Abstract
Objective
To explore the function and mechanism of long noncoding RNA (lncRNA) metastasis associated lung adenocarcinoma transcript 1 (MALAT1) in bronchopulmonary dysplasia.
Methods
Alveolar epithelial cell line BEAS-2B was used as the cell model. The role of MALAT1 and microRNA miR-129-5p in regulating cellular viability and migration were examined by using the CCK-8 and Transwell assays, respectively, in vitro. The luciferase reporter assay and real-time (RT)-PCR were performed to confirm that miR-129-5p was a target of MALAT1. ELISA was conducted to validate MALAT1 and show that miR-129-5p regulated the gene encoding high-mobility group protein 1 (HMGB1).
Results
Overexpression of MALAT1 significantly promoted cellular viability, whereas miR-129-5p had the opposite effect. miR-129-5p was shown to be a target of MALAT1, and HMGB1 could be upregulated by MALAT1 overexpression or miR-129-5p inhibition.
Conclusion
MALAT1 reduced the expression of miR-129-5p, promoting the viability of cells and blocking the development of bronchopulmonary dysplasia. In addition, MALAT1 increased the expression of HMGB1, which contributed to inflammation as the disease progressed.
Keywords: Bronchopulmonary dysplasia, preterm neonate, MALAT1, miR-129-5p, HMGB1, high-mobility group protein 1
Introduction
Bronchopulmonary dysplasia (BPD), a chronic lung disease resulting from mechanical ventilation and long-term use of oxygen, is mainly found in neonates (mostly premature) and infants with respiratory distress syndrome or other diseases requiring long-term mechanical ventilation.1 The incidence of BPD in preterm infants with gestational age <28 weeks is estimated to be 48% to 68%.2,3 The occurrence of BPD is associated with many factors, but essential to BPD is damage to the immature lung caused by factors such as oxygen poisoning, barotrauma, infection, and inflammation. In some cases, BPD is also associated with abnormal repair of lung tissues after injury on the basis of hereditary susceptibility. Underdeveloped lung, acute lung injury, and abnormal repair after injury are the three key factors that together cause BPD.4,5 At present, clinical treatment of BPD mainly involves nutritional support, limiting fluid intake, oxygen therapy, controlling infection, and promoting alveolar development.6,7 Because the molecular mechanism underlying BPD remains unclear, it is important to study the pathogenic factors of BPD to prevent and treat this disease.
Long noncoding RNA (lncRNA) is a type of RNA widely found in mammalian cells. It plays an important role in regulating molecular functions such as RNA processing, gene expression, and transcription.8 There is increasing evidence that lncRNAs are involved in cell growth and development and play key roles in the development and progression of various diseases such as cancer and cardiovascular diseases.9–11 The lncRNA metastasis associated lung adenocarcinoma transcript 1 (MALAT1) was one of the first lncRNAs discovered and associated with human diseases.12 MALAT1 is ubiquitously expressed in almost all tissues of the human body, with the highest expression in pancreas and lung.13 It has been reported that MALAT1 protects premature infants with BPD by inhibiting apoptosis,14 and it also regulates endothelial cell function and blood vessel growth. In vitro experiments indicate that hypoxia induces expression of MALAT1 in endothelial cells.15,16 However, the downstream mechanism of MALAT1 remains unclear and needs further clarification.
MicroRNAs (miRNAs) are a group of endogenous noncoding RNA molecules, about 22 nt long. miRNAs play an important role in cell differentiation, metabolism, proliferation, and apoptosis.17 They are involved in a variety of diseases, but current research on their role in lung development, homeostasis, and disease is still in its early stages.18 miR-129-5p has been shown to prevent UV-induced corneal epithelial damage by upregulating expression of epidermal growth factor receptor (EGFR).19 miR-129-5p can also inhibit the proliferation and invasion of lung cancer cells.20 However, the role of miR-129-5p in the development and progression of BPD requires further research.
High-mobility group box 1 (HMGB1), a protein that plays an important role in the pathogenesis of inflammatory diseases, has a proinflammatory cytokine-like effect and is an important mediator of pulmonary inflammatory response.21,22 Studies have shown that HMGB1 promotes release of the inflammatory mediators and activation and aggregation of neutrophils, thereby aggravating lung injury.23 The expression of HMGB1 is significantly increased after lung injury, and excessive release of HMGB1 may aggravate the inflammatory response, destroy the physiological barrier, and even cause multiple organ failure.21 miR-129-5p has been confirmed to regulate, in a targeted manner, the expression of HMGB1 in a large number of studies. For example, miR-129-5p attenuates the proliferation of gastric cancer cells and epithelial-mesenchymal transition through HMGB1,24 and miR-129-5p overexpression improves neuroinflammation and blood-spinal cord injury after ischemia-reperfusion by inhibiting HMGB1.25 However, the effect of miR-129-5p targeting HMGB1 on BPD remains unknown.
In this study, we investigated the expression, function, and clinical significance of MALAT1, miR-129-5p, and HMGB1 in BPD. The results showed that MALAT1 was upregulated in patients with BPD, whereas miR-129-5p was downregulated. In addition, in vitro studies have shown that both MALAT1 overexpression and miR-129-5p inhibition promote the viability and migration of lung epithelial cells. MALAT1 inhibited the expression of miR-129-5p and increased the expression of HMGB1, thereby inhibiting cell apoptosis, and was involved in the development of BPD, which suggests the significance of a potential MALAT1/miR-129-5p/HMGB1 axis in BPD, providing a theoretical framework for the prevention and treatment of BPD.
Materials and methods
Tissue and blood samples
According to the guidelines of The National Institute of Child Health and Human Development (NICHD), 20 infants diagnosed with BPD and 20 age-matched infants without BPD were selected. These infants were diagnosed in the department of neonatal intensive care unit, Jiaxing Maternity and Child Health Care Hospital, Jiaxing City, Zhejiang Province, China. Blood samples were obtained from all participants at 36 weeks of postmenstrual age. Diagnostic criteria included (1) preterm low-birth-weight infants with respiratory insufficiency who still required oxygen therapy at 36 weeks of postmenstrual age; (2) typical X-ray or computed tomography signs of BPD in the lungs (e.g., enhanced texture, reduced permeability, emphysema, cystic changes). Infants with other diseases such as congenital heart disease, pneumothorax, or infection were excluded. The study was approved by the Clinical Ethics Committee of Jiaxing Maternity and Child Health Care Hospital. Written informed consent was given by patients’ families. The clinical information of the patients is shown in Table 1.
Table 1.
General information of patients with and without bronchopulmonary dysplasia (BPD).
| Gestational age(week) | Birth weight (g) | Mechanical ventilation time (d) | Oxygen inhalation time (d) | |
|---|---|---|---|---|
| BPD patients (n = 20) | 29.85 ± 2.18 | 1328 ± 182.42 | 16.10 ± 4.28 | 46.18 ± 7.56 |
| Non-BPD patients (n = 20) | 30.18 ± 2.07 | 1400 ± 175.59 | 3.42 ± 1.18 | 18.71 ± 3.82 |
| t | 0.491 | 1.272 | 12.954 | 14.504 |
| P | 0.626 | 0.211 | <0.001 | <0.001 |
Cell culture
The human lung epithelial cell line BEAS-2B was purchased from Shanghai Suran Biotechnology Co. Ltd. (Shanghai, China), and cultured in RPMI-1640 medium (Gibco/Invitrogen, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS; Gibco, Shanghai, China) and 100 U/mL penicillin/streptomycin (Gibco/Invitrogen) in a thermostatically controlled incubator at 37°C in 5% CO2.
Total RNA isolation and real-time PCR
Total RNA was isolated from cells or plasma samples of the participants (BPD and non-BPD groups) using TRIzol reagent according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA, USA). RNA purity and concentration were measured using a NanoDrop ND-2000 spectrophotometer (NanoDrop, Wilmington, DE, USA). Moloney murine leukemia virus (M-MLV) reverse transcriptase (Thermo Fisher Scientific Inc., Rockford, IL, USA) was used to generate cDNA. Then, real-time (RT)-PCR was performed on an ABI 7300 system (Applied Biosystems, Foster City, CA, USA) with SYBR Green PCR Master mix (Thermo Fisher Scientific, Waltham, MA, USA). GAPDH was used as internal reference gene to detect MALAT1 and HMGB1, and U6 was used as internal reference gene to detect miR-129-5p. The 2−ΔΔCt method was used to calculate the relative expression of MALAT1, miR-129-5p, and HMGB1. Primers are listed in Table 2.
Table 2.
Primer sequences for quantitative real-time PCR.
| Name | Primer sequences (5′-3′) |
|---|---|
| MALAT1 | Forward: CTATGCTGTTGGCACGACA |
| Reverse: TCCTGAGGTGACTGTGAACC | |
| miR-129-5p | Forward: ACACTCCTTTTTGCGTCTGGGCTTGC |
| Reverse: TGGTGTCGTGGAGTCG | |
| HMGB1 | Forward: GCATGGGCAAAGGAGATCCTAA |
| Reverse: TTAGCAGACATGGTCTTCCAC | |
| GAPDH | Forward: GCCCAATACGACCAAATCC |
| Reverse: AGCCACATCGCTCAGACAC | |
| U6 | Forward: GGTCGGGCAGGAAAGAGGGC |
| Reverse: CTAATCTTCTCTGTATCGTTCC |
ELISA
The HMGB1 protein content in cell supernatant was determined according to the instructions of the HMGB1 ELISA kit (MultiSciences, Hangzhou, China). The optical density value was measured using an BioRad 550 automatic microplate reader (BioRad Laboratories, Hercules, CA, USA) at a wavelength of 450 nm. A standard curve was drawn using the Revelation Spectra MR software (Dynex Technologies, Chantilly, VA, USA), with the standard concentration as the abscissa, and the absorbance value of the optical density as the ordinate. The HMGB1 concentration of the specimen was calculated from the standard curve using the absorbance value of the specimen.
Cell transfection
GenePharma Co. Ltd. (Shanghai, China) provided the pcDNA3.1 vector (normal control, NC), pcDNA3.1-MALAT1, miR-129-5p mimics, and miR-129-5p inhibitors. The BEAS-2B cells were inoculated into a 24-well plate at a density of 3 × 105 cells/well. Cells were cultured at 37°C and 5% CO2 for 24 hours before transfection. In compliance with the supplier’s instructions, BEAS-2B cells were transfected with Lipofectamine 2000 (Invitrogen). The transfection efficiency was determined by RT-PCR.
CCK-8 assay
Cell growth curves were determined using CCK-8 method. First, BEAS-2B cells were harvested in logarithmic phase using trypsin. Cell suspensions were prepared, and 3,000 cells per well were added to wells of a 96-well plate and cultured for 1, 2, 3, and 4 days, respectively. On each day, 10 µL of Enhanced Cell Counting Kit-8 (Beyotime, Beijing, China) was added into each well and incubated at 37°C for 1 hour. Then, the absorbance of cells was measured at 450 nm. Each experiment was repeated three times.
Transwell migration assay
BEAS-2B cells were dispersed with 0.25% trypsin, centrifuged, and resuspended in serum-free medium. Transwell chambers (8 µm pore size; Corning, Beijing, China) were used to detect migration of the cells. In brief, 5 × 104 transfected cells were placed in the upper compartment. Medium containing 10% FBS was added to the lower compartment. After being cultured at 37°C for 24 hours, cells that failed to migrate were removed. Then, the Transwell membrane was fixed with 4% paraformaldehyde for 10 minutes and stained with 0.5% crystal violet. After the membrane was rinsed in tap water, the cells were counted under an inverted microscope. All experiments were repeated three times.
Dual-luciferase reporter assay
The luciferase reporter assay was performed using a dual-luciferase reporter assay system (Promega, Madison, WI, USA). Target fragments of wild-type MALAT1 and mutant MALAT1 were constructed and integrated into pGL3 vector (Promega) to construct pGL3-MALAT1-wild type (MALAT1-wt) and pGL3-MALAT1-mutant (MALAT1-mut) reporter vectors. MALAT1-wt or MALAT1-mut was co-transfected into BEAS-2B cells with miR-129-5p mimics or negative control (NC) miRNA. After 48 hours of transfection, luciferase activity was determined according to the manufacturer’s instructions. All experiments were repeated three times.
Statistical methods
All experiments were repeated three times. All data are expressed as mean ± standard deviations. Statistical comparisons between two groups were performed using the t test, and P < 0.05 was considered statistically significant. All statistical analyses were performed using the SPSS 22.0 software (IBM Corp., Armonk, NY, USA).
Results
MALAT1, miR-129-5p, and HMGB1 expression
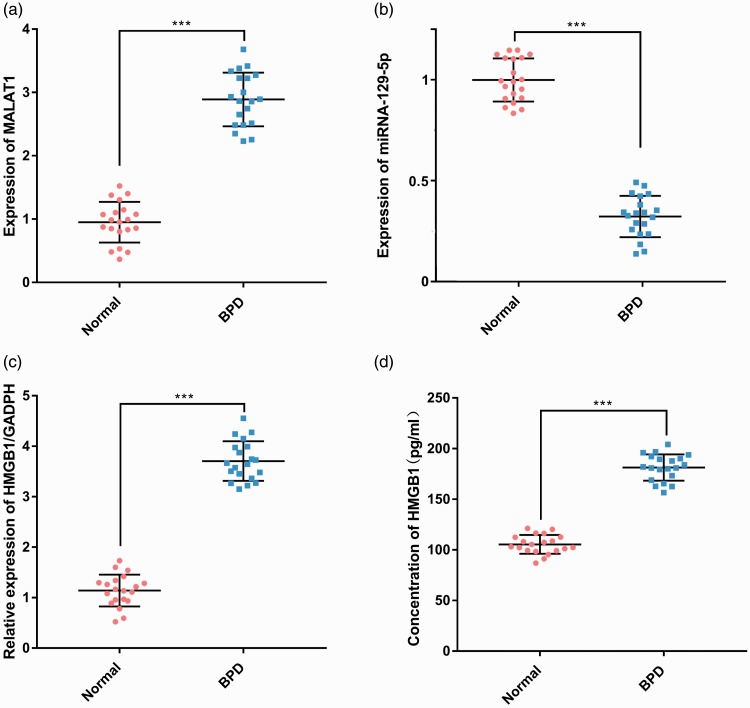
To clarify the expression of MALAT1, miR-129-5p, and HMGB1 in BPD, we used quantitative real-time (qRT)-PCR and ELISA to detect the expression levels of MALAT1, miR-129-5p, and HMGB1 in blood samples from 20 infants with BPD and 20 infants without BPD. Expression of MALAT1 and HMGB1 was significantly upregulated in the BPD group compared with that of the non-BPD group (P < 0.001, Figure 1a, c, and d), whereas expression of miR-129-5p was significantly downregulated (P < 0.001, Figure 1b) .
Figure 1.
Expression characteristics of MALAT1, miR-129-5p, and HMGB1 in blood samples of patients with BPD and healthy infants. The expression of MALAT1 (a) and miR-129-5p (b) in blood samples of the patients was detected by RT-PCR. The relative expression (c) and concentration (d) of HMGB1 in blood samples of the patients was detected by RT-PCR and ELISA, respectively. ***P < 0.001 compared with the control group. MALAT1, metastasis associated lung adenocarcinoma transcript 1; HMGB1, high-mobility group protein 1; BPD, bronchopulmonary dysplasia; RT-PCR, real-time PCR.
MALAT1 overexpression in lung epithelial cells
To further explore and confirm the effect of MALAT1 on lung epithelial cells, we used pcDNA3.1 plasmid to construct BEAS-2B cells with overexpressed MALAT1 (P < 0.001, Figure 2a). The cell viability assay showed that the viability of the MALAT1 overexpression cells was significantly higher than that of the control group on days 2, 3, and 4 (P < 0.01, Figure 2b). Cell migration in the MALAT1 overexpression group was significantly higher than that of the control group (P < 0.01, Figure 2c). To clarify the effect of MALAT1 overexpression on expression of miR-129-5p and HMGB1, we used qRT-PCR and ELISA to detect their expression, respectively. Expression of miR-129-5p in the MALAT1 overexpression group was reduced compared with that in the control group (P < 0.01, Figure 2d), and HMGB1 expression was significantly higher than that of the control group (P < 0.001, Figure 2e and f). These results indicated that overexpression of MALAT1 significantly promoted the viability and migration of lung epithelial cells, inhibited the expression of miR-129-5p, and increased the expression of HMGB1.
Figure 2.
Effect of MALAT1 overexpression on lung epithelial cells and expression of miR-129-5p and HMGB1. (a) BEAS-2B cells overexpressing MALAT1 were constructed and transfection efficiency was detected by RT-PCR. (b) The viability of cells in the two groups (MALAT1 and NC) was detected by CCK-8 assay. (c) The migration of cells in the MALAT1 and NC groups was detected by the Transwell assay. (d) The expression of miR-129-5p in the two groups was detected by RT-PCR. The relative expression (e) and concentration (f) of HMGB1 in blood samples in the two groups was detected by RT-PCR and ELISA, respectively. **P < 0.01 and ***P < 0.001 compared with the NC group. MALAT1, metastasis associated lung adenocarcinoma transcript 1; HMGB1, high-mobility group protein 1; BEAS-2B, alveolar epithelial cell line; OD, optical density; RT-PCR, real-time PCR; NC, negative control (not overexpressing MALAT1).
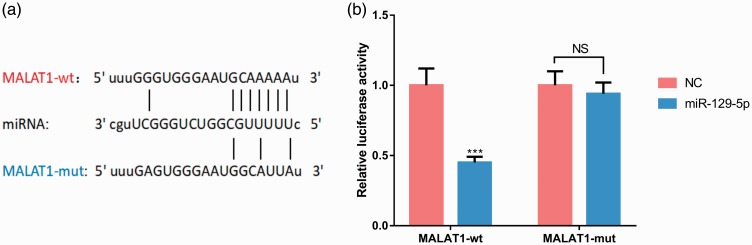
miR-129-5p binding to MALAT1
To further explore the downstream molecular mechanisms of MALAT1, we performed bioinformatics analysis using the StarBase database (http://www.starbase.com), which showed that MALAT1 contained a target site for miR-129-5p (Figure 3a). To verify this prediction, we performed the dual-luciferase reporter assay, which confirmed that miR-129-5p reduced luciferase activity of the reporter carrying wild-type MALAT1 (P < 0.001) but had no effect on the reporter carrying mutant MALAT1 (P < 0.001, Figure 3b). This result indicated that miR-129-5p was directly bound to MALAT1 at the recognition site of miRNA.
Figure 3.
miR-129-5p binds directly to MALAT1 at the recognition site of miRNA. (a) MALAT1 contains a target site for miR-129-5p, which was predicted by StarBase database (http://www.starbase.com). (b) miR-129-5p reduced the luciferase activity of a reporter carrying the wild-type MALAT1 sequence. ***P < 0.001 compared with the NC group; P > 0.05, nonsignificant (NS). MALAT1, metastasis associated lung adenocarcinoma transcript 1; NC, negative control; MALAT1-wt, MALAT1 wild-type; MALAT1-mut, MALAT1 mutant.
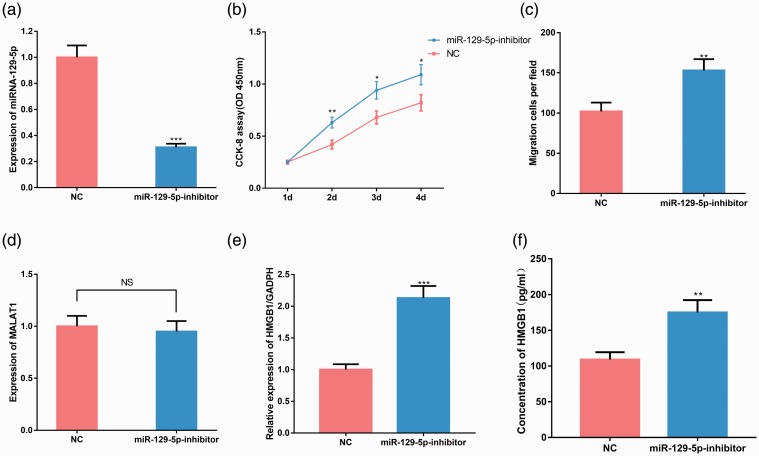
miR-129-5p in lung epithelial cells and expression of HMGB1
To study the effect of miR-129-5p on viability and migration of lung epithelial cells, we constructed BEAS-2B cells with miR-129-5p inhibition (P < 0.001, Figure 4a). Cell viability experiments showed that the viability of BEAS-2B cells with miR-129-5p inhibition was significantly higher than that of the control group (P < 0.05, Figure 4b). The Transwell assay showed that migration of BEAS-2B cells with miR-129-5p inhibition was significantly higher than that of the control group (P < 0.01, Figure 4c). No significant difference was observed in the expression of MALAT1 between the miR-129-5p inhibition group and the control group (Figure 4d), whereas expression of HMGB1 in the miR-129-5p inhibition group was significantly higher than that of the control group (P < 0.01, Figure 4e and f). These results indicated that miR-129-5p significantly inhibited the growth and migration of lung epithelial cells, and its inhibition increased the expression of HMGB1.
Figure 4.
Effect of miR-129-5p inhibition on lung epithelial cells and expression of MALAT1 and HMGB1. (a) BEAS-2B cells with miR-129-5p inhibition was constructed using miR-129-5p inhibitors, and transfection efficiency was validated by RT-PCR. (b) The viability of cells in the two groups (miR-129-5p inhibitor and NC) was detected by CCK-8 assay. (c) The migration of cells in the two groups was detected by Transwell assay. (d) The expression of MALAT1 in the two groups was detected by RT-PCR. The relative expression (e) and concentration (f) of HMGB1 in blood samples in the two groups was detected by RT-PCR and ELISA, respectively. *P < 0.05, **P < 0.01 and ***P < 0.001 compared with the NC group; P > 0.05, nonsignificant (NS). MALAT1, metastasis associated lung adenocarcinoma transcript 1; HMGB1, high-mobility group protein 1; BEAS-2B, alveolar epithelial cell line; RT-PCR, real-time PCR; NC, negative control (not overexpressing MALAT1).
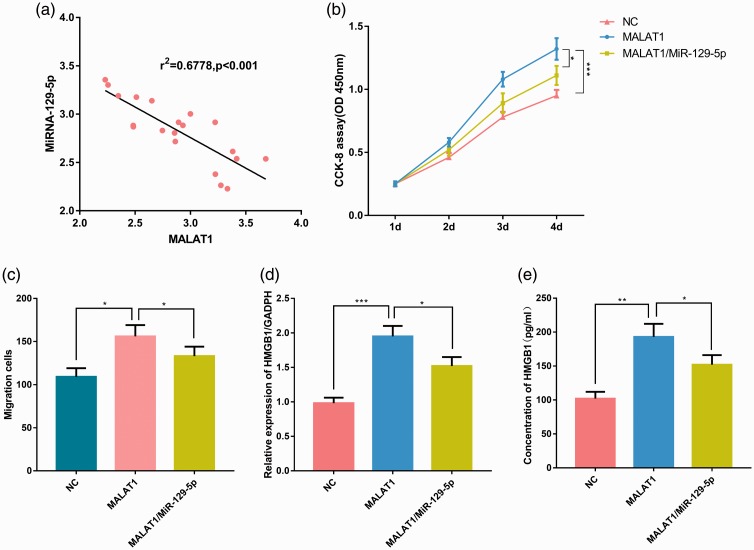
Effect of MALAT1 on lung epithelial cell viability and migration
To investigate whether MALAT1 negatively regulated miR-129-5p expression in BPD, we used qRT-PCR to detect expression of MALAT1 and miR-129-5p in blood samples from 20 infants with BPD. The expression level of MALAT1 in BPD samples was negatively correlated with the expression level of miR-129-5p (P < 0.001, Figure 5a). To further determine the relationship between MALAT1 and miR-129-5p, we constructed two cell lines: one with overexpressed MALAT1 and one with overexpressed MALAT1 and miR-129-5p. The CCK-8 assay showed that the viability of the MALAT1 group was significantly higher than that of the MALAT1/miR-129-5p group or the NC group; and the viability of the MALAT1/miR-129-5p group was significantly higher than that of the NC group, demonstrating that miR-129-5p reduced the proliferation of BEAS-2B cells, whereas overexpressed MALAT1 inhibited the action of miR-129-5p (P < 0.05, Figure 5b). Similarly, the Transwell experiment demonstrated that overexpression increased the migration capacity of BEAS-2B cells, which was offset by miR-129-5p (P < 0.05, Figure 5c). To further investigate the mechanism of action of MALAT1 and miR-129-5p in regulating HMGB1 expression, we used qRT-PCR and ELISA to detect the expression of HMGB1 in the NC, MALAT1, and MALAT1/miR-129-5p groups. Expression of HMGB1 in the MALAT1/miR-129-5p group was lower (P < 0.05) than that in the MALAT1 group, but higher (P < 0.001) than that in the NC group (Figure 5d and e), indicating that HMGB1 was modulated by the MALAT1/miR-129-5p axis.
Figure 5.
The function of MALAT1 is mediated by miR-129-5p. (a) The expression of MALAT1 and miR-129-5p in BPD samples was detected by RT-PCR and was shown to be significantly negatively correlated. (b) MALAT1 and MALAT1/miR-129-5p cell lines were constructed, and cellular viability was compared by CCK-8 proliferation assay. (c) The Transwell assay was used to detect cell migration. The relative expression (d) and concentration (e) of HMGB1 in the three groups (MALAT1, MALAT1/miR-129-5p, and NC) was detected by RT-PCR and ELISA, respectively. *P < 0.05, **P < 0.01 and ***P < 0.001 compared with the MALAT1 group. MALAT1, metastasis associated lung adenocarcinoma transcript 1; BPD, bronchopulmonary dysplasia; RT-PCR, real-time PCR; HMGB1, high-mobility group protein 1; BEAS-2B, alveolar epithelial cell line; NC, negative control.
Discussion
BPD is a chronic lung disease in newborns; it is a highly destructive disease that can develop into multiple organ dysfunctions.26 At present, BPD is a difficult problem in the treatment of premature infants, seriously threatening the life of infants. However, the current research on BPD at the molecular level is still in an early stage.
LncRNAs participate in the occurrence and development of human diseases through multiple mechanisms. They also play important regulatory roles in lung development and pathophysiology. For example, LncRNA LL18/NANCI regulates lung endoderm gene expression and plays an important role in the differentiation of lung epithelial cells and development of the respiratory tract.27,28 Knocking out specific lncRNA in rats could lead to abnormal lung growth and development.29 Importantly, a recent study reports that in the lung tissues of a BPD mice model, compared with the control group, 882 lncRNAs were upregulated and 887 were downregulated, indicating the importance of lncRNAs in BPD development.30 In the present study, we found that MALAT1 expression was significantly increased in blood samples from infants with BPD, and MALAT1 overexpression promoted the proliferation and migration of lung epithelial cells, which is consistent with a previous report.14
Previous studies have found that miRNA also plays a crucial role in lung development. For example, dynamic changes in miRNAs can be observed during lung development;31–33 miR-127 is increased in the late stage of fetal lung development, and its overexpression in the early stage of fetal lung development leads to defective terminal bud formation and uneven development of the lung.31 miR-129-5p has been widely investigated in cancer biology, and in most cases, it is regarded as a pro-apoptotic factor.19,20 In this study, we found that expression of miR-129-5p in lung epithelial cells was significantly decreased when MALAT1 was overexpressed, and there was no significant change in MALAT1 expression after miR-129-5p inhibition. Therefore, we concluded that miR-129-5p was a downstream target of MALAT1 and could be suppressed by it. Our demonstration partly explained the underlying mechanism of MALAT1 in BPD development and highlighted the role of miR-129-5p in the pathogenesis of this disease.
HMGB1, a proinflammatory mediator that maintains a chronic inflammatory state and amplifies inflammatory cascades, is elevated in pathophysiological processes such as infection, injury, and ischemia.34,35 The role of HMGB1 in the pathogenesis of BPD has been studied previously.36 Expression of HMGB1 in lung tissue of mice with hyperoxia-induced bronchopulmonary hypoplasia is significantly increased, and HMGB1 expression increases further with the prolongation of hyperoxic exposure time.36 Another study demonstrated that the neutralizing antibodies against HMGB1 attenuate the damage of lung structure under hyperoxic conditions but, at the same time, HMGB1 eliminates proinflammatory mediators such as interleukins and reduces lung inflammation in the late stage of lung development.37 Previous studies have confirmed that miR-129-5p can inhibit the expression of HMGB1 and affect various diseases such as breast cancer and osteosarcoma.38,39 In this study, HMGB1 was shown to be upregulated in blood samples of infants with BPD. We also validated that HMGB1 was a target gene of miR-129-5p and could be indirectly upregulated by MALAT1. Therefore, we concluded that a MALAT1/miR-129-5p/HMGB1 axis exists in the development and progression of BPD. Based on our data, we hypothesize that even though the upregulation of MALAT1 and inhibition of miR-129-5p reduce apoptosis of lung epithelial cells, their constant dysregulation probably exacerbates the inflammatory response and is involved in the pathophysiological process of BPD by upregulating HMGB1.
To summarize, we demonstrated that MALAT1 participated in the development of BPD by targeting miR-129-5p to increase the expression of HMGB1 in lung epithelial cells. Our results help clarify the mechanism of BPD development and provide a theoretical basis for treatment of this disease.
Data availability statement
The data used to support the findings of this study are available from the corresponding author upon request.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
Rongwe Yangi https://orcid.org/0000-0001-9902-9062
References
- 1.Popova AP. Mechanisms of bronchopulmonary dysplasia. J Cell Commun Signal 2013; 7: 119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hwang JS, Rehan VK. Recent advances in bronchopulmonary dysplasia: pathophysiology, prevention, and treatment. Lung 2018; 196: 129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalikkot TR, Guaman MC, Shivanna B. Bronchopulmonary dysplasia: a review of pathogenesis and pathophysiology. Respir Med 2017; 132: 170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silva DM, Nardiello C, Pozarska A, et al. Recent advances in the mechanisms of lung alveolarization and the pathogenesis of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 2015; 309: L1239–L1272. [DOI] [PubMed] [Google Scholar]
- 5.Hilgendorff A, O’Reilly MA. Bronchopulmonary dysplasia early changes leading to long-term consequences. Front Med (Lausanne) 2015; 2: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iyengar A, Davis JM. Drug therapy for the prevention and treatment of bronchopulmonary dysplasia. Front Pharmacol 2015; 6: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Principi N, Di Pietro GM, Esposito S. Bronchopulmonary dysplasia: clinical aspects and preventive and therapeutic strategies. J Transl Med 2018; 16: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esteller M. Non-coding RNAs in human disease. Nat Rev Genet 2011; 12: 861–874. [DOI] [PubMed] [Google Scholar]
- 9.Mohanty V, Gokmen-Polar Y, Badve S, et al. Role of lncRNAs in health and disease-size and shape matter. Brief Funct Genomics 2015; 14: 115–129. [DOI] [PubMed] [Google Scholar]
- 10.Botti G, Marra L, Malzone MG, et al. LncRNA HOTAIR as prognostic circulating marker and potential therapeutic target in patients with tumor diseases. Curr Drug Targets 2017; 18: 27–34. [DOI] [PubMed] [Google Scholar]
- 11.Lorenzen JM, Thum T. Long noncoding RNAs in kidney and cardiovascular diseases. Nat Rev Nephrol 2016; 12: 360–373. [DOI] [PubMed] [Google Scholar]
- 12.Zhang X, Hamblin MH, Yin KJ. The long noncoding RNA Malat1: its physiological and pathophysiological functions. RNA Biol 2017; 14: 1705–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tano K, Mizuno R, Okada T, et al. MALAT-1 enhances cell motility of lung adenocarcinoma cells by influencing the expression of motility-related genes. Febs Lett 2010; 584: 4575–4580. [DOI] [PubMed] [Google Scholar]
- 14.Cai C, Qiu J, Qiu G, et al. Long non-coding RNA MALAT1 protects preterm infants with bronchopulmonary dysplasia by inhibiting cell apoptosis. BMC Pulm Med 2017; 17: 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michalik KM, You X, Manavski Y, et al. Long noncoding RNA MALAT1 regulates endothelial cell function and vessel growth. Circ Res 2014; 114: 1389–1397. [DOI] [PubMed] [Google Scholar]
- 16.Lelli A, Nolan KA, Santambrogio S, et al. Induction of long noncoding RNA MALAT1 in hypoxic mice. Hypoxia (Auckl) 2015; 3: 45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sayed D, Abdellatif M. MicroRNAs in development and disease. Physiol Rev 2011; 91: 827–887. [DOI] [PubMed] [Google Scholar]
- 18.Ameis D, Khoshgoo N, Iwasiow BM, et al. MicroRNAs in lung development and disease. Paediatr Respir Rev 2017; 22: 38–43. [DOI] [PubMed] [Google Scholar]
- 19.Yang Y, Gong B, Wu ZZ, et al. Inhibition of microRNA-129-5p expression ameliorates ultraviolet ray-induced corneal epithelial cell injury via upregulation of EGFR. J Cell Physiol 2019; 234: 11692–11707. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, An J, Lv W, et al. MiRNA-129-5p suppresses cell proliferation and invasion in lung cancer by targeting microspherule protein 1, E-cadherin and vimentin. Oncol Lett 2016; 12: 5163–5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ding J, Cui X, Liu Q. Emerging role of HMGB1 in lung diseases: friend or foe. J Cell Mol Med 2017; 21: 1046–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang R, Chen R, Zhang Q, et al. HMGB1 in health and disease. Mol Aspects Med 2014; 40: 1–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Souza A, Chirico M, Cartelle CT, et al. High-fat diet increases HMGB1 expression and promotes lung inflammation in mice subjected to mechanical ventilation. Oxid Med Cell Longev 2018; 2018: 7457054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang S, Chen Y, Yu X, et al. MiR-129-5p attenuates cell proliferation and epithelial mesenchymal transition via HMGB1 in gastric cancer. Pathol Res Pract 2019; 215: 676–682. [DOI] [PubMed] [Google Scholar]
- 25.Li XQ, Chen FS, Tan WF, et al. Elevated microRNA-129-5p level ameliorates neuroinflammation and blood-spinal cord barrier damage after ischemia-reperfusion by inhibiting HMGB1 and the TLR3-cytokine pathway. J Neuroinflammation 2017; 14: 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jung EY, Park KH, Han BR, et al. Amniotic fluid infection, cytokine levels, and mortality and adverse pulmonary, intestinal, and neurologic outcomes in infants at 32 weeks’ gestation or less. J Korean Med Sci 2017; 32: 480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herriges MJ, Swarr DT, Morley MP, et al. Long noncoding RNAs are spatially correlated with transcription factors and regulate lung development. Genes Dev 2014; 28: 1363–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herriges MJ, Tischfield DJ, Cui Z, et al. The NANCI-Nkx2.1 gene duplex buffers Nkx2.1 expression to maintain lung development and homeostasis. Genes Dev 2017; 31: 889–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sauvageau M, Goff LA, Lodato S, et al. Multiple knockout mouse models reveal lincRNAs are required for life and brain development. Elife 2013; 2: e1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bao TP, Wu R, Cheng HP, et al. Differential expression of long non-coding RNAs in hyperoxia-induced bronchopulmonary dysplasia. Cell Biochem Funct 2016; 34: 299–309. [DOI] [PubMed] [Google Scholar]
- 31.Bhaskaran M, Wang Y, Zhang H, et al. MicroRNA-127 modulates fetal lung development. Physiol Genomics 2009; 37: 268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang Y, Kai G, Pu XD, et al. Expression profile of microRNAs in fetal lung development of Sprague-Dawley rats. Int J Mol Med 2012; 29: 393–402. [DOI] [PubMed] [Google Scholar]
- 33.Lu Y, Thomson JM, Wong HY, et al. Transgenic over-expression of the microRNA miR-17-92 cluster promotes proliferation and inhibits differentiation of lung epithelial progenitor cells. Dev Biol 2007; 310: 442–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang L, Ji Y, Kang Z, et al. Protocatechuic aldehyde ameliorates experimental pulmonary fibrosis by modulating HMGB1/RAGE pathway. Toxicol Appl Pharmacol 2015; 283: 50–56. [DOI] [PubMed] [Google Scholar]
- 35.Su Z, Yin J, Wang T, et al. Up-regulated HMGB1 in EAM directly led to collagen deposition by a PKCbeta/Erk1/2-dependent pathway: cardiac fibroblast/myofibroblast might be another source of HMGB1. J Cell Mol Med 2014; 18: 1740–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feng J, Deng C, Yu JL, et al. [ Expression of high mobility group protein-B1 in mice with hyperoxia-induced bronchopulmonary dysplasia]. Zhongguo Dang Dai Er Ke Za Zhi 2010; 12: 219–223. [PubMed] [Google Scholar]
- 37.Yu B, Li X, Wan Q, et al. High-Mobility group box-1 protein disrupts alveolar elastogenesis of hyperoxia-injured newborn lungs. J Interferon Cytokine Res 2016; 36: 159–168. [DOI] [PubMed] [Google Scholar]
- 38.Liu K, Huang J, Ni J, et al. MALAT1 promotes osteosarcoma development by regulation of HMGB1 via miR-142-3p and miR-129-5p. Cell Cycle 2017; 16: 578–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luo J, Chen J, He L. Mir-129-5p attenuates irradiation-induced autophagy and decreases radioresistance of breast cancer cells by targeting HMGB1. Med Sci Monit 2015; 21: 4122–4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.