Abstract
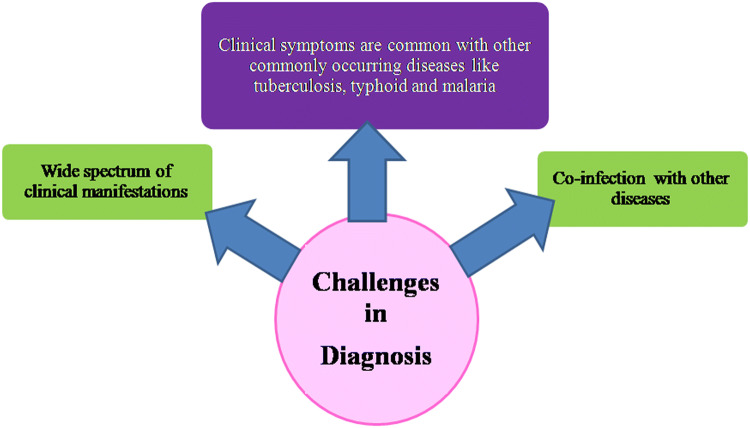
Diagnosis of leishmaniasis has always been a major challenge as its clinical features resemble some other commonly occurring diseases such as tuberculosis, typhoid, and malaria. Reliable laboratory methods become important for differential diagnosis. Demonstration of the parasites in stained preparations of bone marrow and splenic aspirates being risky and invasive is still the gold standard for diagnosis. Serological tests utilizing rapid immunochromatographic formats or rK39 in enzyme linked immune sorbent assay, immunoblotting, direct agglutination test have complications related to high proportions of positive asymptomatic individuals and the inability to diagnose a relapse. Among the molecular techniques, polymerase chain reaction is the most commonly used technique that is successfully implied for diagnosis. This review provides updated information on the recent developments in the field of diagnosis in leishmaniasis, various methods utilized with their advantages and limitations.
Keywords: Leishmania, Diagnosis, Parasitological, Immunological, Molecular
Introduction
The parasitic protozoan of genus Leishmania and family Trypanosomatidae are responsible for causing leishmaniasis. They are characterized by possessing kinetoplast, a distinctive form of mitochondrial DNA (Sharma and Singh 2008). Leishmaniasis is distributed in subtropical and tropical regions affecting 97 countries in America, Africa, Asia and Europe (Steverding 2017). It can occur in three main forms viz., Visceral Leishmaniasis (VL), Cutaneous Leishmaniasis (CL) and Mucocutaneous Leishmaniasis (MCL). The numbers of cases show changes or variations with time and are challenging to estimate. For VL, approximated cases per year may have been reduced to < 100,000, but earlier estimations ranged up to 400,000 or more. For CL, the number of cases has ranged from 700,000 to 1.2 million or more (CDC 2018). The disease is prevalent on every continent except Antarctica and Australia. In the eastern hemisphere, leishmaniasis is found in the Middle East, Africa, Asia, and Southern Europe. In the western region, it is prevalent in Central and South America and Mexico (Chhabra and Singla 2014). It is not seen in Uruguay and Chile. Infrequent cases of CL have been reported in Oklahoma and Texas (WHO 2016). VL moved from Southern India and became endemic in Eastern states of Assam, Bihar, and Bengal until the late 1990s (Singh 2014). More recently, epidemiology of VL was seen mainly in eastern states of the country, including West Bengal, Jharkhand, Uttar Pradesh, and Bihar (Thakur et al. 2018). Bihar is the most distressed state with 90% of the caseload, although the VL elimination target has been successful in eliminating 366 out of 456 blocks in Bihar (Olliaro et al. 2017). Also, VL cases reported in India have declined from 32,803 in 2005 to 6231 in 2016. Besides the states mentioned above with high caseloads, reports on re-emergence of the disease in the states of Assam, Tamil Nadu, and Gujarat along with the finding of newer distressed regions have been outlined during the last decade (Dhiman 2014). Although there is an overall reduction in VL incidence in India as a consequence of the VL elimination program in the highly endemic areas, the new cases from mostly non-endemic regions appear to be increasing, and this is of utmost importance to avoid reemergence and to achieve sustainable elimination of disease from the country (Thakur et al. 2018).
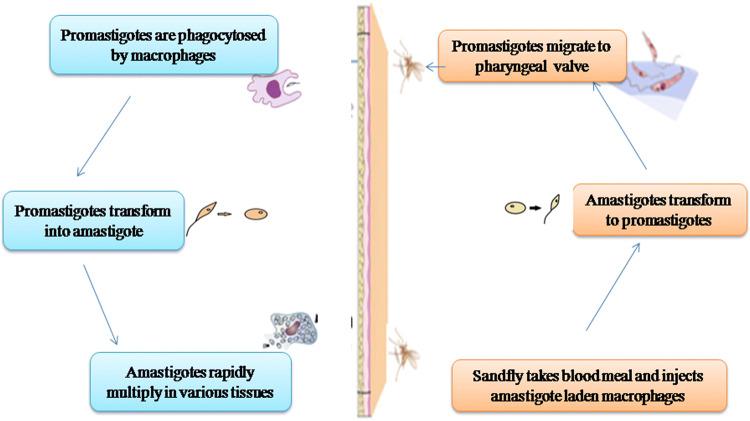
Leishmania comprises of two developmental stages amastigotes and promastigotes. The amastigotes are non-flagellated spherical cells ranging in size from 2 to 4 µm in diameter. Promastigote forms are thin elongate cells with an emergent flagellum and anterior kinetoplast. They are usually lance-like in shape and range in size from 5 to 14 µm in length by 1.5–3.5 µm in width. Different parasite species are usually not differentiated by morphological differences, but rather on the basis of geographical, biological and clinical features (Chhabra and Singla 2014). Both vertebrates and invertebrates are their hosts, the mammal being the final host and sandfly (Phlebotomus in the Old World and Lutzomyia in the New World) (Sharma and Singh 2008), the intermediate host. Promastigotes being propagative forms occur in the female sandfly lumen, and amastigotes are found inside phagolysosomes in different mammalian hosts. The transmission begins when the infected phlebotomine sandfly carrying Leishmania parasite bites its mammalian host (human) (Teixeira et al. 2013).
The disease encounters humans when the reservoir host and flies share the same environment (Lemma et al. 2017). The first form is anthroponotic VL, with transmission of infection from humans to humans and the second form is zoonotic VL, with transmission between animals to humans. Epidemiological studies of VL worldwide have incriminated several animal species as reservoirs for zoonotic VL, including dogs, jackals, rodents, and foxes (Mukhtar et al. 2000; Reithinger et al. 2002; Chappuis et al. 2007). Transmission of disease begins when infected sand fly bites its mammalian host (Fig. 1) (Ready 2013). However, the infection can rarely be transmitted by other means such as needle sharing, blood transfusion, or from mother to child during pregnancy (Alemayehu and Alemayehu 2017). Clinical symptoms of leishmaniasis rely on interactions between immune responses of host and aspects of Leishmania; this results in a spectrum of diseases from localized skin lesions to involvement of reticuloendothelial system (Sharma and Singh 2008).
Fig. 1.
Life cycle of Leishmania donovani
Co-infections
Co-infection of leishmaniasis and Human immunodeficiency virus (HIV) has important diagnostic, clinical and epidemiological implications. HIV is one of the significant health problems worldwide, and about 369 lakh people are living with HIV and 20 lakh new infections are surveyed every year. Overlapping between transmission areas of leishmaniasis and HIV co-infection is very commonly observed (Lindoso et al. 2016). The presence of parasites in peripheral blood of HIV infected patients makes them a source of infection for the vectors (Chappuis et al. 2007). Both diseases target the same immune cells, thus exerting a cumulative effect on the immune system (Pintado et al. 2001). In co-infected patients, HIV modifies the presentation of Leishmania. VL increases the rate of onset of AIDS and decreases the lifetime of HIV infected patients. Also, HIV upregulates the rate of VL to a great extent. This dual response leads to insufficiency in CD4+ T cell immune response, enhancing the severity of disease (Singh 2014).
Intestinal parasitic infections might also affect the disease severely by changing cell-mediated immunity and by damaging effects of malnutrition (Diro et al. 2015). A strong Th2 response, a characteristic feature of helminthic infection (Maizels et al. 2012) is seen to suppress the protective Th1 response in patients with VL contributing to immunosuppression that is characteristic to VL patients (Nylén and Sacks 2007) (Fig. 2).
Fig. 2.
Challenges in diagnosis of Leishmania parasite
CL and malaria are the two most severe vector-borne parasitic diseases (Alvar et al. 2012) affecting millions of people. Overlapping geographical distribution of Leishmania and malaria depicts that susceptibility and severity to both conditions are elevated during co-infection. Since the two parasites do not compete for the same host cells, the interaction between them is not direct and is dependent upon host immune response induced by the pathogen (Coleman et al. 1998).
Co-infection of VL and pulmonary tuberculosis are increasing problems of public health in developing countries. Infection of leishmaniasis changes the protective immune response to BCG vaccine against tuberculosis (Li and Zhou 2013). Although the transmission mechanism and etiology of both diseases are different, yet they share several features. Tuberculosis is an immunosuppressive condition that provokes the progression of latent leishmaniasis to clinical leishmaniasis, and VL can provoke latent tuberculosis (Shweta et al. 2014).
Immune response to Leishmania
The life cycle of Leishmania begins when the infected sandfly carrying the metacyclic promastigote bites the host during its blood meal. Within few minutes the promastigotes are taken up by the phagocytic cells such as macrophages and neutrophils (Antoine et al. 1998). After internalization inside phagolysosomes of macrophages, promastigotes begin to differentiate into small, non-motile amastigote forms, that divide multiple times by binary fission, ultimately rupturing the macrophages to infect surrounding macrophages (Liu and Uzonna 2012).
Once the initial infection starts, monocytes are recruited to infected tissue and begin to differentiate into macrophages (Yang et al. 2014). T cells in lymphoid tissues and spleen are activated both by dendritic cells and the macrophages. The naïve T cells get activated into Th0 cells (intermediate stage) which then travel to the liver and get triggered as Th1 cells on coming in contact with dendritic cells and macrophages in IL-12 environment (Siewe et al. 2016). During this attachment, CD4+ T cells recognize the antigens which are bound to Major Histocompatibility Complex (MHC) (Dhanji and Teh 2003). CD4+ T cells then produce IL-12, which in turn triggers CD8+ T cells and enhance CD4+ T cell multiplication. Both CD8+ T cells and CD4+ T cells yield IFN-γ, which in turn activates the macrophages to wipe out the parasites (Siewe et al. 2016). The advancement of the disease is mainly related to Th 2 type of immune response along with an increase in levels of interleukin-10, IL-5, IL-4 and transforming growth factor (Torres-Guerrero et al. 2017).
Treatment
Treatment remains an overwhelming question as different species of Leishmania show various manifestations, which makes the treatment even more complicated (Kotb Elmahallawy and Agil 2015). Despite the availability of different treatment approaches to treat leishmaniasis, therapeutic tools are not adequate to eradicate this infection. These compounds face high cost, drug resistance, toxicity and some other side effects (Ghorbani and Farhoudi 2018). Pentavalent antimoniate was first introduced 60 years ago, and it was the most effective drug with good results (Torres-Guerrero et al. 2017). Pentavalent antimoniate such as sodium stibogluconate is given at a dose of 20 mg/kg body weight for a month, and these drugs can be used both in combination or alone (Akbari et al. 2017). Treatment with antimonials is affected due to adverse effects such as cardiac arrhythmia, pancreatitis, hepatotoxicity and nephrotoxicity (Freitas-Junior et al. 2012). These are also not recommended for pregnant women due to several limitations like parenteral administration (Akbari et al. 2017). Amphotericin B is an anti-fungal drug that is used as a second line treatment (Sundar et al. 2000). Liposomal formulation of this drug is more useful for the treatment of VL (Copeland and Aronson 2015). Miltefosine is the first oral drug for the treatment of VL. It is given for a month at a dose of 50–100 mg/day. Despite its numerous advantages, it has certain side effects such as nephrotoxicity, hepatotoxicity and being teratogenic (Sundar and Singh 2016). The available treatment for leishmaniasis is overwhelmed with resistance to some of the currently available drugs. Mechanism of drug resistance is often related to lower drug uptake, increased efflux, rapid rate of drug metabolism, modifications of drug targets and over expression of drug transporters. The widespread presence of leishmaniasis and appearance of drug resistance reveals the urge to develop and explore novel, less toxic and more promising therapeutic approaches (Yasinzai et al. 2013). Combination drugs for the treatment of VL can be utilized to combat the drug resistance developed by monotherapy. It can broaden the spectrum, enhance the activity of the drug by additive or synergistic action, decrease the duration and dosage, reduce the side effects, the cost of treatment and the emergence of drug resistance (Musa et al. 2012).
Vaccines
Patients once cured of leishmaniasis, are generally immune to second attack of the disease (Gupta et al. 2013). This fact leads to the development of vaccines to immunize the individuals which are at risk of infection (Reithinger et al. 2007). No vaccines are available for humans against leishmaniasis. However, vaccines such as genetically attenuated live parasites; freeze-thawed/heat-killed parasites, recombinant proteins and DNA vaccines are in the process of development (Thomaz-Soccol et al. 2018). The live attenuated Leishmania parasites can be used to develop a successful vaccine (Ismail et al. 2017). However, chances of reversion of attenuated form of Leishmania to virulent form and possibility of attenuated form, causing the disease in immunocompromised individuals is a major concern (Palatnik-de-Sousa 2008). Vaccines with a dead parasite or with fragments are called “first generation vaccines” against the Leishmania parasites (Thomaz-Soccol et al. 2018). Other vaccine candidates like recombinant proteins or subunit vaccines are weak immunogens that fail to mount an adequate immune response (Dunning 2009). Vaccines made from DNA have many advantages over other vaccine (first and second generation vaccines) strategies as they are cheap, fast, and simpler to produce on a large scale, they do not require low temperature for transport and storage and can give protection against more than one species of Leishmania and for a long time (Donnelly et al. 2000) But the major concern with DNA vaccine is the risk of integrating parasite DNA into the mammalian genome, which can sometimes result in the induction of an autoimmune disease or cancer (Dunning 2009). However, in light of its many advantages over other Leishmania vaccine strategies, DNA vaccination could prove to be the best approach for use in therapy and prophylaxis against human leishmaniasis (Dunning 2009).
Diagnosis
The first sign of infection is small erythema at the site of bite. After this erythema formation, the infection begins, and parasites cause an inflammatory reaction due to which erythema develops into an open ulcer or visceralize to organs like spleen and liver. These inflammatory reactions of parasites depend upon the species of parasite or its host immunity, strain and several other unknown factors (Reithinger and Dujardin 2007). Thus, the early diagnosis of leishmaniasis is of great significance to prevent the development of severe clinical manifestations and mortality in patients with VL. Conventional diagnosis depends upon the microscopical examination of amastigotes in the aspirates from tissues of different organs like spleen, lymph nodes, liver, skin, and can also be done by parasite culturing from these sites. However, this process of aspirate examination is not comfortable for the patients, and the method of isolating the parasite from culture is time-consuming, expensive and difficult to perform (Mugasa et al. 2010).
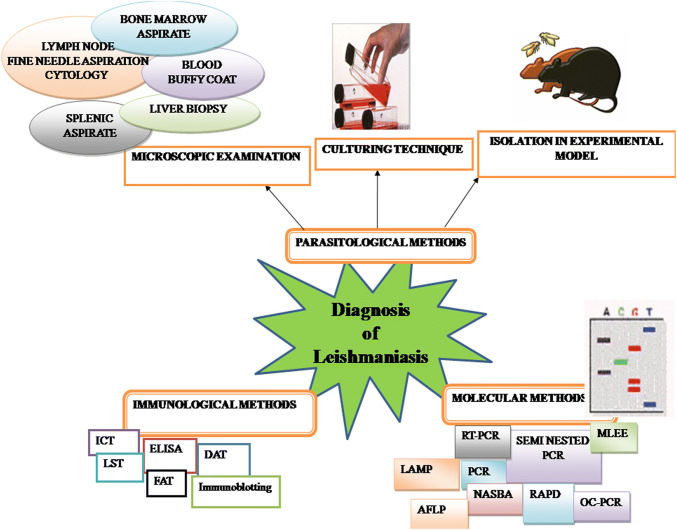
A number of diagnostic methods have been produced with considerable variations in accuracy of diagnosis, including the parasitological examination (histopathology, microscopy and parasite culture), serological technique and molecular diagnostics (Fig. 3) (Goto and Lindoso 2010). Thus, this review aims to highlight the recent progress in the diagnosis of leishmaniasis and its limitation with focus on what can be the most successful methods for developing fast, economic, specific and sensitive diagnostic tests.
Fig. 3.
Diagrammatic representation of different methods used in diagnosis of leishmaniasis
Culture techniques Parasite culturing enhances the detection sensitivity, although it is seldom used for routine clinical practices. Isolating and culturing the parasites gives better diagnostic sensitivity and organisms can also be identified to genotype and species level. Parasite species characterization and identification can be done by combination of culture and multilocus enzyme electrophoresis (Reithinger et al. 2007). Solid NNN (Novy-MacNeal-Nicolle) medium containing 20–30% rabbit blood or liquid Schneider’s insect culture medium can be used for isolating and culturing the promastigote forms (Singh 2006). Some new culture methods which can enhance the sensitivity are micro-culture method (MCM), recent modifications of MCM includes usage of peripheral blood mononuclear cells (PBMC) and buffy coat (WBC rich layer) (Maurya et al. 2010). Mini and microculture techniques are advantageous being cheaper as compared to other cultures because they need small volume of culture medium and are also easy to handle even if the parasite burden is low (Boggild et al. 2008).
Lateral flow biosensors (LFB) LFBs are the devices based on single use paper carrier materials, where dry reagents are activated by putting fluid sample. LFBs are significant for the diagnosis as they are sensitive, specific, affordable, rapid, robust and equipment free (Parolo and Merkoçi 2013). Immunochromatography based assays are easy to use and provide rapid qualitative results (Mettler et al. 2005; Pattabhi et al. 2010). Lateral flow strips for rK39 antigens are available commercially for detection of visceral leishmaniasis, but their performance is still not optimal (Welch et al. 2008; Solano-Gallego et al. 2011). Alternative methodologies include using interdigitated electrodes for detection of antibodies, implementation of paper biosensors, or utilizing nanoparticles for detecting Leishmania antigens and unamplified DNA (Andreadou et al. 2012, 2014). Several lateral flow assays have been proposed for detecting PCR amplified Leishmania specific DNA, including OligoC-TestT PCR-oligochromatographic test; an OligoC-TestT variation for Leishmania typing; a generic lateral flow oligochromatographic dipstick paper (Rivas et al. 2015).
Parasitological methods of diagnosis
Parasitological methods of diagnosis remain the gold standard for diagnosing leishmaniasis (de Vries et al. 2015) and are very crucial in eco-epidemiological studies (Shirian et al. 2014). Diagnosis in the laboratory can be achieved by direct examination of amastigotes in giemsa-stained lesion smears of scrapping, biopsies, or impression smears (Boelaert et al. 2007). The most frequently used samples are bone marrow or splenic aspirates. However, amastigotes can also be identified in other samples like buffy coat of peripheral blood, lymph nodes, and liver biopsies (Elmahallawy et al. 2014). Of all the samples, the highest sensitivity was observed with splenic aspirates (93–99%) (Srivastava et al. 2011). Splenic aspirates are an abundant source of Leishmania parasites, but their examination corresponds to a high risk of hemorrhage in unskilled hands (Barrett and Croft 2012). In a study conducted in India, it was observed that fatal bleeding appeared in two out of 9612 splenic aspirate procedures in a duration of 10 years (Sundar and Rai 2002). Also, one death was reported out of 671 patients that were administered with splenic aspiration in Kenya for a period of 10 years, and three deaths were reported in India out of 3000 (Boelaert et al. 2007). Giemsa-stained bone marrow showed a sensitivity of about 60–80%, but the sensitivity of peripheral blood smears was low particularly in patients with low parasitemia (Elmahallawy et al. 2014) and higher in HIV-coinfected individuals (Cota et al. 2013). Although liver biopsies have little risk of rupture and bleeding but identifying the amastigotes is possible only in case of heavy infections (Barrett and Croft 2012). Lymph node aspiration technique can be used when an increase in the size of a lymph node is seen, such as in VL patients and the sensitivity of this method range from 52 to 58% (Boelaert et al. 2007). However, in vitro, cultures of blood cells and tissue aspirates have shown 100% sensitivity (Hide et al. 2007). Cultures prepared from bone marrow and splenic aspirates show high specificity (Srividya et al. 2012) and the highest sensitivity is observed with splenic aspirates (93–99%). Being very tedious, exorbitant, and time-consuming, it is confined to research laboratories only (Singh and Sundar 2015). In a study, 155 suspicious patients with CL in Mashhad City were investigated by three diagnostic methods, and the results so obtained were compared. Results showed a sensitivity of 44.5% by microscopic investigation of smears and 48.5% with cultures (Shahbazi et al. 2008). In another study, 62 CL samples were diagnosed using kinetoplastid DNA PCR, microscopic evaluations and parasite culture in Diagnosis and Treatment Centre in Turkey. Results indicated a sensitivity of 54.3% with culture and 71.4% by microscopy. However, microscopy and culture technique when combined together, sensitivity was increased to 88.6% (Zeyrek et al. 2018). Parasites can also be inoculated in the laboratory animals, like mice, guinea pigs, hamsters or rodents (Ready 2014), but this method is not considered as a first diagnostic procedure as several months are required to demonstrate the parasites in these animals (Sundar and Rai 2002). Parasitological methods are most favored and first line diagnostics for determining the disease. However, drawback of the parasitological approaches is low sensitivity, requirement of technical expertise for carrying out the procedure and further risks associated with the tests (Reithinger 2008).
Immunological methods of diagnosis
To overcome the limitations of parasitological methods of diagnosis, immunological methods were developed (Singh and Sundar 2015). These methods are based on the presence of specific humoral responses (Elmahallawy et al. 2014). The trademark of mucocutaneous and CL is that the humoral immune response is very low (Singh et al. 2005). Thus, immunological tests are not frequently utilized in areas where CL prevails as circulating antibodies are very low and in regions with cross-reacting parasites like Trypanosoma cruzi exist specificity can be fluctuating (Reithinger and Dujardin 2007). However, in VL, hyperimmunoglobulinemia is observed. Utilizing this interaction between hosts and parasites, many antibody detection methods have been developed for the diagnosis of leishmaniasis (Boelaert et al. 2004). Some of these diagnostic methods are enzyme-linked immunosorbent assay (ELISA), western blot, indirect fluorescent antibody and direct agglutination (Goto and Lindoso 2010). The sensitivity of these immunological tests depends mainly on the assay and its methodology, while specificity depends on the antigen rather than the serological format used (Elmahallawy et al. 2014).
Fluorescent antibody test
In this test, a fluorescent marker is attached to an antibody, which results in a reporter molecule that is rapid, easy to measure and bind to a target molecule with high specificity. This test can be both direct, where the labeled antibody binds the antigen and indirect in which secondary polyclonal antibody binds patient that bind prepared antigen. This test is amongst one of the frequently performed tests for detecting the anti-leishmanial antibodies by using the promastigotes. However, cross-reactions with trypanosomal sera have been observed (Boelaert et al. 2004). To minimize the cross-reactions with trypanosomal sera, promastigote is used as antigens (Elmahallawy et al. 2014; Rezvan and Hamoon Navard 2017). A study conducted in Iran to estimate the performance of enzyme-linked immunosorbent assay (ELISA) for detection of total IgG and IgM, showed sensitivity of 83.6% and 84.7% and specificity of 62.7% and 54.6%, respectively. Sensitivity and specificity of ELISA for detecting IgG1 and IgG4 were 64%, 75% and 85%, 49%, respectively. However, immunodiagnosis of CL showed 91.6% sensitivity and 81% specificity with IFA (Indirect Fluorescent Antibody) (Sarkari et al. 2014). Similarly, in another experiment conducted to compare DAT, IFA, and ELISA, sensitivity, and specificity of IFA was 80.3% and 90.5%, while the sensitivity and specificity of direct agglutination test (DAT) was 70.5% and 100%, and ELISA was 83.6% and 90.5% (Mikaeili et al. 2007).
Direct agglutination test (DAT)
DAT is a simple, reliable, cost-effective, and semi-quantitative test (Srividya et al. 2012). DAT has been approved in various countries including India, Brazil Nepal, Sudan, Bangladesh, Kenya, and Ethiopia. This test is dependent on the agglutination of promastigotes of Leishmania that react with the anti-leishmanial antibodies leading to promastigote agglutination (Elmahallawy et al. 2014). The test is economical and easy to perform; thus, it can be used both in laboratory and fields. The sensitivity and specificity of the direct agglutination test as found by various studies ranges from 70.5–100% and 53–100% (S. Mondal et al. 2010). During a study conducted in Spain, the sensitivity of DAT was observed to be 86.4% for suspected VL patients and for individuals underlying HIV infection, increase in sensitivity of 91.3% was seen (Bangert et al. 2018). DAT can be performed on serum, plasma and whole blood (Moody and Chiodini 2000). But, the main drawback of direct agglutination test is that it doesn’t have prognostic value for calculating the cure of disease, as after several years of treatment they remain positive.
Moreover, the long incubation period of 18 h and requirement of serial dilution of serum or blood makes it disadvantageous (Singh 2006). In order to overcome these problems, promastigotes are used as freeze-dried or suspension (liquid) form. The freeze-dried forms can tolerate heat, thus being more heat stable makes the use of new formulated DAT in fields (Abdallah et al. 2004). However, for quick identification (less than 3 h) of antibodies against Leishmania in blood collected on filter paper and in the serum samples, FAST (Fast Agglutination Screening Test) has been developed (Schoone et al. 2001). The sensitivity and specificity with FAST were reported to be 91.1– 95.4% and 70.5%–88.5%, respectively (Hailu et al. 2006).
Enzyme-linked immunosorbent assay (ELISA)
This test is amongst one of the most sensitive serodiagnostic methods for VL. However, the sensitivity of the test relies on the antigen used (Table 1) (Singh and Sundar 2015). Different antigens used in ELISA are:
The crude soluble antigen of promastigote achieved by freeze-thawing the live promastigotes. Ryan et al., in 2002, developed ELISA that can detect IgG and IgM antibodies in CL and VL patients and the overall sensitivity is shown by this ELISA was 95.1%.
Promastigote of L. donovani liberates excretory, secretory and metabolic antigens within protein-free medium (Martin et al. 1998). Sensitivity and specificity shown by these antigens is 100%, but besides this, retrospective and further multisite interpretations are needed. Romero et al. (2004) described that ELISA showed a sensitivity of 89% using an antigen of Leishmania mexicana and 71% with the antigen of Leishmania braziliensis. In another study, using a sequence-specific peptide antigen in 33 patients infected with CL of L. major observed sensitivity of 67% (Jensen et al. 1999).
In a study conducted by Kaul et al. (2000) for evaluation of diagnositic efficacy of 200kDA amastigote antigen of L. donovani, positive antibody response against this antigen and LASA (Leishmania amastigote soluble antigen) was seen in 96.6% and 100% of VL patients respectively.
Kaur and Kaur (2013) demonstrated the serodiagnostic potential of heat shock proteins Hsp 70 and Hsp 83 in combination through ELISA and western blotting. Occurance of both Hsp 70 and Hsp 83 suggested that these antigens can be useful for serodiagnosis of VL.
Recombinant antigens like rk39, rGBP, Ld-Rgbp, rORFF, etc. have been developed. The surface of amastigotes and promastigotes of L. major express a hydrophilic protein (gene B protein, rGBP) encoded by gene B. L. donovani gene B homolog (Ld-rGBP) encodes a protein that comprises of up to 22 replicas of repetitive element in which 14, 9 are conserved within 2 species (Singh 2006). One more recombinant protein of L. infantum, rORFF has been created for the diagnosis of VL in India (Raj et al. 1999). ELISA utilizing this antigen is reported to be significantly specific and sensitive (Singh 2006). A recombinant antigen, rk39 is demonstrated to be accurate against the antibody that emerges during VL caused by L. donovani. Utilizing this antigen, specificity and sensitivity was noted to be 99% in immunocompetent patients with VL (Singh et al. 2010) and also rk39 ELISA has anticipating values for interpreting VL in co-infected individuals like AIDS (Singh et al. 2005). The rk39 antigen is available commercially now as nitrocellulose papers strip impregnated with antigens and is suitable for using in field conditions also.
Carvalho et al. (2018) in their study, evaluated new antigens that were identified by immunogenic screening for serological diagnosis of human VL. It was found that different antigenic L. infantum peptides, based on linear B cell epitopes, had potential for the development of an immunoassay obtained from isolated or multi antigens with ability to improve the sensitivity and specificity values for the VL serodiagnosis.
Also, two more recombinant proteins rK9 and rK26 apart from rK39 have been generated from the kinesin gene of L. chagasi. (Singh et al. 2005; Mohapatra et al. 2010). An experiment conducted for comparative evaluation of rK9 and rK26 with rK39 and crude soluble antigen observed sensitivity of 78%, 38%, 100%, and 80% respectively while the specificity of rK9, rK26, rK39 and Crude Soluble Antigen (CSA) was 84%, 80%, 96%, and 72% respectively.
Table 1.
Sensitivity of various tissues examined parasitologically
| S. no. | Tissue | Sensitivity | References |
|---|---|---|---|
| 1. | Splenic aspirate | 93–99% | Srivastava et al. (2011), Bhargava and Singh (2012) |
| 2. | Bone marrow aspirate | 60–80% | Srivastava et al. (2011), Elmahallawy et al. (2014) |
| 3. | Lymph node | 52–58% | Srivastava et al. (2011), Zijlstra et al. 1992), Boelaert et al. (2007) |
| 4. | Blood buffy coat | 53% | Singh (2006) |
Thus, it was concluded from the study that rK39 was most appropriate antigen as compared to rK26 and rK9. (Mohapatra et al. 2010). Apart from these, recombinant antigen KE16 has been cloned from Indian L. donovani, and this antigen is found to be 100% specific and sensitive (Sivakumar et al. 2006).
The specificity and sensitivity of ELISA using recombinant HSP83 displayed better results in comparison to using crude L. major antigens for diagnosing a cutaneous form of the disease, next to mucocutaneous and VL (Al-Salem et al. 2014). Although ELISA is a technique with enhanced specificity and sensitivity but the need of expert people, sophisticated technologies and requirement of electricity restricts its use to advanced laboratories only (Elmahallawy et al. 2014) (Table 2).
Table 2.
Sensitivity and Specificity of ELISA using different antigens
| S. no. | Antigens | Sensitivity | Specificity | References |
|---|---|---|---|---|
| 1. | Crude soluble antigen | 80–100% | 84–94% | Ryan et al. (2002) |
| 2. | Secretary, metabolic and excretory, antigens liberated by promastigote of L. donovani | 71–89% | Romero et al. (2004) | |
| 3. | rK39 as antigen | 75–98% | 79–89% | Elmahallawy et al. (2014), Chappuis et al. (2007) |
Immunoblotting
The requirement of sophisticated technique and skilled individuals for performing ELISA leads to the development of the immunoblotting method of diagnosis. Serodiagnosis using immunoblotting of soluble antigens has been reported to be highly sensitive and specific. The sensitivity of the test is 90–98%, and specificity is 98–100% (Elmahallawy et al. 2014). In immunoblotting, the disease stage can be determined by the band pattern obtained (Ravindran et al. 2004). In an experiment, soluble antigens from three strains of L. major from Pakistan and 5 Indian strains of L. donovani were separated by SDS-PAGE, electrotransferred and then western blotting with Indian PKDL patients showed antigenic band of approx 72–74 kDa (Singh and Sivakumar 2003). Increase in sensitivity to a great extent was observed by using commercially available electrochemiluminescent kit 9ECL, (Amershan, UK) (Singh 2006). Antibody response to different antigens can be determined by western blot technique (Ravindran et al. 2004). In a study conducted to check the utility of western blot in the diagnosis of CL, the sensitivity of more than 95% and sensitivity of 70% were observed (Ashrafmansouri et al. 2015). However, it’s high cost, extended tenure and need for sophisticated equipment limits its use (Elmahallawy et al. 2014).
Immunochromatographic test (ICT) or immunochromatographic assay (IC)
IC is an accurate, rapid and straightforward immunochromatographic test based on rK39 antigen (Sundar and Rai 2002). The sensitivity and specificity of rK39 in VL cases was found to be different among varying populations. Sensitivity and specificity were 90% and 100% in Brazil (Carvalho et al. 2003) and 100% and 93–98% in India, respectively (Sundar et al. 2002a, b). A study conducted in Mediterranean region on large sets of patients from rK39 showed the sensitivity of 78% in all VL patients. However, the sensitivity dropped to 67.3% in patients with HIV co-infection (Bangert et al. 2018). These variations in sensitivity were due to the presence of different antibody responses in different groups. This test can be used in fields as well because it is quick, economical and gives positive results (Elmahallawy et al. 2014). Vink et al. (2018) in their experiment evaluated Loopamp™ Leishmania Detection Kit (Loopamp) and CL Detect™ Rapid Test (CL Detect), for Cutaneous Leishmaniais (CL) diagnosis. It was observed that out of 274 CL suspects included in the study, CL Detect test showed 65.4% sensitivity and 100% specificity, while it was 87.6% and 70.6% for Loopamp kit respectively.
Leishmanin skin test (LST)
One of the significant attributes of cutaneous form of leishmaniasis is an occurrence of delayed-type hypersensitivity (DTH), and this can be estimated by leishmanin skin test which is also called Montenegro reaction (Stockdale and Newton 2013). Delayed-type hypersensitivity skin reactions are recognized as positive, when LST > 5 mm and negative, when LST < 5 mm (Passos et al. 2000). After asymptomatic infection and in a period of weeks to months after treatment against VL, results of the test become positive, showing a healing or protective response (Khalil et al. 2005). Thus, this experiment fails to discriminate between the past or present infection. Furthermore, active PKDL, VL, and DCL are signalized by a negative skin test (Neogy et al. 1990; Rezvan and Hamoon Navard 2017). However, in areas endemic to VL, the sensitivity of this test in asymptomatic infections is similar or even higher than that of other serologic tests (Gadisa et al. 2012). LST shows a higher sensitivity of 86.4–100% and is simple to use (Antonio et al. 2014). Thus, LST is becoming a valuable method for identifying the exposure to Leishmania parasites and distinguishing the asymptomatic cases in epidemiologic surveys (Riera et al. 2008). LST is frequently employed as a measure of the occurrence of MCL and CL in animal and human populations and successful cure of VL, as it prevails negative during active VL and will be transformed to positive after treatment. Despite its benefits, this test is not helpful in PKDL patients as the results are not related to the presence of the infection (Zijlstra et al. 2000).
Latex agglutination test (LAT)
This test is amongst one of the latest developed tests for quick detection of anti-leishmanial antibody against A2 antigens obtained from the amastigote form and crude antigens from promastigote forms. When compared with DAT, the sensitivity was 88.4%, and specificity was 93.5% (Akhoundi et al. 2013). In a study designed to evaluate the use of kala-azar latex agglutination test (Katex), the sensitivity of KAtex was observed to be moderate, i.e. 75%, but specificity was 100%. This reduced sensitivity restricts the use of KAtex in resource-limited settings (Salam et al. 2012).
Immunological methods are a landmark in the diagnosis of leishmaniasis. Although many different methods have been developed, yet all are met with few limitations. Moreover, these are too costly to be used in developing countries. The requirement of very advanced apparatus limits their use in field conditions. Some commercially available kits have different sensitivity and are sometimes unable to detect the parasite infections in case of Leishmania-HIV co-infections (Schallig et al. 2004) (Table 3).
Table 3.
Sensitivity and specificity of various immunological tests
| S. no. | Test | Sensitivity | Specificity | Reference |
|---|---|---|---|---|
| 1. | Fluorescent antibody test | 80.3–91.6% | 81–90.5% | Mikaeili et al. (2007), Sarkari et al. (2014) |
| 2. | Direct agglutination test | 70.5–100% | 53–100% | Mondal et al. (2010) |
| 3. | Immunoblotting | 90–98% | 98–100% | Elmahallawy et al. (2014 |
| 4. | Immunochromatographic Test | 90–100% | 93–100% | Carvalho et al. (2003), Sundar et al. (2002a, b) |
| 5. | Latex agglutination test | 75–88.4% | 93.5–100% | Akhoundi et al. (2013), Salam et al. (2012) |
Xenodiagnosis
In this method of diagnosis, the infected lesion or tissues are exposed to the phlebotomine vector, and the gut of vector is examined later for the existence of flagellates of Leishmania (Sadlova et al. 2015). Sadlova et al. (2015) conducted an experiment in which they inoculated BALB/c mice intradermally in the ear pinna with L. donovani. This study showed thateven small number parasites in mice can cause massive infection in Phlebotomus orientalis (vector) and thus constituted a suitable laboratory animal for xenodiagnosis. Xenodiagnosis shows high sensitivity and is comparatively more straightforward than other methods, but it cannot differentiate between species of Leishmania. Furthermore, it is a time-consuming process that is not possible without the insect/animal (Akhoundi et al. 2017).
Molecular methods
The conventional parasitological and serological techniques exhibit certain constraints to the diagnosis of leishmaniasis (de Paiva-Cavalcanti et al. 2015) and these limitations have led to the discovery of molecular methods (Tlamcani 2016). In contrast to regular diagnosis, molecular methods are recommended more for CL because of their excellent accuracy and fast speed (Azizi et al. 2012). Molecular techniques serve as a supplement as well as an alternative method of diagnosis. The main reason for approval of molecular techniques in the routine laboratories worldwide is the feasibility, safety, and reliable molecular tools.
Furthermore, numerous applications and the encouraging outcomes have led to continued approval of these methods (de Paiva-Cavalcanti et al. 2015). Though various molecular methods of diagnosis have been developed viz., multilocus enzyme electrophoresis and pulse-field gel electrophoresis yet, assays based on polymerase chain reactions presently constitute the main way of molecular diagnosis for health professionals and researchers. Information and knowledge of DNA sequencing have been much used for establishing the PCR (polymerase chain reaction) based assay for different utilizations in interpreting the parasite and disease (Singh et al. 2005). In a study conducted to differentiate Leishmania species based on internal transcribed spacers (ITS)-PCR, in contrast to parasitological methods of diagnosing CL, it was observed that ITS-PCR is not only valid for identification of species but also showed a high sensitivity and specificity (98.8% and 100%) as compared to microscopy and culture methods (79.6% and 86.2% sensitivity respectively) (Shahbazi et al. 2008). PCR and its modifications such as quantitative-PCR, semi-nested-PCR, and nested-PCR have significantly been used for diagnostic assays using various samples and target regions (da Silva Solcà et al. 2012; Reis et al. 2013). Initially, the qPCR technique had been used to discern DNA from the causative agent in different samples of human and animals to study host-parasite interactions and to quantitatively estimate parasitic load in infected patients (de Paiva-Cavalcanti et al. 2015).
Moreover, Leishmania species characterization is also a vital function of the PCR (Mohammadiha et al. 2013). Pairing PCR with different methodologies such as Restriction Fragment Length Polymorphism (RFLP) analysis and gene sequencing have helped in the confirmation of different species in epidemiological researches (Wang et al. 2011). With the emergence of recombinant technology and nucleic acid engineering, a variety of schemes have been developed for constructing the recombinant protein for the diagnostic purposes (Singh et al. 2005). Table 2 shows the advantages and disadvantages of various molecular methods for the detection of Leishmania.
Conventional PCR
It facilitates the amplification of RNA or DNA through continually repeated cycles. The elucidation of some DNA fragments that are species-specific made this procedure feasible for diagnosing leishmaniasis (Smyth et al. 1992). Also, PCR techniques are more appropriate than parasitological methods of diagnosis (culturing and microscopy) especially for samples that have lower parasite load (Antinori et al. 2007) thus beneficial in observing the disease progression, estimating the result of anti-leishmanial therapy, describing the species discrimination and drug resistance (Schönian et al. 2011). Moreira et al. (2007) conducted a comparative study of different diagnostic methods and observed sensitivity of 100%, 96%, and 95.65% respectively for symptomatic, oligosymptomatic and asymptomatic groups and specificity of 100%. Similarly, in another study, PCR was targeted against kinetoplastid DNA (kDNA) using Uni21/Lmj4 primers, and the results were compared with parasitological methods, cultures, and smears. The results showed that kDNA PCR was most sensitive, i.e. 100%; however, microscopy and cultures were only 71.4% and 54.3% sensitive respectively (Zeyrek et al. 2018). Abd El-Salam et al. (2014), conducted an experiment in which Leishmania tropica was extracted from CL patients of Kohat and analyzed by PCR, microscopy and culture techniques. Of the 113 samples, PCR, microscopy, and culture showed 87.61%, 53.98%, and 46.90% sensitivity. Although conventional PCR shows enhanced sensitivity when compared to the parasitological method of diagnosis, but an essential drawback of this method is their inability to discriminate between clinically active VL and asymptomatic infections (Sudarshan and Sundar 2014). Also, conventional PCR have a high risk of contamination, which takes a longer time to give the results.
Nested and semi-nested PCR
This form of PCR is significant in differentiating the species (Akhoundi et al. 2017). In this technique, two sets of primers are utilized in two consecutive cycles. The second set of primers amplifies the secondary target in the first product. This step makes sure that the outcomes of the 2nd PCR have very less impurity due to primer dimers, alternative primer targets and hairpins (Akhavan et al. 2010). Shirian et al. (2014) executed this method by using scraped off slides from twenty suspected individuals with MCL and got positive results in 18 individuals (90%). However, through direct microscopy, only eight cases were found positive (44.4%). In another study, a combination of the parasitological, immunological and molecular technique was used to discern the course of infection in dogs after sporadic exposure to L. infantum and nested PCR which showed 100% sensitivity as compared to all other methods (Oliva et al. 2006). Although this method is highly sensitive, yet it has some limitations. After achieving the first stage, the product should be transferred to a new tube or material of the second stage are added to it. This step increases the chances of contamination.
Multiplex PCR
In this method, different DNA targets can be amplified simultaneously, and different PCR reactions are performed in a single experiment. Amplicons of specific DNA targets having varying sizes can be produced by using numerous primers set in one PCR mixture only. The annealing temperature of all sets of primers can be developed to perform accurately in one reaction tube and size of amplicons should be distinct enough to be anticipated by gel electrophoresis. Different types of markers like kDNA minicircle (de Pita-Pereira et al. 2008) and multicopy SL RNAs (Spliced Leader RNAs) (Jorquera et al. 2005) have been utilized in this method for diagnosis of leishmaniasis. This method can be used for clinical laboratories, but a relative decline in sensitivity is one the drawbacks of this method as compared to single PCR method. Utilizing this technique for the detection of infectious agents need accurate primer design and extensive optimization of the test.
Real-time PCR (quantitative PCR)
This method depends upon analysis by fluorescent signals that are generated during amplification. Fluorescent dye and fluorescent tubes are used to create the fluorescence (Galluzzi et al. 2018). RT-PCR is fast and has an extensive dynamic range as there is no requirement of opening the reaction tube; also, cross-contamination is decreased. RT-PCR is convenient for diagnosis of PKDL (Ghosh et al. 2018) and canine leishmaniasis where it helps in monitoring parasitic load in different tissues throughout and after the treatment (Manna et al. 2008; Pennisi et al. 2005). In an experiment, 91 PKDL patients from Bangladesh went for skin biopsy, and both real-time PCR and microscopy was performed. RT-PCR showed the sensitivity of 91.2% and microscopy showed 50.6% (Ghosh et al. 2018).
Similarly, another study was conducted to detect and quantify L. donovani for diagnosis of VL and monitoring its response to treatment. RT-PCR showed a sensitivity of 93.33% and specificity of 100% (Hossain et al. 2017). Quantitative estimation of products, reduced contamination, and high speed are among the significant advantages of this method but high cost and need of an expert skilled person to deduce the results are its current significant drawbacks.
Nucleic acid sequence-based amplification (NASBA)
This method is a transcription-based amplification and isothermal complex constructed to discern the RNA target (van der Meide et al. 2005). NASBA has its different modifications such as quantitative-NASBA (QT-NASBA) and paired with oligochromatography (NASBA-OC). In an experiment conducted on 50 samples from healthy control and 30 samples from VL, sensitivity and specificity of NASBA-OC was 93.3% and 100% respectively (Mugasa et al. 2010). Similarly, in another study, QT-NASBA method was used to evaluate Leishmania parasites in samples of a skin biopsy from CL patients. This study demonstrated that QT-NASBA could detect parasite level 100 fold lower than detected by conventional PCR. Sensitivity and specificity observed, in this case, was 97.5% and 100%, respectively (van der Meide et al. 2005). NASBA is the only isothermal amplification method that utilizes RNA as starting material, but the main limitation of this method is that they are prone to contamination of ribonuclease, which can degrade the target RNA (Zanoli and Spoto2013).
Oligochromatography-PCR (OC-PCR)
It is a rapid and straightforward scheme for detecting nucleic acid-based amplification (NASBA) or PCR outcomes anticipated on dipstick by hybridization with a gold-conjugated probe that allows the detection of sequence-specific products. Although this method is greatly sensitive, yet it does not provide discrimination between different species of Leishmania (Mugasa et al. 2010; Saad et al. 2010). Leishmania OligoC-Test and NASBA, coupled to oligochromatography, are used as low tech molecular diagnostic methods (Mugasa et al. 2010). In a study conducted to calculate specificity and sensitivity of NASBA-OC and OligoC-TestT from blood specimen of 84 VL patients. NASBA-OC showed specificity and sensitivity of 100% and 79.8% while Leishmania OligoC-TestT showed specificity and sensitivity of 88.8% and 96.4% respectively (Basiye et al. 2010). Saad et al. (2010) experimented with checking the diagnostic accuracy of Leishmania OligoC-TestT and NASBA-OC for diagnosis of leishmaniasis in Sudan and found the sensitivity of OligoC-TestT and NASBA-OC on lymph node to be 96.8%, blood 96.2% and on the bone marrow to be 96.9%. Despite its high sensitivity, Oligochromatography-PCR is not an option for routine diagnosis in primary health centers due to the requirements of basic molecular laboratories.
Randomly amplified polymorphic DNA (RAPD)
In this method, DNA amplification by PCR is performed using a short primer that is randomly determined. This short primer can be thus utilized for any organism even without early knowledge of the target sequence. RAPD (Randomly amplified polymorphic DNA) can be performed in combination with some other techniques or used alone to check genetic intra-specific and inter-specific differences in species of Leishmania (Botilde et al. 2006). In a study conducted for identification of Leishmania species causing CL in Kharve, Iran, RAPD-PCR was found successful in identifying the causative species, i.e. L. tropica. However, its use is limited due to its demand for pure leishmanial DNA and accurate polymerase chain reactions conditions that assure the specificity (Akhoundi et al. 2017).
Amplified fragment length polymorphism (AFLP)
This is a very convenient method for quick visualization of polymorphic DNA fragment from organisms with no previous information of the sequence. Amplified fragments so obtained are isolated and then seen on PAGE, and recorded an occurrence or lack of the polymorphism (Kumar et al. 2010). It is a reproducible technique as it combines the sensitivity of PCR reaction with the specificity of RFLP. AFLP is greatly used in ecology, biology, genetics, and phylogenetics of many organisms (Bensch and Åkesson 2005). AFLP is a fast and compelling technique for the identification of marker and identifying closely linked species of Leishmania and genetic variations in strains (Kumar et al. 2010). Kumar et al. (2010) used the test to differentiate the species of Leishmania causing CL and VL, and showed clear cut variation in genetic distance between L. major and L. tropica. Similarly, Restrepo et al. (2013) also used AFLP to characterize genetic variability of Leishmania parasites isolated from Panamanian CL patients. The technique was found to be successful in clearly separating some groups of L. panamensis and highly related species like L. panamensis and L. guyanensis.
Loop-mediated isothermal amplification (LAMP)
LAMP is a parasite species-specific amplification of DNA, rapid and needs only basic laboratory equipment (Kothalawala and Karunaweera 2016). LAMP is a helpful tool for diagnosis is sometimes used as a substitute technique for PCR because it is an economical, more sensitive and faster method. As the reaction is isothermal in nature, there is no requirement of a thermal cycler, except for heat block and water bath (Khan et al. 2012). All these features of LAMP make it suitable to use under field conditions. In this method, each initial single stranded DNA product supplements subsidiary template for chain-reaction utilizing 2nd outer or inner primer with the help of stem-loop intermediate structure. This assay was developed for detection of L. donovani in patients with PKDL and VL. The sensitivity and specificity were found to be good in both the cases reaching a sensitivity of 96.4–98.5% in VL blood samples, 96.8–98.5% in samples of tissue biopsy and 96.8% for PKDL cases (Verma et al. 2013). In a study conducted on 31 CL patients, LAMP was found to be positive for 19 out of 23 microscopically positive patients, yielding a sensitivity of 82.6%. And specificity 100% (Kothalawala and Karunaweera 2016). In another experiment conducted for evaluation of VL in Sudan, Loopamp (Leishmania detection kit) showed a specificity of 99.01% and sensitivity of 100% (Mukhtar et al. 2018). In another study, LAMP assay was designed for CL on 105 patients in South-West Colombia. Sensitivity and specificity shown by LAMP was 95%, and 86% for the diagnosis of CL and for VL sensitivity and specificity was 92% and 100% (Adams et al. 2018). Despite high sensitivity and specificity, the major disadvantage of LAMP is need of non-extreme GC strand, the possibility of the presence of secondary structures and required temperature range. (Akhoundi et al. 2017).
Multilocus enzyme electrophoresis (MLEE)
This method allows identification of microorganisms by electrophoretic mobility of various intracellular enzymes (Ovalle-Bracho et al. 2018). Isoenzymes play a significant role in metabolic processes in different cellular components and are produced by multiple genes. The typical kind or strain-specific mobility pattern can be confirmed by using the defined set of isoenzymes. MLEE is regarded as a reference standard for identifying species of Leishmania and can also discriminate between species of Old World and new world. However, MLEE cannot differentiate between populations because of lower marker number, homoplasy or destined gene heterozygosity that cause more than one mobility scheme (Jamjoom et al. 2004). MLEE is not suggested because of the requirement of specialized equipment, laborious parasite culturing and due to lack of power to discriminate between some species and populations (e.g., L. infantum MON-1) (Schönian et al. 2011) (Table 4).
Table 4.
Advantages and disadvantages of different molecular methods
| S. no. | Assay | Types of leishmaniasis | Specificity | Sensitivity | Advantage | Disadvantage | References |
|---|---|---|---|---|---|---|---|
| 1. | Conventional PCR | Cutaneous leishmaniasis (CL) | 87.61–100% | 100% |
Precise results, high specificity, and sensitivity. Uncomplicated Diagnostic interpretations |
Time consuming and incompetent to evaluate the destined DNA. Qualitative Approach. Restricted detection range | Moreira et al. (2007), Zeyrek et al. (2018), Abd El-Salam et al. (2014), De Paiva-Cavalcanti et al. (2015) |
| 2. | Nested PCR | Cutaneous Leishmaniasis (CL), Visceral Leishmaniasis (VL) | 90–100% | – | Shows higher sensitivity and specificity. A convenient method for investigating the molecular epidemiology in the field | Qualitative test. Incompetent to evaluate the target DNA requires prolonged time and is expensive | Shirian et al. (2014), Oliva et al. (2006), De Paiva-Cavalcanti et al. (2015) |
| 3. | Real Time-PCR | PKDL, VL | 91.2–93.33% | 100% | Elevated specificity and sensitivity, Numerical potential and rapid results. Differentiation of species can be achieved by melting Temperature |
Complexity in elucidating the outcomes, Requires a skilled operator Presence of thermocycler makes it costly |
Hossain et al. (2017, Ghosh et al. (2018), De Paiva-Cavalcanti et al. (2015) |
| 4. | NASBA | VL | 93.3–97.5% | 100% | NASBA is the only isothermal amplification method that utilizes RNA as starting material. There is no requirement of the complicated laboratory structure. Shows higher specificity and rapid results | Prone to contamination of ribonuclease, which can degrade the target RNA | van der Meide et al. (2005), Mugasa et al. (2010), Zanoli and Spoto (2013), De Paiva-Cavalcanti et al. (2015) |
Diagnosis of leishmania-hiv co-infection
In AIDS patients, VL is an opportunistic infection, and unusual pathological presentations of VL in HIV-infected patients create a challenge for diagnosis (Srivastava et al. 2011). However, regardless of the status of HIV, parasitological mode of diagnosis is the gold standard because of its high specificity (Singh 2014). For HIV-infected and non-infected individuals, spleen tissues have the best sensitivity, followed by bone marrow and lymph nodes. However, bone marrow aspiration is most used because of its excellent sensitivity (67–94%) and reduced complication than splenic aspiration (Alvar et al. 2008; Lima et al. 2013). Also, culture can improve sensitivity, but it requires a particular medium and is generally not available in endemic areas. Cota et al. (2013) observed specificity and sensitivity of parasitological methods to be 100% and 93.2%. Concerning immunological diagnosis of HIV-infected cases, there are few pieces of evidence of the accomplished tests with significant variety of studies (Cota et al. 2012). Serological tests, in this case, are less reliable. Also, there is confusion about which technique is better than other (Lindoso et al. 2014). As far as a demonstration of antibodies are concerned immunoblotting, enzyme immunoassays, direct agglutination tests (DAT) (Singh 2014; Cota et al. 2012), immunofluorescence assay (Antinori et al. 2012) can be performed with varying sensitivities. Cota et al. (2012) found that in comparison to ELISA, immunoblotting, and DAT had enhanced sensitivity (84% and 81%). Also, Cota et al. (2012) demonstrated in their review which included 33 studies and 1489 patients that serological tests have limited sensitivity and immunoblotting and DAT show better results when compared to IFA and enzyme-linked immunosorbent assay. In another study, including 113 patients of HIV-infected symptoms, DAT gave excellent performance with the positivity of 93%. However, recombinant K39 antigen-based immunochromatographic test and IFA showed low sensitivity, i.e. 60.9% and 45.6% (Cota et al. 2013). Katex (latex agglutination test) demonstrating the antigens in urine sample displayed better specificity and sensitivity in immunocompetent patients of VL, but low sensitivity in HIV-coinfected patients (Singh 2014; Attar et al. 2001). In a study conducted in Latin America on 13 individuals with HIV coinfection, only five were positive with Katex, while DAT showed 100% positivity (Barbosa Junior et al. 2015). The molecular mode of leishmaniasis diagnosis utilizing different Leishmania gene target sequence is becoming essential in both HIV-infected and non-HIV-infected patients (Singh 2014; Lindoso et al. 2016). Benefits of molecular methods are elevated specificity and sensitivity, chances of using bone marrow and peripheral blood (Lindoso et al. 2014). Positivity of PCR differs, keeping in mind the use of bone marrow (93–100%) and whole blood (83–98%) (Cota et al. 2012). PCR assays depending on the amplification of kinetoplast are the most analytical method to detect Leishmania DNA (Cota et al. 2013; Lindoso et al. 2016). In their study, Khatun et al. (2017) used MK1F/R primer for targeting the kDNA sequences and found the sensitivity and specificity to be 98% and 100%. Real-time qPCR is a substitute for diagnosis and investigation of infection and shows positivity of 85.7% in HIV-infected individuals in Latin America (Cota et al. 2013; Bossolasco et al. 2003). Despite the fact that this method is a useful tool for diagnosing co-infected patients, it is essential to note that asymptomatic patients can also show positive results (Cota et al. 2013) which makes it relevant for active disease diagnosis in areas endemic to high transmission of leishmaniasis (Lindoso et al. 2016).
Conclusion
The reason why leishmaniasis is a diagnostic challenge is because of a wide spectrum of clinical manifestations present. Moreover, overlapping clinical symptoms with diseases like tuberculosis, typhoid, and malaria further complicate the disease and diagnosis.
Three major diagnostic methods have been discussed in this review with variations in accuracy of diagnosis; including the parasitological examination (histopathology, microscopy and parasite culture), serology and molecular diagnostics. In parasitological methods, the amastigote stage is demonstrated in spleen, liver or lymph node aspirates. Although, parasitological methods of diagnosis remain the best method for leishmaniasis diagnosis but the presence of inevitable disadvantages delays this diagnosis in the field. The serological methods are sensitive, specific and economical. Diagnosis based on antibodies, like rK39 strip test is used in affected countries over the world despite their limitation of remaining positive in healthy individuals for very long periods even after cure. Molecular methods are even more sensitive and are a powerful technique that provides early detection of the parasites. Many molecular methods have been successfully implemented for leishmaniasis diagnosis, but the technique is used mostly in research laboratories, and its application in clinical practices and health facilities requires skilled persons who are generally not available in poor and developing countries. Compared to other diagnostic techniques, molecular approaches remain expensive and require technical expertise. However, efforts should be made to make diagnosis more user-friendly and cost-effective, especially in the remote areas where leishmaniasis is endemic. Leishmaniasis could be prevented by reducing human contact with infected phlebotomine sandflies (the vector), or by reducing the number of infected animals (the reservoir). Although no published studies on the effectiveness of diagnosis for prevention and management of the diseases are present but early diagnosis allow fast treatment and could contribute to the therapeutic success. This can be achieved by training the physicians working in primary health centres for proper identification, diagnosis and treatment of leishmaniasis.
Acknowledgements
We would like to thank Prof. V.K. Walia, Department of Zoology, Panjab University, Chandigarh, India for critically editing the review.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abd El-Salam NM, Ayaz S, Ullah R. PCR and microscopic identification of isolated Leishmania tropica from clinical samples of cutaneous leishmaniasis in human population of Kohat region in Khyber Pakhtunkhwa. Biomed Res Int Sudarshan. 2014;2014:2014. doi: 10.1155/2014/861831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdallah KA, Nour BY, Schallig HD, Mergani A, Hamid Z, Elkarim AA, Saeed OK, Mohamadani AA. Evaluation of the direct agglutination test based on freeze-dried Leishmania donovani promastigotes for the serodiagnosis of visceral leishmaniasis in Sudanese patients. Trop Med Int Health. 2004;9(10):1127–1131. doi: 10.1111/j.1365-3156.2004.01308.x. [DOI] [PubMed] [Google Scholar]
- Adams ER, Schoone G, Versteeg I, Gomez MA, Diro E, Mori Y, Perlee D, Downing T, Saravia N, Assaye A, Hailu A. Development and evaluation of a novel LAMP assay for the diagnosis of cutaneous and visceral leishmaniasis. J Clin Microbiol. 2018 doi: 10.1128/JCM.00386-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbari M, Oryan A, Hatam G. Application of nanotechnology in treatment of leishmaniasis: a review. Acta Trop. 2017;172:86–90. doi: 10.1016/j.actatropica.2017.04.029. [DOI] [PubMed] [Google Scholar]
- Akhavan AA, Mirhendi H, Khamesipour A, Alimohammadian MH, Rassi Y, Bates P, Kamhawi S, Valenzuela JG, Arandian MH, Abdoli H, Jalali-zand N. Leishmania species: detection and identification by nested PCR assay from skin samples of rodent reservoirs. Exp Parasitol. 2010;126(4):552–556. doi: 10.1016/j.exppara.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhoundi B, Mohebali M, Shojaee S, Jalali M, Kazemi B, Bandehpour M, Keshavarz H, Edrissian GH, Eslami MB, Malekafzali H, Kouchaki A. Rapid detection of human and canine visceral leishmaniasis: assessment of a latex agglutination test based on the A2 antigen from amastigote forms of Leishmania infantum. Exp Parasitol. 2013;133(3):307–313. doi: 10.1016/j.exppara.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Akhoundi M, Downing T, Votýpka J, Kuhls K, Lukeš J, Cannet A, Ravel C, Marty P, Delaunay P, Kasbari M, Granouillac B. Leishmania infections: molecular targets and diagnosis. Mol Asp Med. 2017;57:1–29. doi: 10.1016/j.mam.2016.11.012. [DOI] [PubMed] [Google Scholar]
- Alemayehu B, Alemayehu M. Leishmaniasis: a review on parasite, vector and reservoir host. Health Sci J. 2017;11(4):1. [Google Scholar]
- Al-Salem WS, Ferreira DM, Dyer NA, Alyamani EJ, Balghonaim SM, Al-Mehna AY, Al-Zubiany S, Ibrahim EK, Al Shahrani AM, Alkhuailed H, Aldahan MA. Detection of high levels of anti-α-galactosyl antibodies in sera of patients with Old World cutaneous leishmaniasis: a possible tool for diagnosis and biomarker for cure in an elimination setting. Parasitology. 2014;141(14):1898–1903. doi: 10.1017/S0031182014001607. [DOI] [PubMed] [Google Scholar]
- Alvar J, Aparicio P, Aseffa A, Den Boer M, Canavate C, Dedet JP, Gradoni L, Ter Horst R, López-Vélez R, Moreno J. The relationship between leishmaniasis and AIDS: the second 10 years. Clin Microbiol Rev. 2008;21(2):334–359. doi: 10.1128/CMR.00061-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvar J, Velez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J, den Boer M, WHO Leishmaniasis Control Team Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE. 2012;7(5):e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreadou M, Liandris E, Kasampalidis IN, Taka S, Antoniou M, Ntais P, Vaiopoulou A, Theodoropoulos G, Gazouli M, Ikonomopoulos J. Evaluation of the performance of selected in-house and commercially available PCR and real-time PCR assays for the detection of Leishmania DNA in canine clinical samples. Exp Parasitol. 2012;131(4):419–424. doi: 10.1016/j.exppara.2012.05.012. [DOI] [PubMed] [Google Scholar]
- Andreadou M, Liandris E, Gazouli M, Taka S, Antoniou M, Theodoropoulos G, Tachtsidis I, Goutas N, Vlachodimitropoulos D, Kasampalidis I, Ikonomopoulos J. A novel non-amplification assay for the detection of Leishmania spp. in clinical samples using gold nanoparticles. J Microbiol Methods. 2014;96:56–61. doi: 10.1016/j.mimet.2013.10.011. [DOI] [PubMed] [Google Scholar]
- Antinori S, Calattini S, Longhi E, Bestetti G, Piolini R, Magni C, Orlando G, Gramiccia M, Acquaviva V, Foschi A, Corvasce S. Clinical use of polymerase chain reaction performed on peripheral blood and bone marrow samples for the diagnosis and monitoring of visceral leishmaniasis in HIV-infected and HIV-uninfected patients: a single-center, 8-year experience in Italy and review of the literature. Clin Infect Dis. 2007;44(12):1602–1610. doi: 10.1086/518167. [DOI] [PubMed] [Google Scholar]
- Antinori S, Schifanella L, Corbellino M. Leishmaniasis: new insights from an old and neglected disease. Eur J Clin Microbiol Infect Dis. 2012;31(2):109–118. doi: 10.1007/s10096-011-1276-0. [DOI] [PubMed] [Google Scholar]
- Antoine JC, Prina E, Lang T, Courret N. The biogenesis and properties of the parasitophorous vacuoles that harbour Leishmania in murine macrophages. Trends Microbiol. 1998;6(10):392–401. doi: 10.1016/s0966-842x(98)01324-9. [DOI] [PubMed] [Google Scholar]
- Antonio LD, Fagundes A, Oliveira RV, Pinto PG, Bedoya-Pacheco SJ, Vasconcellos ÉD, Valete-Rosalino MC, Lyra MR, Passos SR, Pimentel MI, Schubach AD. Montenegro skin test and age of skin lesion as predictors of treatment failure in cutaneous leishmaniasis. Rev Inst Med Trop Sao Paulo. 2014;56(5):375–380. doi: 10.1590/S0036-46652014000500002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashrafmansouri M, Sarkari B, Hatam G, Habibi P, Khabisi SA. Utility of western blot analysis for the diagnosis of cutaneous leishmaniasis. Iran J Parasitol. 2015;10(4):599. [PMC free article] [PubMed] [Google Scholar]
- Attar ZJ, Chance ML, el-Safi S, Carney J, Azazy A, El-Hadi M, Dourado C, Hommel M. Latex agglutination test for the detection of urinary antigens in visceral leishmaniasis. Acta Trop. 2001;78(1):11–16. doi: 10.1016/s0001-706x(00)00155-8. [DOI] [PubMed] [Google Scholar]
- Azizi K, Soltani A, Alipour H. Molecular detection of Leishmania isolated from cutaneous leishmaniasis patients in Jask County, Hormozgan Province, Southern Iran, 2008. Asian Pac J Trop Med. 2012;5(7):514–517. doi: 10.1016/S1995-7645(12)60090-X. [DOI] [PubMed] [Google Scholar]
- Bangert M, Flores-Chávez MD, Llanes-Acevedo IP, Arcones C, Chicharro C, García E, Ortega S, Nieto J, Cruz I. Validation of rK39 immunochromatographic test and direct agglutination test for the diagnosis of Mediterranean visceral leishmaniasis in Spain. PLoS Negl Trop Dis. 2018;12(3):e0006277. doi: 10.1371/journal.pntd.0006277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa Junior WL, Ramos de Araujo PS, Dias de Andrade L, et al. Rapid tests and the diagnosis of visceral leishmaniasis and human immunodeficiency virus/acquired immunodeficiency syndrome coinfection. Am J Trop Med Hyg. 2015;93(5):967–969. doi: 10.4269/ajtmh.14-0798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett MP, Croft SL. Management of trypanosomiasis and leishmaniasis. Br Med Bull. 2012;104:175–196. doi: 10.1093/bmb/lds031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basiye FL, Mbuchi M, Magiri C, Kirigi G, Deborggraeve S, Schoone GJ, Saad AA, El-Safi S, Matovu E, Wasunna MK. Sensitivity and specificity of the Leishmania OligoC-TesT and NASBA oligochromatography for diagnosis of visceral leishmaniasis in Kenya. Trop Med Int Health. 2010;15(7):806–810. doi: 10.1111/j.1365-3156.2010.02548.x. [DOI] [PubMed] [Google Scholar]
- Bensch S, Åkesson M. Ten years of AFLP in ecology and evolution: why so few animals? Mol Ecol. 2005;14:2899–2914. doi: 10.1111/j.1365-294X.2005.02655.x. [DOI] [PubMed] [Google Scholar]
- Bhargava P, Singh R. Developments in diagnosis and antileishmanial drugs. Interdiscip Perspect Infect Dis. 2012;2012:2012. doi: 10.1155/2012/626838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boelaert M, Rijal S, Regmi S, Singh R, Karki B, Jacquet D, Chappuis F, Campino L, Desjeux P, Le Ray D, Koirala S. A comparative study of the effectiveness of diagnostic tests for visceral leishmaniasis. Am J Trop Med Hyg. 2004;70(1):72–77. [PubMed] [Google Scholar]
- Boelaert M, Bhattacharya S, Chappuis F, El Safi SH, Hailu A, Mondal D, Rijal S, Sundar S, Wasunna M, Peeling RW. Evaluation of rapid diagnostic tests: visceral leishmaniasis. Nat Rev Microbiol. 2007;5:S30–S39. [Google Scholar]
- Boggild AK, Miranda-Verastegui C, Espinosa D, Arevalo J, Martinez-Medina D, Llanos-Cuentas A, Low DE. Optimization of microculture and evaluation of miniculture for the isolation of Leishmania parasites from cutaneous lesions in Peru. Am J Trop Med Hyg. 2008;79(6):847–852. [PubMed] [Google Scholar]
- Bossolasco S, Gaiera G, Olchini D, Gulletta M, Martello L, Bestetti A, Bossi L, Germagnoli L, Lazzarin A, Uberti-Foppa C, Cinque P. Real-time PCR assay for clinical management of human immunodeficiency virus-infected patients with visceral leishmaniasis. J Clin Microbiol. 2003;41(11):5080–5084. doi: 10.1128/JCM.41.11.5080-5084.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botilde Y, Laurent T, Tintaya WQ, Chicharro C, Cañavate C, Cruz I, Kuhls K, Schönian G, Dujardin JC. Comparison of molecular markers for strain typing of Leishmania infantum. Infect Genet Evol. 2006;6(6):440–446. doi: 10.1016/j.meegid.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Carvalho SF, Lemos EM, Corey R, Dietze R. Performance of recombinant K39 antigen in the diagnosis of Brazilian visceral leishmaniasis. Am J Trop Med Hyg. 2003;68(3):321–324. [PubMed] [Google Scholar]
- Carvalho AM, de Oliveira Mendes TA, Coelho EA, Duarte MC, Menezes-Souza D. New antigens for the serological diagnosis of human visceral leishmaniasis identified by immunogenomic screening. PLoS ONE. 2018;13(12):e0209599. doi: 10.1371/journal.pone.0209599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (2018) Leishmania epidemilogy and risk factors. https://www.cdc.gov/parasites/leishmaniasis/epi.html. Accessed 26 July 2019
- Chappuis F, Sundar S, Hailu A, Ghalib H, Rijal S, Peeling RW, Alvar J, Boelaert M. Visceral leishmaniasis: what are the needs for diagnosis, treatment and control? Nat Rev Microbiol. 2007;5(11supp):S7. doi: 10.1038/nrmicro1748. [DOI] [PubMed] [Google Scholar]
- Chhabra MB, Singla LD. Leishmaniasis. In: Garg SR, editor. Zoonosis: parasitic and mycotic diseases. New Delhi: Daya Publishing House; 2014. pp. 134–147. [Google Scholar]
- Coleman RE, Edman JD, Semprevivo LH. Interactions between Plasmodium yoelii and Leishmania mexicana amazonensis in Leishmania resistant C57B1/6 mice. Am J Trop Med Hyg. 1998;39(6):540–544. doi: 10.4269/ajtmh.1988.39.540. [DOI] [PubMed] [Google Scholar]
- Copeland NK, Aronson NE. Leishmaniasis: treatment updates and clinical practice guidelines review. Curr Opin Infect Dis. 2015;28(5):426–437. doi: 10.1097/QCO.0000000000000194. [DOI] [PubMed] [Google Scholar]
- Cota GF, De Sousa MR, Demarqui FN, Rabello A. The diagnostic accuracy of serologic and molecular methods for detecting visceral leishmaniasis in HIV infected patients: meta-analysis. PLoS Negl Trop Dis. 2012;6(5):e1665. doi: 10.1371/journal.pntd.0001665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota GF, de Sousa MR, de Freitas Nogueira BM, Gomes LI, Oliveira E, Assis TS, de Mendonça AL, Pinto BF, Saliba JW, Rabello A. Comparison of parasitological, serological, and molecular tests for visceral leishmaniasis in HIV-infected patients: a cross-sectional delayed-type study. Am J Trop Med Hyg. 2013;89(3):570–577. doi: 10.4269/ajtmh.13-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva Solcà M, Guedes CE, Nascimento EG, de Sá vOliveira GG, dos Santos WL, Fraga DB, Veras PS. Qualitative and quantitative polymerase chain reaction (PCR) for detection of Leishmania in spleen samples from naturally infected dogs. Vet Parasitol. 2012;184(2–4):133–140. doi: 10.1016/j.vetpar.2011.08.026. [DOI] [PubMed] [Google Scholar]
- de Paiva-Cavalcanti M, de Morais RC, Pessoa-e-Silva R, Silva LA, da Cunha Gonçalves-de-Albuquerque S, Tavares DD, Brelaz-de-Castro MC, Silva RD, Pereira VR. Leishmaniases diagnosis: an update on the use of immunological and molecular tools. Cell Biosci. 2015;5(1):31. doi: 10.1186/s13578-015-0021-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pita-Pereira D, Cardoso MA, Alves CR, Brazil RP, Britto C. Detection of natural infection in Lutzomyia cruzi and Lutzomyia forattinii (Diptera: Psychodidae: Phlebotominae) by Leishmania infantum chagasi in an endemic area of visceral leishmaniasis in Brazil using a PCR multiplex assay. Acta Trop. 2008;107(1):66–69. doi: 10.1016/j.actatropica.2008.04.015. [DOI] [PubMed] [Google Scholar]
- de Vries HJ, Reedijk SH, Schallig HD. Cutaneous leishmaniasis: recent developments in diagnosis and management. Am J Clin Dermatol. 2015;16(2):99–109. doi: 10.1007/s40257-015-0114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanji S, Teh HS. IL-2-activated CD8+CD44high cells express both adaptive and innate immune system receptors and demonstrate specificity for syngeneic tumor cells. J Immunol. 2003;171(7):3442–3450. doi: 10.4049/jimmunol.171.7.3442. [DOI] [PubMed] [Google Scholar]
- Dhiman RC. Emerging vector-borne zoonoses: eco-epidemiology and public health implications in India. Front Public Health. 2014;2:168. doi: 10.3389/fpubh.2014.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diro E, Lynen L, Gebregziabiher B, Assefa A, Lakew W, Belew Z, Hailu A, Boelaert M, van Griensven J. Clinical aspects of paediatric visceral leishmaniasis in North-west Ethiopia. Trop Med Int Health. 2015;20(1):8–16. doi: 10.1111/tmi.12407. [DOI] [PubMed] [Google Scholar]
- Donnelly JJ, Liu MA, Ulmer JB. Antigen presentation and DNA vaccines. American journal of respiratory and critical care medicine. 2000;162(supplement_3):S190–S193. doi: 10.1164/ajrccm.162.supplement_3.15tac10. [DOI] [PubMed] [Google Scholar]
- Dunning N. Leishmania vaccines: from leishmanization to the era of DNA technology. Biosci Horiz. 2009;2(1):73–82. [Google Scholar]
- Elmahallawy EK, Martínez AS, Rodriguez-Granger J, Hoyos-Mallecot Y, Agil A, Mari JM, Fernández JG. Diagnosis of leishmaniasis. J Infect Dev Ctries. 2014;8(08):961–972. doi: 10.3855/jidc.4310. [DOI] [PubMed] [Google Scholar]
- Freitas-Junior LH, Chatelain E, Kim HA, Siqueira-Neto JL. Visceral leishmaniasis treatment: what do we have, what do we need and how to deliver it? Int J Parasitol Drugs Drug Resist. 2012;2:11–19. doi: 10.1016/j.ijpddr.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadisa E, Custodio E, Canavate C, Sordo L, Abebe Z, Nieto J, et al. Usefulness of the rK39-immunochromatographic test, direct agglutination test, and leishmanin skin test for detecting asymptomatic Leishmania infection in children in a new visceral leishmaniasis focus in Amhara State, Ethiopia. Am J Trop Med Hyg. 2012;86(5):792–798. doi: 10.4269/ajtmh.2012.11-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Ceccarelli M, Diotallevi A, Menotta M, Magnani M. Real-time PCR applications for diagnosis of leishmaniasis. Parasit Vectors. 2018;11(1):273. doi: 10.1186/s13071-018-2859-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghorbani M, Farhoudi R. Leishmaniasis in humans: drug or vaccine therapy? Drug Des Dev Ther. 2018;12:25. doi: 10.2147/DDDT.S146521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh P, Hasnain MG, Hossain F, Khan MA, Chowdhury R, Faisal K, Mural MA, Baker J, Nath R, Ghosh D, Maruf S. Evaluation of real-time PCR for diagnosis of post-kala-azar dermal leishmaniasis in endemic foci of Bangladesh. Open Forum Infect Dis. 2018;5(10):ofy234. doi: 10.1093/ofid/ofy234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto H, Lindoso JA. Current diagnosis and treatment of cutaneous and mucocutaneous leishmaniasis. Expert Rev Anti-infect Ther. 2010;8(4):419–433. doi: 10.1586/eri.10.19. [DOI] [PubMed] [Google Scholar]
- Gupta G, Oghumu S, Satoskar AR. Mechanisms of immune evasion in leishmaniasis. Appl Microbiol Biotechnol. 2013;82:155–184. doi: 10.1016/B978-0-12-407679-2.00005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hailu A, Schoone GJ, Diro E, Tesfaye A, Techane Y, Tefera T, Assefa Y, Genetu A, Kebede Y, Kebede T, Schallig HD. Field evaluation of a fast anti-Leishmania antibody detection assay in Ethiopia. Trans R Soc Trop Med Hyg. 2006;100(1):48–52. doi: 10.1016/j.trstmh.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Hide M, Singh R, Kumar B, Bañuls AL, Sundar S. A microculture technique for isolating live Leishmania parasites from peripheral blood of visceral leishmaniasis patients. Acta Trop. 2007;102(3):197–200. doi: 10.1016/j.actatropica.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Hossain F, Ghosh P, Khan MA, Duthie MS, Vallur AC, Picone A, Howard RF, Reed SG, Mondal D. Real-time PCR in detection and quantitation of Leishmania donovani for the diagnosis of Visceral Leishmaniasis patients and the monitoring of their response to treatment. PLoS ONE. 2017;12(9):e0185606. doi: 10.1371/journal.pone.0185606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail N, Kaul A, Bhattacharya P, Gannavaram S, Nakhasi HL. Immunization with live attenuated Leishmania donovani Centrin−/− parasites is efficacious in asymptomatic infection. Front Immunol. 2017;8:1788. doi: 10.3389/fimmu.2017.01788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamjoom MB, Ashford RW, Bates PA, Chance ML, Kemp SJ, Watts PC, Noyes HA. Leishmania donovani is the only cause of visceral leishmaniasis in East Africa; previous descriptions of L. infantum and “L. archibaldi” from this region are a consequence of convergent evolution in the isoenzyme data. Parasitology. 2004;129(4):399–409. doi: 10.1017/s0031182004005955. [DOI] [PubMed] [Google Scholar]
- Jensen AT, Gasim S, Moller T, Ismail A, Gaafar A, Kemp M, El Hassan AM, Kharazmi A, Alce TM, Smith DF, Theander TG. Serodiagnosis of Leishmania donovani infections: assessment of enzyme-linked immunosorbent assays using recombinant L. donovani gene B protein (GBP) and a peptide sequence of L. donovani GBP. Trans R Soc Trop Med Hyg. 1999;93(2):157–160. doi: 10.1016/s0035-9203(99)90291-2. [DOI] [PubMed] [Google Scholar]
- Jorquera A, González R, Marchán-Marcano E, Oviedo M, Matos M. Multiplex-PCR for detection of natural Leishmania infection in Lutzomyia spp. captured in an endemic region for cutaneous leishmaniasis in state of Sucre, Venezuela. Mem Inst Oswaldo Cruz. 2005;100(1):45–48. doi: 10.1590/s0074-02762005000100008. [DOI] [PubMed] [Google Scholar]
- Kaul P, Malla N, Kaur S, Mahajan RC, Ganguly NK. Evaluation of a 200-kDa amastigote-specific antigen of L. donovani by enzyme-linked immunosorbent assay (ELISA) for the diagnosis of visceral leishmaniasis. Trans R Soc Trop Med Hyg. 2000;94(2):173–175. doi: 10.1016/s0035-9203(00)90264-5. [DOI] [PubMed] [Google Scholar]
- Kaur J, Kaur S. ELISA and western blotting for the detection of Hsp70 and Hsp83 antigens of Leishmania donovani. J Parasit Dis. 2013;37(1):68–73. doi: 10.1007/s12639-012-0133-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil EA, Ayed NB, Musa AM, Ibrahim ME, Mukhtar MM, Zijlstra EE, et al. Dichotomy of protective cellular immune responses to human visceral leishmaniasis. Clin Exp Immunol. 2005;140(2):349–353. doi: 10.1111/j.1365-2249.2005.02768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MG, Bhaskar KR, Salam MA, Akther T, Pluschke G, Mondal D. Diagnostic accuracy of loop-mediated isothermal amplification (LAMP) for detection of Leishmania DNA in buffy coat from visceral leishmaniasis patients. Parasit Vectors. 2012;5(1):280. doi: 10.1186/1756-3305-5-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatun M, Alam SS, Khan AH, Hossain MA, Haq JA, Jilani MS, Rahman MT, Karim MM. Novel PCR primers to diagnose visceral Leishmaniasis using peripheral blood, spleen or bone marrow aspirates. Asian Pac J Trop Med. 2017;10(8):753–759. doi: 10.1016/j.apjtm.2017.08.002. [DOI] [PubMed] [Google Scholar]
- Kotb Elmahallawy E, Agil A. Treatment of leishmaniasis: a review and assessment of recent research. Curr Pharm Des. 2015;21(17):2259–2275. doi: 10.2174/1381612821666141231163053. [DOI] [PubMed] [Google Scholar]
- Kothalawala HS, Karunaweera ND. Loop-mediated isothermal amplification assay as a sensitive diagnostic tool for Leishmania donovani infections in Sri Lanka. Ceylon Med J. 2016 doi: 10.4038/cmj.v61i2.8286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Boggula VR, Misra P, Sundar S, Shasany AK, Dube A. Amplified fragment length polymorphism (AFLP) analysis is useful for distinguishing Leishmania species of visceral and cutaneous forms. Acta Trop. 2010;113(2):202–206. doi: 10.1016/j.actatropica.2009.10.006. [DOI] [PubMed] [Google Scholar]
- Lemma W, Bizuneh A, Tekie H, Belay H, Wondimu H, Kassahun A, Shiferaw W, Balkew M, Abassi I, Baneth G, Hailu A. Preliminary study on investigation of zoonotic visceral leishmaniasis in endemic foci of Ethiopia by detecting Leishmania infections in rodents. Asian Pac J Trop Dis. 2017;10(4):418–422. doi: 10.1016/j.apjtm.2017.03.018. [DOI] [PubMed] [Google Scholar]
- Li XX, Zhou XN. Co-infection of tuberculosis and parasitic diseases in humans: a systematic review. Parasit Vectors. 2013;6(1):79. doi: 10.1186/1756-3305-6-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima IP, Müller MC, Holanda TA, Harhay M, Costa CH, Costa DL. Human immunodeficiency virus/Leishmania infantum in the first foci of urban American visceral leishmaniasis: clinical presentation from 1994 to 2010. Rev Soc Bras Med Trop. 2013;46(2):156–160. doi: 10.1590/0037-8682-0033-2012. [DOI] [PubMed] [Google Scholar]
- Lindoso JA, Cota GF, da Cruz AM, Goto H, Maia-Elkhoury AN, Romero GA, de Sousa-Gomes ML, Santos-Oliveira JR, Rabello A. Visceral leishmaniasis and HIV coinfection in Latin America. PLoS Negl Trop Dis. 2014;8(9):e3136. doi: 10.1371/journal.pntd.0003136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindoso JA, Cunha MA, Queiroz IT, Moreira CH. Leishmaniasis–HIV coinfection: current challenges. HIV/AIDS (Auckland, NZ) 2016;8:147. doi: 10.2147/HIV.S93789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Uzonna JE. The early interaction of Leishmania with macrophages and dendritic cells and its influence on the host immune response. Front Cell Infect Microbiol. 2012;2:83. doi: 10.3389/fcimb.2012.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maizels RM, Hewitson JP, Smith KA. Susceptibility and immunity to helminth parasites. Curr Opin Immunol. 2012;24(4):459–466. doi: 10.1016/j.coi.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manna L, Reale S, Vitale F, Picillo E, Pavone LM, Gravino AE. Real-time PCR assay in Leishmania-infected dogs treated with meglumine antimoniate and allopurinol. Vet J. 2008;177(2):279–282. doi: 10.1016/j.tvjl.2007.04.013. [DOI] [PubMed] [Google Scholar]
- Martin SK, Thuita-Harun L, Adoyo-Adoyo M, Wasunna KM. A diagnostic ELISA for visceral leishmaniasis, based on antigen from media conditioned by Leishmaniadonovani promastigotes. Ann Trop Med Parasitol. 1998;92(5):571–577. doi: 10.1080/00034989859267. [DOI] [PubMed] [Google Scholar]
- Maurya R, Mehrotra S, Prajapati VK, Nylén S, Sacks D, Sundar S. Evaluation of blood agar microtiter plates for culturing Leishmania parasites to titrate parasite burden in spleen and peripheral blood of patients with visceral leishmaniasis. J Clin Microbiol. 2010;48(5):1932–1934. doi: 10.1128/JCM.01733-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettler M, Grimm F, Capelli G, Camp H, Deplazes P. Evaluation of enzyme-linked immunosorbent assays, an immunofluorescent-antibody test, and two rapid tests (immunochromatographic-dipstick and gel tests) for serological diagnosis of symptomatic and asymptomatic Leishmania infections in dogs. J Clin Microbiol. 2005;43(11):5515–5519. doi: 10.1128/JCM.43.11.5515-5519.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikaeili F, Fakhar M, Sarkari B, Motazedian MH, Hatam G. Comparison of serological methods (ELISA, DAT and IFA) for diagnosis of visceral leishmaniasis utilizing an endemic strain. Iran J Immunol. 2007;4(2):116–121. doi: 10.22034/iji.2007.17188. [DOI] [PubMed] [Google Scholar]
- Mohammadiha A, Mohebali M, Haghighi A, Mahdian R, Abadi AR, Zarei Z, Yeganeh F, Kazemi B, Taghipour N, Akhoundi B. Comparison of real-time PCR and conventional PCR with two DNA targets for detection of Leishmania (Leishmania) infantum infection in human and dog blood samples. Exp Parasitol. 2013;133(1):89–94. doi: 10.1016/j.exppara.2012.10.017. [DOI] [PubMed] [Google Scholar]
- Mohapatra TM, Singh DP, Sen MR, Bharti K, Sundar S. Comparative evaluation of rK9 rK26 and rK39 antigens in the serodiagnosis of Indian visceral leishmaniasis. J Infect Dev Ctries. 2010;4:114–117. doi: 10.3855/jidc.544. [DOI] [PubMed] [Google Scholar]
- Mondal S, Bhattacharya P, Ali N. Current diagnosis and treatment of visceral leishmaniasis. Expert Rev Anti-infect Ther. 2010;8(8):919–944. doi: 10.1586/eri.10.78. [DOI] [PubMed] [Google Scholar]
- Moody A, Chiodini P. Methods for the detection of blood parasites. Clin Lab Haematol. 2000;22(4):189–201. doi: 10.1046/j.1365-2257.2000.00318.x. [DOI] [PubMed] [Google Scholar]
- Moreira MA, Luvizotto MC, Garcia JF, Corbett CE, Laurenti MD. Comparison of parasitological, immunological and molecular methods for the diagnosis of leishmaniasis in dogs with different clinical signs. Vet Parasitol. 2007;145(3–4):245–252. doi: 10.1016/j.vetpar.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Mugasa CM, Deborggraeve S, Schoone GJ, Laurent T, Leeflang MM, Ekangu RA, El Safi S, Saad AF, Basiye FL, De Doncker S, Lubega GW. Accordance and concordance of PCR and NASBA followed by oligochromatography for the molecular diagnosis of Trypanosoma brucei and Leishmania. Trop Med Int Health. 2010;15(7):800–805. doi: 10.1111/j.1365-3156.2010.02547.x. [DOI] [PubMed] [Google Scholar]
- Mukhtar MM, Sharief AH, El Saffi SH, Harith AE, Higazzi TB, Adam AM, Abdalla HS. Detection of antibodies to Leishmania donovani in animals in a Kala-Azar Endemic Region in Eastern Sudan: a preliminary report. Trans R Soc Trop Med Hyg. 2000;94:33–36. doi: 10.1016/s0035-9203(00)90429-2. [DOI] [PubMed] [Google Scholar]
- Mukhtar M, Ali SS, Boshara SA, Albertini A, Monnerat S, Bessell P, Mori Y, Kubota Y, Ndung’u JM, Cruz I. Sensitive and less invasive confirmatory diagnosis of visceral leishmaniasis in Sudan using loop-mediated isothermal amplification (LAMP) PLoS Negl Trop Dis. 2018;12(2):e0006264. doi: 10.1371/journal.pntd.0006264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musa A, Khalil E, Hailu A, Olobo J, Balasegaram M, Omollo R, et al. Sodium stibogluconate (SSG) and paromomycin combination compared to SSG for visceral leishmaniasis in East Africa: a randomised controlled trial. PLoS Negl Trop Dis. 2012;6:e1674. doi: 10.1371/journal.pntd.0001674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neogy AB, Nandy A, Chowdhury AB. Leishmanin test in post-kala-azar dermal leishmaniasis. Trans R Soc Trop Med Hyg. 1990;84(1):58. doi: 10.1016/0035-9203(90)90381-n. [DOI] [PubMed] [Google Scholar]
- Nylén S, Sacks D. Interleukin-10 and the pathogenesis of human visceral leishmaniasis. Trends Immunol. 2007;28(9):378–384. doi: 10.1016/j.it.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Oliva G, Scalone A, Manzillo VF, Gramiccia M, Pagano A, Di Muccio T, Gradoni L. Incidence and time course of Leishmania infantum infections examined by parasitological, serologic, and nested-PCR techniques in a cohort of naive dogs exposed to three consecutive transmission seasons. J Clin Microbiol. 2006;44(4):1318–1322. doi: 10.1128/JCM.44.4.1318-1322.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olliaro PL, Shamsuzzaman TA, Marasini B, Dhariwal AC, Be-Nazir A, Mondal D, Banjara MR, Das P, Sundar S, Rijal S, Arana B. Investments in research and surveillance are needed to go beyond elimination and stop transmission of Leishmania in the Indian subcontinent. PLoS Negl Trop Dis. 2017;11:e0005190. doi: 10.1371/journal.pntd.0005190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovalle-Bracho C, Camargo C, Díaz-Toro Y, Parra-Muñoz M. Molecular typing of Leishmania (Leishmania) amazonensis and species of the subgenus Viannia associated with cutaneous and mucosal leishmaniasis in Colombia: a concordance study. Biomédica. 2018;38(1):86–95. doi: 10.7705/biomedica.v38i0.3632. [DOI] [PubMed] [Google Scholar]
- Palatnik-de-Sousa CB. Vaccines for leishmaniasis in the fore coming 25 years. Vaccine. 2008;26(14):1709–1724. doi: 10.1016/j.vaccine.2008.01.023. [DOI] [PubMed] [Google Scholar]
- Parolo C, Merkoçi A. Paper-based nanobiosensors for diagnostics. Chem Soc Rev. 2013;42:450–457. doi: 10.1039/c2cs35255a. [DOI] [PubMed] [Google Scholar]
- Passos V, Barreto SM, Romanha AJ, Krettli AU, Volpini ÂC, Costa MF. American cutaneous leishmaniasis: use of a skin test as a predictor of relapse after treatment. Bull World Health Organ. 2000;78:968–974. [PMC free article] [PubMed] [Google Scholar]
- Pattabhi S, Whittle J, Mohamath R, El-Safi S, Moulton GG, Guderian JA, Colombara D, Abdoon AO, Mukhtar MM, Mondal D, Esfandiari J, Kumar S, Chun P, Reed SG, Bhatia A. Design, development and evaluation of rK28-based point-of-care tests for improving rapid diagnosis of visceral leishmaniasis. PLoS Negl Trop Dis. 2010;14(9):e822. doi: 10.1371/journal.pntd.0000822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennisi MG, Reale S, Giudice SL, Masucci M, Caracappa S, Vitale M, Vitale F. Real-time PCR in dogs treated for leishmaniasis with allopurinol. Vet Res Commun. 2005;29(2):301–303. doi: 10.1007/s11259-005-0067-4. [DOI] [PubMed] [Google Scholar]
- Pintado V, MartÍn-rabadÁn P, Rivera ML, Moreno S, Bouza E. Visceral leishmaniasis in human immunodeficiency virus (HIV)-infected and non-HIV-infected patients: a comparative study. Medicine. 2001;80(1):54–73. doi: 10.1097/00005792-200101000-00006. [DOI] [PubMed] [Google Scholar]
- Raj VS, Ghosh A, Dole VS, Madhubala RE, Myler PJ, Stuart KD. Serodiagnosis of leishmaniasis with recombinant ORFF antigen. Am J Trop Med Hyg. 1999;61(3):482–487. doi: 10.4269/ajtmh.1999.61.482. [DOI] [PubMed] [Google Scholar]
- Ravindran R, Anam K, Bairagi BC, Saha B, Pramanik N, Guha SK, Goswami RP, Banerjee D, Ali N. Characterization of immunoglobulin G and its subclass response to Indian kala-azar infection before and after chemotherapy. Infect Immun. 2004;72(2):863–870. doi: 10.1128/IAI.72.2.863-870.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ready PD. Biology of phlebotomine sand flies as vectors of disease agents. Annu Rev Entomol. 2013;58:227–250. doi: 10.1146/annurev-ento-120811-153557. [DOI] [PubMed] [Google Scholar]
- Ready P. Epidemiology of visceral leishmaniasis. Clin Epidemiol. 2014;6:147–154. doi: 10.2147/CLEP.S44267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis LE, Coura-Vital W, Roatt BM, Bouillet LÉ, Ker HG, de Brito RC, de Melo Resende D, Carneiro M, Giunchetti RC, Marques MJ, Carneiro CM. Molecular diagnosis of canine visceral leishmaniasis: a comparative study of three methods using skin and spleen from dogs with natural Leishmania infantum infection. Vet Parasitol. 2013;197(3–4):498–503. doi: 10.1016/j.vetpar.2013.07.006. [DOI] [PubMed] [Google Scholar]
- Reithinger R. Diagnosis and treatment of cutaneous leishmaniasis. Expert Rev Dermatol. 2008;3(3):315–327. [Google Scholar]
- Reithinger R, Dujardin JC. Molecular diagnosis of leishmaniasis: current status and future applications. J Clin Microbiol. 2007;45(1):21–25. doi: 10.1128/JCM.02029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reithinger R, Quinnell RJ, Alexander B, Davies CR. Rapid detection of Leishmania infantum infection in dogs: comparative study using an immunochromatographic dipstick test, enzyme-linked immunosorbent assay, and PCR. J Clin Micro-Biol. 2002;40:2352–2356. doi: 10.1128/JCM.40.7.2352-2356.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reithinger R, Dujardin JC, Louzir H, Pirmez C, Alexander B, Brooker S. Cutaneous leishmaniasis. Lancet Infect Dis. 2007;7(9):581–596. doi: 10.1016/S1473-3099(07)70209-8. [DOI] [PubMed] [Google Scholar]
- Restrepo CM, De La Guardia C, Sousa OE, Calzada JE, Fernández PL, Lleonart R. AFLP polymorphisms allow high resolution genetic analysis of American Tegumentary Leishmaniasis agents circulating in Panama and other members of the Leishmania genus. PLoS ONE. 2013;8(9):e73177. doi: 10.1371/journal.pone.0073177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezvan H, Hamoon Navard S. An overview on Leishmania diagnosis. J Med Microbiol Infect Dis. 2017;5(1):1. [Google Scholar]
- Riera C, Fisa R, Lopez-Chejade P, Serra T, Girona E, Jimenez M, et al. Asymptomatic infection by Leishmania infantum in blood donors from the Balearic Islands (Spain) Transfusion. 2008;48(7):1383–1389. doi: 10.1111/j.1537-2995.2008.01708.x. [DOI] [PubMed] [Google Scholar]
- Rivas L, de la Escosura-Muñiz A, Serrano L, Altet L, Francino O, Sánchez A, Merkoçi A. Triple lines gold nanoparticle-based lateral flow assay for enhanced and simultaneous detection of Leishmania DNA and endogenous control. Nano Res. 2015;8(11):3704–3714. [Google Scholar]
- Romero LI, Paz HM, Ortega-Barría E, et al. Evaluation of serological assays based on a novel excreted antigen preparation for the diagnosis of Cutaneous Leishmaniasis in Panama. J Microbiol Methods. 2004;57(3):391–397. doi: 10.1016/j.mimet.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Ryan JR, Smithyman AM, Rajasekariah GH, Hochberg L, Stiteler JM, Martin SK. Enzyme-linked immunosorbent assay based on soluble promastigote antigen detects immunoglobulin M (IgM) and IgG antibodies in sera from cases of visceral and cutaneous leishmaniasis. J Clin Microbiol. 2002;40(3):1037–1043. doi: 10.1128/JCM.40.3.1037-1043.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad AA, Ahmed NG, Osman OS, Al-Basheer AA, Hamad A, Deborggraeve S, Büscher P, Schoone GJ, Schallig HD, Laurent T, Haleem A. Diagnostic accuracy of the Leishmania OligoC-TesT and NASBA-Oligochromatography for diagnosis of leishmaniasis in Sudan. PLoS Negl Trop Dis. 2010;4(8):e776. doi: 10.1371/journal.pntd.0000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadlova J, Seblova V, Votypka J, Warburg A, Volf P. Xenodiagnosis of Leishmania donovani in BALB/c mice using Phlebotomusorientalis: a new laboratory model. Parasit Vectors. 2015;8(1):158. doi: 10.1186/s13071-015-0765-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salam MA, Khan MG, Bhaskar KR, Afrad MH, Huda MM, Mondal D. Peripheral blood buffy coat smear: a promising tool for diagnosis of visceral leishmaniasis. J Clin Microbiol. 2012;50(3):837–840. doi: 10.1128/JCM.05067-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkari B, Ashrafmansouri M, Hatam G, Habibi P, AbdolahiKhabisi S. Performance of an ELISA and indirect immunofluorescence assay in serological diagnosis of zoonotic cutaneous leishmaniasis in Iran. Interdiscip Perspect Infect Dis. 2014 doi: 10.1155/2014/505134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schallig HD, Cardoso L, Hommers M, Kroon N, Belling G, Rodrigues M, Semiao-Santos SJ, Vetter H. Development of a dipstick assay for detection of Leishmania-specific canine antibodies. J Clin Microbiol. 2004;42(1):193–197. doi: 10.1128/JCM.42.1.193-197.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönian G, Kuhls K, Mauricio IL. Molecular approaches for a better understanding of the epidemiology and population genetics of Leishmania. Parasitology. 2011;138(4):405–425. doi: 10.1017/S0031182010001538. [DOI] [PubMed] [Google Scholar]
- Schoone GJ, Hailu A, Kroon CC, Nieuwenhuys JL, Schallig HD, Oskam L. A fast agglutination screening test (FAST) for the detection of anti-Leishmania antibodies. Trans R Soc Trop Med Hyg. 2001;95(4):400–401. doi: 10.1016/s0035-9203(01)90196-8. [DOI] [PubMed] [Google Scholar]
- Shahbazi F, Shahabi S, Kazemi B, Mohebali M, Abadi AR, Zare Z. Evaluation of PCR assay in diagnosis and identification of cutaneous leishmaniasis: a comparison with the parasitological methods. Parasitol Res. 2008;103(5):1159–1162. doi: 10.1007/s00436-008-1111-4. [DOI] [PubMed] [Google Scholar]
- Sharma U, Singh S. Insect vectors of Leishmania: distribution, physiology and their control. J Vector Borne Dis. 2008;45(4):255–272. [PubMed] [Google Scholar]
- Shirian S, Oryan A, Hatam GR, Panahi S, Daneshbod Y. Comparison of conventional, molecular, and immunohistochemical methods in diagnosis of typical and atypical cutaneous leishmaniasis. Arch Pathol Lab Med. 2014;138(2):235–240. doi: 10.5858/arpa.2013-0098-OA. [DOI] [PubMed] [Google Scholar]
- Shweta SB, Gupta AK, Murti K, Pandey K. Co-infection of visceral leishmaniasis and pulmonary tuberculosis: a case study. Asian Pac J Trop Med. 2014;4(1):57. [Google Scholar]
- Siewe N, Yakubu AA, Satoskar AR, Friedman A. Immune response to infection by Leishmania: a mathematical model. Math Biosci. 2016;276:28–43. doi: 10.1016/j.mbs.2016.02.015. [DOI] [PubMed] [Google Scholar]
- Singh S. New developments in diagnosis of leishmaniasis. Indian J Med Res. 2006;123(3):311. [PubMed] [Google Scholar]
- Singh S. Changing trends in the epidemiology, clinical presentation, and diagnosis of Leishmania-HIV co-infection in India. Int J Infect Dis. 2014;29:103–112. doi: 10.1016/j.ijid.2014.07.011. [DOI] [PubMed] [Google Scholar]
- Singh S, Sivakumar R. Recent advances in the diagnosis of leishmaniasis. J Postgrad Med. 2003;49(1):55. doi: 10.4103/0022-3859.927. [DOI] [PubMed] [Google Scholar]
- Singh OP, Sundar S. Developments in diagnosis of visceral leishmaniasis in the elimination era. J Parasitol Res. 2015;2015:2015. doi: 10.1155/2015/239469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Dey A, Sivakumar R. Applications of molecular methods for Leishmania control. Expert Rev Mol Diagn. 2005;5(2):251–265. doi: 10.1586/14737159.5.2.251. [DOI] [PubMed] [Google Scholar]
- Singh DP, Goyal RK, Singh RK, Sundar S, Mohapatra TM. In search of an ideal test for diagnosis, prognosis of kala-azar. J Health Popul Nutr. 2010;28:281–285. doi: 10.3329/jhpn.v28i3.5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivakumar R, Sharma P, Chang KP, Singh S. Cloning, expression, and purification of a novel recombinant antigen from Leishmania donovani. Protein Expr Purif. 2006;46(1):156–165. doi: 10.1016/j.pep.2005.07.027. [DOI] [PubMed] [Google Scholar]
- Smyth AJ, Ghosh A, Hassan MQ, Basu D, De Bruijn MHL, Adhya S, Mallik KK, Barker DC. Rapid and sensitive detection of Leishmania kinetoplast DNA from spleen and blood samples of kala-azar patients. Parasitology. 1992;105(2):183–192. doi: 10.1017/s0031182000074096. [DOI] [PubMed] [Google Scholar]
- Solano-Gallego L, Miró G, Koutinas A, Cardoso L, Pennisi MG, Ferrer L, Bourdeau P, Oliva G, Baneth G, The LeishVet Group LeishVet guidelines for the practical management of canine leishmaniosis. Parasit Vectors. 2011;4:86. doi: 10.1186/1756-3305-4-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava P, Dayama A, Mehrotra S, Sundar S. Diagnosis of visceral leishmaniasis. Trans R Soc Trop Med Hyg. 2011;105(1):1–6. doi: 10.1016/j.trstmh.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srividya G, Kulshrestha A, Singh R, Salotra P. Diagnosis of visceral leishmaniasis: developments over the last decade. Parasitol Res. 2012;110:1065–1078. doi: 10.1007/s00436-011-2680-1. [DOI] [PubMed] [Google Scholar]
- Steverding D. The history of leishmaniasis. Parasit Vectors. 2017;10(1):82. doi: 10.1186/s13071-017-2028-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockdale L, Newton R. A review of preventative methods against human leishmaniasis infection. PLoS Negl Trop Dis. 2013;7(6):e2278.58. doi: 10.1371/journal.pntd.0002278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudarshan M, Sundar S. Parasite load estimation by qPCR differentiate between asymptomatic and symptomatic infection in Indian visceral leishmaniasis. Diagn Microbiol Infect Dis. 2014;80(1):40–42. doi: 10.1016/j.diagmicrobio.2014.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundar S, Rai M. Laboratory diagnosis of visceral leishmaniasis. Clin Diagn Lab Immunol. 2002;9(5):951–958. doi: 10.1128/CDLI.9.5.951-958.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundar S, Singh A. Recent developments and future prospects in the treatment of visceral leishmaniasis. Ther Adv Infect Dis. 2016;3–4:98–109. doi: 10.1177/2049936116646063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundar S, Gupta LB, Rastogi V, Agrawal G, Murray HW. Short-course, cost-effective treatment with amphotericin B-fat emulsion cures visceral leishmaniasis. Trans R Soc Trop Med Hyg. 2000;94:200–204. doi: 10.1016/s0035-9203(00)90277-3. [DOI] [PubMed] [Google Scholar]
- Sundar S, Jha TK, Thakur CP, Engel J, Sindermann H, Fischer C, et al. Oral miltefosine for Indian visceral leishmaniasis. N Engl J Med. 2002;347:1739–1746. doi: 10.1056/NEJMoa021556. [DOI] [PubMed] [Google Scholar]
- Sundar S, Pai K, Sahu M, Kumar V, Murray HW. Immunochromatographic strip-test detection of anti-K39 antibody in Indian visceral leishmaniasis. Ann Trop Med Parasitol. 2002;96(1):19–23. doi: 10.1179/000349802125000466. [DOI] [PubMed] [Google Scholar]
- Teixeira DE, Benchimol M, Rodrigues JC, Crepaldi PH, Pimenta PF, de Souza W. The cell biology of Leishmania: how to teach using animations. PLoS Pathog. 2013;9(10):e1003594. doi: 10.1371/journal.ppat.1003594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur L, Singh KK, Shanker V, Negi A, Jain A, Matlashewski G, Jain M. Atypical leishmaniasis: a global perspective with emphasis on the Indian subcontinent. PLoS Negl Trop Dis. 2018;12(9):e0006659. doi: 10.1371/journal.pntd.0006659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomaz-Soccol V, Ferreira da Costa ES, Karp SG, Junior Letti LA, Soccol FT, Soccol CR. Recent advances in vaccines against Leishmania based on patent applications. Recent Pat Biotechnol. 2018;12(1):21–32. doi: 10.2174/1872208311666170510121126. [DOI] [PubMed] [Google Scholar]
- Tlamcani Z. Visceral leishmaniasis: an update of laboratory diagnosis. Asian Pac J Trop Dis. 2016;6(7):505–508. [Google Scholar]
- Torres-Guerrero E, Quintanilla-Cedillo MR, Ruiz-Esmenjaud J, Arenas R. Leishmaniasis: a review. F1000 Res. 2017 doi: 10.12688/f1000research.11120.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Meide WF, Schoone GJ, Faber WR, Zeegelaar JE, de Vries HJ, Özbel Y, Fat RFLA, Coelho LI, Kassi M, Schallig HD. Quantitative nucleic acid sequence-based assay as a new molecular tool for detection and quantification of Leishmania parasites in skin biopsy samples. J Clin Microbiol. 2005;43(11):5560–5566. doi: 10.1128/JCM.43.11.5560-5566.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma S, Avishek K, Sharma V, Negi NS, Ramesh V, Salotra P. Application of loop-mediated isothermal amplification assay for the sensitive and rapid diagnosis of visceral leishmaniasis and post-kala-azar dermal leishmaniasis. Diagn Microbiol Infect Dis. 2013;75(4):390–395. doi: 10.1016/j.diagmicrobio.2013.01.011. [DOI] [PubMed] [Google Scholar]
- Vink MM, Nahzat SM, Rahimi H, Buhler C, Ahmadi BA, Nader M, Zazai FR, Yousufzai AS, van Loenen M, Schallig HD, Picado A. Evaluation of point-of-care tests for cutaneous leishmaniasis diagnosis in Kabul, Afghanistan. E Bio Medicine. 2018;37:453–460. doi: 10.1016/j.ebiom.2018.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JY, Ha Y, Gao CH, Wang Y, Yang YT, Chen HT. The prevalence of canine Leishmania infantum infection in western China detected by PCR and serological tests. Parasit Vector. 2011;4(1):69. doi: 10.1186/1756-3305-4-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch RJ, Anderson BL, Litwin CM. Rapid immunochromatographic strip test for detection of anti-K39 immunoglobulin G antibodies for diagnosis of visceral leishmaniasis. Clin Vaccine Immunol. 2008;15(9):1483–1484. doi: 10.1128/CVI.00174-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2016) Leishmaniasis epidemilogical situations. https://www.who.int/leishmaniasis/en. Accessed 20 May 2018
- Yang J, Zhang L, Yu C, Yang XF, Wang H. Monocyte and macrophage differentiation: circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomark Res. 2014;2(1):1. doi: 10.1186/2050-7771-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasinzai M, Khan M, Nadhman A, Shahnaz G. Drug resistance in leishmaniasis: current drug-delivery systems and future perspectives. Future Med Chem. 2013;5(15):1877–1888. doi: 10.4155/fmc.13.143. [DOI] [PubMed] [Google Scholar]
- Zanoli L, Spoto G. Isothermal amplification methods for the detection of nucleic acids in microfluidic devices. Biosensors. 2013;3(1):18–43. doi: 10.3390/bios3010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeyrek FY, Töz S, Yuksel F, Turgay N, Ozbel Y. The comparison of kinetoplastid DNA PCR and parasitological methods in the diagnosis of cutaneous leishmaniasis using clinical samples of suspected patients in Turkey. Int J Infect Dis. 2018;73:317. [Google Scholar]
- Zijlstra EE, Ali MS, El-Hassan AM, El-Toum IA, Satti M, Ghalib HW, Kager PA. Kala-azar: a comparative study of parasitological methods and the direct agglutination test in diagnosis. Trans R Soc Trop Med Hyg. 1992;86(5):505–507. doi: 10.1016/0035-9203(92)90086-r. [DOI] [PubMed] [Google Scholar]
- Zijlstra EE, Khalil EA, Kager PA, El-Hassan AM. Post-kalaazar dermal leishmaniasis in the Sudan: clinical presentation and differential diagnosis. Br J Dermatol. 2000;143(1):136–143. doi: 10.1046/j.1365-2133.2000.03603.x. [DOI] [PubMed] [Google Scholar]