Abstract
Cold atmospheric plasma sources are emerging as new potent tools in different fields of medicine, from oncology to dermatology. Psoriasis is a chronic inflammatory disease characterized by the presence of itchy red plaque on skin, known as psoriatic plaques. In this case report, we evaluate the effectiveness of a cold atmospheric plasma treatment on a psoriatic plaque on the hand of a 20-year-old woman. Two quick applications of the procedure led to a complete disappearance of the cutaneous lesion in 14 days. The results of this case show a potential application of this new technique in the dermatological field, as palmo-plantar psoriasis is usually resistant to traditional treatments. A clinical trial would be necessary in order to evaluate the real effectiveness of this plasma.
Keywords: Psoriasis, cold atmospheric plasma, plasma tool, case report, innovative treatment, dermatology
Introduction
Psoriasis is a chronic autoimmune disease characterized by red and scaly patches on the skin often associated with itching. Symptoms are most commonly seen on the scalp, elbows, knees, hands, and feet, but can also affect areas such as nails and soft genital tissues.1 Current therapies are limited and include topical drugs, phototherapy, and systemic therapy. Other treatments, such as topical oxygen therapy, have also been proposed.2 However, none of these has proved to solve the disease, without mentioning the considerable side effects of the most effective therapies, such as biological drugs and immunosuppressants.3
Plasma medicine is an emerging field studying the use of low temperature plasmas for different biomedical applications. A portable cold atmospheric plasma (CAP), named plasma coagulation controller (PCC), has been recently developed, and it has been tested for accelerating blood coagulation and disinfection.4,5 On PCC, repetitive high voltage pulses are applied on two close electrodes separated by dielectric layers (dielectric barrier discharge scheme). Plasma is formed when a neutral gas (helium in this case) flows in the duct between the electrodes (Figure 1). Due to the short duration of each pulse (<1 μs), PCC produces a cold (similar to room temperature) stable plasma jet that can be directly applied on the substrate to be treated without any uncomfortable feeling for patients. Low-power UV radiation and different reactive oxygen and nitrogen species (RONS) are also produced upon the plasma formation and contribute to the therapeutic PCC action.
Figure 1.
Plasma tool schematic representation: a schematic representation of PCC instrument used for the treatment.
Scientific evidence, still preliminary and limited, has shown how plasma instruments, including PCC, are able to increase blood coagulation, activate fibroblasts, and have an antibacterial effect; suggesting an enormous potential for these tools in wounds healing as well as dermatological diseases treatment.6,7
Case presentation
A 20-year-old female patient showing itchy psoriasis plaques on a small area (about 5%, 4 cm2) of the right hand diagnosed by an expert dermatologist came to our unit (U.O. of Dermatology, AOU “Mater Domini” of Catanzaro) for clinical evaluation; psoriasis was present since 2016 on elbows, knees, and hands, and it was treated with various topical ointments based on vitamin D derivatives, corticosteroids, and salicylic acids. The manifestation on the left hand was not responsive to topical treatment. The Psoriasis Area Severity Index of the patient was low (3.4).8 The patient scored itch gravity before treatment with a 6 out of 10 on a Visual Analogue Scale.9 After informed written consent, the patient was enrolled for the study and treated with PCC plasma source. The treatment was applied for 30″ only in the left-hand psoriatic plaque at standard conditions (voltage of 7 kV; frequency of 5 kHz; distance of 1 cm) on day 0 and on day 3; the effective applied power on the substrate, measured on the bench, is of the order of tens of mW.5
A picture has been taken right before the PCC application, and 3, 7, and 14 days after treatment (Figure 2). The patient did not use any topical or systemic medication for 4 weeks before the beginning of the treatment.
Figure 2.
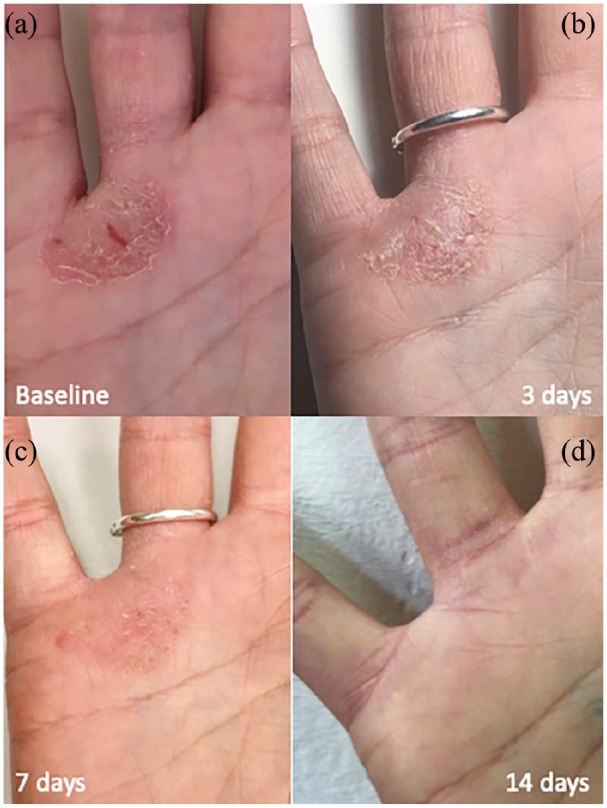
Healing progress: (a) area presenting psoriasis plaque prior treatments, (b) same area at 3 days from the treatment, (c) same area at 7 days from the first treatment, and (d) same area 14 days post-treatment.
The patient came on day 3, 7, and 14 to check the healing progress. Clinically, a gradual redness, dimensions, and scale reduction was observed, up to complete disappearance (Figure 2). Itch related to the lesion disappeared, scoring 0 after treatment.
Discussion
Psoriasis is a chronic disease with great negative impact on patients’ quality of life. According to the World Health Organization (WHO) reports, its prevalence ranges between 0.09% and 11.4% in different countries with an apparent upward trend, making psoriasis a serious global problem.
Despite there is evidence for genetic predisposition, the etiology of psoriasis remains unclear. Treatment of psoriasis is still based on controlling the symptoms. Even with the advent of more highly targeted therapeutic agents such as biologics, the use of alternative therapies for psoriasis is still very interesting for those patients who are not eligible or responsive to treatments.
Lately, the use of plasma sources for biomedical application have become a very attractive alternative for biomedical applications. In physics, “plasma” is considered the fourth state of matter next to solids, liquids, and gases. In the past, only the thermal properties of plasmas (>80°C) were utilized (i.e. cauterization, sterilization), while current research is directed primarily at the non-thermal effects of plasma. Indeed, it has already been demonstrated how CAP can interact with living matter with no significant deleterious effects. However, several studies have demonstrated a significant effect of CAP instruments on wound healing10 and disinfection on both bacteria and fungi.4,11 To date, the mechanism by which the plasma delivers its therapeutic effect and interacts with living matter is not completely understood; it is known that numerous components, such as RONS, charged particles, electric fields, and even UV light can be involved in mediating its effects.12
Because of their potential, the use of CAP instruments in dermatology has aroused much interest. For instance, it has been suggested that the anti-staphylococcal activity of a plasma jet on human skin could be helpful in atopic dermatitis,13 as well as in erythema treatment.14 However, more data are needed for the introduction of CAP instruments into the clinical practice.
In our study, a recently developed plasma tool, named PCC4,5,15 has been used for the first time on treating psoriasis. The use of this plasma source has shown a good response in the case of a 20-year-old female patient with only two administrations of 30″, with no side effects observed up to 14 days follow-up.
The great importance of this report is due to the characteristics of the treatment, which appears to be quick, non-invasive and efficient. Since, it is known that side effects are proportional to the treatment duration,15 this preliminary study makes the use tools very appealing for the clinical practice, especially in patients that have manifestations of hands and feet, characteristically less responsive to canonical treatments.
Of course, this report represents a very preliminary study, mainly based on the observation of disease progression in one patient; further studies in a bigger cohort of patients are needed to confirm the potential effect of these plasma tools on psoriasis.
Conclusion
The case reported in this article highlights the safety and the effectiveness of the PCC plasma tool in the treatment of psoriasis, and paves the way to a bigger trial for the possibility of an innovative way in the management of that disease.
Acknowledgments
The authors are grateful to Dr L Cordaro and Dr A Fassina for the realization of the PCC device, and to Dr E Martines for inspiring the whole research project. The authors thank also the BioNEM Laboratory of the Department of Experimental and Clinical Medicine (University “Magna Graecia” of Catanzaro) for its technical support in the 3D printing activity of the PCC source.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics approval: Ethical approval to report this case was obtained from the ethics review board of the Magna Graecia University of Catanzaro (n. 215 dated 18 July 2019), and the study was conducted in accordance with the Declaration of Helsinki.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by the “Fondazione Con Il Sud” within the call “Brains2South.”
Informed consent: Written informed consent was obtained from the patient for its anonymized information to be published in this article.
ORCID iD: Clarice Gareri  https://orcid.org/0000-0002-4859-8780
https://orcid.org/0000-0002-4859-8780
References
- 1. Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol 2008; 58(5): 826–850. [DOI] [PubMed] [Google Scholar]
- 2. Bennardo L, Del Duca E, Dastoli S, et al. Potential applications of topical oxygen therapy in dermatology. Dermatol Pract Concept 2018; 8(4): 272–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dattola A, Silvestri M, Bennardo L, et al. Update of calcineurin inhibitors to treat inverse psoriasis: a systematic review. Dermatol Ther 2018; 31(6): e12728. [DOI] [PubMed] [Google Scholar]
- 4. De Masi G, Gareri C, Cordaro L, et al. Plasma coagulation controller: a low-power atmospheric plasma source for accelerated blood coagulation. Plasma Med 2018; 8(3): 245–254. [Google Scholar]
- 5. Cordaro L, De Masi G, Fassina A, et al. On the electrical and optical features of the plasma coagulation controller low temperature atmospheric plasma jet. Plasma 2019; 2(2): 156–167. [Google Scholar]
- 6. Metelmann HR, Vu TT, Do HT, et al. Scar formation of laser skin lesions after cold atmospheric pressure plasma (CAP) treatment: a clinical long term observation. Clin Plasma Med 2013; 1(1): 30–35. [Google Scholar]
- 7. Wang L, Yang X, Yang C, et al. The inhibition effect of cold atmospheric plasma-activated media in cutaneous squamous carcinoma cells. Future Oncol 2019; 15(5): 495–505. [DOI] [PubMed] [Google Scholar]
- 8. Gerdes S, Korber A, Biermann M, et al. Absolute and relative psoriasis area and severity index (PASI) treatment goals and their association with health-related quality of life. J Dermatolog Treat. Epub ahead of print 13 April 2020. DOI: 10.1080/09546634.2020.1746734. [DOI] [PubMed] [Google Scholar]
- 9. Reich A, Riepe C, Anastasiadou Z, et al. Itch assessment with visual analogue scale and numerical rating scale: determination of minimal clinically important difference in chronic itch. Acta Derm Venereol 2016; 96(7): 978–980. [DOI] [PubMed] [Google Scholar]
- 10. Heartel B, von Woedtke T, Weltmann KD, et al. Non-thermal atmospheric-pressure plasma possible application in wound healing. Biomol Ther 2014; 22: 477–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brun P, Vono M, Venier P, et al. Disinfection of ocular cells and tissues by atmospheric-pressure cold plasma. PLoS ONE 2012; 7(3): e33245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tanaka H, Ishikawa K, Mizuno M, et al. State of the art in medical applications using non-thermal atmospheric pressure plasma. Rev Mod Plasma Phys 2017; 1: 3. [Google Scholar]
- 13. Daeschlein G, Darm K, Niggemeier M, et al. Selective antistaphylococcal activity of atmospheric pressure plasma jet (APPJ) on human skin. In: Second international conference on plasma medicine, San Antonio, TX, 16–20 March 2009. [Google Scholar]
- 14. Mertens N, Helmke A, Goppold A, et al. Low temperature plasma treatment of human tissue. In: Second international conference on plasma medicine, San Antonio, TX, 16–20 March 2009. [Google Scholar]
- 15. Cordaro L, De Masi G, Fassina A, et al. The role of thermal effects in plasma medical applications: biological and calorimetric analysis. Appl Sci 2019; 9: 5560. [Google Scholar]



