Abstract
Purpose
Venous thromboembolism (VTE) in injured children is rare, but sequelae can be morbid and life-threatening. Recent trauma society guidelines suggesting that all children over 15 years old should receive thromboprophylaxis may result in overtreatment. We sought to evaluate the efficacy of a previously published VTE prediction algorithm and compare it to current recommendations.
Methods
Two institutional trauma registries were queried for all pediatric (age < 18 years) patients admitted from 2007 to 2018. Clinical data were applied to the algorithm and the area under the receiver operating characteristic (AUROC) curve was calculated to test algorithm efficacy.
Results
A retrospective review identified 8271 patients with 30 episodes of VTE (0.36%). The VTE prediction algorithm classified 51 (0.6%) as high risk (> 5% risk), 322 (3.9%) as moderate risk (1–5% risk) and 7898 (95.5%) as low risk (< 1% risk). AUROC was 0.93 (95% CI 0.89–0.97). In our population, prophylaxis of the ‘moderate-’ and ‘high-risk’ cohorts would outperform the sensitivity (60% vs. 53%) and specificity (96% vs. 77%) of current guidelines while anticoagulating substantially fewer patients (373 vs. 1935, p < 0.001).
Conclusion
A VTE prediction algorithm using clinical variables can identify injured children at risk for venous thromboembolic disease with more discrimination than current guidelines. Prospective studies are needed to investigate the validity of this model.
Level of evidence
III—Clinical decision rule evaluated in a single population.
Electronic supplementary material
The online version of this article (10.1007/s00383-019-04613-y) contains supplementary material, which is available to authorized users.
Keywords: Venous thromboembolism, Pediatric trauma, Thromboprophylaxis, Guidelines
Background
Morbidity from deep vein thrombosis (DVT) and its consequences, collectively referred to as venous thromboembolism (VTE), is a significant problem in trauma, but the specific risk of such events in children is poorly understood. Clear evidence has demonstrated that the high rate of DVT (40–80%) [1] and pulmonary embolism (PE) (1–2%) [2] in the adult trauma population are associated with significant rates of mortality, as high as 18–50% in those with PE [3, 4]. While the incidence of VTE is substantially lower in the pediatric population—0.2–0.4% of all pediatric trauma patients and 6–10% of those requiring ICU care [5, 6]—the potential morbidity of such events remains substantial. Sequelae of VTE in children include prolonged hospitalization, higher medical costs, post-thrombotic syndrome, paradoxical embolic stroke and death [7–10].
Conversely, prophylaxis for the prevention of VTE is not without its own risks. Anticoagulation in any form increases the risk of bleeding. This is especially problematic given the known increased risk of bleeding in the peritraumatic period [11]. Fortunately, recommendations to address VTE prophylaxis in children have recently been published as a joint practice management guideline from the Pediatric Trauma Society and the Eastern Association for the Surgery of Trauma (PTS/EAST) [12]. While these guidelines provide the first formal recommendations to address this clinical dilemma, implementation has been variable due to their basis in ‘very low’ level evidence and logistical challenges arising from reliance on retrospectively assigned Injury Severity Score (ISS). To bridge this gap, a group developed an algorithm [13] to predict VTE in pediatric trauma, derived from risk factors that are identified at admission or shortly thereafter. This VTE prediction tool was developed from 536,423 pediatric trauma patients sourced from the National Trauma Data Bank (NTDB) and was internally validated. Subsequently, a similar analysis of the NTDB was independently performed [14] using the same risk factors for VTE in children, confirming the utility of this approach. Despite internal validation and independent agreement, the VTE prediction algorithm has yet to be broadly implemented in clinical practice, likely due to the limitations of conclusions derived solely from large database studies.
In this study, we aim to evaluate the efficacy of the VTE prediction algorithm through retrospective application in two locally maintained institutional trauma registries with the ability for data verification. Specifically, we review traumatically injured children who were admitted to two American College of Surgeons (ACS) Verified Level I Pediatric Trauma Centers over a 12-year period. Additionally, we compare the efficacy of the VTE prediction algorithm against PTS/EAST prophylaxis recommendations. We hypothesize that the VTE prediction algorithm will more effectively and efficiently risk stratify patients compared to current guidelines.
Methods
Data source and patient selection
After institutional review board approval, two institutional trauma registries were queried for pediatric (age < 18 years) trauma admissions during the study period (January 2007–June 2018). Registries were retrospectively reviewed for clinical variables associated with VTE risk, discharge ISS, pharmacologic thromboprophylaxis use and adverse events including mortality. After identification of all episodes of VTE, the registry data for all cases were validated with dedicated data abstraction directly from the electronic medical record. All traumatically injured children admitted to the two medical centers during the study period were included; there were no exclusion criteria.
VTE prediction algorithm development and application
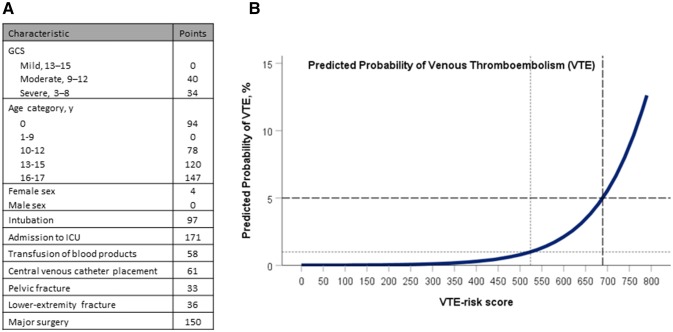
Development of a ten-point VTE risk tool was described previously by Connelly et al. [13], who developed multiple mixed-effects logistic regression models of varying complexity from a data set of 536,423 pediatric trauma patients sourced from the NTDB 2007–2012. This model (Fig. 1) was applied to our established population, and the VTE risk score for each patient was calculated. Patient GCS, age, sex, intubation, ICU admission, blood product transfusion, CVL placement, pelvic or lower extremity fracture, or major surgical procedure each contributed to their VTE risk score. Pairwise deletion was employed for missing values. Patients were categorized as low, moderate, or high risk for VTE, based on the previously described risk category score cutoffs.
Fig. 1.
Venous thromboembolism (VTE) prediction tool in pediatric trauma patients. A scoring system to predict VTE in pediatric trauma patients was previously developed from the National Trauma Data Bank and reported in Connelly et al. [13], recreated with permission above. a VTE prediction model with assigned point value to each clinical characteristic. The cumulative VTE risk score is tabulated and applied to the prediction curve. b VTE risk scores of 0–523 corresponds with low risk (< 1%), scores of 524–688 correspond with moderate risk (1–5%), and scores of 689–797 correspond to high risk (> 5%) of VTE. Cutoff values for the above risk categories are identified by dashed lines. GCS Glasgow Coma Score, Y year, ICU intensive care unit
Definitions, outcomes and procedures
Both institutional trauma registries were maintained on the TraumaOne platform (Lancet Technology, Inc., Boston, MA, USA) and collected demographic, injury and clinical data on all admitted trauma patients during the course of their hospitalization. Each institution contributed data to the NTDB during the study. Inclusion criteria into both trauma registries were defined by the National Trauma Data Standard (NTDS) patient inclusion criteria [15]. Diagnoses and procedures were defined according to specific listed criteria or according to their International Classification of Diseases, Ninth or Tenth Revision, Clinical Modification (ICD-9/10) code (eSupplemental Appendix 1). The first recorded hospital GCS was used in the risk score calculation. Intubation was determined according to ICD-9/10 codes corresponding to endotracheal intubation or the presence of at least one ventilator day. Transfusions were similarly determined based on ICD-9/10 codes corresponding to blood product transfusion or the specific reporting of product transfusion in the registry. Admission to the ICU was determined based on registry-defined length of stay in the ICU or reported disposition from the emergency department to the ICU. Central venous line placement, presence of a pelvic or lower extremity fracture and major surgical procedures were determined based on ICD-9/10 codes reported in the registries. Central venous lines contributing to the VTE risk score include upper and lower extremity centrally inserted catheters as well as peripherally inserted central venous catheters. Major surgery was inclusive of neurologic, thoracic, cardiovascular, hematologic, spleen, gastrointestinal, genitourinary, orthopedic or spine procedures and is enumerated in eSupplemental Appendix 1. Venous thromboembolism was defined according to the NTDS definitions [15] and determined based on registry defined reporting or an ICD-9/10 code corresponding to deep vein thrombosis or pulmonary embolus. During the period of the study, each trauma center provided thromboprophylaxis based on admitting service; children greater than 12 years or 14 years, depending on the facility, were admitted to the adult trauma service and managed according to the adult VTE screening and prophylaxis protocol, all other children were admitted to the pediatric trauma service (ward or ICU) and received no standard management. The pediatric trauma services did not routinely provide VTE screening or prophylaxis to any patient, whereas the adult trauma services employed both ultrasound screening and pharmacologic prophylaxis to institutionally defined ‘high-risk’ patients. ‘High-risk’ patients on the adult trauma service were categorized as such if they suffered traumatic brain injury, spinal cord injury, a pelvic fracture, multiple or open extremity fractures, multiple rib fractures or flail chest, or required prolonged mechanical ventilation, multiple blood product transfusions, or femoral vein catheterization. These patients wore bilateral lower extremity sequential compression devices, began prophylactic anticoagulation with enoxaparin 40 mg subcutaneous injection daily, and received bilateral lower extremity screen venous duplex studies on hospital day 3 and every 7 days thereafter.
The primary outcomes of interest in this study were the VTE risk score, incidence of VTE and predictive ability of the model. Secondary outcomes include the number of children who would receive thromboprophylaxis and mortality.
Statistical analysis
Descriptive statistics were tabulated. Non-parametric data was reported as medians with inter-quartile ranges (IQR). Continuous variables were not normally distributed, and therefore differences between VTE groups were compared using a Wilcoxon rank-sum test. Categorical variables were analyzed with a Chi-square test for independence. Risk prediction scores were calculated for all participants, and sensitivity and specificity were calculated. Receiver operating characteristics (ROC) curves were constructed, and the area under the ROC curve (AUROC) was used to evaluate the ability of the VTE risk prediction score to discriminate between patients who did and did not have VTE. Significance was defined as p < 0.05. Variables were defined and coded using SAS 9.4 (SAS Inc, Cary, NC, USA) and analyses were performed using IBM SPSS Statistics for Windows, version 25 (IBM Corp., Armonk, NY, USA).
Predictive accuracy and potential patient impact of our VTE algorithm were compared to current PTS/EAST guidelines in a real-world analysis, only employing pieces of the guidelines that could be prospectively implemented. We compared the impact of these recommendations to the impact called for by our VTE algorithm for moderate- and high-risk children. An additional analysis to define alternative VTE risk score cutoffs for intervention was performed. A Youden’s analysis was performed to maximize the sensitivity and specificity of the prognostic test and a similar analysis was used to identify a cutoff that maximizes positive predictive value.
Results
Demographic data
A retrospective review identified 8271 patients with 30 episodes (0.36%) of VTE during the study period. There were 27 (0.33%) patients with DVT only, 2 (0.02%) with PE only and 1 (0.01%) patient with both DVT and PE. Data regarding prophylaxis usage could be ascertained for 2715 patients (32.8%). Of these, 279 (10.3%) patients received prophylactic anticoagulation. Median time to anticoagulation was 2 days (1.0–3.0, IQR, n = 266) and median time to VTE diagnosis was 5.5 days (4.0–8.75, IQR, n = 14). Of those patients diagnosed with VTE where prophylaxis data was available (n = 14), 43% (n = 6) received prophylactic anticoagulation prior to VTE diagnosis on extremity venous duplex ultrasound.
Children who were greater than 13 years of age accounted for 38.9% (n = 3221) of the entire cohort. Children with VTE were older, more severely injured, with lower GCS on admission and more likely to have pelvic or lower extremity fractures than those without VTE (Table 1). Similarly, children with VTE were more likely to be admitted to the ICU, intubated, undergo CVL placement, receive blood product transfusions, and undergo major surgery. However, there was no observed statistical difference in sex or mortality between those with and without VTE.
Table 1.
Patient characteristics
| Total (%) N = 8271 |
No VTE N = 8241 |
VTE N = 30 |
p | |
|---|---|---|---|---|
| Age, median (IQR), years | 10.0 (4.0–15.0) | 10.0 (4.0–15.0) | 15.1 (11.6–16.7) | 0.001 |
| Female | 2817 (34.2) | 2807 (34.2) | 10 (33.3) | 0.923 |
| ISS, median (IQR) | 9 (4–14) | 9 (4–14) | 24.5 (14–29) | < 0.001 |
| GCS, median (IQR) | 15 (15–15) | 15 (15–15) | 15 (3–15) | < 0.001 |
| Intubation | 901 (10.9) | 886 (10.8) | 15 (50.0) | < 0.001 |
| Admission to ICU | 2707 (32.7) | 2684 (32.6) | 23 (76.7) | < 0.001 |
| Transfusion of blood products | 398 (4.8) | 378 (4.6) | 20 (66.7) | < 0.001 |
| Central venous line placement | 1408 (17.0) | 1386 (16.8) | 22 (73.3) | < 0.001 |
| Pelvic fracture | 257 (3.1) | 251 (3.0) | 6 (20.0) | < 0.001 |
| Lower extremity fracture | 1079 (13.0) | 1070 (13.0) | 9 (30.0) | 0.006 |
| Major surgery | 1490 (18.0) | 1463 (17.8) | 27 (90.0) | < 0.001 |
| DVT | 28 (0.3) | – | 28 (93.3) | – |
| PE | 3 (0.04) | – | 3 (10.0) | – |
| Mortality | 124 (1.5) | 123 (1.5) | 1 (3.3) | 0.408 |
Continuous variables are reported with medians and inter-quartile ranges while dichotomous variables are reported as counts with percentages. Significance is considered at p < 0.05
VTE venous thromboembolism, IQR inter-quartile range, ISS Injury Severity Score, GCS Glasgow Coma Score, ICU intensive care unit, DVT deep vein thrombosis, PE pulmonary embolism
Outcomes
In our retrospective population, the VTE prediction algorithm classified 51 (0.6%) patients as high risk, 322 (3.9%) patients as moderate risk and 7898 (95.5%) patients as low risk (Table 2). Of the 30 patients who were diagnosed with VTE, the prediction tool classified 18 (60%) of them as moderate or high risk. The false-positive rate of moderate- or high-risk classification was 4.3% and the false-negative rate was 40.0%. Patients who were classified as moderate or high risk for VTE were older, more severely injured, with lower GCS on admission, more likely to have pelvic or lower extremity fractures and more likely to be diagnosed with DVT than those who were classified as low risk. Similarly, moderate- or high-risk patients were more likely to be admitted to the ICU, intubated, receive a blood product transfusion, have a CVL placed, undergo major surgery, or die as a result of their injuries. Statistically significant differences in sex or incidence of PE were not observed between the low- or moderate-/high-risk groups.
Table 2.
Venous thromboembolism (VTE) prediction algorithm results in a retrospective population
| Low risk (%) N = 7898 |
Moderate or high risk (%) N = 373 |
p | |
|---|---|---|---|
| Age, median (IQR), years | 10.0 (3.8–15.0) | 15.1 (11.4–16.8) | < 0.001 |
| Female | 2701 (34.3) | 116 (31.1) | 0.201 |
| ISS, median (IQR) | 8 (4–13) | 26 (17–35) | < 0.001 |
| GCS, median (IQR) | 15 (15–15) | 7 (3–15) | < 0.001 |
| Intubation | 583 (7.4) | 318 (85.3) | < 0.001 |
| Admission to ICU | 2334 (29.6) | 373 (100) | < 0.001 |
| Transfusion of blood products | 196 (2.5) | 202 (54.2) | < 0.001 |
| Central venous line placement | 1178 (14.9) | 230 (61.7) | < 0.001 |
| Pelvic fracture | 200 (2.5) | 57 (15.3) | < 0.001 |
| Lower extremity fracture | 983 (12.4) | 96 (25.7) | < 0.001 |
| Major surgery | 1139 (14.4) | 351 (94.1) | < 0.001 |
| DVT | 10 (0.1) | 18 (4.8) | < 0.001 |
| PE | 3 (0.04) | 0 | 0.707 |
| Mortality | 93 (1.2) | 31 (8.3) | < 0.001 |
Patient characteristics and clinical outcomes reported in those categorized as low or moderate/high risk for venous thromboembolic disease according to the VTE prediction algorithm. Significance is considered at p < 0.05
IQR inter-quartile range, ISS Injury Severity Score, GCS Glasgow Coma Score, ICU intensive care unit, DVT deep vein thrombosis, PE pulmonary embolism
Venous thromboembolism risk scores were used to construct a ROC curve. The AUROC was then calculated to determine the discriminatory ability of the algorithm (Fig. 2). The VTE prediction algorithm demonstrated excellent fidelity to differentiate between those with and without VTE with an AUROC of 0.93 (95% CI 0.89–0.97). Calculated AUROC for a prospective application of current PTS/EAST guidelines (age greater than 15 years) was 0.63 (95% CI 0.53–0.74), demonstrating fair discriminatory ability.
Fig. 2.
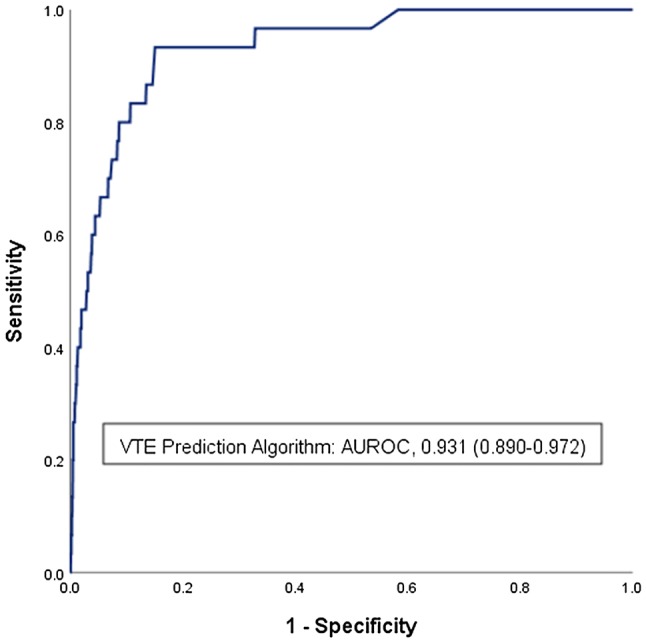
Receiver operating characteristic curve for the VTE prediction algorithm. Retrospective application of the VTE prediction algorithm was performed in a population of 8271 children collected from institutional trauma registries. The VTE risk score was calculated and plotted against the outcome of VTE, the area under the receiver operating characteristic (AUROC) curve was calculated and reported with a 95% confidence interval. An AUROC of 0.931 demonstrates excellent fidelity of the model to predict VTE. VTE: venous thromboembolism. AUROC area under the receiver operating characteristic curve
In a real-world analysis of our population, prophylaxis of the moderate- and high-risk cohorts would outperform the sensitivity (60% vs. 53%) and specificity (96% vs. 77%) of current guidelines while anticoagulating substantially fewer patients (373 vs. 1935, p < 0.001). Additional VTE risk score cutoffs for hypothetical prophylactic intervention were calculated based on an optimized sensitivity and specificity or an optimized positive predictive value (Table 3).
Table 3.
Predictive statistics
| Age > 15 years (PTS/EAST) | VTE prediction model: moderate or high risk | VTE prediction model: maximized Sen/Sp | VTE prediction model: maximized PPV | |
|---|---|---|---|---|
| Sensitivity, % (95% CI) | 53.3 (34.3–71.7) | 60.0 (40.6–77.3) | 93.3 (77.9–99.2) | 26.7 (12.3–45.9) |
| Specificity, % (95% CI) | 76.7 (75.8–77.6) | 95.7 (95.2–96.1) | 85.0 (84.2–85.7) | 99.5 (99.3–99.6) |
| Positive predictive value, % (95% CI) | 0.83 (0.6–1.2) | 4.8 (3.6–6.5) | 2.2 (2.0–2.5) | 15.1 (8.4–25.6) |
| Negative predictive value, % (95% CI) | 99.8 (99.7–99.9) | 99.9 (99.8–99.9) | 100.0 (99.9–100.0) | 99.7 (99.7–99.8) |
| Accuracy, % (95% CI) | 76.6 (75.7–77.5) | 95.6 (95.1–96.0) | 85.0 (84.2–85.8) | 99.2 (99.0–99.4) |
| False-positive rate (%) | 23.3 | 4.3 | 15 | 0.5 |
| False-negative rate (%) | 46.7 | 40 | 6.7 | 73.3 |
| Suggested prophylaxis cohort (%) | 23.4 | 4.5 | 15.3 | 0.6 |
Diagnostic statistics and suggested prophylaxis intervention size are reported for thromboprophylaxis regimens based on current society guidelines or moderate- or high-risk classification by the VTE prediction algorithm. Additionally, these statistics are reported for two alternative analyses of the VTE prediction algorithm where either sensitivity/specificity are maximized (VTE risk score: 332) or positive predictive value is maximized (VTE risk score: 687)
PTS/EAST Pediatric Trauma Society/Eastern Association of the Surgery for Trauma management guidelines, VTE venous thromboembolism, Sen/sp sensitivity/specificity, PPV positive predictive value, 95% CI 95% confidence interval
Discussion
In this retrospective pilot study, we provide dual-center evaluation of our VTE prediction algorithm through an analysis of locally maintained, institutional trauma registries with excellent model fidelity as demonstrated by an AUROC of 0.93. Additionally, in a direct comparison of the VTE prediction algorithm to the current PTS/EAST guidelines, we show that not only would our algorithm outperform current recommendations, but it would do so while also anticoagulating 19% fewer children.
The reported rising incidence of venous thromboembolism in injured children [7] has garnered significant scientific debate. While the overall incidence of VTE in pediatric trauma is relatively rare and variably reported [16], it has become increasingly clear that older children and adolescents have risk approaching that of their adult counterparts (estimated incidence in adolescents of 1.0–5.1%) [17]. Regardless, a void of high-level evidence in the pediatric surgical literature remains. In the only reported prospective study of VTE in critically injured children, Hanson et al. [18] describe the successful implementation of a clinical guideline to reduce the incidence of venous thromboembolic disease suggesting the possible efficacy of a screening and prophylaxis regimen. However, due to the non-randomized quality improvement nature of this initiative—including the implementation of selective ultrasound surveillance, mechanical and pharmacologic prophylaxis—the efficacy of any individual intervention is difficult to determine. Recently, following an exhaustive systematic review on the topic, a joint practice management guideline published by PTS/EAST recommended “pharmacologic prophylaxis be considered for children older than 15 years old and in younger postpubertal children with Injury Severity Score (ISS) greater than 25”. Unfortunately, due to the inherent limitations in studying this population, these recommendations were developed from ‘very low’ level evidence and reliant upon calculation of the ISS for implementation, making them difficult to employ clinically.
In the wake of this void, prediction algorithms [13, 14] were developed to help provide clinically actionable information for children at risk of VTE. Our analysis demonstrates strong agreement with these prior studies, all of which reported an AUROC of 0.90 or greater. Nevertheless, these algorithms have yet to be largely adopted, likely due to the criticisms surrounding large database studies. In the past, the NTDB has been criticized for underreporting complications [19] and is noted for having a considerable amount of missing data [20]. To address these concerns, our retrospective study demonstrates the efficacy of the VTE prediction algorithm in an external cohort of more closely maintained registries from two Level I trauma centers, each of whom separately report complications. This practice suggests higher fidelity of data in these registries over historical norms [20, 21]. Notably, our reported incidence of VTE (0.36%) is nearly double that seen in a comparable NTDB population, suggesting better data fidelity in this subset.
We performed an additional analysis identifying alternative cutoffs for prophylactic intervention in an effort to demonstrate the utility of our algorithm. While a sensitivity of 60% is considerably low for a potential screening test, various VTE risk score cutoffs can be determined to optimize the value of the tool and localize the ideal point of prophylactic intervention. For instance, the risk of major bleeding following pharmacologic thromboprophylaxis in children has been reported at 2.3% [22]. If this is the most significant counterpoint to the use of pharmacologic prophylaxis, a VTE risk score cutoff corresponding to this risk could be set as the point of prophylaxis. We offer two additional possible points for intervention: (1) maximizing sensitivity and specificity—an ideal screening test, but sacrificing a decreased positive predictive value, or (2) maximizing positive predictive value—identifying those who are the highest risk for VTE, but sacrificing sensitivity. Currently, prospective implementation of the VTE risk tool is ongoing at our institution using the moderate- or high-risk cutoffs (VTE risk score > 523) published in the index study [13] as an inflection point for prophylactic intervention. Use of the moderate-risk cutoff was chosen for future implementation of VTE risk score at our institution, because the positive predictive value at that cutoff is approximately 5%, which approximates the risk of complications with prophylactic anticoagulation in this population.
The use of pharmacologic prophylaxis in our population confounds some of our results. Notably, in patients where data are available, approximately 10% of all injured children received pharmacologic prophylaxis. Among these, the incidence of VTE was 2%, considerably higher than the incidence within the entire cohort. Presumably, this is a highly selected cohort, predominantly based on the criteria of age and clinical risk factors. Nevertheless, as prophylaxis is used to prevent future occurrences, we can assume that at least some proportion of those who received prophylaxis were prevented from developing VTE leading to an underrepresentation of incidence in our cohort. The exact proportion of prevented occurrences, however, is debatable. Multiple randomized control trials [23] have failed to demonstrate effectiveness of pharmacologic prophylaxis against thrombosis in the pediatric population. Data in adults however are clear, demonstrating that low-molecular-weight heparin (LMWH) reduces clinically significant VTE by 70% [24]. If we assume that the effectiveness of pharmacologic prophylaxis in children is somewhere in the middle of these, then we might suspect that at the most, prophylaxis provides 35% relative risk reduction in VTE meaning that 3.1% (rather than 2%) of those who received prophylaxis would develop VTE, meaning a total of 26 patients rather than 16, or 10 additional patients would have VTE. Ultimately, inclusion of patients receiving pharmacologic prophylaxis in our study might have led to underestimation of the incidence of VTE by 30% at most.
We recognize that there are a number of limitations with this study notably a relatively small number of patients diagnosed with VTE making this study insufficiently powered to completely evaluate the efficacy of a ten-variable prediction model. Furthermore, given the low incidence of VTE in the population, the analysis of AUROC, as we performed, tends to overestimate the efficacy of such diagnostic tests. However, despite the small sample size, we demonstrate a number of important findings that should provoke future investigation. Additionally, since both institutions in this study contributed data to the NTDB during the study period, it is likely that some of the patients evaluated in the application study were used to derive the model. This could be viewed as a circular analysis but because both institutions were not ACS-verified pediatric trauma centers until 2015, pediatric data used to create the model from these centers were likely incomplete. Our analysis, with electronic medical record data abstraction for all cases, additionally verified all of the case-level data in the registries, reflecting a more real-world application of the model. Specific VTE screening protocols by each adult trauma service create an additional source of confounding, as there is significant overlap in the ‘high-risk’ criteria at each institution and the clinical characteristics used to support our model, leading to possible artificial inflation of the AUROC. Another limitation of our study is the use of institutional trauma registries to gather data. Trauma registries are known for surveillance bias, the inability to temporally associate risk factors and outcomes, large proportion of missing data and for significant error rates, from 0.4–5.2% [25] in a recent study. These limitations likely contribute to both underreporting of risk factors as well as incidence of VTE, limiting the application of our analysis. However, given the low incidence of pediatric traumatic VTE, a retrospective review of institutional trauma registries, with selected case validation through dedicated data abstraction, represents the best forum for analysis of this data, prior to a safe prospective validation study. Lastly, as the institutional usage of anticoagulation in our population has varied based on the patients’ age and a sizable portion of this data is missing (66%), the true incidence of VTE in this population is likely masked by a variable use of prophylaxis.
Conclusions
Quantifying the risk of VTE in injured children has been difficult, but recent analyses have proposed a VTE prediction algorithm using limited, easily collected, clinical variables that can identify those at significant risk. We provide the first application of our VTE prediction algorithm to a retrospective institutionally controlled database, demonstrating its potential utility in identifying moderate- and high-risk injured children. This tool has the unique potential to provide clinically meaningful information to guide decision making regarding thromboprophylaxis in pediatric trauma. Additionally, we demonstrate the potential value of this algorithm over current society guidelines while also theoretically exposing substantially fewer children to anticoagulation. Prior to implementation, larger studies are needed, perhaps through a multicenter retrospective review of more regional data where the criticisms of large database studies can be addressed through case validation. If successful, this algorithm could be used to risk stratify pediatric trauma patients and ultimately provide evidence-based thromboprophylaxis guidelines.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- VTE
Venous thromboembolism
- DVT
Deep vein thrombosis
- PE
Pulmonary embolism
- ISS
Injury Severity Score
- GCS
Glasgow Coma Score
- ICU
Intensive care unit
- CVL
Central venous line
- NTDB
National Trauma Data Bank
- ICD-9/10
International Classification of Diseases, Ninth or Tenth Revision, Clinical Modification
Author contributions
All authors attest that they meet the current ICMJE criteria for Authorship. Specific contributions are listed here: study conception and design: AC, ED, CC, MS, MJ. Data acquisition: AC, LM, KD, MS, MJ. Analysis and data interpretation: AC, ED, SL, KH, EB, SK, NH, MS, MJ. Drafting of the manuscript: AC, ED, SL, NH, SK, MJ. Critical revision: AC, ED, SL, KH, EB, CC, LM, KD, NH, SK, MS, MJ.
Funding
This research did not receive any specific funding or grant support from agencies in the public, commercial, or not-for-profit sectors.
Compliance with ethical standards
Conflict of interest
The following authors AC, ED, SL, KH, EB, CC, LM, KD, NH, SK, MS, MJ have no financial disclosures.
Patient consent
This study was exempted from obtaining individual patient consent as approved by our Institutional Review Board. This report does not contain any personal information that could lead to identification of any patients. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Aaron J. Cunningham, Email: cunninaa@ohsu.edu
Elizabeth Dewey, Email: deweye@ohsu.edu.
Saunders Lin, Email: lasau@ohsu.edu.
Kristina M. Haley, Email: haley@ohsu.edu
Erin C. Burns, Email: comer@ohsu.edu
Christopher R. Connelly, Email: conchris@med.umich.edu
Lori Moss, Email: mossl@ohsu.edu.
Katie Downie, Email: kdownie@lhs.org.
Nicholas A. Hamilton, Email: hamilnic@ohsu.edu
Sanjay Krishnaswami, Email: krishnas@ohsu.edu.
Martin A. Schreiber, Email: schreibm@ohsu.edu
References
- 1.Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR, et al. Prevention of venous thromboembolism. Chest. 2008;133(6):381S–453S. doi: 10.1378/chest.08-0656. [DOI] [PubMed] [Google Scholar]
- 2.Knudson MM, Ikossi DG. Venous thromboembolism after trauma. Curr Opin Crit Care. 2004;10(6):539–548. doi: 10.1097/01.ccx.0000144941.09650.9f. [DOI] [PubMed] [Google Scholar]
- 3.Geerts WH, Code KI, Jay RM, Chen E, Szalai JP. A prospective study of venous thromboembolism after major trauma. N Engl J Med. 1994;331(24):1601–1606. doi: 10.1056/NEJM199412153312401. [DOI] [PubMed] [Google Scholar]
- 4.Knudson MM, Ikossi DG, Khaw L, Morabito D, Speetzen LS. Thromboembolism after trauma: an analysis of 1602 episodes from the American College of Surgeons National Trauma Data Bank. Ann Surg. 2004;240(3):490–498. doi: 10.1097/01.sla.0000137138.40116.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanson SJ, Punzalan RC, Greenup RA, Liu H, Sato TT, Havens PL. Incidence and risk factors for venous thromboembolism in critically ill children after trauma. J Trauma Acute Care Surg. 2010;68(1):52–56. doi: 10.1097/TA.0b013e3181a74652. [DOI] [PubMed] [Google Scholar]
- 6.Mahajerin A, Branchford BR, Amankwah EK, Raffini L, Chalmers E, van Ommen CH, et al. Hospital-associated venous thromboembolism in pediatrics: a systematic review and meta-analysis of risk factors and risk-assessment models. Haematologica. 2015;100(8):1045–1050. doi: 10.3324/haematol.2015.123455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raffini L, Huang YS, Witmer C, Feudtner C. Dramatic increase in venous thromboembolism in children’s hospitals in the United States from 2001 to 2007. Pediatrics. 2009;124(4):1001–1008. doi: 10.1542/peds.2009-0768. [DOI] [PubMed] [Google Scholar]
- 8.Candrilli SD, Balkrishnan R, O’Brien SH. Effect of injury severity on the incidence and utilization-related outcomes of venous thromboembolism in pediatric trauma inpatients. Pediatr Crit Care Med. 2009;10(5):554–557. doi: 10.1097/PCC.0b013e3181a705d3. [DOI] [PubMed] [Google Scholar]
- 9.Engel E, Albisetti M, Brandao LR, Amankwah E, Nguyen A, Goldenberg NA, et al. Predictors of post-thrombotic syndrome (PTS) in pediatric thrombosis: a systematic review and meta-analysis of the literature. Blood. 2018;132(Suppl 1):3806–3806. doi: 10.1182/blood-2018-99-114999. [DOI] [PubMed] [Google Scholar]
- 10.Fang Z, Tang L, Zhou S. Ischemic stroke caused by paradoxical embolism after an unsuccessful transcatheter atrial septal defect closure procedure: a word of caution. Pediatr Cardiol. 2012;33(2):366–369. doi: 10.1007/s00246-011-0150-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brohi K, Cohen MJ, Ganter MT, Schultz MJ, Levi M, Mackersie RC, et al. Acute coagulopathy of trauma: hypoperfusion induces systemic anticoagulation and hyperfibrinolysis. J Trauma Acute Care Surg. 2008;64(5):1211–1217. doi: 10.1097/TA.0b013e318169cd3c. [DOI] [PubMed] [Google Scholar]
- 12.Mahajerin A, Petty JK, Hanson SJ, Thompson AJ, O’Brien SH, Streck CJ, et al. Prophylaxis against venous thromboembolism in pediatric trauma: a practice management guideline from the Eastern Association for the Surgery of Trauma and the Pediatric Trauma Society. J Trauma Acute Care Surg. 2017;82(3):627–636. doi: 10.1097/TA.0000000000001359. [DOI] [PubMed] [Google Scholar]
- 13.Connelly CR, Laird A, Barton JS, Fischer PE, Krishnaswami S, Schreiber MA, et al. A clinical tool for the prediction of venous thromboembolism in pediatric trauma patients. JAMA Surg. 2016;151(1):50–57. doi: 10.1001/jamasurg.2015.2670. [DOI] [PubMed] [Google Scholar]
- 14.Yen J, Van Arendonk KJ, Streiff MB, McNamara L, Stewart FD, Conner KG, et al. Risk factors for venous thromboembolism in pediatric trauma patients and validation of a novel scoring system: the risk of clots in kids with trauma score. Pediatr Crit Care Med. 2016;17(5):391–399. doi: 10.1097/PCC.0000000000000699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American College of Surgeons. Committee on Trauma. National Trauma Data Standard: Data Dictionary; 2016.
- 16.Petty JK. Venous thromboembolism prophylaxis in the pediatric trauma patient. Semin Pediatr Surg. 2017;26(1):14–20. doi: 10.1053/j.sempedsurg.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Liras IN, Rahbar E, Harting MT, Holcomb JB, Cotton BA. When children become adults and adults become most hypercoagulable after trauma: An assessment of admission hypercoagulability by rapid thrombelastography and venous thromboembolic risk. J Trauma Acute Care Surg. 2016;80(5):778–782. doi: 10.1097/TA.0000000000000985. [DOI] [PubMed] [Google Scholar]
- 18.Hanson SJ, Punzalan RC, Arca MJ, Simpson P, Christensen MA, Hanson SK, et al. Effectiveness of clinical guidelines for deep vein thrombosis prophylaxis in reducing the incidence of venous thromboembolism in critically ill children after trauma. J Trauma Acute Care Surg. 2012;72(5):1292–1297. doi: 10.1097/TA.0b013e31824964d1. [DOI] [PubMed] [Google Scholar]
- 19.Kardooni S, Haut ER, Chang DC, Pierce CA, Efron DT, Haider AH, et al. Hazards of benchmarking complications with the national trauma data bank: numerators in search of denominators. J Trauma Inj Infect Crit Care. 2008;64(2):273–279. doi: 10.1097/TA.0b013e31816335ae. [DOI] [PubMed] [Google Scholar]
- 20.Fransman R, Kent AJ, Haut ER, Reema Kar A, Sakran JV, Stevens K, et al. Facility disparities in reporting comorbidities to the National Trauma Data Bank. Am J Surg. 2018;216(3):401–406. doi: 10.1016/j.amjsurg.2018.01.031. [DOI] [PubMed] [Google Scholar]
- 21.Robles AJ, Conroy AS, Cohen MJ, Callcut RA. Is it time to measure complications from the National Trauma Data Bank? A longitudinal analysis of recent reporting trends. J Trauma Acute Care Surg. 2019;86(2):282–288. doi: 10.1097/TA.0000000000002133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bidlingmaier C, Kenet G, Kurnik K, Mathew P, Manner D, Mitchell L, et al. Safety and efficacy of low molecular weight heparins in children: a systematic review of the literature and meta-analysis of single-arm studies. Semin Thromb Hemost. 2011;37(07):814–825. doi: 10.1055/s-0031-1297173. [DOI] [PubMed] [Google Scholar]
- 23.Vidal E, Sharathkumar A, Glover J, Faustino EVS. Central venous catheter-related thrombosis and thromboprophylaxis in children: a systematic review and meta-analysis. J Thromb Haemost. 2014;12(7):1096–1109. doi: 10.1111/jth.12598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gould MK, Garcia DA, Wren SM, Karanicolas PJ, Arcelus JI, Heit JA, et al. Prevention of VTE in nonorthopedic surgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e227S–e277S. doi: 10.1378/chest.11-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dente CJ, Ashley DW, Dunne JR, Henderson V, Ferdinand C, Renz B, et al. Heterogeneity in trauma registry data quality: implications for regional and national performance improvement in trauma. J Am Coll Surg. 2016;222(3):288–295. doi: 10.1016/j.jamcollsurg.2015.11.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.