–1126. doi: 10.1016/j.jglr.2018.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordell JR, Bollens SM, Draheim R, Sytsma M. Asian copepods on the move: recent invasions in the Columbia-Snake River system, USA. ICES Journal of Marine Science. 2008;65:753–758. doi: 10.1093/icesjms/fsm195. [DOI] [Google Scholar]
- Cuhel RL, Aguilar C. Ecosystem transformations of the Laurentian Great Lake Michigan by nonindigenous biological invaders. Annual Review of Marine Science. 2013;5:289–320. doi: 10.1146/annurev-marine-120710-100952. [DOI] [PubMed] [Google Scholar]
- Davies JB, Sandström S, Shorrocks A, Wolff E. The global pattern of household wealth: the global pattern of household wealth. Journal of International Development. 2009;21:1111–1124. doi: 10.1002/jid.1648. [DOI] [Google Scholar]
- Davis MA, Thompson K. Eight ways to be a colonizer; two ways to be an invader: a proposed nomenclature scheme for invasion ecology. Bulletin of the Ecological Society of America. 2000;81:226–230. [Google Scholar]
- de Leon, J. R., H. G. de Vera, E. J. A. Giron, S. Chambord, A. Souissi, S. Souissi, & R. D. S. Papa. 2016. Temporal Variability of Abundance, Morphological and Reproductive Traits of the Invasive Arctodiaptomus dorsalis (Marsh 1907) (Copepoda: Calanoida: Diaptomidae) in Relation to the Reduction of Aquaculture in Lake Taal (2008 & 2013). 145: 12.
- Decaestecker E, Gaba S, Raeymaekers JAM, Stoks R, Kerckhoven LV, Ebert D, Meester LD. Host–parasite ‘Red Queen’ dynamics archived in pond sediment. Nature. 2007;450:870–873. doi: 10.1038/nature06291. [DOI] [PubMed] [Google Scholar]
- Dexter E, Bollens SM, Rollwagen-Bollens G, Emerson J, Zimmerman J. Persistent vs. ephemeral invasions: 8.5 years of zooplankton community dynamics in the Columbia River. Limnology and Oceanography. 2015;60:527–539. doi: 10.1002/lno.10034. [DOI] [Google Scholar]
- Dexter E, Bollens SM, Cordell J, Soh HY, Rollwagen-Bollens G, Pfeifer SP, Goudet J, Vuilleumier S. A genetic reconstruction of the invasion of the calanoid copepod Pseudodiaptomus inopinus across the North American Pacific Coast. Biological Invasions. 2018;20:1577–1595. doi: 10.1007/s10530-017-1649-0. [DOI] [Google Scholar]
- Duggan IC, Green JD, Burger DF. First New Zealand records of three non-indigenous Zooplankton species: Skistodiaptomus pallidus, Sinodiaptomus valkanovi, and Daphnia dentifera. New Zealand Journal of Marine and Freshwater Research. 2006;40:561–569. doi: 10.1080/00288330.2006.9517445. [DOI] [Google Scholar]
- Duggan I, Robinson K, Burns C, Banks J, Hogg I. Identifying invertebrate invasions using morphological and molecular analyses: North American Daphnia ‘pulex’ in New Zealand fresh waters. Aquatic Invasions. 2012;7:585–590. doi: 10.3391/ai.2012.7.4.015. [DOI] [Google Scholar]
- Duggan, I. C., M. W. Neale, K. V. Robinson, P. Verburg & N. T. N. Watson, 2014. Skistodiaptomus pallidus (Copepoda: Diaptomidae) establishment in New Zealand natural lakes, and its effects on zooplankton community composition. 9: 195–202.
- Ebert, D. 2005. Ecology, Epidemiology, and Evolution of Parasitism in Daphnia [ebook], National Library of Medicine (US), National Center for Biotechnology Information. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=Books.
- Ebert D. Host–parasite coevolution: insights from the Daphnia–parasite model system. Current Opinion in Microbiology. 2008;11:290–301. doi: 10.1016/j.mib.2008.05.012. [DOI] [PubMed] [Google Scholar]
- Estoup A, Guillemaud T. Reconstructing routes of invasion using genetic data: why, how and so what? Molecular Ecology. 2010;19:4113–4130. doi: 10.1111/j.1365-294X.2010.04773.x. [DOI] [PubMed] [Google Scholar]
- Ferrari FD, Orsi J. Oithona davisae, New Species, and Limnoithona sinensis (Burckhardt, 1912) (Copepoda: Oithonidae) from the Sacramento-San Joaquin Estuary, California. Journal of Crustacean Biology. 1984;4:106–126. doi: 10.2307/1547900. [DOI] [Google Scholar]
- Frisch D, Green AJ, Figuerola J. High dispersal capacity of a broad spectrum of aquatic invertebrates via waterbirds. Aquatic Sciences. 2007;69:568–574. doi: 10.1007/s00027-007-0915-0. [DOI] [Google Scholar]
- García-Llorente M, Martín-López B, González JA, Alcorlo P, Montes C. Social perceptions of the impacts and benefits of invasive alien species: implications for management. Biological Conservation. 2008;141:2969–2983. doi: 10.1016/j.biocon.2008.09.003. [DOI] [Google Scholar]
- Ghabooli S, Zhan A, Paolucci E, Hernandez MR, Briski E, Cristescu ME, MacIsaac HJ. Population attenuation in zooplankton communities during transoceanic transfer in ballast water. Ecology and Evolution. 2016;6:6170–6177. doi: 10.1002/ece3.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn TC. Field guide to next-generation DNA sequencers. Molecular Ecology Resources. 2011;11:759–769. doi: 10.1111/j.1755-0998.2011.03024.x. [DOI] [PubMed] [Google Scholar]
- Gollasch S. Overview on introduced aquatic species in European navigational and adjacent waters. Helgoland Marine Research. 2006;60:84–89. doi: 10.1007/s10152-006-0022-y. [DOI] [Google Scholar]
- Goodwin S, McPherson JD, McCombie WR. Coming of age: ten years of next-generation sequencing technologies. Nature Reviews Genetics. 2016;17:333. doi: 10.1038/nrg.2016.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigorovich Igor A., Dovgal Igor V., MacIsaac Hugh J., Monchenko Vladislav I. Acineta nitocrae: A new suctorian epizooic on nonindigenous harpacticoid copepods, Nitocra hibernica and N. incerta, in the Laurentian Great Lakes. Fundamental and Applied Limnology. 2001;152(1):161–176. doi: 10.1127/archiv-hydrobiol/152/2001/161. [DOI] [Google Scholar]
- Hamel RE. The dominance of English in the international scientific periodical literature and the future of language use in science. AILA Review. 2007;20:53–71. doi: 10.1075/aila.20.06ham. [DOI] [Google Scholar]
- Hansson H. Ctenophores of the Baltic and adjacent Seas – the invader Mnemiopsis is here! Aquatic Invasions. 2006;1:295–298. doi: 10.3391/ai.2006.1.4.16. [DOI] [Google Scholar]
- Hoegh-Guldberg O, Bruno JF. The impact of climate change on the world’s marine ecosystems. Science. 2010;328:1523. doi: 10.1126/science.1189930. [DOI] [PubMed] [Google Scholar]
- IPCC. 2014. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. IPCC.
- Javidpour J. First record of Mnemiopsis leidyi A. Agassiz 1865 in the Baltic Sea. Aquatic Invasions. 2006;1:299–302. doi: 10.3391/ai.2006.1.4.17. [DOI] [Google Scholar]
- Kideys AE, Roohi A, Eker-Develi E, Mélin F, Beare D. Increased Chlorophyll Levels in the Southern Caspian Sea Following an Invasion of Jellyfish. International Journal of Ecology. 2008 doi: 10.1155/2008/185642. [DOI] [Google Scholar]
- Lennon JT, Smith VH, Williams K. Influence of temperature on exotic Daphnia lumholtzi and implications for invasion success. Journal of Plankton Research. 2001;23:425–433. doi: 10.1093/plankt/23.4.425. [DOI] [Google Scholar]
- Li J, Wang M-H, Ho Y-S. Trends in research on global climate change: a Science Citation Index Expanded-based analysis. Global and Planetary Change. 2011;77:13–20. doi: 10.1016/j.gloplacha.2011.02.005. [DOI] [Google Scholar]
- Macisaac HJ. Potential abiotic and biotic impacts of zebra mussels on the inland waters of North America. Integrative and Comparative Biology. 1996;36:287–299. [Google Scholar]
- Mack RN, Simberloff D, Mark Lonsdale W, Evans H, Clout M, Bazzaz FA. Biotic invasions: causes, epidemiology, global consequences, and control. Ecological Applications. 2000;10:689–710. doi: 10.1890/1051-0761(2000)010[0689:BICEGC]2.0.CO;2. [DOI] [Google Scholar]
- May RM. The scientific wealth of nations. Science. 1997;275:793–796. doi: 10.1126/science.275.5301.793. [DOI] [Google Scholar]
- Minchin D. Aquaculture and transport in a changing environment: overlap and links in the spread of alien biota. Marine Pollution Bulletin. 2007;55:302–313. doi: 10.1016/j.marpolbul.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Nalepa TF, Schloesser DW. Quagga and Zebra Mussels: Biology, Impacts, and Control. 2. Boca Raton: CRC Press; 2013. [Google Scholar]
- Nandini S, Sarma SSS, Gulati RD. A seasonal study reveals the occurrence of exotic rotifers, the River Antigua, Veracruz, close to the Gulf of Mexico. River Research and Applications. 2017;33:970–982. doi: 10.1002/rra.3140. [DOI] [Google Scholar]
- Papa RDS, Li H, Tordesillas DT, Han B, Dumont HJ. Massive invasion of Arctodiaptomus dorsalis (Copepoda, Calanoida, Diaptomidae) in Philippine lakes: a threat to Asian zooplankton biodiversity? Biological Invasions. 2012;14:2471–2478. doi: 10.1007/s10530-012-0250-9. [DOI] [Google Scholar]
- Papa RDS, Tordesillas DT, Mamaril AC. An updated taxonomic account of limnetic crustacean zooplankton in Lake Taal, Philippines. Philippine Journal of Science. 2012;141:11. [Google Scholar]
- Penk M, Irvine K, Donohue I. Ecosystem-level effects of a globally spreading invertebrate invader are not moderated by a functionally similar native. Journal of Animal Ecology. 2015;84:1628–1636. doi: 10.1111/1365-2656.12402. [DOI] [PubMed] [Google Scholar]
- Peterson BK, Weber JN, Kay EH, Fisher HS, Hoekstra HE. Double digest RADseq: an inexpensive method for de novo SNP discovery and genotyping in model and non-model species. PLoS ONE. 2012;7:e37135. doi: 10.1371/journal.pone.0037135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimentel D, Zuniga R, Morrison D. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol. Econ. 2005;52:273–288. doi: 10.1016/j.ecolecon.2004.10.002. [DOI] [Google Scholar]
- Pothoven SA, Grigorovich IA, Fahnenstiel GL, Balcer MD. Introduction of the Ponto-Caspian Bloody-red Mysid Hemimysis anomala into the Lake Michigan Basin. Journal of Great Lakes Research. 2007;33:285–293. doi: 10.3394/0380-1330(2007)33[285:IOTPBM]2.0.CO;2. [DOI] [Google Scholar]
- Ruiz GM, Rawlings TK, Dobbs FC, Drake LA, Mullady T, Huq A, Colwell RR. Global spread of microorganisms by ships. Nature. 2000;408:49–50. doi: 10.1038/35040695. [DOI] [PubMed] [Google Scholar]
- Schlötterer C, Tobler R, Kofler R, Nolte V. Sequencing pools of individuals – mining genome-wide polymorphism data without big funding. Nature Reviews Genetics. 2014;15:749–763. doi: 10.1038/nrg3803. [DOI] [PubMed] [Google Scholar]
- Scott R, Zhan A, Brown EA, Chain FJJ, Cristescu ME, Gras R, MacIsaac HJ. Optimization and performance testing of a sequence processing pipeline applied to detection of nonindigenous species. Evol. Appl. 2018;11:891–905. doi: 10.1111/eva.12604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segers H. Zoogeography of the Southeast Asian Rotifera. Rotifera IX. 2001 doi: 10.1007/978-94-010-0756-6_32. [DOI] [Google Scholar]
- Simberloff D. How common are invasion-induced ecosystem impacts? Biol. Invasions. 2011;13:1255–1268. doi: 10.1007/s10530-011-9956-3. [DOI] [Google Scholar]
- Simberloff D, Gibbons L. Now you see them, Now you don’t! – population crashes of established introduced species. Biological Invasions. 2004;6:161–172. doi: 10.1023/B:BINV.0000022133.49752.46. [DOI] [Google Scholar]
- Sommer U, Adrian R, Domis LDS, et al. Beyond the Plankton Ecology Group (PEG) model: mechanisms driving plankton succession. Annual Review of Ecology, Evolution, and Systematics. 2012;43:429–448. doi: 10.1146/annurev-ecolsys-110411-160251. [DOI] [Google Scholar]
- Stachowicz JJ, Terwin JR, Whitlatch RB, Osman RW. Linking climate change and biological invasions: ocean warming facilitates nonindigenous species invasions. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:15497–15500. doi: 10.1073/pnas.242437499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taberlet P, Coissac E, Hajibabaei M, Rieseberg LH. Environmental DNA. Molecular Ecology. 2012;21:1789–1793. doi: 10.1111/j.1365-294X.2012.05542.x. [DOI] [PubMed] [Google Scholar]
- U.N. Statistics Division . United Nations Statistics Division – Methodology. New York: U.N. Statistics Division; 2019. [Google Scholar]
- Walsh JR, Carpenter SR, Zanden MJV. Invasive species triggers a massive loss of ecosystem services through a trophic cascade. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:4081–4085. doi: 10.1073/pnas.1600366113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winder M, Jassby AD, Mac Nally R. Synergies between climate anomalies and hydrological modifications facilitate estuarine biotic invasions. Ecology Letters. 2011;14:749–757. doi: 10.1111/j.1461-0248.2011.01635.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.