Abstract
Pseudorabies virus (PRV) primarily infects swine but can infect cattle, dogs, and cats. Several studies have reported that PRV can cross the specie barrier and induce human encephalitis, but a definitive diagnosis of human PRV encephalitis is debatable due to the lack of PRV DNA detection. Here, we report a case of human PRV encephalitis diagnosed by the next-generation sequencing (NGS) of PRV sequences in the cerebrospinal fluid (CSF) of a patient. A male pork vendor developed fever and seizures for 6 days. NGS results showed PRV sequences in his CSF and blood. Sanger sequencing showed that PRV DNA in the CSF and PRV antibodies in both the CSF and blood were positive. MRI results revealed multiple inflammatory lesions in the bilateral hemisphere. Based on the clinical and laboratory data, we diagnosed the patient with PRV encephalitis. This case suggests that PRV can infect humans, causing severe viral encephalitis. People at risk of PRV infection should improve their self-protection awareness.
Keywords: Pseudorabies virus, Human encephalitis, Next-generation sequencing
Introduction
Pseudorabies virus (PRV), also called suid herpesvirus 1, primarily infects swine but can infect cattle, dogs, and cats (Pomeranz et al., 2005). Mravak et al. reported suspected cases of human PRV infection with positive PRV antibodies in plasma (Mravak et al., 1987). In 2018, Ai J-W et al. reported a case of human PRV endophthalmitis diagnosed by the identification of unique PRV sequences in vitreous humor (Ai et al., 2018). These cases demonstrate cross-species PRV transmission from domestic animals to humans. We reported a case of human encephalitis caused by PRV.
Medical history
The patient was a 44-year-old man who worked as a pork vendor in Anhui Province of China. His daily work duties were to cut and sell pork. Two weeks before illness onset, he obtained some minor cuts on his fingers, but he still engaged in direct contact with the pork at work.
On January 1, 2019, he developed a cough; runny nose; and quick, single jerks of the arm muscles that last for a few seconds. Four days later, he developed a fever of 41 °C, and at about 22:00, he presented with upward rolling of the eyes and rhythmic muscle contractions in the arms, face, legs, and body for approximately 1 min. On the way to a nearby hospital, he had three more seizures, between which he did not regain consciousness. One day later, he was transferred to the First Affiliated Hospital of the University of Science and Technology of China (Hefei, China). After a cranial computerized tomography (CT) scan showing no obvious abnormalities, he was preliminarily diagnosed with viral encephalitis with status epilepticus and admitted to the intensive care unit.
Physical examination
In our department, he remained comatose with a Glasgow Coma Scale (GCS) of 3 (E1, VT, M1). Pupils were both 5 mm in diameter and sluggishly reactive to light. Corneal reflexes were absent, all muscle tone and deep tendon reflexes normal, neck stiffness absent, and Babinski signs negative bilaterally. There were some minor cuts on his fingers (see Appendix 1 figure 3).
Fig 3.
Cuts in patient's fingers (white arrow).
Laboratory examinations
A routine blood test revealed a total white blood cell count of 11.08 109/L (normal range 4–10), a neutrophil count of 8.69 109/L (normal range 2–7), a red blood cell count of 4.27 1012/L (normal range 4–5.5), a hemoglobin concentration of 124 g/L (normal range 120–160), and a blood platelet count of 109 109/L (normal range 100–300).
Biochemical examination revealed an alanine transaminase level of 119 U/L (normal range 0–50), an aspartate transaminase level of 401 U/L (normal range 0–40), a mitochondria aspartate aminotransferase level of 87 U/L (normal range 0–15.0), a creatinine level of 39 μmol/L (normal range 40–120), and a glucose level of 6.62 mmol/L (normal range 3.9–6.1). The C-reactive protein level was 18.4 mg/L (normal range 0–10), and the procalcitonin level was 0.56 ng/ml (normal range 0–0.1).
The cerebrospinal fluid (CSF) was colorless and clear with an opening pressure of 220 mmH2O (normal range 80–180). A CSF examination revealed 5 cells/mm3 (normal range 0–5) with lymphocytic predominance (90%), 4.50 mmol/L of glucose (normal range 2.8–4.5), 116.0 mmol/L of chloride (normal range 120–130), 0.2 g/L of protein (normal range 0.15–0.45), 24.8 mg/l of IgG (normal range 0–34), 1.91 mg/l of IgA (normal range 0–5), and 0.33 mg/l of IgM (normal range 0–1.3). CSF bacterial smear and culture, staining for cryptococcus, and acid-fast staining for tuberculosis were all negative. CSF antibodies for autoimmune encephalitis were all negative.
Blood nucleic acid tests for rubella virus, cytomegalovirus, herpes simplex virus type 2, and Epstein-Barr virus were all negative.
The serological assays for hepatitis B antibodies and antigens, hepatitis C antibodies, syphilis antibodies, and HIV antibodies were all negative. The serological assays for rubella virus, cytomegalovirus, and Epstein-Barr virus IgGs were all positive, whereas the IgMs for all three viruses were all negative. The serological assays for herpes simplex virus type 2 IgG and IgM were all negative. Plasma 1-3-β-D glucan and galactomannan antigen tests were both normal.
The serological assays for antinuclear antibodies, anti-glomerular basement membrane (GBM) antibodies, P-anti neutrophil cytoplasmic antibodies (P-ANCAs), C-ANCA, myeloperoxidase, and serine proteinase were all negative.
Electroencephalogram
EEG showed generalized moderate-amplitude theta (4–7 Hz) or delta (1–3 Hz) frequency waves diffusely throughout the background.
MRI
The magnetic resonance imaging (MRI) images revealed abnormal signals distributed symmetrically in bilateral frontal lobe, temporal lobe, insula lobe, basal ganglia, and hippocampus. (Fig. 1).
Fig. 1.
A–F show MRI images at 1 week after admission. T2-weighted and fluid-attenuated inversion recovery (FLAIR) images shown hyperintense signal distributed symmetrically in bilateral frontal lobe (A, B, D, and E; white arrow), temporal lobe (B and E; green arrow), insula lobe (B and E; yellow arrow), basal ganglia (B and E; red arrow), and hippocampus (C and F; blue arrow)
Treatment and diagnosis
According to the clinical manifestations, CSF analysis, and MRI findings, we diagnosed the patient with viral encephalitis and immediately began treatment with ganciclovir (300 mg/day), 20% mannitol (125 ml q8 h), cefuroxime (1.5 g bid), diazepam, and propofol.
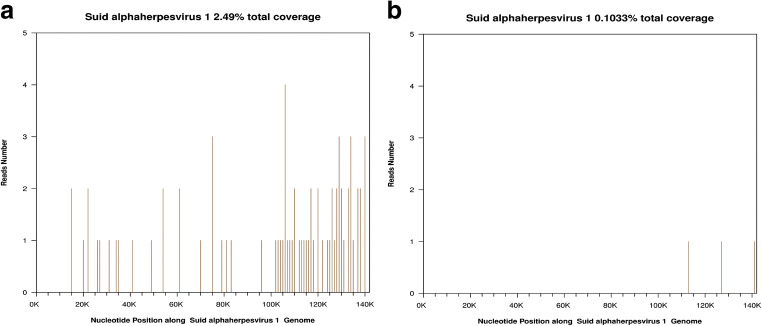
We suspected that this case of encephalitis might have been caused by a rare virus. To identify the causative virus, we subjected 1.5 ml of CSF to next-generation sequencing (NGS) on hospital day 3. On hospital day 5, the NGS results showed a total of 74 PRV reads in the CSF, accounting for 2.49% (3544/142298) of the whole genome (Fig. 2). Considering that the encephalitis was probably caused by PRV infection, we adjusted the treatment to acyclovir (10 mg/kg/8 h), dexamethasone (10 mg bid), sodium valproate, and sodium phenobarbital.
Fig. 2.
Sequence reads mapped to PRV from NGS results. A Sequence reads of PRV in CSF with a total coverage of 2.49%. B Sequence reads of PRV in blood with a total coverage of 0.10%
On hospital day 6, we sampled 2 ml of blood for NGS, and the result showed 3 unique PRV sequences in the blood, accounting for 0.103% of the total nucleotide sequences on hospital day 8 (Fig. 2).
To validate the NGS results, we performed polymerase chain reaction (PCR) analysis and Sanger sequencing on the CSF. The gel electrophoresis results of the PCR products and Sanger sequencing data are shown in Appendix 2.
To confirm the diagnosis of PRV encephalitis, we sent a blood sample for a serological examination of the PRV antibody. On hospital day 14, tests for PRV antibodies were both negative in the plasma and CSF. On hospital days 23 and 56, tests for PRV antibodies were positive in the plasma and CSF, indicating a recent PRV infection.
Follow-up and prognosis
After the initial treatment, we performed three lumbar punctures on hospital days 14, 23, and 55; the CSF examination results are shown in Table 1. MRI images revealed multiple inflammatory lesions in the bilateral hemisphere at 2 weeks and 3 weeks after admission.
Table 1.
Cerebrospinal fluid examination
| Nucleated cells count (106/L) | Major cell type | Glucose (mmol/L) | Chloride (mmol/L) | Protein (g/L) | |
|---|---|---|---|---|---|
| Day 0 | 5 | lymphocyte | 4.50 | 116.0 | 0.2 |
| Day 3 | 11 | lymphocyte | 4.18 | 121.8 | 0.5 |
| Day 14 | 6 | lymphocyte | 2.98 | 121.0 | 0.3 |
| Day 23 | 1 | lymphocyte | 3.21 | 116.0 | 0.5 |
| Day 56 | 12 | lymphocyte | 3.02 | 121.9 | 0.3 |
After 1 month of treatment, the patient gradually gained consciousness, with an increased GCS of 7 (E4, VT, M2). After 2 months of treatment, he had been weaned off the ventilator, with a GCS of 9 (E4, VT, M4) and was voluntarily discharged to a hospital in his hometown. At the 6-month follow-up after discharge from our hospital, the patient had the ability to perform eye movements and simple body movements in response to verbal commands.
Discussion
PRV infection is highly prevalent among pigs. Recently, it has been reported that PRV can cause cross-species transmission and induce human infection. The first two suspected cases of human PRV infection were reported in 1914 (Mravak et al., 1987), but those cases were debatable because they lacked antibodies or PRV sequences. Three suspected cases of human PRV infection with positive antibodies were reported in 1987 (Mravak et al., 1987), strongly indicating that PRV could infect humans. All three patients initially developed fever, sweating, weakness, and tiredness 1–3 weeks after contact with animals and other domestic animals. Later, they complained of difficulty swallowing; dysgeusia; dryness and tension in the nose, mouth, and throat, suggesting that the central nervous system may be involved. The three patients were all positive for PRV antibodies.
In 2018, Ai J-W et al. reported a case of human PRV endophthalmitis (Ai et al., 2018). The patient, who was a swine herder, developed fever, headache, and visual impairment after her eyes were splashed by sewage containing pig excrement on a hog farm. NGS showed unique PRV sequences, and real-time PCR showed PRV DNA in the patient’s vitreous humor. PRV antibodies were detected in the plasma at 4 months after disease onset, confirming PRV infection. This is the first case of human PRV endophthalmitis and the first case of PRV detected by NGS and PCR in humans.
In 2018, Zhao et al. first reported human encephalitis caused by PRV (Zhao et al., 2018). This report identified four human cases of PRV encephalitis from patients with infectious encephalitis of unknown etiology in China. All four patients were occupationally exposed to raw pork. The patients all presented sudden onset of high fever, headaches, and chills and rapidly developed seizures, coma, and respiratory failure in 1 to 4 days. CSF analyses showed slight increases in leukocyte counts (6–14 cells/mm3), protein levels (0.18–0.41 g/L) and glucose levels (4.8–6.39 mmol/L) in all four patients. Cranial MRI of one patient showed a high T2-weighted value widely distributed in the bilateral areas of the temporal lobe, insula, cingulate gyrus, basal ganglia, thalamus, frontal lobe, parietal lobe, and midbrain. Cranial MRI of another patient showed a high T2-weighted value in the bilateral temporal lobe and bilateral basal ganglia. The CSF samples of two patients were available for NGS, and PRV sequences were detected. In addition, three patients underwent serological analysis, and PRV antibodies were detected in all three patients. All the patients received antiviral (acyclovir) and corticosteroid therapy (dexamethasone or methylprednisolone). Three patients remained unconscious 3 to 4 months after treatment, and one patient died. One patient regained consciousness but was blind at the 1-year follow-up. The results of this study indicated that PRV could infect humans and induce encephalitis. However, CSF specimens from these patients were not sent for PRV antibody detection.
In 2019, Yang H N et al. reported a human case of PRV encephalitis in China (Yang et al., 2019). The 43-year-old patient presented with high-grade fever 4 days after his hands were cut by a knife when dissecting dead swine. He successively developed headache, seizures, and coma within 3 days. Plain CT brain imaging showed hypodensity in the left limbic lobe and bilateral region of the basal ganglia and bilateral occipital lobe. PRV nucleic acid sequences were detected with NGS, and PRV gB and gE antibodies were positive in the CSF, confirming PRV encephalitis. The patient received antiviral therapy (acyclovir, 10 mg/kg/8 h) for 2 weeks and another therapeutic intervention in the ICU and was weaned off mechanical ventilation after a month.
In our case study, the patient was a pork vendor in Meng Cheng County of Anhui Province. His hands directly contacted pork and pork products and his fingers had often been cut in the course of his daily work. He presented with fever, seizures, and coma within 1 day. The CSF analysis showed slightly elevated white blood cell counts, no autoimmune encephalitis antibodies, negative gram-stains, and a negative bacterial culture. It is reported that generalized moderate-amplitude theta (4–7 Hz) or delta (1–3 Hz) frequency waves in EEG result are often seen in drowsiness of a normal adult (St. Louis et al., 2016). EEG was conducted when the patient had been in antiepileptic drug therapy, which may explain the EEG result of the patient. Cranial MRI showed high T2-weighted values in the frontal lobe, temporal lobe, limbic system, basal ganglia, and thalamus. NGS and antibody tests of the blood and CSF confirmed PRV infection of the central nervous system. According to the Infectious Diseases Society of America (IDSA) guidelines, acyclovir, with an A-level strength, is recommended for the empirical treatment of suspected encephalitis and corticosteroids are weakly recommended for the treatment of encephalitis in the absence of good-quality evidence (Tyler, 2018). In this case, we initiated the empirical antiviral treatment with ganciclovir. After diagnosis of PRV encephalitis, according to IDSA guidelines and treatment regimen in four PRV encephalitis cases reported by Zhao WL et al., we changed the treatment to acyclovir for 14 days and dexamethasone for 4 days. The clinic characteristics of the patient, including a history of exposure to pork; signs of fever, seizures, and loss of consciousness; a typical CSF profile of viral encephalitis; and MRI findings, were consistent with those in the five PRV encephalitis cases previously reported.
NGS is an accurate and time-saving technology for the molecular diagnosis of infectious diseases. NGS is a powerful platform to amplify and sequence total DNA from a single sample without any probes or primers, so it can unbiasedly identify all the potential pathogens in a single assay (Guan et al., 2015). NGS has revolutionized the diagnosis of infectious diseases, especially diseases caused by rare or newly discovered pathogens. In our case, PRV sequences were detected in the CSF within 2 days. Although the PRV coverage was relatively low, the mapped reads were distributed uniformly in the whole PRV genome. Based on the clinical characteristics and NGS results, we cautiously suspected that the encephalitis was caused by PRV infection. Considering that PRV encephalitis is rare, a PRV antibody test was conducted, and the positive results in the CSF and blood confirmed the diagnosis.
A large-scale etiological investigation showed an average PRV positivity rate of 8.27% in pigs during 2012 and 2017 in most regions in China. In Eastern China, which includes Anhui Province, the average PRV positivity rate was 11.6% in pigs, which was higher than that in other regions in China (Sun et al., 2018).
Regarding this case of PRV encephalitis, the patient was a pork vendor in Meng Cheng County in Anhui Province. His hands directly contacted pork and pork products during the course of his daily work, and he was probably infected with PRV from raw pork through a break in the skin. Therefore, in regions with a high PRV positivity rate in pigs, it is necessary to improve awareness about PRV prevention in those with occupations associated with a high risk of infection.
Conclusion
In summary, this paper reported a case of human PRV encephalitis, which manifested as seizures and rapidly developed to severe viral encephalitis. The treatment included antiviral drugs, corticosteroids, antiepileptic drugs, and organ function support. NGS is a rapid and accurate method for the detection of PRV infection. This case indicates that PRV can infect humans, causing viral encephalitis, and demonstrates the need to increase self-protection awareness in workers with high-risk occupations.
Acknowledgments
We thank Professor Songhe Zhan of the Anhui Provincial Center for Animal Disease Control and Prevention for his help in the PRV antibody examinations and Dr. Yang Zhou of BGI Group for her help in the PCR analysis and Sanger sequencing.
Appendix 1
Appendix 2
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ai JW, Weng SS, Cheng Q et al (2018) Human endophthalmitis caused by pseudorabies virus infection, China, 2017. Emerg Infect Dis. 10.3201/eid2406.171612 [DOI] [PMC free article] [PubMed]
- Guan H, Shen A, Lv X et al (2015) Detection of virus in CSF from the cases with meningoencephalitis by next-generation sequencing:1–6. 10.1007/s13365-015-0390-7 [DOI] [PubMed]
- Mravak S, Bienzle U, Feldmeier H, et al. Pseudorabies in man. Lancet. 1987;1:501–502. doi: 10.1016/s0140-6736(87)92105-2. [DOI] [PubMed] [Google Scholar]
- Pomeranz LE, Reynolds AE, Hengartner CJ. Molecular biology of pseudorabies virus: impact on neurovirology and veterinary medicine. Microbiol Mol Biol Rev. 2005;69:462–500. doi: 10.1128/mmbr.69.3.462-500.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. Louis E, Frey L, Britton J et al (2016) Electroencephalography (EEG): an introductory text and atlas of normal and abnormal findings in adults. Children, and Infants [PubMed]
- Sun Y, Liang W, Liu Q, Zhao T, Zhu H, Hua L, Peng Z, Tang X, Stratton CW, Zhou D, Tian Y, Chen H, Wu B. Epidemiological and genetic characteristics of swine pseudorabies virus in mainland China between 2012 and 2017. PeerJ. 2018;6:e5785. doi: 10.7717/peerj.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler KL. Acute viral encephalitis. N Engl J Med. 2018;379:557–566. doi: 10.1056/NEJMra1708714. [DOI] [PubMed] [Google Scholar]
- Yang H, Han H, Wang H, et al. A case of human viral encephalitis caused by pseudorabies virus infection in China. Front Neurol. 2019;10:1–5. doi: 10.3389/fneur.2019.00534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao WL, Wu YH, Li HF, Li SY, Fan SY, Wu HL, Li YJ, Lü YL, Han J, Zhang WC, Zhao Y, Li GL, Qiao XD, Ren HT, Zhu YC, Peng B, Cui LY, Guan HZ. Clinical experience and next-generation sequencing analysis of encephalitis caused by pseudorabies virus. Zhonghua Yi Xue Za Zhi. 2018;98:1152–1157. doi: 10.3760/cma.j.issn.0376-2491.2018.15.006. [DOI] [PubMed] [Google Scholar]