Abstract
Purpose of the Review
Present an overview of perioperative considerations specific to endoscopic skull base surgery necessary to maximize successful outcomes.
Recent Findings
The majority of perioperative considerations for endoscopic skull base surgery lack strong supporting evidence and frequently have varied use or implementation amongst institutions. A notable exception comes from a recent randomized controlled trial demonstrating the benefit of lumbar drainage in high-risk cerebrospinal fluid leaks.
Summary
Skull base surgeons must consider a multitude of perioperative factors. While many components of perioperative management are extrapolated from related fields such as endoscopic sinus surgery or open cranial base surgery, additional high-quality studies are needed to delineate best practices specific to endoscopic skull base surgery.
Keywords: Endoscopic, Skull base surgery, Perioperative, Antibiotics, Lumbar drain
Introduction
Over the past three decades, the management of patients with anterior skull base disease has rapidly evolved. Advances in endoscopic visualization and instrumentation have greatly expanded the ablative and reconstructive potential of transnasal approaches while concurrently minimizing morbidity and mortality [1]. Although endoscopic skull base surgery has its origins in pituitary and sellar pathology, it is now regularly employed in a vast array of other disease processes. Complex skull base tumors, once reserved exclusively for open craniofacial resection, are often routinely addressed via purely endoscopic techniques. Furthermore, the variety of anatomical sites which are accessible via transnasal approaches has vastly increased. Approaches to the cavernous sinus, clivus, pterygopalatine fossa, and petrous apex are well described in the literature with excellent outcomes [2–5].
Although much success has been appreciated in the field of endoscopic skull base surgery as a whole, there remains a need for a high degree of fidelity in operative planning. By virtue of its inherent anatomy, skull base surgery is an exceedingly complex endeavor and requires a high level of familiarity and knowledge of critical neurovascular structures in the craniofacial skeleton. However, beyond the technical mastery needed to achieve success, adequate and complete care of patients hinges on several important factors. The purpose of the present review is to highlight select perioperative considerations to maximize success during endoscopic skull base surgery and assess supporting evidence if available.
Preoperative Considerations
Establishment of a Skull Base Team
While more of a “big-picture” consideration, establishment of a dedicated skull base team is an important component for any institution seeking to manage the complexities of a skull base patient. Multidisciplinary care is integral with otolaryngology and neurosurgery typically playing central roles. Collaboration with critical care, endocrinology, neuroradiology, interventional radiology, ophthalmology, medical, and radiation oncology enables the most comprehensive management of these complex patients and disorders. Learning curves for various procedures and pathologies ranging from endoscopic pituitary surgery to transclival resection of chordomas have been reported, and while there is significant variation amongst the varied pathologies or approaches, increased experience by a surgical team routinely translates into improved outcomes and decreased complications [6, 7].
Defining Goals of Surgery and Informed Consent
Recognizing and defining surgical goals prior to intervention is critically important. Goals may vary based upon underlying pathology, patient age/general health, as well as potential morbidity. Evaluating the tumor anatomy is fundamental. Given the relationship to vital structures, total resection is sometimes not technically feasible, or surgical risks may outweigh the benefits [8, 9]. For instance, in sellar-based neoplasms, tumors that extend laterally beyond the cavernous sinus, superiorly through the plane of the hypothalamus and floor of the third ventricle, and/or those with retrosellar extension must be critically assessed given associations with increased morbidity [10]. An exhaustive review of every subsite of the skull base has been addressed in various other studies [11••, 12]. As a general tenet, endoscopic approaches are ill-suited to pathology that is deep to critical neurologic and vascular structures (that would require transgressing), and alternative approaches should be sought. Beyond anatomical constraints, the natural biology of the tumor must also be considered. Notably for malignant disease, tumor extent may be more significant than appreciated based on imaging alone, and consideration of potential perineural spread may alter the operative plan. In the case of vascular tumors, subtotal resection may increase the possibility of postoperative bleeding and edema leading to mass effect [10]. Despite limitations, indications for proceeding with endoscopic skull base surgery over open craniofacial resection (CFR) continue to expand. Open CFR may be advocated for in select lesions; however, it is worth noting that in the endoscopic era, complication rates for CFR may be higher than historically stated numbers [13].
The potential impact on quality of life after any intervention has become a major consideration in surgical planning [15]. Joint decision-making between the physician and patient has become more central in ultimately determining these goals of surgery and is based on honest discussions regarding the likelihood of success as well as potential risks and morbidities of endoscopic skull base procedures. Given the close proximity of the surgical corridor to critical nerves and arteries, there exists the potential for serious intraoperative injury leading to significant debilitation and/or death. The overall risk for complication following endoscopic skull base surgery is reported to be 10–20%, with more common complications including cerebrospinal fluid (CSF) leak, visual changes, and pituitary dysregulation [14–19].
Risk Stratification
General preoperative risk stratification of the endoscopic skull base patient follows common guidelines for other surgical patients with regard to complete evaluation of their underlying cardiopulmonary function and assessment of their major medical comorbidities. Further delineation of a risk profile may require specialty-specific consultation as dictated by patient need (e.g., cardiology, pulmonary medicine). Several specific considerations to endoscopic skull base surgery are detailed below.
The complexity of surgery correlates with the potential for complications and morbidity. Varying levels of difficulty of endoscopic skull base surgery (ranging from basic sinus procedures to endonasal management of aneurysms) have been previously described as they relate to surgical skill acquisition in training [20]. This concept was recently validated by the same group, demonstrating increased complications/morbidity with progressively more challenging approaches or lesions [21]. It is important that skull base teams are cognizant of their level of experience and limitations and proceed in an incremental fashion up the levels of complexity in order to potentially optimize surgical outcomes.
Formal angiography may be considered in select cases with the potential to serve several different roles. First is a more accurate delineation of tumor vascular supply with the potential for mitigation of subsequent operative hemorrhage via transarterial or direct puncture embolization. While not necessary in the majority of cases, the benefit for highly vascular tumors such as juvenile nasopharyngeal angiofibromas has been clearly demonstrated in multiple studies [22]. The second notable role for angiography is in the assessment of adequate intracranial collateral circulation. In cases where the internal carotid artery is deemed to be at particularly high risk (e.g., adjacent malignancy, prior radiation, revision surgery), balloon occlusion testing can help to stratify those patients that would be unlikely to tolerate carotid sacrifice and may push either towards a more conservative resection or possibly vascular bypass surgery in the setting of aggressive disease. Angiography, tumor embolization, and balloon occlusion testing carry an inherent level of risk and must be weighed against the potential benefit on a case-by-case basis.
Revision procedures may be indicated to remove residual disease, decompress neurovascular structures, or as a planned second stage procedure. Individuals who have undergone previous endoscopic skull base procedures require special consideration, as they may be at increased risk for complications [23]. The basis for such complications may be related to distorted anatomical landmarks, extensive scarring, and/or limited reconstructive options.
Intraoperative Considerations
While the approach and surgical technique tend to be the major focus of the operating room, there are several important peripheral considerations. Specific consideration of reconstructive technique or materials, variations on approach, and other surgery-specific variations is beyond this review and is detailed elsewhere.
Operating Suite Design and Setup
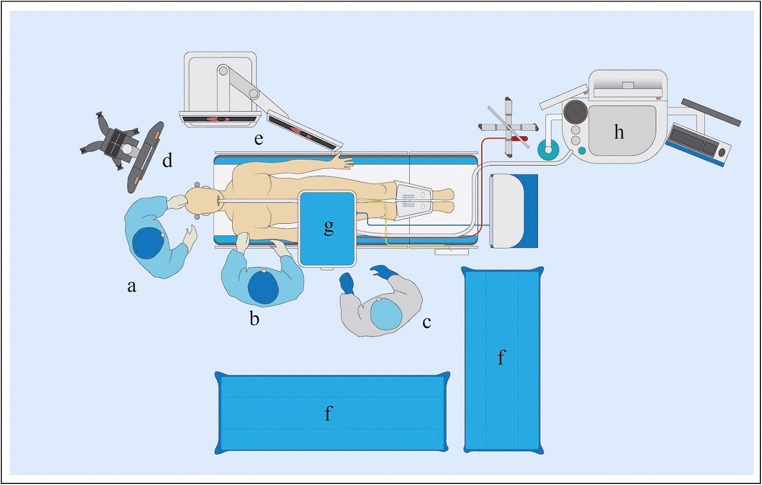
The operating room is the heart of any skull base program. An endoscopic suite which is designed with a two-surgeon team in mind can optimize interaction between surgical assistants, improve efficiency of instrument handoffs, and offer enhanced visualization for surgeons and observers. At the very minimum, an endoscopic skull base room should include the following: (1) an operating room table capable of being turned 180° away from the anesthetist and ventilator as well as providing adequate incline for reverse Trendelenberg positioning to decrease bleeding, (2) at least one, though preferably two, high-resolution endoscopic monitors, (3) stereotactic navigation, (4) one to two organized instrument tables, (5) and most importantly, enough room to accommodate all of the preceding in a comfortable manner. Figure 1 depicts an example setup with both surgeons on the same side, though some may find a preference for operating on opposite sides. While ergonomics has been a topic of great interest in many surgical subspecialties, optimization of surgeon comfort and efficacy has been poorly studied in endoscopic skull base surgery and would be of significant interest in future studies given the often long and taxing nature of many skull base procedures.
Fig. 1.
Typical operating room setup for endoscopic skull base cases. a otolaryngologist, b neurosurgeon, c surgical technician, d stereotactic navigation tower, e high-resolution endoscopic monitors, f instrument tables, g Mayo stand, h anesthesiologist and ventilator
Equipment
The very principle of endoscopic skull base surgery relies on adequate and reliable visualization of the operative field. The Hopkins rod telescope has been the workhorse of the field, with numerous iterations and advances over the past decade, including the introduction of ultra-high-resolution 4K, variable angled lens, and three-dimensional capable endoscopes [24]. Although advanced scopes can offer superior visualization, they are not mandatory to proceed with skull base surgery. All cases can be typically performed with basic 0°, 30°, 45°, and 70° endoscopes, and no studies have demonstrated superior outcomes from any newer endoscope capabilities. Access to specifically designed skull base instrumentation enables more effective dissection, and in some cases, the ability to access deeper areas that shorter common sinus instruments lack the ability to reach. Endoscopically designed bipolar forceps are frequently crucial for focal control of bleeding vessels.
Stereotactic Navigation
Stereotactic navigation is frequently used during endoscopic skull base surgery and allows for high-resolution and panoramic visualization of the complex anatomy within the sinonasal and intracranial cavities. Survey studies report that more than 80% of skull base surgeons use stereotactic navigation for every case performed [25]. Surgical tracking is achieved through various means, including infrared and electromagnetic technologies, though no system has been shown to be more accurate or superior than others [26]. While navigation is commonly employed, no studies have demonstrated a definite benefit in terms of outcomes or complications [27]. One notable limitation of stereotactic navigation is that the preoperative images may not correlate with intraoperative changes [28].
Neurophysiological Monitoring
As previously mentioned, there is an inherent risk to critical neurovascular structures including the carotid artery, anterior cerebral arteries, and various cranial nerves. Injuries and their neurological sequelae may be prevented by using neurophysiological monitoring. Various options include somatosensory evoked potentials (SSEP), brainstem auditory evoked potentials (BAEP), and electromyography (EMG), though the decision to use monitoring depends largely on the procedure being performed and the specific structures at risk. Numerous studies have proven the efficacy of monitoring during cranial base surgery, and routine use is strongly advocated for complex and high-risk cases [29, 30].
Prophylactic Antibiotics
There exists no definitive preoperative antibiotic regimen for endoscopic skull base cases. Antibiotic choice is largely dictated by skull base team preferences and is widely variable. In a survey of otolaryngologists who perform endoscopic skull base surgery, Wannemuehler et al. assessed intraoperative antibiotic preferences and found that the majority of respondents utilized either a first-generation or third-generation cephalosporin [31]. At our institution, cephalexin is typically used for extradural cases while ceftriaxone is preferred for those cases that extend intradurally. Despite wide variation in reported protocols, the incidence of meningitis remains low. A systematic review of the topic found few published observational studies that precluded meta-analysis and concluded that a large randomized controlled study is necessary to identify the role and optimal antibiotic choice [32].
Postoperative Considerations
General Considerations
Although much emphasis is placed on preoperative planning and intraoperative techniques, the postoperative period for the skull base patient is equally as critical. In order to maximize the success of a skull base ablation and reconstruction, special care must be taken to carefully monitor patients for critical neurological changes and signs of insufficient repair. The immediate postoperative period, particularly the first 24 h, is extremely critical [33].
With the exception of straightforward CSF leak repairs and relatively small lesions, postoperative patients should be admitted for close monitoring in an intensive care unit (ICU), or at the very least, a neurological step-down unit. Routine neurological checks vary by institution, and nurse-driven protocols at our institution are performed on an hourly basis for the first 24–48 h. For pituitary pathologies, strict recording of hourly intakes and outputs is performed to monitor for development of diabetes insipidus and is supplemented by serial laboratory chemistries [34]. With regard to preventing undue pressure on skull base reconstructions, patients are generally kept with their head elevated at 30° or higher. Additionally, sinonasal precautions are employed, and patients are instructed to avoid nose blowing, sneeze with their mouths open, restrict the use of straws, and refrain from straining. Supplemental oxygen is provided via humidified face tent as opposed to direct injection into the nasal cavity with a pronged cannula in order to minimize the drying effect on nasal mucosa as well as the theoretical risk of pneumocephalus [35].
Postoperative Antibiotics
Variability exists in the use, type, and duration of postoperative antibiotics. In a recent survey study, roughly 50% of surgeons did not employ the use of postoperative oral antibiotics when no nasal packing was placed. When absorbable packing was used, approximately 40% of surgeons prescribed oral antibiotics for 7 days postoperatively. When non-absorbable packing was placed, over 60% prescribed oral antibiotics until packing removal [31]. Our current practice is congruent with these findings, as we typically prescribe oral antibiotics only when non-absorbable packing is placed and cease antibiotic use upon packing removal. A recent systematic review evaluating the need for systemic antibiotic use with nasal packing after sinus surgery found few studies adequately addressing the issue. While no significant benefit (prevention of local nasal infection or toxic shock syndrome) was identified with antibiotic use, all studies evaluated were underpowered to identify a difference [36].
Nasal Packing
The type of nasal packing and duration of use vary significantly amongst surgeons. The overwhelming majority, close to 90%, will use some form of packing for intradural pathology in order to buttress the skull base reconstruction [31]. Various types may be used including absorbable or non-absorbable subtypes. No particular one has been demonstrated to be more efficacious. The duration of packing is also highly variable and typically ranges from 5 to 7 days, though it is important to note that the optimal duration of packing has not been determined by any published studies. Our current institutional practice is for 7 days of non-absorbable tampon sponge packing for any moderate dural defect. Pinhole leaks will typically be managed with 5 days of non-absorbable packing or use of dissolvable packing in select cases. In the absence of any intraoperative leak, postoperative packing is not utilized.
Lumbar Drain Use
CSF leaks are a known complication of endoscopic sinus and skull base surgery. During sinus surgery, they are typically iatrogenic in nature; however, in ablative endoscopic skull base surgery, they are often an intentional consequence of achieving adequate exposure for tumor resection. The overall incidence of CSF rhinorrhea following expanded endonasal surgery has generally been accepted to be around 5% [37]. Leaks may be classified as low-flow or high-flow, with the latter referring to those leaks associated with a large dural defect or violation of a cistern or ventricle [38]. With the advent and increased use of vascularized pedicled flaps for reconstruction, postoperative CSF leak rates have decreased. Consequently, the routine use of lumbar drains in endoscopic skull base surgery has fallen out of favor [39]. A prior meta-analysis demonstrated overall poor quality of available data and no significant benefit of postoperative lumbar drainage [40]. However, a recent large randomized controlled trial demonstrated that patients with a high-flow leak (as defined by a dural defect greater than 1 cm, arachnoid dissection, and/or entry into a cistern or ventricle) were 2.9 times more likely to have a postoperative CSF leak if no lumbar drain was placed immediately postoperatively [41••]. Our current practice has evolved from the rare use of lumbar drains to more selective use with very high-risk defects.
Postoperative Nasal Care Regimen
Specific postoperative nasal regimens vary widely and are in large part determined by surgeon preference. The benefit of nasal saline on reduced crusting and improved re-mucosalization has been demonstrated by multiple randomized controlled trials following endoscopic sinus surgery and extrapolated to the care of skull base patients [42]. Nasal saline is essentially used by all surgeons, though the method of application (saline spray vs. irrigations) and timing of initiation (immediately vs. starting at 1/2/2+ weeks) varies significantly and is affected by the extent of approach. Repeated postoperative debridements until healing is complete are commonly performed by the majority of surgeons with the initial limited nasal debridement typically occurring around 1.5 weeks postoperatively and initial skull base debridement reserved until approximately 3 weeks postoperatively [31]. The benefit of regular debridement has been called into question in the sinus surgery literature with conflicting reports as to whether this practice positively impacts patient outcomes and raises the question of the ultimate need for postoperative debridements following endoscopic skull base cases [42].
Postoperative Imaging
The role of postoperative imaging is frequently debated. In particular, best practices for timing and modality vary by institution and have not been clearly defined. The advocates for early imaging, which is often conducted several hours postprocedure, highlight benefits such as early detection of life-threatening intracranial hemorrhage and/or tension pneumocephalus [43]. At our own institution, we follow a similar protocol, with a postoperative MRI or CT performed within 24 h of surgery. Overall, the utility of early postoperative scans depends on its ability to rule out catastrophic events as well as establish a baseline following surgery which may help to differentiate postsurgical changes from tumor recurrence on subsequent imaging. Additionally, evidence of residual disease may prompt re-resection for select pathology.
Obstructive Sleep Apnea and CPAP Use
Patients with obstructive sleep apnea (OSA) frequently require continuous positive airway pressure (CPAP) for effective management of their disease. These patients pose a unique challenge in postoperative management, especially in those with reconstructed dural defects. Concerns for increased risk of pneumocephalus, meningitis, and CSF leak have typically led to delayed reinstitution of CPAP postoperatively. Unfortunately, no definitive studies have identified the necessary required time of non-use to minimize complications. Survey studies have shown that most practicing skull base surgeons will hold CPAP use for at least 2 weeks in the event of an intraoperative leak and closer to 3 weeks for larger dural defects [25, 44].
Conclusion
A multidisciplinary skull base team represents the gold standard of care in patients with complex neurological disease. Numerous perioperative factors require consideration for optimizing chances of a successful outcome. Unfortunately, many of the considerations reviewed above lack strong supporting data, and additional future research is required to validate and determine their optimal use. Multi-institutional trials may be required to adequately power the necessary studies capable of answering many of these lingering questions.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical collection on Skull Base Surgery
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Adnan S. Hussaini, Email: adnan.s.hussaini@gunet.georgetown.edu
Christine M. Clark, Email: christine.m.clark@gunet.georgetown.edu
Timothy R. DeKlotz, Email: timothy.r.deklotz@medstar.net
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
- 1.Turri-Zanoni M, Zocchi J, Lambertoni A, Giovannardi M, Karligkiotis A, Battaglia P, et al. Endoscopic endonasal reconstruction of anterior skull base defects: what factors really affect the outcomes? World Neurosurg. 2018;116:e436–e443. doi: 10.1016/j.wneu.2018.04.225. [DOI] [PubMed] [Google Scholar]
- 2.Di Somma A, Torales J, Cavallo LM, Pineda J, Solari D, Gerardi RM, et al. Defining the lateral limits of endoscopic endonasal transtuberculum transplanum approach: anatomical study with pertinent quantitative analysis. J Neurosurg. 2018;130(3):848–860. doi: 10.3171/2017.9.JNS171406. [DOI] [PubMed] [Google Scholar]
- 3.Raithatha R, McCoul ED, Woodworth GF, Schwartz TH, Anand VK. Endoscopic endonasal approaches to the cavernous sinus. Int Forum Allergy Rhinol. 2012;2(1):9–15. doi: 10.1002/alr.20097. [DOI] [PubMed] [Google Scholar]
- 4.Zanation AM, Snyderman CH, Carrau RL, Gardner PA, Prevedello DM, Kassam AB. Endoscopic endonasal surgery for petrous apex lesions. Laryngoscope. 2009;119(1):19–25. doi: 10.1002/lary.20027. [DOI] [PubMed] [Google Scholar]
- 5.Plzák J, Kratochvil V, Kešner A, Šurda P, Vlasák A, Zvěřina E. Endoscopic endonasal approach for mass resection of the pterygopalatine fossa. Clinics (Sao Paulo) 2017;72(9):554–561. doi: 10.6061/clinics/2017(09)06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koutourousiou M, Gardner PA, Tormenti MJ, Henry SL, Stefko ST, Kassam AB, Fernandez-Miranda JC, Snyderman CH. Endoscopic endonasal approach for resection of cranial base chordomas: outcomes and learning curve. Neurosurgery. 2012;71(3):614–624. doi: 10.1227/NEU.0b013e31825ea3e0. [DOI] [PubMed] [Google Scholar]
- 7.Shikary T, Andaluz N, Meinzen-Derr J, Edwards C, Theodosopoulos P, Zimmer LA. Operative learning curve after transition to endoscopic transsphenoidal pituitary surgery. World Neurosurg. 2017;102:608–612. doi: 10.1016/j.wneu.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Sanusi O, Arnaout O, Rahme RJ, Horbinski C, Chandler JP. Surgical resection and adjuvant radiation therapy in the treatment of skull base chordomas. World Neurosurg. 2018;115:e13–e21. doi: 10.1016/j.wneu.2018.02.127. [DOI] [PubMed] [Google Scholar]
- 9.Mendenhall WM, Friedman WA, Amdur RJ, Foote KD. Management of benign skull base meningiomas: a review. Skull Base. 2004;14(1):53–60. doi: 10.1055/s-2004-821364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zada Gabriel, Du Rose, Laws Edward R. Defining the “edge of the envelope”: patient selection in treating complex sellar-based neoplasms via transsphenoidal versus open craniotomy. Journal of Neurosurgery. 2011;114(2):286–300. doi: 10.3171/2010.8.JNS10520. [DOI] [PubMed] [Google Scholar]
- 11.Wang EW, Zanation AM, Gardner PA, Schwartz TH, Eloy JA, Adappa ND, Bettag M, Bleier BS, Cappabianca P, Carrau RL, Casiano RR, Cavallo LM, Ebert CS Jr, el-Sayed IH, Evans JJ, Fernandez-Miranda JC, Folbe AJ, Froelich S, Gentili F, Harvey RJ, Hwang PH, Jane JA Jr, Kelly DF, Kennedy D, Knosp E, Lal D, Lee JYK, Liu JK, Lund VJ, Palmer JN, Prevedello DM, Schlosser RJ, Sindwani R, Solares CA, Tabaee A, Teo C, Thirumala PD, Thorp BD, de Arnaldo Silva Vellutini E, Witterick I, Woodworth BA, Wormald PJ, Snyderman CH. ICAR: endoscopic skull-base surgery. Int Forum Allergy Rhinol. 2019;9(S3):S145–S365. doi: 10.1002/alr.22326. [DOI] [PubMed] [Google Scholar]
- 12.Rangel-Castilla L, Russin JJ, Spetzler RF. Surgical management of skull base tumors. Rep Pract Oncol Radiother. 2016;21(4):325–335. doi: 10.1016/j.rpor.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller JD, Taylor RJ, Ambrose EC, Laux JP, Ebert CS, Zanation AM. Complications of open approaches to the skull base in the endoscopic era. J Neurol Surg B Skull Base. 2017;78(1):11–17. doi: 10.1055/s-0036-1583948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirkman MA, Borg A, Al-Mousa A, Haliasos N, Choi D. Quality-of-life after anterior skull base surgery: a systematic review. J Neurol Surg B Skull Bae. 2014;75(2):73–89. doi: 10.1055/s-0033-1359303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicolai P, Battaglia P, Bignami M, Bolzoni Villaret A, Delù G, Khrais T, et al. Endoscopic surgery for malignant tumors of the sinonasal tract and adjacent skull base: a 10-year experience. Am J Rhinol. 2008;22(3):308–316. doi: 10.2500/ajr.2008.22.3170. [DOI] [PubMed] [Google Scholar]
- 16.Batra PS, Citardi MJ, Worley S, Lee J, Lanza DC. Resection of anterior skull base tumors: comparison of combined traditional and endoscopic techniques. Am J Rhinol. 2005;19(5):521–528. doi: 10.1177/194589240501900517. [DOI] [PubMed] [Google Scholar]
- 17.Suh JD, Ramakrishnan VR, Chi JJ, Palmer NJ, Chiu AG. Outcomes and complications of endoscopic approaches for malignancies of the paranasal sinuses and anterior skull base. Am Otol Rhinol Laryngol. 2013;122(1):54–59. doi: 10.1177/000348941312200110. [DOI] [PubMed] [Google Scholar]
- 18.Dave SP, Bared A, Casiano RR. Surgical outcomes and safety of transnasal endoscopic resection for anterior skull tumors. Otolaryngol Head Neck Surg. 2007;136(6):920–927. doi: 10.1016/j.otohns.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 19.Naunheim MR, Sedaghat AR, Lin DT, Bleier BS, Holbrook EH, Curry WT, Gray ST. Immediate and delayed complications following endoscopic skull base surgery. J Neurol Surg B Skull Base. 2015;76(5):390–396. doi: 10.1055/s-0035-1549308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Snyderman C, Kassam A, Carrau R, Mintz A, Gardner P, Prevedello DM. Acquisition of surgical skills for endonasal skull base surgery: a training program. Laryngoscope. 2007;117(4):699–705. doi: 10.1097/MLG.0b013e318031c817. [DOI] [PubMed] [Google Scholar]
- 21.Lavigne P, Faden D, Gardner PA, Fernandez-Miranda JC, Wang EW, Snyderman CH. Validation of training levels in endoscopic endonasal surgery of the skull base. Laryngoscope. 2019;129(10):342–376. doi: 10.1002/lary.27895. [DOI] [PubMed] [Google Scholar]
- 22.Khoueir Nadim, Abou Hamad Walid. Response to “Re: Khoueir N et al, Otolaryngol Head Neck Surg, 2014;150(3):350-358”. Otolaryngology–Head and Neck Surgery. 2014;151(1):184–184. doi: 10.1177/0194599814536367. [DOI] [PubMed] [Google Scholar]
- 23.Tajudeen BA, Mundi J, Suh JD, Bergsneider M, Wang MB. Endoscopic endonasal surgery for recurrent pituitary tumors: technical challenges to the surgical approach. J Neurol Surg B Skull Base. 2015;76(1):50–56. doi: 10.1055/s-0034-1383856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riley CA, Soneru CP, Tabaee A, Kacker A, Anand VK, Schwartz TH. Technological and ideological innovations in endoscopic skull base surgery. World Neurosurg. 2019;S1878–8750(19):30220–30227. doi: 10.1016/j.wneu.2019.01.120. [DOI] [PubMed] [Google Scholar]
- 25.Roxbury Christopher R., Lobo Brian C., Kshettry Varun R., D'Anza Brian, Woodard Troy D., Recinos Pablo F., Snyderman Carl H., Sindwani Raj. Perioperative management in endoscopic endonasal skull-base surgery: a survey of the North American Skull Base Society. International Forum of Allergy & Rhinology. 2017;8(5):631–640. doi: 10.1002/alr.22066. [DOI] [PubMed] [Google Scholar]
- 26.Chang CM, Jaw FS, Lo WC, Fang KM, Cheng PW. Three-dimensional analysis of the accuracy of optic and electromagnetic navigation systems using surface registration in live endoscopic sinus surgery. Rhinology. 2016;54(1):88–94. doi: 10.4193/Rhin15.131. [DOI] [PubMed] [Google Scholar]
- 27.Ramakrishnan VR, Kingdom TT. Does image-guided surgery reduce complications? Otolaryngol Clin N Am. 2015;48(5):851–859. doi: 10.1016/j.otc.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 28.Tabaee A. Perioperative adjuncts in endoscopic skull base surgery. Oper Tech Otolaryngol Head Neck Surg. 2011;22:200–205. doi: 10.1016/j.otot.2011.08.007. [DOI] [Google Scholar]
- 29.Elangovan C, Singh SP, Gardner P, Snyderman C, Tyler-Kabara EC, Habeych M, et al. Intraoperative neurophysiological monitoring during endoscopic endonasal surgery for pediatric skull base tumors. J Neurosurg Pediatr. 2016;17(2):147–155. doi: 10.3171/2015.7.PEDS14403. [DOI] [PubMed] [Google Scholar]
- 30.Thirumala PD, Kassam AB, Habeych M, Wichman K, Chang YF, Gardner P, et al. Somatosensory evoked potential monitoring during endoscopic endonasal approach to skull base surgery: analysis of observed changes. Neurosurgery. 2011;69(1 Suppl Operative):64–76. doi: 10.1227/NEU.0b013e31821606e4. [DOI] [PubMed] [Google Scholar]
- 31.Wannemuehler TJ, Rabbani CC, Burgeson JE, Illing EA, Walgama ES, Wu AW, Ting JY. Survey of endoscopic skull base surgery practice patterns among otolaryngologists. Laryngoscope Investig Otolaryngol. 2018;3(3):143–155. doi: 10.1002/lio2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosen SA, Getz AE, Kingdom T, Youssef AS, Ramakrishnan VR. Systematic review of the effectiveness of perioperative prophylactic antibiotics for skull base surgeries. Am J Rhinol Allergy. 2016;30(2):e10–e16. doi: 10.2500/ajra.2016.30.4298. [DOI] [PubMed] [Google Scholar]
- 33.Tien DA, Stokken JK, Recinoes PF, Woodard TD, Sindwani R. Comprehensive postoperative management after endoscopic skull base surgery. Otolaryngol Clin N Am. 2016;49(1):253–263. doi: 10.1016/j.otc.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 34.Hui C, Radbel JM. Diabetes insipidus. In: StatPearls. StatPearls Publishing; 2019. [PubMed] [Google Scholar]
- 35.Nyquist GG, Rosen MR, Friedel ME, Beahm DD, Farrell CJ, Evans JJ. Comprehensive management of the paranasal sinuses in patients undergoing endoscopic endonasal skull base surgery. World Neurosurg. 2014;82(6 Suppl):S54–S58. doi: 10.1016/j.wneu.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 36.Lange JL, Peeden E, Stringer SP. Are prophylactic systemic antibiotics necessary with nasal packing? A systematic review. Am J Rhinol Allergy. 2017;31(4):240–247. doi: 10.2500/ajra.2017.31.4454. [DOI] [PubMed] [Google Scholar]
- 37.Alexander A, Mathew J, Varghese AM, Ganesan S. Endoscopic repair of CSF fistulae: a ten year experience. J Clin Diagn Res. 2016;10(8):MC01–MC04. doi: 10.7860/JCDR/2016/18903.8390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patel MR, Stadler ME, Snyderman CR, Carrau RL, Kassam AB, Germanwala AV, Gardner P, Zanation AM. How to choose? Endoscopic skull base reconstructive options and limitations. Skull Base. 2010;20(6):397–404. doi: 10.1055/s-0030-1253573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zanation AM, Carrau RL, Snyderman CR, Germanwala AV, Gardner PA, Prevedello DM, Kassam AB. Nasoseptal flap reconstruction of high flow intraoperative cerebral spinal fluid leaks during endoscopic skull base surgery. Am J Rhinol Allergy. 2009;23(5):518–521. doi: 10.2500/ajra.2009.23.3378. [DOI] [PubMed] [Google Scholar]
- 40.D’Anza B, Tien D, Stokken JK, Recinos PF, Woodard TR, Sindwani R. Role of lumbar drains in contemporary endonasal skull base surgery: meta-analysis and systematic review. Am J Rhinol Allergy. 2016;30(6):430–435. doi: 10.2500/ajra.2016.30.4377. [DOI] [PubMed] [Google Scholar]
- 41.Zwagerman NT, Wang EW, Shin SS, Chang YF, Fernandez-Miranda JC, Snyderman CH, et al. Does lumbar drainage reduce postoperative cerebrospinal fluid leak after endoscopic endonasal skull base surgery? A prospective, randomized controlled trial. J Neurosurg. 2018;1:1–7. doi: 10.3171/2018.4.JNS172447. [DOI] [PubMed] [Google Scholar]
- 42.Eloy P, Andrews P, Poirrier AL. Postoperative care in endoscopic sinus surgery: a critical review. Curr Opin Otolaryngol Head Neck Surg. 2017;25(1):35–42. doi: 10.1097/MOO.0000000000000332. [DOI] [PubMed] [Google Scholar]
- 43.Carrau RL, Weissman JL, Janecka IP, Snyderman CH, Curtin HD, Sekhar L, Lee HS. Computerized tomography and magnetic resonance imaging following cranial base surgery. Laryngoscope. 1991;101(9):951–959. doi: 10.1288/00005537-199109000-00005. [DOI] [PubMed] [Google Scholar]
- 44.Choi DL, Reddy K, Weitzel EK, Rotenberg BW, Vescan A, Algird A, Sommer DD. Postoperative continuous positive airway pressure use and nasal saline rinses after endonasal endoscopic skull base surgery in patients with obstructive sleep apnea: a practice pattern survey. Am J Rhinol Allergy. 2019;33(1):51–55. doi: 10.1177/1945892418804987. [DOI] [PubMed] [Google Scholar]