Abstract
Despite limited scientific evidence, there is an increasing interest in soft robotic gloves to optimize hand- and finger-related functional abilities following a neurological event. This review maps evidence on the effects and effectiveness of soft robotic gloves for hand rehabilitation and, whenever possible, patients’ satisfaction. A systematized search of the literature was conducted using keywords structured around three areas: technology attributes, anatomy, and rehabilitation. A total of 272 titles, abstracts, and keywords were initially retrieved, and data were extracted out of 13 articles. Six articles investigated the effects of wearing a soft robotic glove and eight studied the effect or effectiveness of an intervention with it. Some statistically significant and meaningful beneficial effects were confirmed with the 29 outcome measures used. Finally, 11 articles also confirmed users’ satisfaction with regard to the soft robotic glove, while some articles also noticed an increased engagement in the rehabilitation program with this technology. Despite the heterogeneity across studies, soft robotic gloves stand out as a safe and promising technology to improve hand- and finger-related dexterity and functional performance. However, strengthened evidence of the effects or effectiveness of such devices is needed before their transition from laboratory to clinical practice.
Keywords: Exoskeleton, hand, neurorehabilitation, soft robotic glove, technology
Introduction
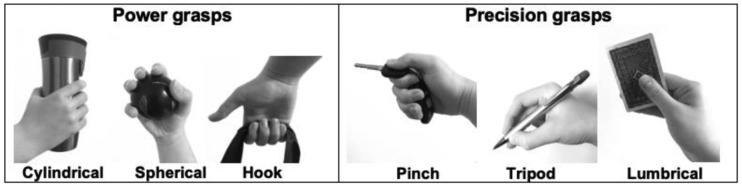
The hand and fingers are essential organs to perform a multitude of functional tasks in daily life, particularly to grasp and handle objects. In fact, the movements performed with the hand to grasp and handle objects, which can solicit up to 19 articulations driven by 29 muscles,1 can be grouped into two broad categories: power and precision grasps. Power grasping requires an individual performing gross motor tasks to generate large forces to firmly hold an object. In contrast, precision grasping requires an individual performing fine motor tasks to generate multiple levels of force to hold an object. The power grasps can be further characterized into cylindrical, spherical, or hook grasps whereas the precision grasps can be further categorized into pinch, tripodal, or lumbrical grasps (Figure 1).2 Whenever sensorimotor impairments of the hand and fingers develop as a result of a neurological event (e.g. stroke, spinal cord injury, Parkinson’s disease),3 the ability to grasp becomes jeopardized to various extents and may negatively impact functional abilities, as well as social participation and life satisfaction.4
Figure 1.
Different types of power and precision grasps.
Despite intensive neurorehabilitation efforts, the likelihood of regaining optimal hand and finger-related functional abilities remains low following a neurological event. For examples, three months after a stroke, only 12% of survivors say they have no problem at all whereas 38% report major difficulties with hand and finger-related functional abilities,5,6 while 75% of individuals with a spinal cord injury at the cervical vertebral level (i.e. tetraplegia), who were asked which function they would most like to have restored, chose upper extremity function,7 with improvement in hand function being their highest-ranked goal.8 Therefore, it is no surprise that one of the most commonly expressed goals of individuals who have sustained a neurological event (i.e. stoke, tetraplegia) and rehabilitation professionals is to engage in neurorehabilitation interventions that can reduce hand and finger sensorimotor impairments, thus improving related functional abilities that are crucial for optimal social participation and life satisfaction.
Rehabilitation strategies designed to maximize hand and finger-related functional abilities are predominantly founded on activity-based therapy, integrating the principles of neuroplasticity.9 Such an approach requires these individuals to engage in meaningful hand- and finger-specific exercises that they must repeat intensively on a daily basis.10,11 In fact, to expect beneficial neuroplastic adaptations, animal studies focusing on gait suggest that up to 1000 to 2000 steps must be taken daily, whereas human studies focusing on grasping in stroke survivors suggest that at least 100 repetitions need to be completed daily.12 Although the evidence suggests the need, adhering to these principles13 remains challenging in clinical practice, especially given various time and productivity constraints. Indeed, it is common to observe in clinical practice that exercise programs are performed individually with direct supervision by a rehabilitation professional, which leads to productivity issues and limits the possibility of implementing interventions at high intensity.14,15 In fact, evidence suggests that the number of repetitions observed for upper extremity work in stroke survivors undergoing neurorehabilitation typically ranges between 12 and 60 repetitions per session, which is far below the number required to expect neuroplastic adaptations.16,17 In addition, recovery may be limited by lack of treatment time, due to the elevated demand for neurorehabilitation services and increased therapists’ workload, especially in publicly funded healthcare environments.18 As a result, individuals with sensorimotor deficits undergoing intensive functional rehabilitation may not achieve the full potential of their hand and fingers sensorimotor and related functional recovery and may reach a ‘recovery plateau’ earlier than expected during the rehabilitation process.
To overcome this challenge, the last decade has seen substantial progress in the development of soft robotic gloves that can facilitate hand and finger movements when performing activities of daily living (ADL) and instrumental activities (iADL) that require grasping objects.19 Moreover, these soft robotic gloves are predicted to be a promising adjunct neurorehabilitation intervention to potentiate the effects of conventional rehabilitation interventions and are now about to be introduced into clinical practice; their effects, however, remain uncertain due to a paucity of evidence. In this context, the present review aims to map, for the first time, the evidence of the effects of the soft robotic glove on the performance of hand- and finger-related functional activities (i.e. with vs. without the technology) and on hand and finger sensorimotor and related functional abilities (i.e. before vs. after an intervention using the technology), among individuals with hand and finger sensorimotor impairments and related disabilities and, whenever investigated, patients’ satisfaction related to the use of the soft robotic glove. Specifically, this review seeks to address the following objectives: (1) determine the effects of rehabilitation interventions using soft robotic gloves; and (2) determine the acceptability and the perceived usefulness of this technology.
Methods
Data sources and searches
This systematic review was based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses reference framework (PRISMA).20 A review of the literature using a combination of search terms was conducted in Medline, EMBASE, and Cumulative Index of Nursing and Allied Health Literature (CINAHL) using specific strategies (Appendix I: Full database searches). Overall, the search strategy was structured around search terms articulated around three key domains and combined together using ‘AND’: technology attributes AND anatomy AND rehabilitation (Table 1). Some search terms were used (e.g. amputation) to avoid retrieving articles that relate to other fields. Given the paucity of available literature on the use of soft robotic glove in neurorehabilitation, and to ensure consideration of all relevant studies, all articles published in English or French from January 2000 to October 2019, specifically investigating human subjects, and using various research designs [randomized controlled trials (RCTs), non-randomized controlled trials (non-RCTs) and other research designs (cohort studies, pre- and post-case interventions, case series, case-control studies and case reports)] were considered. Moreover, articles reporting details regarding the users’ satisfaction and stakeholder views on its use were considered.
Table 1.
Key domains and terms used to support the development of the literature search strategies (1 and 2 and 3 not 4).
| 1. TECHNOLOGY ATTRIBUTE | 2. ANATOMY | 3. REHABILITATION |
|---|---|---|
| Robotic*,Bionics, Exoskeleton device, Robot*,Exoskelet*,Motorized, Motor-driven,Motor Assisted | Hand*, Wrist*, Finger*, Prehension, Dexterity | Rehabilitation,Exercise,Exercise therapy,Physical therapy modalities, Physical therapy speciality, Physical therapists, Occupational therapy, Occupational therapists, Therap*, Exercise*,Physiotherap* |
NOT : Amputee*, Amputation Stumps, Amputation*, Amputation Traumatic, Surgery Computer-assisted, Specialties surgical, Surger*, Surgical*, Teleoperation*.
Eligibility criteria
Inclusion criteria
All scientific manuscripts that investigated the effects of soft robotic gloves for activity-based rehabilitation following at least one or more training session(s) in individuals with hand and finger sensorimotor impairments following a neurological event (i.e. only human subjects), who experienced reduced hand- and finger-related functional abilities and manual dexterity, were deemed eligible. In order to be considered a soft robotic glove for the hand, the technology had to have the capability to generate at least a pinching or grasping movement by combining movements of the thumb with the movement of at least one additional finger. Rehabilitation interventions using a soft robotic glove for the hand may have been performed as part of a rehabilitation program in a hospital, rehabilitation center or at home, with the direct or indirect supervision of a rehabilitation professional. The use of the soft robotic glove could also be combined with other technologies (e.g. virtual reality) or concurrent interventions. All outcome measures characterizing sensorimotor impairments or activity limitations were considered as well as those highlighting users’ satisfaction and stakeholder views.
Exclusion
Research articles that did not include participants with sensorimotor impairments were excluded. All scientific articles focusing on an upper limb exoskeleton targeting the elbow or shoulder joint were excluded.
Article selection
All retrieved articles were imported into EndNote X8, where duplicates were first removed. Thereafter, all articles were uploaded into Covidence, an online systematic review software,21 to conduct the screening process. While doing so, two reviewers screened the titles and abstracts of all articles according to the above-mentioned inclusion and exclusion criteria before removing any articles that were duplicated or did not meet the selection criteria. Whenever a conflict arose between the two reviewers about the eligibility of an article, a third reviewer resolved the conflict. The remaining articles underwent full-text review that was performed by two independent reviewers. Articles not meeting the above-mentioned inclusion and exclusion criteria were excluded.
Data extraction
All selected articles for the full review were read by two reviewers. While doing so, the reviewers completed project-specific data extraction tables developed within an Excel file. The data extraction tables contained information about the source of the article (author-related information, country, and year of publication), study designs and populations, soft robotic glove model, intervention, outcome measures and statistics, user’s satisfaction, and level of evidence. Thereafter, the repertoire of outcome measures documented was classified into two groups (i.e. impairments and activity limitations) according to the International Classification of Functioning, Disability and Health (ICF).22 Finally, to establish if the use of a soft robotic glove yielded significant and meaningful positive, neutral or negative effect(s), the p-value and effect size were computed with the Cohen’s d (small effect ≤ 0.2; medium effect size 0.2–0.5; large effect size ≥ 0.8) of each outcome measure from each article.23
Evidence level assessment
The Oxford Centre for Evidence-Based Medicine 2011 was used to classify the evidence provided by each study. This hierarchical system classifies evidence into five levels, where level 1 is the highest standard of evidence. This classification was chosen mostly because it is usable with a wide range of research designs and allows researchers to answer a range of clinical questions regarding diagnosis, prognosis, therapy, and adverse effects based on the level of evidence quoted.24
Results
Selected articles
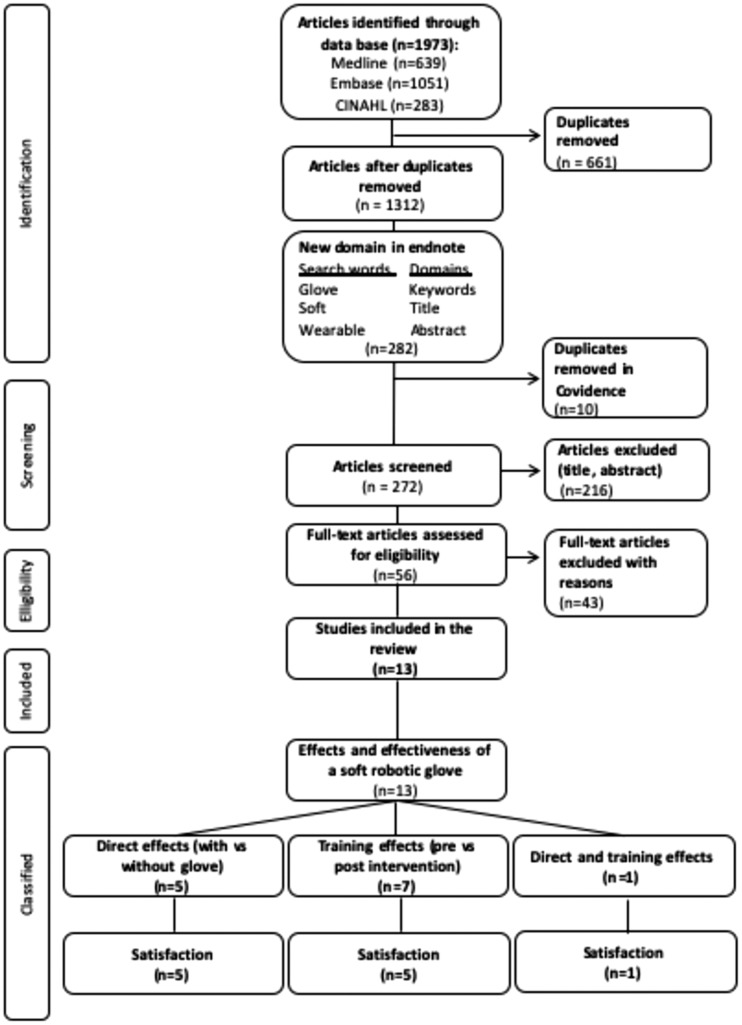
The selection of the articles is summarized in Figure 2. A total of 1973 articles were identified through the search strategy applied to three databases: Medline (n = 639), Embase (n = 1051) and CINAHL (n= 283). First, all identified articles were imported into Endnote to generate a single project-specific library and remove all duplicates (n = 661). Second, to further refine the search, the project-specific library (n= 1312) was searched to select only articles in which the word(s) glove, soft, or wearable appeared in the title, abstract, or key words (n = 282). Third, the 282 articles selected were exported into Covidence where 10 additional duplicates were found and a total of 272 articles were selected for initial screening. Fourth, following the initial screening, a total of 56 articles were selected for full-text review. Finally, a total of 13 articles were selected for this systematic review of the literature, while 43 articles were excluded for various reasons (e.g. wrong study design, wrong patient population, wrong technology).
Figure 2.
Flux diagram and study classification.
Characteristics
Of the 13 articles included in this study, the majority (6/13) originated from the USA,25–30 whereas the others came from Italy (3/13),31–33 the UK (2/13),15,18 Netherlands (1/13),34 and Canada (1/13).35 One study was published in 2016,29 while most of these studies (4/13) were published in 2017,15,18,27,32 in 2018 (4/13)26,28,31,34 or 2019 (3/13).30,33,35 Only one study, published in 2011,25 was more than three years old.
Study designs and populations
Both experimental (3/13)15,29,32 and quasi-experimental study designs (10/13)18,25–28,30,31,33–35 were adopted. The mean sample sizes of the 13 studies was 11.9 participants (min = 2; max = 30), with a median of 10, with most studies (11/13) investigating individuals with hemiparesis following a stroke.15,18,25,27,29–35 Two articles investigated individuals with a traumatic spinal cord injury.26,28 Overall, a total of 106 participants with hand hemiparesis or paralysis following a stroke and of 23 participants with a spinal cord injury have trained at least once with a soft robotic glove.
Intervention
Five studies18,25,26,34,35 used a clinical case series (n > 4) or reports (n ≤ 4) study design to assess the direct effects on hand function of using a soft robotic glove device by comparing measures with and without the use of the glove, whereas seven studies used an experimental or quasi-experimental study design to compare hand sensorimotor integrity and functional abilities before and after an intervention with the soft robotic glove.27–33 One study assessed both the direct and training effects of using a soft robotic glove.15 Concomitant therapy (e.g. physiotherapy and occupational therapy) was used in one study.33 The intervention protocols investigated varied in length from three to eight weeks, in frequency from three to six times a week, and in training session duration from 30 to 90 min.
Soft robotic gloves
Ten different models of soft robotic gloves were investigated across the scientific manuscripts reviewed: HandSOME,25,27 FES Hand Glove 200,28 Gloreha Light Glove,31 Gloreha Professional,32,33 VAEDA,29 HandinMind,15,34 The Hand of Hope,30 HERO Glove35 and two unnamed models.18,26 These gloves provided different types of assistance (i.e. motor,15,28–35 elastic,25,27 or pneumatic18,26). All robotic exoskeletons of the hand had the capability to generate passive hand and finger movements, of various complexity levels, produced entirely by the robotic exoskeletons, while some allowed active-assisted movement (n = 5).15,18,28–30,34 The form of the active-assisted mode of assistance differed from one robotic exoskeleton to another.
Outcome measures, effects, and effectiveness
Numerous outcome measures classified according to the International Classification of Functioning, Disability and Health (ICF)22 were used across the selected scientific manuscripts and are summarized in Table 2. These impairment outcome measures included: range of motion,25,27,35 grip strength,25,27,29–32,35 pinch strength,15,29,32,35 Motricity index,31,32 reach path ratio to assess motor control of the arm,27 hand pain visual analog scale,31 Modified Ashworth scale27,33 or Ashworth Spasticity Index31 and edema.31 The activity limitation outcome measures included were: Box and blocks test,25,30,35 Nine hole peg test (NHPT),31–33 Jebsen-Taylor Hand Function Test (JHFT),15 Wolf Motor Function Test (WMFT),29 Activity of Daily Living (ADL) task,18,34 Stroke Upper Limb Capacity Scale (SULCS),30 the Arm Motor Ability Test (AMAT),30 velocity of movements,25 Quick-DASH,32 Stroke Impact scale,30 Toronto Rehabilitation Institute Hand Function Test (TRI-HFT),26 Action Research Arm Test (ARAT),27,29 Motor Activity Log,27 Chedoke McMaster Stroke Assessment Hand (CMSAH),29,35 Barthel Index,31 Fugl-Meyer Assessment of Upper Extremity (FMA-UE),27,29,30 Fugl-Meyer Hand (FMH),29 and the Functional Independence Measure (FIM).33,36
Table 2.
Summary of outcomes measures.
| DIRECT EFFECTS | Brokaw et al.25 | Prange-Lasonder et al.15 | Yap et al.18 | Cappello et al.26 | Radder et al.34 | Yurkewichet al.35 | TRAINING EFFECTS | Thielbaret al.29 | Chen et al.27 | Prange-Lasonder15 | Vanoglioet al.32 | Bernocchiet al.31 | Scott et al.28 | Kimet al.30 | Milia,etal.33 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Impairments | Impairments | ||||||||||||||||
| • Joint mobility | |||||||||||||||||
| Range of motion (extension) | + | + | – | ||||||||||||||
| Range of motion (flexion) | – | ||||||||||||||||
| Thumb abduction | – | ||||||||||||||||
| • Muscular Strength | |||||||||||||||||
| Grip Strength | – | ? | – | – | + | + | – | ||||||||||
| Pinch Strength | – | ? | – | – | ? | + | |||||||||||
| Motricity Index | + | + | |||||||||||||||
| •Arm motor control | |||||||||||||||||
| Reach path ratio of the hand | + | ||||||||||||||||
| •Hand pain | |||||||||||||||||
| Visual analog Scale | – | ||||||||||||||||
| • Spasticity | |||||||||||||||||
| Modified Ashworth scale a) Elbowb) Wrist c) Finger | |||||||||||||||||
| – | – | ||||||||||||||||
| – | |||||||||||||||||
| – | |||||||||||||||||
| Ashworth Spasticity Indexa) Finger flexorsb) Opponents of the thumbc) Wrist flexors | |||||||||||||||||
| – | |||||||||||||||||
| – | |||||||||||||||||
| – | |||||||||||||||||
| • Edema | |||||||||||||||||
| Circumferencea) Forearmb) Wristc) Finger | |||||||||||||||||
| – | |||||||||||||||||
| – | |||||||||||||||||
| – | |||||||||||||||||
| Activity Limitation | Activity Limitation | ||||||||||||||||
| Box and block test | + | + | – | ||||||||||||||
| Nine Hole Peg Test | + | + | + | ||||||||||||||
| Jebsen–Taylor Hand Function Testa) Picking up small objectsb) Lift cane | |||||||||||||||||
| + | ? | ||||||||||||||||
| – | ? | ||||||||||||||||
| Wolf Motor Function Test | ? | + | |||||||||||||||
| Activity of daily living tasks | ? | ||||||||||||||||
| Stroke Upper Limb Capacity Scale | – | ||||||||||||||||
| Arm Motor Ability Test | + | ||||||||||||||||
| Finger velocitya) in flexionb) in extension | |||||||||||||||||
| + | |||||||||||||||||
| – | |||||||||||||||||
| Quick-Dash | + | ||||||||||||||||
| Stroke Impact scalea) Hand subscaleb) Recovery subscale | |||||||||||||||||
| + | + | ||||||||||||||||
| – | – | ||||||||||||||||
| Toronto Rehabilitation Institute Hand Function Testa) manipulation of small objectsb) uplift strength | |||||||||||||||||
| + | |||||||||||||||||
| + | |||||||||||||||||
| Action Research Arm Test | – | + | |||||||||||||||
| Motor Activity Log | – | ||||||||||||||||
| Chedoke McMaster Stroke Assessment Hand | ? | + | |||||||||||||||
| Barthel Index | + | ||||||||||||||||
| Fugl-Meyer Assessment of Upper Extremity | ? | – | + | – | |||||||||||||
| Fugl-Meyer Hand | – | ||||||||||||||||
| Functional Independence Measure | + | + | |||||||||||||||
+Significant p < 0.05; – not significant with p > 0.05; ? information not available on the p-value. Effect size <0.2; effect size ≥0.2; effect size ≥0.5; effect size ≥0.8.
All scientific articles selected investigated the immediate effects of wearing a soft robotic glove (n= 6/13) or the immediate effect or effectiveness of an activity-based intervention with the soft robotic glove (n= 8/13), as summarized in Tables 3 and 4, respectively. Only three studies27,29,30 investigated the carry-over effects of the latest interventions over time (up to three months post-intervention). Overall, the use of a soft robotic glove increased finger mobility and reduced the time needed to complete functional tasks (e.g. Box and block test; NHPT; JTHFT; AMAT and TRI-HFT). As for joint mobility, muscular strength and other measures of activity limitation (e.g. WMFT; SULCS; ARAT; CMSAH; FMA-UE; and FMH), the results are heterogeneous. The results are inconclusive for the level of pain, spasticity and oedema.
Table 3.
Summary of studies investigating the direct effects of a robotic glove (with vs. robotic glove effects).
| Outcomes |
|||||||
|---|---|---|---|---|---|---|---|
| Authors, Year Soft robotic glove model | Participants characteristics | Research design & Intervention | Measurement instruments | Without the soft robotic glove M (SD) or percentage | With the soft robotic glove, M (SD) or percentage | p-Value | Effect sizes or percentage difference |
| Brokaw et al.,25 2011HandSOME | n = 8M = 4F = 4Age range: not mentionedDx: subacute and chronic stroke | Clinical case series:Measures with and without the soft robotic glove (one visit) | 1) Active range of motion in extension (deg)2) Velocity in flexion (deg/sec)3) Velocity in extension (deg/sec)4) Box and Blocks (inch)5) Grip strength with JAMAR (N) | 1) not reported2) 26.9 (13.9)3) 11.30 (4.45)4) not reported5) 29.9 (1.9) | 1) + 48.7 (1.0)2) 93.10 (24.76)3) 59.40 (22.34)4) not reported5) 26.2 (1.8) | 1) p < 0.001*2) p = 0.004*3) p = 0.0534) p = 0.002*5) p = 0.17 | 1) NA2) d = 3.303) d = 2.994) NA5) d = -2.00 |
| Prange-Lasonder et al.,15 2017 HandinMind | n = 5M = 3F = 2Age range: 58–76 years Dx : chronic stroke | Case-Control series:Measure with and without the soft robotic glove (one visit) | 1) Pinch Gauge (kg)2) Jebsen-Taylor Hand Function Testa) Picking up small objects (sec)b) Lift cane (sec) | 1) Increase by 11% to 27% with the glove2) a) Slower by 6% to 40% with glove in all participantsb) Faster by 2% to 24% with glove in 3/5 participants | Not reported | 1) Not significant2)a) p ≤ 0.043* b) Not significant | NA |
| Yap et al.,18 2017Not mentionned | n = 2M = 1F = 1Age: 40 and 50 yearsDx: chronic stroke | Clinical case reports:Measure with and without the soft robotic glove (one visit) | Grasp an empty bottle, lift it and put it down (sec)Grasp a tin can, lift it and put it down (sec) | Participant 1 = 9.0 (1.4)Participant 2 = > not finish within 90 sec; exact time not reported | Participant 1 = 8.0 (0.7)Participant 2 = 12.3 (2.7) | Participant 1:p = 0.06Participant 2:p = 0.02* | Participant 1: d = −0.90Participant 2 :NA |
| Cappello et al.,26 2018Not mentioned | n = 9M = 8F = 1Age range: 20–68 yearsDx: spinal cord injury C4-C7 | Clinical case series:TRI-HTF performed with and without the soft robotic glove (one visit) | Toronto Rehabilitation Institute Hand Function Test (TRI-HFT)1) Manipulation of objects2) Lift force (N) | 1) 3.77 (SD not reported)2) 1.76 (4.32) | 1) 6.11 (SD not reported)2) 2.76 (5.18) | 1) p < 0.0005*2) p < 0.0135* | 1) NA2) d = 0.21 |
| Radder et al.,34 2018HandinMind | n = 5M = 3F = 2Age range: 45–69 yearsDx: chronic stroke | Clinical case series:Five ADL tasks performed three times with and once without the soft robotic glove (Two visits) | 1) Functional task performance test (sec)a) Drinkingb) Eatingc) Household cleaningd) Readinge) Dressingf) Door opening | Overall, median changes showed a small difference between performance with and without glove ranging from –1.1 to 2.5 s, except for the drinking task in session 1 (median difference of 5.8), door opening task (median difference of 5.4) and the drinking task in session 2 (median difference of 4.1) in favor of performance without glove. | Not reported | Not reported | NA |
| Yurkewich et al., 201935HERO Glove | n = 5M = 2F = 3Age: 57-83Dx: acute and chronic stroke | Clinical case series:Measures with and without the soft robotic glove (one visit) | 1) Finger extension (deg)2) Range of motion (deg)3) Grip and pinch strength (kg)4) Box and Block Test (number of block)5) Chedoke Arm and Hand activity Inventory: water bottle task | 1) 46.25 (31.1)2) 32.5 (53.44)3) not reported4) 0.4 (0.8)5) 1.6 (1.2) | 1) 143.75 (22.18) 2) 78.75 (30.9)3) not reported4) 3.2 (1.17)5) 2.4 (1.02) | 1) p = 0.0002*2) Not significant3) Not significant4) p = 0.004*5) Not reported | 1) d = 3.612) d = 1.063) NA4) d = 2.795) d = 0.72 |
M: mean score; SD: standard deviation; Dx: diagnosis.
*Statistically significant at p < 0.05.
Table 4.
Summary of studies investigating the training effects of a soft robotic glove (pre vs. post intervention).
| Outcomes |
|||||||
|---|---|---|---|---|---|---|---|
| Author, Year & Soft robotic glove model | Participants caracteristics | Research design & Intervention & | Measurements instruments | Pre M (SD) or percentage | Post M (SD) or percentage | p-value | Effect sizes or percentage difference |
| Thielbar et al.29 2016VAEDA | n = 22M = 15F = 7Control group: n = 11Experimental group: n = 11Age range: 32–77 yearsDx: chronic stroke | Randomized controlled pilot study: 3 × 60 min/week for 18 treatments of upper limb functional activities (e.g. reach-to-grasp) with an occupational therapist. Control group performed all the tasks without using the soft robotic glove and experimental group performed the same tasks with the soft robotic gloveMeasures taken pre and post intervention (T) and 10 weeks post-intervention (carry-over). (T10) | 1) Action Research Arm Test (ARAT) 2) Wolf Motor Function Test (WMFT) (sec)3) Fugl-Meyer Upper extremity Motor assessment (FMUE)4) Chedoke McMaster Stroke Assessment – Hand (CMSA-H)5) Grip strength (N)6) Pinch gauge (palmar) (N)7) Pinch gauge (lateral) (N)8) Fugl-Meyer Hand (FMH) | 1) 33.7 (13.5) 2) 31.2 (26.0)3) 34.8 (9.7) 4) 4.0 (0)5) 38.6 (12.6)6) 7.3 (3.3)7) 11.7 (3.7) 8) 9.2 (3.5) | 1) T: 36.3 (11.4)T10: 37.1 (11.9)2) T: 21.1 (13.8)T10: 22.6 (17.9)3) T: 34.8 (9.6)T10: 35.5 (9.3)4) T: 3.9 (0.3) T10: 4.3 (0.5)5) T: 37.8 (13.3)T10: 39.7 (13.1)6) T: 7.7 (3.7)T10: 8.1 (2.9) 7) T: 13.8 (6.9)T10: 14.2 (8.4)8) T: 9.1 (4.3)T10: 9.6 (4.1) | 1) T: p = 0.0722) T: p = 0.012*3) T: p = 0.9604) T: p = 0.038*5) T: p = 0.6376) T: p = 0.5887) T: p = 0.8418) T: p = 0.965 | 1) d = 0.202) d = −0.493) d = 0.004) NA5) d = 0.066) d = 0.117) d = 0.388) d = 0.03 |
| Chen et al.27 2017HandSOME | n = 10(post n = 7)M = 7F = 3Age range = 36–69 yearsDx: chronic stroke | Nonrandomized, pre–post-intervention study:5 × 90 min/week for four weeksUnimanual and bimanual manipulation of objects, lift and move objectsMeasures taken pre and post intervention (T) and three-month post-intervention (carry over) (T3) | 1) The Fugl-Meyer assessment of the upper extremity2) The Action Research Arm Test3) The Motor Activity Log4) The Modified Ashworth Scalea) Elbowb) Wristc) Fingers5) Finger extension (deg)6) thumb abduction (deg)7) Grip Strength with JAMAR (lbs)8) Reach path ratio of the hand | 1) 38.6 (6.9) 2) 23.1 (8.5)3) 1.3 (0.7)4)a) 1.1 (0.6)b) 0.9 (0.9)c) 1.4 (1.1) 5) 139.7 (46.0)6) 51.9 (17.7)7) 24.7 (15.6)8) 1.8 (0.3) | 1) T: + 4.9 (4.1)T3: + 4.0 (4.7)2) T: + 3.3 (2.6) T3: +0.6 (3.3)3) T: +0.23 (0.28)T3: +0.33 (0.32)4) a) T: +0.3 (0.7)T3: +0.1 (0.4)b) T: −0.1 (0.5) T3: 0.0 (0.6)c) T: −0.3 (0.8) T3: 0.0 (0.5)5) T: +6.5 (24.2)T3: +1.2 (23.9)6) T: 8.1 (18.9)T3: −0.5 (25.8)7) T: +3.0 (4.0)T3: 0.0 (9.6)8) T: −0.4 (0.3)T3: −0.4 (0.5) | 1) T: p = 0.039*T3: p = 0.1332) T: p = 0.032*T3: p = 1.0003) T: p = 0.145T3: p = 0.070*4) a) T: p = 0.642T3: p = 1.000b) T: p = 0.914T3: p = 1.000c) T: p = 0.772T3: p = 1.0005) T: p = 1.000T3: p = 1.0006) T: p = 0.598T3: p = 1.0007) T: p = 0.189T3: p = 1.0008) T: p = 0.038*T3: p = 0.340 | 1) NA2) NA3) NA4) a) NAb) NAc) NA 5) NA6) NA7) NA8) NA |
| Prange-Lasonder et al.15 2017HandinMind (HiM) | n = 4M = 3F = 2Assistive support (AS): n = 2Training support (TS): n = 2Age range: 58–76 yearsDx: chronic stroke | Randomized controlled pilot study: AS: 180 min per week of ADL with the glove at homeTS: 3 × 60 min a week through games with the glove in clinical settingTotal duration of six weeks. | 1) Pinch Gauge and grip strength2) Jebsen-Taylor Hand Function Testa) Turning cardsb) Picking up small objectsc) Simulated feedingd) Stacking checkerse) Lifting empty cansf) Lifting full cans | 1) AS: two participants improvement range between +15% in grip strength and +43% in pinch strength per participantTS: individual changes from −3% (grip) to +11% (pinch)2)AS: from +7% and +15% (slower)TS: −4% and −20% (faster)a) AS: −10% and −4% TS: −2% and −35% b) AS: −35% and +16%TS: +34% and −9%c) AS: +65% and +28%TS: −8% and −21%d)AS: +21% to −2%TS: −39% and −19%e) AS:−9% and +25% TS: +14% and −17%f) AS: −16% and +39%TS: −16% and −10% | Not reported | Not reported | NA |
| Vanoglio et al.32 2017Gloreha Professional | n = 30M = 14F = 16Experimental group (E) n = 15(post n = 14)Control group (C) n = 15(post n = 13)Age: mean 72 (T)-73 (C) yearsDx: acute stroke | Randomized controlled pilot study: Total of 30 sessions of 40 min (5× / week) for six weeks.E: passive mobilization with glove: flexion, extension, grasping, forceps.C: conventional physiotherapy | 1) Motricity Index2) Nine Hole Peg Test (pegs/sec)3) Grip strength with JAMAR (Kg/BMI)4) Pinch Gauge (Kg/GMI)5) Quick-DASH | 1) E: 37.4 (26.5)C: 28.1 (29.8) 2) E: 0.014 (0.03)C: 0.017 (0.03)3) E: 0.14 (0.22)C: 0.19 (0.43)4) E: 0.07 (0.08)C: 0.04 (0.07)5) E; 59.7 (24.2)C: 65.6 (11.5) | 1) E: 60.4 (25.7)C: 33.2 (27.5)2) E: 0.17 (0.16)C: 0.04 (0.07)3) E: 0.41 (0.31)C: 0.22 (0.48)4) E: 0.14 (0.10)C: 0.05 (0.09)5) E: 44 (29)C: 65.1 (16.4) | 1) E: p = 0.0004*C: p = 0.06652) E: p = 0.0024*C: p = 0.27923) E: p = 0.005*C: p = 0.08544) E: p = 0.0040*C: p = 0.08695) E: p = 0.0048*C: p = 0.8212 | 1) E: d = 0.881C: d = 0.1782) E: d = 1.36C: d = 0.433) E: d = 1.00C: d = 0.0664) E: d = 0.77C: d = 0.1245) E: d = −0.59C: d = −0.036 |
| Bernocchi et al.31 2018Gloreha Lite Glove | n = 21(post n = 17)M = 14F = 7Mean age: 69 yearDx: chronic stroke | Nonrandomized, pre–post-intervention study: 45 min, 6×/week, for eight weeks. Passive finger mobility exercises. | 1) Modified Ashworth Scalea) Finger flexorsb) Opponents of the thumb c) Wrist flexors2) Circumference (cm)a) Forearmb) Wristc) Fingers3) Pain Visual Analog Scale4) Barthel Index5) Motricity index6) Nine-hole peg test (pegs/sec)7) Grip strength (kg/BMI) | 1)a) 0.6 (0.92)b) 0.4 (0.54)c) 0.5 (1.04)2)a) 18.6 (1.52)b) 18.5 (1.29)c) 21.3 (2.34)3) 1.3 (2.64)4) 32.7 (25.13)5) 44 (24.08)6) 0.05 (0.11)7) 0.005 (0.009) | 1)a) 1.0 (1.26)b) 0.4 (0.63)c) 0.8 (0.74)2)a) 18.6 (1.58)b) 18.3 (1.37)c) 21.2 (1.68)3) 1.8 (3.04)4) 54.2 (29.56)5) 56 (24.09)6) 0.10 (0.16)7) 0.009 (0.012) | 1)a) p = 0.0781b) p = 0.5625c) p = 0.13092)a) p = 0.1475b) p = 0.2661c) p = 0.89843) p = 1.00004) p = 0.0001*5) p = 0.002*6) p = 0.0156*7) p = 0.0024* | 1)a) d = 0.36b) d = 0c) d = 0.332)a) d = 0b) d = −0.15c) d = −0.053) d = 0.184) d = 0.785) d = 0.506) d = 0.367) d = 0.38 |
| Scott et al.28 2018FES Hand Glove 200 | n = 14(post n = 11)M = 14Age: 19–66 yearsDx: Spinal cord injury C4–C6 | Nonrandomized, pre–post-intervention study:30 min PROM with glove followed by 30 min PROM with FES(Total = 1h of treatment / session) four session week for six weeks. | 1) Functional Independence Measure (FIM) | 74.26 (22.52) | 80.55 (24.22) | p = 0.02* | 1) d = 0.27 |
| Kim et al.30 2019Hand of Hope | n = 12M = 2F = 10Age range: 39–79 yearsDx: chronic stroke | Nonrandomized, pre–post-intervention study:18 session of intensive robotic hand therapy over six weeks. Treatments three times a week. Measures taken pre and post intervention (T) and six weeks post-intervention (carry-over) (T6). | 1) Box and Block Test 2) Grip Strength (Kg)3) Arm Motor Ability Test (AMAT)4) Stroke Upper Limb Capacity Scale (SULCS)5) Fugel-Meyer Assessmenta) Wrist and handb) shoulder and elbowc) total6) Stroke Impact Scale a) Hand subscale b) Recovery subscale | 1) 5.67 (10.36)2) 7.96 (6.24) 3) 2.13 (0.50) 4) 5.33 (2.15) 5)a) 8.67 (6.27)b) 12.08 (2.68) c) 20.75 (7.90)6)a) 11.50 (4.91) b) 58.75 (18.48) | 1) T: 6.08 (10.00) T6: 5.92 (10.41)2) T: 8.23 (6.18)T6: 7.44 (6.78)3) T: 2.35 (0.58)T6: 2.39 (0.66)4) T: 5.17 (2.17) T6: 5.00 (2.17)5) a) T: 9.42 (6.43)T6: 9.17 (6.34) b) T: 13.08 (3.26)T6: 13.92 (4.12)c) T: 22.50 (9.47)T6: 23.08 (9.82)6)a) T: 13.33 (5.48)T6: 14.42 (4.64)b) T: 66.25 (15.24)T6: 66.25 (17.47) | 1) p = 0.9592) p = 0.3933) p = 0.016*4) p = 0.5935)a) p = 0.649b) p = 0.273c) p = 0.2476)a) p = 0.008*b) p = 0.098 | 1) d = 0.042) d = 0.043) d = 0.044) d = 0.075) a) d = 0.1b) d = 0.04c) d = 0.26) a) d = 0.35 b) d = 0.44 |
| Milia et al.33 2019Gloreha Professional | n = 12M = 8F = 4Age range: 42–82 yearsDx: acute stroke | Nonrandomized, pre-post-intervention study: For three weeks, participants were exposed to Gloreha device rehabilitation (30 min/day), physiotherapy (1.5 h/day), and OT (30 min/day). | 1) Modified Ashworth scale2) Functional Independence measure3) Nine-Hole-Peg-Test | 1) 1.25 (0.87)2) 88.33 (12.34)3) 51.58 (5.40) | 1) 1.08 (0.9)2) 117.2 (7.59)3) 36.33 (3.67) | 1) p = 0.01*2) p = 0.63) p = 0.01* | 1) d = 0.1922) d = 2.8183) d = 3.303 |
M: mean score; SD: standard deviation; Dx: diagnosis.
*Statistically significant at p < 0.05.
Acceptability and the perceived usefulness
Numerous satisfaction measures were used across the 11 articles, that also assessed the feasibility, usability, safety or satisfaction of the users after trying the soft robotic glove,15,18,25–28,30–32,34,35 and are summarized in Table 5. One study took into consideration the cost analysis of using a robotic device to assess its feasibility.32 To evaluate the usability and the user’s satisfaction, standardized questionnaires were used, such as the Usefulness-Satisfaction-and-Ease-of-Use questionnaire,18 the System Usability Scale15,34 and the Intrinsic Motivation Inventory,34 in addition to patients’ informal and formal feedback.18,25–27,30,31,34,35 Also, compliance rate was used as a measure of satisfaction, based on the fact that participants who did not like using the glove would be less likely to attend treatment.15,27,30–32 Safety was mostly determined by the absence of side-effects or adverse events.28,30,32 Studies concluded that soft robotic gloves are generally seen as being easy to use, safe, feasible and acceptable by individuals with neurological impairments15,18,25–28,30–32,34,35 and increase motivation to engage in an intensive rehabilitation program.18,34 However, the robotic glove was found to be more useful when performing gross motor tasks (e.g. lifting cans) than when performing fine motor tasks (e.g. handling small objects).15 Putting on or activating the glove appeared to be a difficulty in more than one study,15,26,27,31,34 and the choice of material, especially its thickness, was found to interfere with hand and finger sensations.34 A preference for the rental of these devices has been voiced.18 The most important features highlighted in the studies included ease of cleaning, comfort, and ease of putting on and taking off. Finally, a decrease in rehabilitation cost may be anticipated, linked to the use of a soft robotic device at home.32
Table 5.
Summary of user’s satisfaction and acceptability of studies in this review.
| Author, Year & soft robotic glove model | Outcome measures | Results & feedbacks |
|---|---|---|
| Brokaw et al.252011HandSOME | Participant feedbacks | Positive feedbacks; glove generally comfortable; majority of participants reported that they would be interested in using the glove at home. However, due to shoulder weakness, the added weight due to the glove restricted upper limb mobility due to increased relative muscular demand. |
| Chen et al.272017HandSOME | 1) Number of participants who completed the program 2) Participant feedbacks | 1) Three participants dropped out because of difficulties donning and doffing the glove and an absence of caregiver at home to assist.2) Participants generally positive about the treatment and report an increased use of their hand after the program. |
| Prange-Lasonder et al.15 2017HandinMind | 1) System usability scale2) Use time | 1) Mean score (SD) 73.1 (24.2) 2) Assistive support group: one participant used the glove 30 min a day (∼200 min per week) whereas the other participant used the glove only once a week because she felt it was too cumbersome donning the glove by herself relative to its corresponding gains.Training support group: Participants followed scheduled (180 min per week). |
| Vanoglio et al.32 2017Gloreha Professionnal | 1) Number of participants who completed the program2) Side effects3) Perceived operator difficulty using a visual analog scale (VAS)4) Cost analysis | 1) Three participants did not complete the program in the control group due to acute hospital transfer for infection and one participant in the treatment group due to reactivated rheumatoid arthritis.2) All participants accepted to use the glove.3) Mean value reported for the first three days 5.13 (1.6) and 1.16 (0.26) for the last 27 days.4) Treatment group: 237.20 euro/participant for 30 days and control group: 480 euro/participant. |
| Yap et al.18 2017 Not reported | 1) Usefulness-Satisfacation-and-Ease-of-use questionnaire (USE)a) Usefulness b) Ease of use c) Ease of learningd) Satisfaction 2) Participant feedbacksa) Comfort levelb) Desire to usec) Desire to purchase | 1) a) Mean score (SD) 5.9 (0.3)b) Mean score (SD) 6.4 (0.4)c) Mean score (SD) 6.6 (0.2)d) Mean score (SD) 6.6 (0.5) 2)a) Mean score (SD) 6.0 (1.4)b) Mean score (SD) 6.5 (0.7) c) Mean score (SD) 5.0 (1.4) |
| Bernocchi et al.31 2018 | 1) Number of participants who completed the program2) Minutes of exercise and number of sessions/patients performed3) Participant feedbacks | 1) Seventeen participants completed the program. Four patients interrupted the program: one died one had a new stroke event, one was transferred to a rest home and one withdrew consent.2) Over a mean period of 56.1 (17.18) days, participants completed a total of 1699 (808.97) min/participant divided in 5.1 (1.75) days/week of home exercises with the glove.3) Difficulties in donning the glove by caregivers, because of edema two gloves have been replaced. The glove was well tolerated by participants. |
| Cappello et al.26 2018Not reported | Participant feedbacks | No discomfort associated to the use of the glove was reported. All participants stated that they could benefit of using the glove during the performance of their daily domestic activities; the glove is light weight; the glove is difficult to don independently. |
| Radder et al.,34 2018HandinMind | 1) Participant feedbacks2) System Usability Scale (SUS)3) Intrinsic Motivation Inventory (IMI) | 1) All participants could don and doff the glove, closing the zips was not possible for all participants; the thickness of the fabric reduced sensation was experienced. Difficulties performing fine motor subtasks with the glove; appreciation of grip support during gross motor activities. For some participants, their hand became warm and sweaty when using the glove.2) The median score at session 1 was 80.0 (Interquartile range 70.0–88.8) and the median score at session 2 was 77.5 (interquartile range 75.0–87.5). The lowest SUS score was 65. 3) Each part of the IMI was rated very positively by all participants with a total score between 6.1 and 6.3/7. |
| Scott et al.28 2018FES Hand Glove 200 | 1) Skin integrity2) Wrist/finger joints deformity3) Hand pain during intervention, Scale 0–10 (location)4) Occurrence of Automatic Dysreflexia | 1) Intact or unchanged after protocol.2) No wrist/finger joints deformity after protocol.3) No increased pain documented except for one participant out of 14 but unrelated to the use of the glove.4) No occurrence of autonomic dysreflexia. |
| Kim et al.30 2019Hand of Hope | 1) Participant feedbacks2) Adverse events3) Compliance rates | 1) Hand feels less tight; increase the perceived ease of use of the hand after training; increase in attention; not changed with the hand after the program; increase in mobility; need longer therapy.2) Skin pinching or rubbing near the proximal interphalangeal joints on the dorsal side of the hand for 58% of participants. Muscle fatigue at the shoulder was reported for 50% of participants and cognitive fatigue for 25%.3) All participants tolerated and completed the program. |
| Yurkewich et al.35 2019HERO Glove | Participant feedbacks | Participants saw the glove as an affordable assistive and rehabilitative device for performing daily tasks with more independence and ease. Its light weight, portability, ease of donning and use were appreciated by the participants. However, participants reported that its robustness, grip strength comfort and aesthetic should be improved to be use during daily tasks at home. |
SD: standard deviation.
Evidence level of studies
Only three articles present the results of a randomized control trial and reached level 2 of evidence, following the criteria proposed by the OCEBM 2011.15,29,32 All other articles (n = 10) were rated at a level 4 of evidence, mostly due to the lack of a control group and the risk of bias.18,25–28,30,31,33–35
Discussion
This systematic review of the literature includes a selection of 13 articles on soft robotic gloves, all published between 2011 and 2019. This confirms an increased interest over the last decade in the development, testing, and use of this technology for rehabilitation of individuals with sensorimotor impairments of the hand following a neurological event. Although the evidence of the effectiveness of soft robotic gloves in improving the function of the hand is promising, the strength of the currently available evidence remains limited, given the wide variety of soft robotic glove models and their attributes, the study designs and interventions, and the outcome measures, as well as the small sample sizes tested. It is, therefore, impossible to highlight which soft robotic glove or intervention protocol would be the most appropriate to obtain the best clinical results.
Optimal intervention – no consensus
The interventions described in the selected articles had two main goals: (1) compare finger and hand range of motion and strength as well as finger- and hand-specific and global functional abilities, with or without the use of a soft robotic glove or (2) quantify the effects or effectiveness of an intervention involving the use of a soft robotic glove on finger and hand pain, oedema, strength, and spasticity as well as on finger- and hand-specific and global functional abilities. These two different approaches adopted in the literature reflect the fact that the soft robotic glove can be perceived both as a dynamic orthosis for those with a chronic neurological event who have a poor prognosis for recovery of hand function and manual dexterity, or as a neurorehabilitation adjunct intervention for those with a recent neurological event who have a good prognosis for recovery of these abilities.
Based on the present results, the amount of training required for the soft robotic glove to become an effective dynamic orthosis remains unclear, although different beneficial effects were reported after only one training session under the supervision of a therapist.15,18,25,26,34,35 Likewise, the optimal therapeutic dosage of interventions, based solely on or combined with the use of a soft robotic glove, remains unclear. Despite different dosages of the interventions across the selected studies, in terms of length, frequency and training session duration, immediate beneficial effects were found after three weeks,33 with some carry-over effects up to a period of three months.27,30 Nonetheless, the dosages of all selected studies remain far from the number of repetitions that are recommended to anticipate neuroplastic adaptations and will need additional consideration in future studies.12 Finally, the fact that only three studies investigated the medium-term effects with outcomes measured two to three months after the end of the intervention27,29,31 remains disconcerting, especially as they reported discordant findings; this supports the need for future longitudinal studies.
Overall, despite the generally low level of evidence, the results of the selected articles are somewhat promising; further clinical research on the superiority, non-inferiority, and equivalence37 of soft robotic gloves is warranted before formulating recommendations for its eventual incorporation into neurorehabilitation programs. On one hand, the majority of articles that have investigated individuals with hand and finger sensorimotor impairments following a stroke reported meaningful changes with moderate to high effect size on at least one key outcome measure, although very few confirmed statistically significant results. On the other hand, for the articles that have investigated individuals with a spinal cord injury, only medium effect sizes were calculated despite statistical significance. For both populations studied, these results are explained, for the most part, by the fact that the majority of articles (10/13 = 77%) included less than 15 participants, out of which 54% (7/13) involved less than 10 participants (i.e. very small sample size), resulting in a negative impact on the statistical power (i.e. underpowered study). In fact, if a study aiming to assess the superior effectiveness of a neurorehabilitation intervention integrating the soft robotic glove among individuals with no voluntary finger extension38,39 following a recent stroke (i.e., randomization completed within three weeks) was to be designed, considering a normally distributed outcome, a 95% two-sided confidence level, a statistical power set at 80%, a ratio of 1:1 between the control and experimental groups, and including 10% to account for dropouts, it is expected that a total sample size of about 64 participants (i.e. 32 participants per group) would be required if using, as the main outcome measure, the upper extremity subscore of the Fugl-Meyer Assessment of Motor Recovery (mean (SD): pre = 7.86 (7.84); post = 9.62 (9.62); pooled SD= 8.77; minimal detectable change = 5.7 points)) or of 24 participants (i.e. 12 participants per group) if using the Action Research Arm Test (mean (SD): pre = 0.82 (1.98); post = 2.48; pooled SD = 4.54, minimal detectable change = 6.6 points).40 Thus, it remains impossible to generalize the present results to the population under study and the implementation of this technology in clinical practice at the present time would be premature.
Aside from the small sample size, out of 140 participants with a neurological disease included in all selected articles, a total of 109 (77%) represented individuals with chronic sensorimotor impairments following a neurological event (≥3 months). Knowing that the greatest neuroplastic adaptation potential is available within the first three months following a neurological event, it is plausible that some of the selected articles, especially the sub-sample targeting neurorehabilitation (n = 112; 80%), underestimate the potential beneficial effects of the soft robotic glove during neurorehabilitation.
Surprisingly, a consensus has not yet been reached on a minimal data set of outcome measures to evaluate the effectiveness of any intervention aiming to improve hand and finger abilities, including an intervention integrating a soft robotic glove. Hence, it is no surprise that the selected articles have used a total of 29 different outcome measures or measurement instruments to quantify changes. Such a large number makes it very difficult to compare results across articles and to conduct a meta-analysis. The two most commonly assessed domains were muscle strength, evaluated with a hand-held dynamometer or a pinch gauge, and functional abilities, evaluated with the upper extremity subscore of the Fugl-Meyer Assessment of Motor Recovery and The Action Research Arm Test. Ideally, future studies should integrate these outcome measures or measurement instruments to eventually facilitate comparisons across studies and generate aggregate data for meta-analyses.
The soft robotic glove represents a relevant adjunct intervention to intensify activity-based therapy, integrating the principle of neuroplasticity with the intensity of treatment. For improved adherence to these principles, rehabilitation interventions using the soft robotic glove may benefit from being coupled with virtual reality to ensure the user remains cognitively engaged during the exercises, as proposed with the Gloreha glove for example, and this also opens the door to telerehabilitation. Independently of the future development and advancement of this technology, it remains clear that the soft robotic glove is clearly not intended as a replacement for current therapy practices. In fact, the training, expertise, and experience of rehabilitation professionals, especially occupational therapists, who are highly involved in hand and finger neurorehabilitation remain essential to deliver personalized rehabilitation interventions with the greatest potential to positively impact social participation and life satisfaction for individuals with sensorimotor impairments following a neurological event.
Accessibility and perceived usefulness
Acceptability becomes a very important step when integrating a new technology into clinical practice in rehabilitation.41 This most likely explains why the majority of selected articles (n = 11; 85%) documented it.25–27,30,31,34,35 Yet, from those articles only three used standardized questionnaires15,18,34 and seven gathered general feedback focusing in most part on comfort among end users (i.e., participants).25–27,30,31,34,35 Two articles attempted to judge the acceptability simply by relying on the absence or presence of side effects which, instead, relates to the safety of the technology.28,32 From those that have used standardized questionnaires and feedbacks, individuals with neurological impairments who have had the opportunity to train with a soft robotic glove generally expressed high satisfaction levels in terms of comfort and perceived usefulness with regard to this technology, which can address a critical need in the field of neurorehabilitation. Moreover, many participants felt that the use of a soft robotic glove would facilitate the performance of ADL and iADL at home26,27,35 and even increase engagement into an intensive rehabilitation program.18,34 However, despite all positive feedback identified across studies, some aspects of the soft robotic gloves tested still represent a challenge for participants, especially the ease of use. Indeed, difficulties in donning and doffing the glove independently, represented an obstacle for many participants,15,26,27,31,34 and affected the compliance rate, and even led to abandonment of the technology, in some studies.15,27 Finally, depending on the context of use, opinions may differ on satisfaction with regards to the soft robotic glove. In order to meet stakeholders’ expectations, it would be important to consider their preferences when developing new soft robotic glove models and the expected context of their use when users are asked to comment: for example, it is important to distinguish between therapeutic use during rehabilitation and use as a robotic orthosis in everyday life.
Study limitations and mitigation strategies
The present systematic search and review of the literature, which mostly included studies adopting a research design ranking low on the hierarchy of scientific evidence (i.e. case study, case series, quasi-experimental study), limits the ability to draw strong conclusions regarding the effects or effectiveness of the soft robotic glove. The numerous challenges encountered when reviewing the selected articles (e.g. use of numerous robotic gloves with different attributes; recruitment of small and heterogeneous samples; adoption of numerous intervention protocols, predominantly realized within a research laboratory; selection of diverse outcome measures) further limits this capability.
Nonetheless, one may conclude, based on the currently available evidence, that the soft robotic glove represents a safe, feasible, and positively perceived intervention needing to be investigated further. To strengthen the current level of evidence regarding its potential effectiveness among individuals with sensorimotor impairments of the hand and fingers, particularly those recuperating from hand paresis or paralysis following a recent stroke (≤3 months), there is a need to conduct larger-scale pragmatic clinical trials with multiple baseline measurement times or randomized controlled clinical trials in which an appropriate comparator intervention is selected. Moreover, establishing a consensus on a minimal data set of outcome measures to evaluate the effects or effectiveness of the soft robotic glove could eventually facilitate a meta-analysis.
Conclusion
The present systematic search and review of the literature maps currently available evidence on the effect and effectiveness of different soft robotic gloves on hand and finger impairments and related functional disabilities among individuals who have had a neurological event and are engaged in rehabilitation intervention. The soft robotic glove stands as a promising assistive technology or adjunct rehabilitation intervention to optimize sensorimotor impairments and hand- and upper limb-related functional abilities, mainly among individuals with hand paresis or paralysis following a stroke. Moreover, the acceptability and the perceived usefulness of the soft robotic glove reaches satisfactory levels, although improvements still remain possible. This being said, the current level of evidence needs to be substantially strengthened before encouraging the use of the soft robotic glove to optimize functional abilities in daily life or confirming its effectiveness and formulating recommendations for eventual incorporation into neurorehabilitation programs, especially those offered during intensive functional rehabilitation, when the best neuroplastic adaptations may be anticipated.
Acknowledgement
CEP holds a scholarship from INSPIRE and the School of Rehabilitation (Université de Montréal) and DHG holds a senior research salary award from the Fonds de la recherche du Québec – Santé (FRQS).
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The project is supported by the Initiative for the development of new technologies and innovative practices in rehabilitation (INSPIRE) and the University of Montreal.
ORCID iDs
Florian Klug https://orcid.org/0000-0001-9147-7362
Mario Kupnik https://orcid.org/0000-0003-2287-4481
Dany H. Gagnon https://orcid.org/0000-0003-3464-4667
References
- 1.Neumann DA. Kinesiology of the musculoskeletal system; Foundation for rehabilitation. Maryland Heights, MO: Mosby Elsevier; 2010. [Google Scholar]
- 2.Yang Y, Fermuller C, Li Y, et al. Grasp type revisited: A modern perspective on a classical feature for vision. IEEE Conference on Computer Vision and Pattern Recognition 2015; 400–408.
- 3.Chu C-Y, Patterson RM. Soft robotic devices for hand rehabilitation and assistance: a narrative review. J Neuroengineering Rehabil 2018; 15: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Almenara M, Cempini M, Gómez C, et al. Usability test of a hand exoskeleton for activities of daily living: an example of user-centered design. Disabil Rehabil Assist Technol 2017; 12: 84–96. [DOI] [PubMed] [Google Scholar]
- 5.Kwakkel G, Kollen BJ, van der Grond J, et al. Probability of regaining dexterity in the flaccid upper limb: impact of severity of paresis and time since onset in acute stroke. Stroke 2003; 34: 2181–2186. [DOI] [PubMed] [Google Scholar]
- 6.Duncan PW, Bode RK, Lai SM, et al. Glycine Antagonist in Neuroprotection Americas investigators. Rasch analysis of a new stroke-specific outcome scale: the Stroke Impact Scale. Arch Phys Med Rehabil 2003; 84: 950–963. [DOI] [PubMed] [Google Scholar]
- 7.Bednar MS, Woodside JC. Management of upper extremities in tetraplegia: current concepts. JAAOS-J Am Acad Orthop Surg 2018; 26: e333–e341. [DOI] [PubMed] [Google Scholar]
- 8.Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma 2004; 21: 1371–1383. [DOI] [PubMed] [Google Scholar]
- 9.Chollet F, DiPiero V, Wise R, et al. The functional anatomy of motor recovery after stroke in humans: a study with positron emission tomography. Ann Neurol Off J Am Neurol Assoc Child Neurol Soc 1991; 29: 63–71. [DOI] [PubMed] [Google Scholar]
- 10.Dimyan MA, Cohen LG. Neuroplasticity in the context of motor rehabilitation after stroke. Nat Rev Neurol 2011; 7: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dobkin BH. Training and exercise to drive poststroke recovery. Nat Rev Neurol 2008; 4: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nudo RJ, Wise BM, SiFuentes F, et al. Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science 1996; 272: 1791–1794. [DOI] [PubMed] [Google Scholar]
- 13.Hubbard IJ, Parsons MW, Neilson C, et al. Task‐specific training: evidence for and translation to clinical practice. Occup Ther Int 2009; 16: 175–189. [DOI] [PubMed] [Google Scholar]
- 14.Schweighofer N, Choi Y, Winstein C, et al. Task-oriented rehabilitation robotics. Am J Phys Med Rehabil 2012; 91: S270–S279. [DOI] [PubMed] [Google Scholar]
- 15.Prange-Lasonder GB, Radder B, Kottink AI, et al. Applying a soft-robotic glove as assistive device and training tool with games to support hand function after stroke: preliminary results on feasibility and potential clinical impact. 2017 International Conference on Rehabilitation Robotics (ICORR), London, 2017, pp. 1401–1406. [DOI] [PubMed]
- 16.Kimberley TJ, Samargia S, Moore LG, et al. Comparison of amounts and types of practice during rehabilitation for traumatic brain injury and stroke. J Rehabil Res Dev 2010; 47: 851–861. [DOI] [PubMed]
- 17.Lang CE, MacDonald JR, Gnip C. Counting repetitions: an observational study of outpatient therapy for people with hemiparesis post-stroke. J Neurol Phys Ther 2007; 31: 3–10. [DOI] [PubMed] [Google Scholar]
- 18.Yap HK, Lim JH, Nasrallah F, et al. Design and preliminary feasibility study of a soft robotic glove for hand function assistance in stroke survivors. Front Neurosci 2017; 11: 547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shahid T, Gouwanda D, Nurzaman SG. Moving toward soft robotics: a decade review of the design of hand exoskeletons. Biomimetics 2018; 3: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009; 151: 264–269. [DOI] [PubMed] [Google Scholar]
- 21.Covidence. Melbourne: Covidence, 2017, https://www.covidence.org/
- 22.World Health Organization. International classification of functioning, disability and health: ICF. Geneva: World Health Organization, 2001. [Google Scholar]
- 23.Cohen J. Statistical power analysis for the behavioral sciences. Abingdon, UK: Routledge, 2013. [Google Scholar]
- 24.OCEBM Levels of Evidence Working Group. The Oxford 2011 Levels of Evidence Oxford Centre for Evidence-Based Medicine. http://www.cebm.net/index.aspx?o=5653
- 25.Brokaw EB, Black I, Holley RJ, et al. Hand Spring Operated Movement Enhancer (HandSOME): a portable, passive hand exoskeleton for stroke rehabilitation. IEEE Trans Neural Syst Rehabil Eng 2011; 19: 391–399. [DOI] [PubMed] [Google Scholar]
- 26.Cappello L, Meyer JT, Galloway KC, et al. Assisting hand function after spinal cord injury with a fabric-based soft robotic glove. J Neuroengineering Rehabil 2018; 15: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen J, Nichols D, Brokaw EB, et al. Home-based therapy after stroke using the hand spring operated movement enhancer (HandSOME). IEEE Trans Neural Syst Rehabil Eng 2017; 25: 2305–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scott S, Yu T, White KT, et al. A robotic hand device safety study for people with cervical spinal cord injury. Fed Pract 2018; 35: S21. [PMC free article] [PubMed] [Google Scholar]
- 29.Thielbar KO, Triandafilou KM, Fischer HC, et al. Benefits of using a voice and EMG-driven actuated glove to support occupational therapy for stroke survivors. IEEE Trans Neural Syst Rehabil Eng 2016; 25: 297–305. [DOI] [PubMed] [Google Scholar]
- 30.Kim GJ, Taub M, Creelman C, et al. Feasibility of an electromyography-triggered hand robot for people after chronic stroke. Am J Occup Ther 2019; 73: 7304345040p1–9. [DOI] [PubMed] [Google Scholar]
- 31.Bernocchi P, Mulè C, Vanoglio F, et al. Home-based hand rehabilitation with a robotic glove in hemiplegic patients after stroke: a pilot feasibility study. Top Stroke Rehabil 2018; 25: 114–119. [DOI] [PubMed] [Google Scholar]
- 32.Vanoglio F, Bernocchi P, Mulè C, et al. Feasibility and efficacy of a robotic device for hand rehabilitation in hemiplegic stroke patients: a randomized pilot controlled study. Clin Rehabil 2017; 31: 351–360. [DOI] [PubMed] [Google Scholar]
- 33.Milia P, Peccini MC, De Salvo F, et al. Rehabilitation with robotic glove (Gloreha) in poststroke patients. Digit Med 2019; 5: 62. [Google Scholar]
- 34.Radder B, Prange-Lasonder GB, Kottink AI, et al. Feasibility of a wearable soft-robotic glove to support impaired hand function in stroke patients. J Rehabil Med 2018; 50: 598–606. [DOI] [PubMed] [Google Scholar]
- 35.Yurkewich A, Hebert D, Wang RH, et al. Hand Extension Robot Orthosis (HERO) Glove: development and testing with stroke survivors with severe hand impairment. IEEE Trans Neural Syst Rehabil Eng 2019; 27: 916–926. [DOI] [PubMed] [Google Scholar]
- 36.Duncan SF, Saracevic CE, Kakinoki R. Biomechanics of the hand. Hand Clin 2013; 29: 483–492. [DOI] [PubMed] [Google Scholar]
- 37.Bokai W, Hongyue W, Xin M, et al. Comparisons of superiority, non-inferiority, and equivalence trials. Shanghai Arch Psychiatr 2017; 29: 385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nijland RH, van Wegen EE, Harmeling-van der Wel BC, et al. Presence of finger extension and shoulder abduction within 72 hours after stroke predicts functional recovery: early prediction of functional outcome after stroke: the EPOS cohort study. Stroke 2010; 41: 745–750. [DOI] [PubMed] [Google Scholar]
- 39.Stinear C. Prediction of recovery of motor function after stroke. Lancet Neurol 2010; 9: 1228–1232. [DOI] [PubMed] [Google Scholar]
- 40.Winters C, Heymans MW, van Wegen EE, et al. How to design clinical rehabilitation trials for the upper paretic limb early post stroke? Trials 2016; 17: 468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hong Y, Goldberg D, Dahlke DV, et al. Testing usability and acceptability of a web application to promote physical activity (iCanFit) among older adults. JMIR Hum Factors 2014; 1: e2. [DOI] [PMC free article] [PubMed] [Google Scholar]