Abstract
A 56-year-old man diagnosed with non-Hodgkin’s lymphoma underwent autologous bone marrow transplantation. He was subsequently admitted to the hospital with fever, and his symptoms were initially controlled by multiple antibiotics, including tigecycline. He then developed a generalized body rash that improved after treatment with anti-allergy drugs and steroids. Furthermore, tigecycline treatment for a second time resulted in a severe skin reaction with systemic symptoms, suggesting toxic epidermal necrolysis syndrome (TEN). The patient was shown to have the slow-metabolizing cytochrome P450 2C19 allele, denoted CYP2C19*2. He was transferred to a laminar flow ward and given strict mucosal care, together with corticosteroids and intravenous immunoglobulin. He recovered after 3 weeks of treatment. Tigecycline-induced Stevens–Johnson syndrome (SJS)/TEN has rarely been reported in the Chinese population. However, our experience suggests that Asians are more likely to have adverse reactions to drugs metabolized by the cytochrome P450 enzyme. Early identification of drug reactions and immediate cessation of the suspected drug is essential. Additionally, a combined therapy scheme and a clean laminar flow environment may improve the cure rate of SJS/TEN.
Keywords: Adverse event, Stevens–Johnson syndrome, toxic epidermal necrolysis, tigecycline, CYP2C19, skin rash
Introduction
Stevens–Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are rare but severe cutaneous adverse reactions (SCARs) associated with high mortality. SJS and TEN represent different stages of the same disease characterized by blistering lesions and epithelial detachment. Many drugs have been reported to cause these conditions.1 Tigecycline is a broad-spectrum glycylcycline antibiotic that is widely used because of its satisfactory antibacterial activity against multidrug-resistant bacteria.2 However, tigecycline has been associated with several adverse events. Here, we present a case of tigecycline-induced TEN syndrome in a patient with diffuse large B-cell lymphoma (DLBCL) who underwent autologous stem cell transplantation (ASCT).
Case report
A 56-year-old male patient was admitted to the hospital with primary central nervous system malignant lymphoma. The pathology was DLBCL. He underwent excision of the primary lesion and six phases of chemotherapy. Autologous hematopoietic stem cells were collected between the 3rd and 4th chemotherapy phases and he underwent ASCT approximately 33 days after completion of the above treatment. He was then discharged with successful hematopoietic reconstitution. However, on the 18th day after transplantation, he developed a fever without shivering or chills and was admitted to hospital. Routine blood test results were almost normal, but his procalcitonin was increased to 4.55 ng/mL, and serum albumin was decreased to 24.5 g/L. Other laboratory tests were normal, including negative findings for mycoplasma IgM and herpes simplex virus IgM.
The patient’s cellular and particularly their humoral immune functions failed to recover within 2 months after ASCT. Additionally, empiric antibiotic therapy is the best choice for serious infections with no definite etiology or drug-sensitivity results. We therefore initiated a comprehensive anti-infective regimen consisting of sulperazone, teicoplanin, voriconazole, and ganciclovir. However, the patient’s fever persisted after 5 days of active treatment. Teicoplanin was replaced with tigecycline (50 mg every 12 hours by intravenous drip) because of suspected drug-resistant bacterial infection. The patient’s temperature rapidly normalized after starting the new therapy, but he developed widespread erythema within 72 hours after the change in the treatment scheme (Figure 1a). Based on the opinion of a dermatologist, the patient was diagnosed with a drug allergy, and all potentially responsible drugs were stopped immediately. Prednisone, loratadine, and calamine were given to control the rash. There was no new erythema and the skin lesions darkened. The patient was finally discharged after his temperature had remained normal for 1 week. He continued to take oral prednisone at a dose of 20 mg/day.
The patient experienced fever again with cough and phlegm on the 7th post-discharge day, with a peak temperature of 39.1°C. The results of etiological examinations were similar to the previous examinations, and similar therapy, including tigecycline at the same dose, was given to control his temperature. However, the erythematous rash unexpectedly covered up to 95% of his body surface area during the following 12 hours. Skin detachment occurred over most of the body, with minimal shearing forces causing the epidermis to peel back (with a positive Nikolsky sign) (Figure 1b). Skin biopsy from a lesion on the abdomen showed superficial and deep perivascular infiltrates of lymphocytes and histiocytes, in accordance with inflammatory changes (Figure 2). The patient was diagnosed with toxic epidermal necrolysis (TEN) syndrome. There was no evidence of any related drug–drug interactions, and notably, some drugs, including sulperazone, teicoplanin, voriconazole, and ganciclovir, had been used multiple times in previous treatment schemes with no adverse cutaneous reactions. After reviewing the patient’s treatment history and calculating the ALDEN scores (an algorithm for assessment of drug causality in SJS and TEN) for all the related drugs, tigecycline was suspected to be the responsible drug, with a high score suggesting that its involvement was “very probable” (Table 1).3 The patient subsequently experienced ocular inflammation and involvement of the mucous membranes of the mouth and genitalia (Figure 1c). He was transferred to the laminar flow ward to reduce the risk of infection and provided with comprehensive strict mucosal care. Calamine lotion, Vaseline, and ethacridine were applied to protect the skin surface and prevent skin infection. Injected corticosteroids (methylprednisolone, 40 mg/day) and intravenous immunoglobulin (20 g/day) were administered. The patient underwent whole-body excoriation and skin integrity was maintained, except for epidermal discoloration (Figure 3). He was finally discharged after 3 weeks, with a favorable prognosis. We analyzed the patient’s cytochrome P450 (CYP) 2C19 status and showed the presence of the slow-metabolizing allele, CYPC219*2.
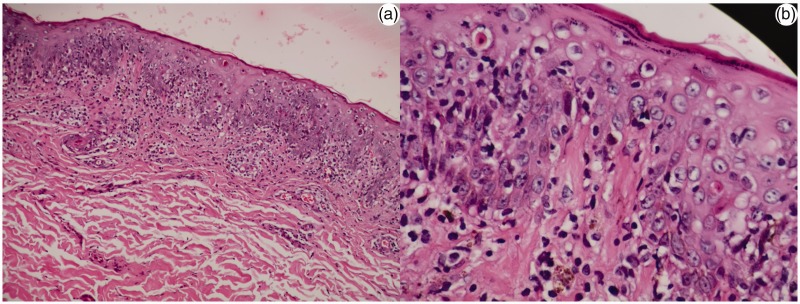
Figure 2.
Histopathology of skin biopsy showing epidermal mild hyperplasia and superficial and deep perivascular infiltrates of lymphocytes and histiocytes (hematoxylin and eosin; a, ×10; b, ×40).
Table 1.
Drugs potentially responsible for SJS/TEN according to ALDEN score.
| Drug | Tigecycline | Voriconazole | Ganciclovir | Teicoplanin | Sulperazone | Prednisone |
|---|---|---|---|---|---|---|
| Treatment period (days) | 4 | 31 | 29 | 22 | 30 | 30 |
| Final score* | 7 | 2 | 1 | 2 | 4 | 1 |
*Final score <0, very unlikely; 0–1, unlikely; 2–3, possible; 4–5, probable; ≥6, very probable.
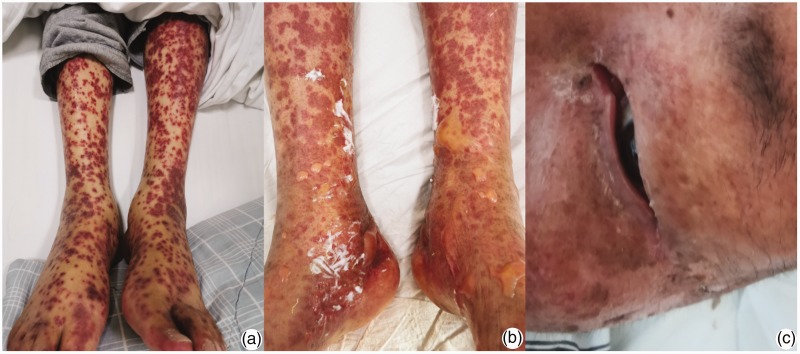
Figure 1.
(a) Rashes before the second antibiotic treatment; (b) multiple central necrosis and flaccid bullae on the lower limbs with positive Nikolsky signs; (c) mucosal damage to the eyes.
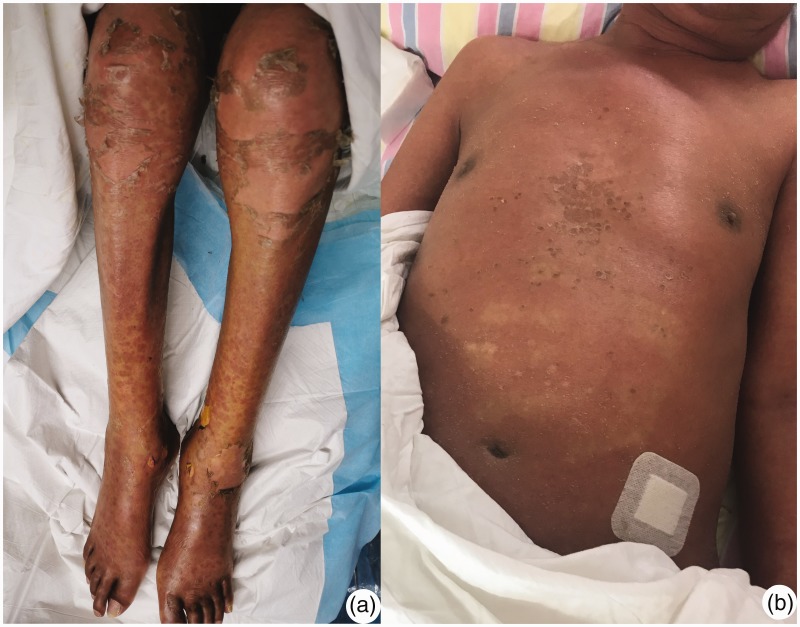
Figure 3.
Skin integrity following epidermal necrosis and exfoliation was maintained by combined therapy.
Case reports do not need to be approved by a review board. The patient described in this report provided informed consent for the use of his data.
Discussion
SJS and TEN are rare drug reactions, with an incidence of approximately one to two cases per million per year. However, affected patients might experience significant mortality, according to the TEN-specific severity of illness score (SCORTEN) system.4 This validated prognostic system was established to predict individual prognosis based on several important clinical factors, including age, malignancy, tachycardia, and epidermal-detachment area, together with the results of some relevant laboratory tests, such as serum urea, glucose, and bicarbonate levels.4 The characteristics of SJS/TEN are widespread epithelial necrosis caused by drug-induced cytotoxic T lymphocytes. SJS is typically diagnosed when <10% of the skin surface is involved, TEN is characterized by >30% involvement, and SJS/TEN overlap is defined as 10% to 30% skin detachment.5 The SCORTEN score in the current patient was 3, with an approximately 32% probability of death, and he was diagnosed with TEN because more than 30% of his body was covered by the skin lesions. However, direct immunofluorescence to exclude autoimmune bullous disorders was not performed in this case. Nevertheless, SJS/TEN is often induced by some kinds of drugs, such as allopurinol, carbamazepine, and non-steroidal anti-inflammatory drugs, and early identification of the culprit drug is critical to prevent progression of the disease.2
A correlation between human leucocyte antigen (HLA) and SJS/TEN has recently been identified, and the incidence of this adverse event was found to be reduced in individuals of certain ethnicities. A previous study reported that patients of Southeast Asian descent carrying the HLA-B*1502 allele were at increased risk of SJS/TEN caused by some antiepileptic drugs, such as carbamazepine and phenytoin.6 Moreover, SJS/TEN might be associated with the HLA-B*5701 allele in patients receiving abacavir, and with the HLA-B*5801 allele in patients using allopurinol.7 Genetic polymorphisms of cytochrome P450 have also been proposed to be involved in the predisposition to drug-related SCARs. CYP2C9*3, encoded by the CYP2C9*3 mutant allele, might be an independent predictor of the risk of phenytoin-induced SJS/TEN based on several studies in Asia.8 Similarly, CYP2C9*2 was suggested to be related to phenobarbital-induced skin reaction syndrome.9 In the current case, we identified the CYP2C19*2/*3 polymorphism in the patient, indicating poor metabolism of relevant drugs.
Tigecycline is a broad-spectrum antibiotic that belongs to the glycylcycline family, with activity against a wide range of Gram-positive and Gram-negative bacterial families.10 Kadoyama et al. analyzed 248 tigecycline-associated spontaneous adverse event reports submitted to the U.S. Food and Drug Administration from 2004 to 2009, and noted that SJS/TEN was rare, but had been reported as a related adverse drug reaction.11 However, there have been few reports of SCARs in the Chinese population. Tigecycline was not metabolized by CYP enzymes according to the results of in vitro studies,12 suggesting a weak relationship between CYP polymorphisms and tigecycline-induced TEN. However, it is possible that there may be some complex and important correlations between tigecycline and drug metabolic pathways.13
Comprehensive management and treatment is recommended for SJS/TEN because of the severe and multisystemic injuries to the skin and membranes. Management involves careful and aseptic skin handling, similar to that for burn care, as well as strict fluid balance and nutritional support. Some mucosal protection of related systems, including ocular and gastrointestinal care, is also important, together with temperature management, pain control, and monitoring/treatment of super-infections. Furthermore, considering potential immune system involvement, immunomodulatory therapies, including corticosteroids, cyclosporine, tumor necrosis factor inhibitors, intravenous immunoglobulins, plasmapheresis, and hemoperfusion, have also been used in individual cases of SJS/TEN, but with insufficient evidence to support their use.2 In the current case, the patient recovered after transfer to a laminar flow ward and systemic treatment, suggesting that a clean laminar flow environment may help to prevent infections and improve the prognosis.
Immediate cessation of the suspected drug is essential for all treatments. This was not performed in the current case, and it is possible that TEN could have been prevented if the culprit drug had been identified and discontinued sooner. In patients with a previous history of drug allergies, drugs with similar structures should be avoided. Furthermore, drugs associated with a high risk of inducing SJS/TEN should be monitored carefully during the medication period. For patients with a previous history of a rash or epidermal necrolysis, anti-infection schemes must be chosen cautiously according to the source of the infection and pathogens present in the patient. It is essential to check the drug instructions, and to consult reviews and pharmacovigilance databases, such as the Food and Drug Administration Adverse Event Reporting System (FAERS),14 to identify possible risks.
We report a rare case of SJS/TEN induced by tigecycline in a Chinese patient. Asians might be more likely to have adverse skin reactions because of genetic polymorphisms of cytochrome P450; however, further studies are needed to clarify this correlation. Use of a laminar flow environment and strict skin and mucosa care might improve the prognosis of patients with SJS/TEN. This case highlights the need to pay close attention to the use of drugs likely to cause this condition.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
Huachao Zhu https://orcid.org/0000-0003-0941-2418
References
- 1.Schwartz RA, Mcdonough PH, Lee BW. Toxic epidermal necrolysis: part II. Prognosis, sequelae, diagnosis, differential diagnosis, prevention, and treatment. J Am Acad Dermatol 2013; 69: 187.e1–e16. [DOI] [PubMed] [Google Scholar]
- 2.Nicolau DP. Management of complicated infections in the era of antimicrobial resistance: the role of tigecycline. Expert Opin Pharmacother 2009; 10: 1213–1222. [DOI] [PubMed] [Google Scholar]
- 3.Sassolas B, Haddad C, Mockenhaupt M, et al. ALDEN, an algorithm for assessment of drug causality in Stevens–Johnson syndrome and toxic epidermal necrolysis: comparison with case–control analysis. Clin Pharmacol Ther 88: 60–68. [DOI] [PubMed] [Google Scholar]
- 4.Bastuji-Garin S, Fouchard N, Bertocchi M, et al. SCORTEN: a severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol 2000; 115: 149–153. [DOI] [PubMed] [Google Scholar]
- 5.Bastuji-Garin S, Rzany B, Stern RS, et al. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol 1993; 129: 92–96. [PubMed] [Google Scholar]
- 6.Nguyen DV, Chu HC, Nguyen DV, et al. HLA-B * 1502 and carbamazepine-induced severe cutaneous adverse drug reactions in Vietnamese. Asia Pac Allergy 2015; 5: 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caudle KE, Rettie AE, Whirl-Carrillo M, et al. Clinical pharmacogenetics implementation consortium guidelines for CYP2C9 and HLA-B genotypes and phenytoin dosing. Clin Pharmacol Ther 2014; 96: 542–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung WH, Chang WC, Lee YS, et al. Genetic variants associated with phenytoin-related severe cutaneous adverse reactions. JAMA 2014; 312: 525–534. [DOI] [PubMed] [Google Scholar]
- 9.Laska AJ, Han MJ, Lospinoso JA, et al. CYP2C19*2 status in patients with Stevens-Johnson syndrome and toxic epidermal necrolysis. Pharmgenomics Pers Med 2017; 10: 183–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slover CM, Rodvold KA, Danziger LH. Tigecycline: a novel broad-spectrum antimicrobial. Ann Pharmacother 2007; 41: 965–972. [DOI] [PubMed] [Google Scholar]
- 11.Kadoyama K, Sakaeda T, Tamon A, et al. Adverse event profile of tigecycline: data mining of the public version of the U.S. Food and Drug Administration adverse event reporting system. Biol Pharm Bull 2012; 35: 967–970. [DOI] [PubMed] [Google Scholar]
- 12.Livermore DM. Tigecycline: what is it, and where should it be used? J Antimicrob Chemother 2005; 56: 611–614. [DOI] [PubMed] [Google Scholar]
- 13.Wallace RJ, Jr, Brown-Elliott BA, Crist CJ, et al. Comparison of the in vitro activity of the glycylcycline tigecycline (formerly GAR-936) with those of tetracycline, minocycline, and doxycycline against isolates of nontuberculous mycobacteria. Antimicrob Agents Chemother 2002; 46: 3164–3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin YF, Yang CH, Sindy H, et al. Severe cutaneous adverse reactions related to systemic antibiotics. Clin Infect Dis 2014; 58: 1377–1385. [DOI] [PubMed] [Google Scholar]