Abstract
Microscopic colitis (MC) is a treatable cause of chronic, non-bloody, watery diarrhoea, but physicians (particularly in primary care) are less familiar with MC than with other causes of chronic diarrhoea. The colon in patients with MC is usually macroscopically normal. MC can only be diagnosed by histological examination of colonic biopsies (subepithelial collagen band >10 µm (collagenous colitis) or >20 intraepithelial lymphocytes per 100 epithelial cells (lymphocytic colitis), both with lamina propria inflammation). The UK National Health Service exerts downward pressure to minimise colonoscopy referrals. Furthermore, biopsies are often not taken according to guidelines. These factors work against MC diagnosis. In this review, we note the high incidence of MC (comparable to ulcerative colitis and Crohn’s disease) and its symptomatic overlap with irritable bowel syndrome. We also highlight problems with the recommendation by National Health Service/National Institute for Health and Care Excellence guidelines for inflammatory bowel diseases that colonoscopy referrals should be based on a faecal calprotectin level of ≥100 µg/g. Faecal calprotectin is <100 µg/g in over half of individuals with active MC, building into the system a propensity to misdiagnose MC as irritable bowel syndrome. This raises important questions—how many patients with MC have already been misdiagnosed, and how do we address this silent burden? Clarity is needed around pathways for MC management; MC is poorly acknowledged by the UK healthcare system and it is unlikely that best practices are being followed adequately. There is an opportunity to identify and treat patients with MC more effectively.
Keywords: inflammatory bowel disease, collagenous colitis, lymphocytic colitis, colonoscopy, histopathology
Key messages.
Microscopic colitis (MC) is a prevalent (comparable to ulcerative colitis and Crohn’s disease) but treatable cause of chronic, non-bloody, watery diarrhoea.
The colon is macroscopically normal in most patients with MC; diagnosis thus requires histological examination of colonic biopsies.
MC is less familiar to physicians (particularly in primary care) than other causes of chronic diarrhoea and may be misdiagnosed because of symptomatic overlap with irritable bowel syndrome (IBS), downward pressure not to refer patients for colonoscopy and low adherence to biopsy guidelines.
The UK National Health Service recommends colonoscopy when faecal calprotectin is ≥100 µg/g, based on National Institute for Health and Care Excellence guidelines for inflammatory bowel diseases; however, faecal calprotectin is <100 µg/g in over 50% of patients with active MC.
In most cases it is possible to distinguish between IBS and MC by taking a thorough history, though a more pragmatic approach is to refer patients for colonoscopy with biopsy to assess MC if they have chronic (>4 weeks), mainly watery diarrhoea.
A large, hidden burden of undiagnosed and untreated MC likely exists in the UK population owing to systemic misdiagnosis of MC as IBS.
Pathways for the management of MC in the UK are unclear and need to be clarified so that they can be amended and standardised where necessary.
Introduction
The underdiagnosis of microscopic colitis (MC) represents a lost opportunity for patients and clinicians. In many national settings, because of downward pressure to minimise specialist referrals and colonoscopies with histology in patients with seemingly normal endoscopic appearances, the systems for diagnosing and managing patients with gastrointestinal problems work against a diagnosis of MC and its effective management.
When first described in 1980, MC was thought to be a rare condition.1 We now know that MC is a common form of inflammatory bowel disease (IBD), with one recent study putting the incidence in the UK at around 18 cases per 100 000 population/year.2 The incidence of MC in the USA and the UK has been increasing, though it may have stabilised in the USA.2–4 Reasons for the growing incidence of MC may include greater awareness and the ageing population. Importantly, MC accounts for approximately 10% of individuals who present with non-bloody diarrhoea,5 but physicians, particularly in primary care, are not as aware of MC as a cause of this symptom as they are of other IBDs and irritable bowel syndrome (IBS). This is perhaps not surprising, considering that the UK National Health Service (NHS)6 refers to MC as a ‘less common type of IBD’, despite the UK incidence being comparable to ulcerative colitis and Crohn’s disease (both around 10 cases per 100 000 population/year).7 8
The defining symptom of MC is chronic or recurrent, non-bloody, watery diarrhoea.9 Known risk factors include older age,3 female sex,3 smoking10 and the use of some drugs.11 The natural course of MC is generally benign, with similar mortality12 and colorectal cancer risk13–15 as the general population. However, chronic diarrhoea, combined with other possible symptoms such as abdominal pain, fatigue, arthralgia, myalgia, urgency, faecal incontinence, nocturnal defecation and weight loss,16–19 significantly impairs health-related quality of life in patients with MC.17 Treatment of symptomatic MC focuses on improving quality of life by targeting the underlying inflammation that is presumed to cause symptoms.
The underestimation of MC as a cause of diarrhoea is creating a legacy of accumulated misdiagnoses—a situation made even more unpalatable by the fact that there is a clear path for the diagnosis and treatment of this burdensome disease. In this article, we provide a brief overview of current best practice for the diagnosis and treatment of MC, explore the clinical implications of the overlap between MC symptoms and those of IBS, particularly in relation to current guidance in the UK healthcare system for the management of IBDs, and ask the question ‘what needs to be done now?’
MC diagnosis requires histological examination of colonic biopsies
Histological assessment of MC needs to be based on stepped biopsies of the colon, with a minimum of two biopsies taken from each of the ascending, transverse and descending/sigmoid colon in separate specimen containers. MC occurs in two histologically distinct forms: collagenous colitis (CC) and lymphocytic colitis (LC). In both CC and LC, inflammation in the lamina propria is present throughout the entire colon.20 The key histological feature distinguishing CC is the presence of a broad subepithelial collagen band, >10 µm in thickness, immediately underneath the surface epithelium.20 The key histological feature that distinguishes LC is an increased number of intraepithelial lymphocytes (IEL), with >20 IELs per 100 epithelial cells generally considered diagnostic.20 Another MC group may also exist, referred to variably in the literature as MC not otherwise specified,21 paucicellular LC 22 or, most recently, MC incomplete (MCi).23 In patients with MCi, the collagen layer (>5 µm) or number of IELs (>5 per 100 epithelial cells) is abnormal, but below the threshold for CC or LC, though there is still inflammation in the lamina propria.20
Patients with CC, LC and MCi cannot be distinguished from each other based on their demographic features, clinical characteristics or symptom presentation.23–25 Treatment guidelines do not distinguish between CC and LC,9 because differences in treatment outcomes have not been observed between these subgroups.26 In addition, uncontrolled data from a retrospective analysis indicate that MCi has a similar response to treatment compared with CC and LC.23 Interestingly, in patients with CC, the degree of lamina propria inflammation has been found to be a significant predictor of the degree of symptom response,27 leading to speculation that this feature may be of more diagnostic value than the criteria used to distinguish CC, LC and MCi. It is important to note that patients with MCi currently do not receive a diagnosis of MC. If randomised, controlled data can confirm that the response of MCi to treatment is similar to CC and LC, then it seems clear that patients with MCi should be classified as MC and treated accordingly. This would also simplify diagnosis, because pathologists have more trouble consistently discriminating MCi from CC and LC than they do discriminating between MC/MCi and non-MC.28
Several approaches have been used to try and minimise the need for colonoscopy with biopsy for the diagnosis of MC. One approach is the development of scoring systems that use demographic and symptom data to identify patients at ‘low’ risk of having MC. Some of these scoring systems have shown promise.29–32 However, as pointed out in a recent review, none have been fully validated and their utility thus remains conceptual.33 Numerous potential biomarkers for MC have been explored but none are diagnostic.34 Similarly, macroscopic findings in patients with MC have been described, and are present in around one-third of patients,35 but are not specific to the condition. Histological assessment of colonic biopsies thus remains the only way to diagnose MC. Importantly, a major barrier to the assessment of colonic biopsies in patients with MC is its symptomatic overlap with IBS.
MC can be misdiagnosed as IBS
Concomitant abdominal pain is often present in patients with MC (41%–52% overall, and 74%–82% in patients with active disease16–18) and around one-third to one-half36 37 of patients who present with MC meet symptom criteria for IBS. The hidden burden of MC among patients incorrectly diagnosed with (and treated for) IBS is potentially very large. The prevalence of IBS across North America, Europe and Australasia is estimated at around 7% of the general population.38 Based on previous estimates, around 40% of these patients would have diarrhoea-predominant IBS (IBS-D),39 or around 1.7 million cases in the UK. The prevalence of MC in patients meeting diagnostic criteria for IBS-D is nearly 10%,37 or 170 000 patients if applied to the UK. A lack of familiarity with MC compared with IBS, particularly in primary care where most IBS diagnoses are made, means that many of those 170 000 MC cases might have been diagnosed as IBS-D. Patients with MC without concomitant abdominal pain (obligatory for an IBS diagnosis)40 may still receive an IBS diagnosis or the diagnosis of ‘functional diarrhoea’ if their physician’s familiarity with both MC and diagnostic criteria for functional bowel diseases is low. The latter may be quite common, with only 2%–36% of primary care physicians in a recent review reporting awareness of Rome or Manning IBS criteria.41 In addition, some treating physicians may be unfamiliar with MC, but sufficiently familiar with IBS criteria to exclude an IBS diagnosis. Given the wide range of conditions that can present with chronic diarrhoea,42 patients in this situation may face a long and frustrating road to a correct diagnosis and treatment, though this may still be preferable to being locked into a diagnosis of IBS, which excludes the possibility of more appropriate treatment. It is worth noting that UK primary care physicians frequently diagnose IBS-D without specialist referral.43 Once an IBS-D diagnosis is made, empirical treatment is often initiated without further consideration of potential organic causes.44
Patients with MC have a clinical history that is usually distinct from that of patients with IBS (table 1). In contrast to IBS, MC is most common in older individuals (>50 years) and produces diarrhoea that is consistently watery/soft, leading to imperative urgency that can result in faecal incontinence.45 46 Nocturnal stools may occur and weight loss is common. MC is often accompanied by autoimmune diseases such as rheumatic disease, thyroid disease, diabetes mellitus and coeliac disease.45 46 Indeed, patients with coeliac disease and MC can still have diarrhoea even if they are on a gluten-free diet, as long as MC remains untreated.47 In contrast to MC, IBS occurs most commonly in younger individuals (<50 years), produces stool of varying consistency, and faecal incontinence and nocturnal stools are rare.40 45 46 Unlike MC, IBS is also frequently associated with feelings of fullness/bloating and incomplete bowel evacuation.45 46 In most cases, by taking a thorough history (table 1) and using bowel diaries for 1–2 weeks, it is possible to distinguish between IBS and MC. Nevertheless, the most pragmatic approach is to refer patients for a colonoscopy with biopsy for the assessment of MC if they have chronic (>4 weeks), mainly watery diarrhoea, irrespective of their age and gender. As already discussed, histological examination of colonic biopsies is still the only way to confirm a diagnosis of MC.
Table 1.
Differences in clinical history between patients with irritable bowel syndrome and those with microscopic colitis
| Clinical history variable | Irritable bowel syndrome | Microscopic colitis |
| First occurrence of disease | Usually before 50 years of age | Usually after 50 years of age |
| Stool consistency | Soft‒variable‒hard | Watery/soft |
| Abdominal pain/discomfort | Obligatory | Variable |
| Nocturnal diarrhoea | Very unlikely | Possible |
| Feeling of incomplete bowel evacuation | Common | No |
| Weight loss | Rare | Common |
| Faecal incontinence | Rare | Common |
| Feeling of fullness/bloating | Common | Rare |
| Accompanying autoimmune disease | Rare | Common |
Downward pressure on referral is a barrier to MC diagnosis
It is a problem for the diagnosis of MC that NHS primary care guidelines for referral to colonoscopy for IBDs,48 developed in light of updated National Institute for Health and Care Excellence (NICE) recommendations,49 are currently based on faecal calprotectin (FC) levels, and make no mention of MC. These recommendations state that patients with an FC concentration below 100 µg/g should initially be treated as having IBS, and yet median concentrations of FC are below this level in active (48–80 µg/g)50 51 and inactive (26 µg/g)50 cases of MC. A high proportion of these MC cases (>50% of those with active or treated/inactive CC)50 will be below the FC threshold required for colonoscopy (figure 1). This is not surprising because inflammation in MC mostly involves lymphocytes,20 rather than neutrophils that produce FC. Further adding to the diagnostic inadequacy of FC is variation in the cut-offs that must be applied, depending on the FC assay method used.52 Even in patients with MC who do happen to have FC levels above the threshold for referral to colonoscopy, MC may not be correctly investigated, especially without prior suspicion of MC. Indeed, according to a recent study, only 19.5% of colonoscopies performed at two UK hospitals for the investigation of chronic diarrhoea were conducted according to British Society of Gastroenterology biopsy guidelines for the exclusion of MC, with the rate increasing to 48% when an indication for MC was present.53
Figure 1.
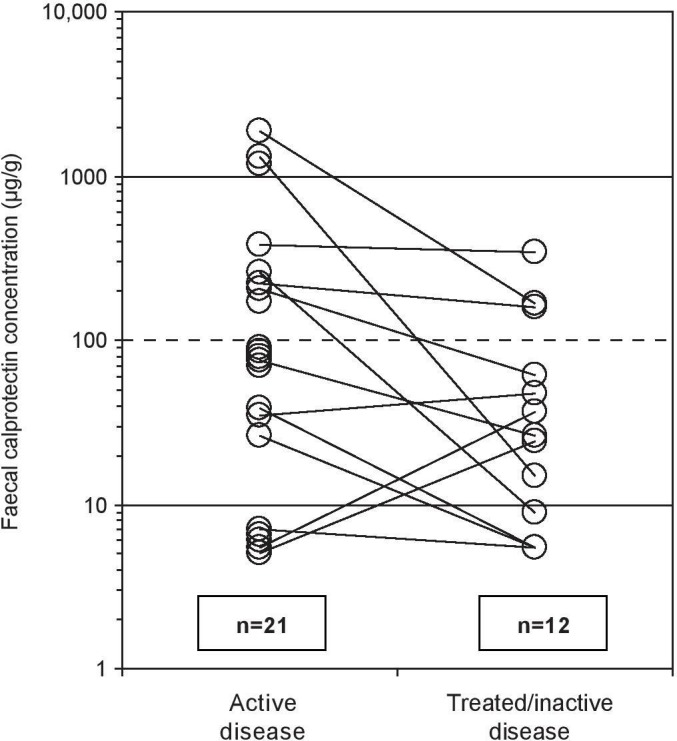
Per patient faecal calprotectin concentrations during active and treated/inactive collagenous colitis. Dashed line indicates the UK National Health Service (NHS) recommended cut-off for referral to colonoscopy,48 which is based on National Institute for Health and Care Excellence (NICE) guidelines for inflammatory bowel diseases (adapted from Wildt et al [50]).
Guidelines for the management of lower gastrointestinal symptoms in the UK reflect a general downward pressure on physicians, particularly general practitioners, to minimise patient referrals. Current pathways restrict referral and colonoscopies for what are perceived to be non-cancer and non-IBD symptoms. Even where NHS guidelines do support referral, such as for suspected colorectal cancer, perceived pressure not to refer appears substantial. A recent survey of primary care physicians in the UK identified resource pressure, perceived as coming from clinical commissioning groups, as one of the barriers preventing referral of patients meeting NICE guidelines for urgent referral for suspected colorectal cancer.54
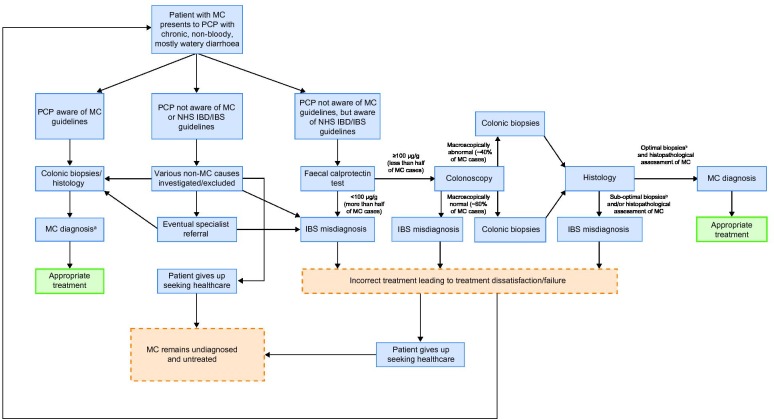
While there is a need to conserve resources in publicly funded healthcare systems, this should not be at the expense of effective patient care. As it currently stands, the UK system militates against diagnosing MC (figure 2). The social and economic (eg, work productivity, repeat visits to the doctor) costs of this likely outweigh those of achieving a rapid histological diagnosis of MC, followed by treatment, which can be effective.
Figure 2.
Pathways to misdiagnosis of microscopic colitis as irritable bowel syndrome in patients who present to a UK primary care physician with chronic diarrhoea. aOutcome assumes biopsiesb and histopathological assessment of MC is performed optimally. bOptimal biopsies for MC detection defined as stepped biopsies of the colon, with a minimum of two biopsies taken from each of the ascending, transverse and descending/sigmoid colon in separate specimen containers. CD, Crohn’s disease; IBD, inflammatory bowel disease; IBS, irritable bowel syndrome; MC, microscopic colitis; NHS, National Health Service; PCP, primary care physician; UC, ulcerative colitis.
MC is a highly treatable condition
Initial management of MC should focus on eliminating (where possible) known risk factors, namely smoking10 and certain medications (proton pump inhibitors, non-steroidal anti-inflammatory drugs, selective serotonin reuptake inhibitors and histamine-2 receptor antagonists).11 Failing this approach, pharmacological interventions should be explored.
Current guidelines recommend treatment with the corticosteroid budesonide for induction and maintenance of remission of symptomatic MC.9 45 The estimated rate of clinical remission combined with histological improvement in patients with symptomatic MC treated with budesonide is 81%.45 Relapse occurs in around 60%‒80% of patients on cessation of budesonide treatment.45 In most cases, however, reinitiating budesonide treatment leads to clinical remission, which can be maintained with a lower dose.45
Drugs used to treat other types of IBD are also likely to have a role in the treatment of MC. Non-biologic (eg, azathioprine and 6-mercaptopurine) immunosuppressant drugs have been used in clinical practice in patients with refractory symptoms or steroid dependence, and may be of benefit, but clinical trials are lacking.45 Similarly, biologic (anti-tumour necrosis factor α) immunosuppressant drugs have been used in patients with refractory symptoms, with potentially encouraging results, but again these have not been assessed in clinical trials.45 The most recent clinical trials of mesalamine in patients with CC and LC have shown this drug to be no more effective than placebo.55 56
In summary, treatment options for patients with symptomatic MC have much to offer and should improve as greater awareness of MC drives new clinical trials to support the use of other drugs in patients with refractory symptoms or who are steroid dependent.
What needs to be done now
The issue of undiagnosed MC is mainly one of familiarity versus better known causes of chronic diarrhoea such as IBS and other IBDs, and downward pressure on colonoscopy investigation and specialist referral. In patients who present with chronic diarrhoea, MC is often not considered or investigated because it is not at the forefront of doctors’ minds and because of pressure to conserve resources. Improving the detection of MC moving forward requires a concerted effort to appraise physicians of the key aspects of this condition, especially in the primary care setting where the fate of these patients is most likely to be determined. The key take home messages are as follows:
MC is an IBD with an incidence similar to ulcerative colitis and Crohn’s disease.
The defining symptom of MC is chronic (>4 weeks), mainly watery diarrhoea.
When this defining symptom is present, an appropriate course of action is colonoscopy with biopsy (minimum of two biopsies taken from each of the ascending, transverse and descending/sigmoid colon in separate tubes) for histological assessment of MC.
FC should not be used as the basis for referral to colonoscopy in patients with chronic, non-bloody, mainly watery diarrhoea because it is not diagnostic for MC.
MC is highly treatable.
In the UK, a good starting point for spreading these messages would include updating NICE recommendations and the information on MC currently provided by the NHS. A dialogue between primary care organisations and secondary care providers is also needed to facilitate greater awareness of patients with MC.
Consideration needs to be given to patients who have already sought care for established symptoms of MC, but who have not received a diagnosis. One approach is to use patient records and healthcare databases to identify and follow-up on those patients most likely to have had MC misdiagnosed as IBS (eg, IBS-D with symptoms and factors suggestive of MC, no record of colonoscopy, multiple switching between different IBS therapies). Whatever the approach, an initial increase in costs related to colonoscopy referrals would be expected. However, these would probably recede as the accumulated burden of undiagnosed MC is gradually relieved, and be offset by reductions in the known direct and indirect costs of chronic diarrhoea in the general population.42
In addition to activities aimed at increasing the awareness of MC, clarification of current management practices in the UK is required so that they can be amended and standardised where necessary. Examples where practices are unclear for MC include responsibility of care (does it lie with primary care physicians or gastroenterologists?), the use of stool diaries and disease activity criteria, the degree of patient follow-up and regularity of exclusion of coeliac disease, and whether histopathologists are sufficiently alert to MC. Given that MC is poorly acknowledged by the UK healthcare system as a prevalent cause of chronic diarrhoea, it seems unlikely that best practices are being consistently or adequately monitored.
Conclusion
The hidden burden of MC has crept up on us. Awareness of MC as a prevalent, yet highly treatable cause of chronic diarrhoea needs to increase and the pathways for its management clarified. Current NHS guidelines do not adequately acknowledge or accommodate for this condition. The ability to identify and treat patients with MC provides an excellent opportunity to improve care.
Footnotes
Contributors: AM and DSS contributed to the study concept, provided relevant literature and reviewed and revised the manuscript for important intellectual content. MM-B performed literature searches, drafted the manuscript and provided editorial support. APSH contributed to the study concept and reviewed and revised the manuscript for important intellectual content. All authors read and approved the final manuscript version for submission.
Funding: Funding for the study was provided by Tillotts Pharma, the manufacturer of budesonide, which is used to treat patients with microscopic colitis.
Competing interests: MM-B is an independent contractor for Oxford PharmaGenesis, Melbourne, Australia, which received funding for this study from Tillotts Pharma, the manufacturer of budesonide (used to treat patients with microscopic colitis). AM has received honoraria for consultancy, and speaker fees and research grants from Vifor Pharma, Tillotts Pharma, Dr Falk Pharma and Ferring Pharmaceuticals. DSS has received educational grants from Tillotts Pharma for investigator-led research into coeliac disease and microscopic colitis. APSH has served on advisory boards and received funding from Allergan, Shire (now part of Takeda), Tillotts Pharma and Danone within the last 3 years.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Read NW, Krejs GJ, Read MG, et al. Chronic diarrhea of unknown origin. Gastroenterology 1980;78:264–71. 10.1016/0016-5085(80)90575-2 [DOI] [PubMed] [Google Scholar]
- 2. Lewis NR, Archer T, Kaye P. Epidemiology of microscopic colitis in nottingham: a contemporary cohort study. Gut 2017;66(Suppl 2). [Google Scholar]
- 3. Gentile NM, Khanna S, Loftus EV, et al. The epidemiology of microscopic colitis in Olmsted County from 2002 to 2010: a population-based study. Clin Gastroenterol Hepatol 2014;12:838–42. 10.1016/j.cgh.2013.09.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pardi DS, Loftus EV, Smyrk TC, et al. The epidemiology of microscopic colitis: a population based study in Olmsted County, Minnesota. Gut 2007;56:504–8. 10.1136/gut.2006.105890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Olesen M, Eriksson S, Bohr J, et al. Microscopic colitis: a common diarrhoeal disease. An epidemiological study in Orebro, Sweden, 1993-1998. Gut 2004;53:346–50. 10.1136/gut.2003.014431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. National Health Service UK NHS UK website, 2019. Available: https://www.nhs.uk/conditions/inflammatory-bowel-disease/ [Accessed 23 May 2019].
- 7. National Institute for Health and Care Excellence Ulcerative colitis: management [NG130]. NICE, 2019. Available: https://www.nice.org.uk/guidance/ng130/documents/draft-guideline [Accessed 23 May 2019]. [PubMed]
- 8. Thompson NP, Fleming DM, Charlton J, et al. Patients consulting with Crohn's disease in primary care in England and Wales. Eur J Gastroenterol Hepatol 1998;10:1007–12. 10.1097/00042737-199812000-00005 [DOI] [PubMed] [Google Scholar]
- 9. Nguyen GC, Smalley WE, Vege SS, et al. American Gastroenterological Association Institute guideline on the medical management of microscopic colitis. Gastroenterology 2016;150:242–6. 10.1053/j.gastro.2015.11.008 [DOI] [PubMed] [Google Scholar]
- 10. Vigren L, Sjöberg K, Benoni C, et al. Is smoking a risk factor for collagenous colitis? Scand J Gastroenterol 2011;46:1334–9. 10.3109/00365521.2011.610005 [DOI] [PubMed] [Google Scholar]
- 11. Verhaegh BPM, de Vries F, Masclee AAM, et al. High risk of drug-induced microscopic colitis with concomitant use of NSAIDs and proton pump inhibitors. Aliment Pharmacol Ther 2016;43:1004–13. 10.1111/apt.13583 [DOI] [PubMed] [Google Scholar]
- 12. Nyhlin N, Bohr J, Eriksson S, et al. Systematic review: microscopic colitis. Aliment Pharmacol Ther 2006;23:1525–34. 10.1111/j.1365-2036.2006.02913.x [DOI] [PubMed] [Google Scholar]
- 13. Bonderup OK, Folkersen BH, Gjersøe P, et al. Collagenous colitis: a long-term follow-up study. Eur J Gastroenterol Hepatol 1999;11:493–5. [PubMed] [Google Scholar]
- 14. Chan JL, Tersmette AC, Offerhaus GJ, et al. Cancer risk in collagenous colitis. Inflamm Bowel Dis 1999;5:40–3. 10.1097/00054725-199902000-00006 [DOI] [PubMed] [Google Scholar]
- 15. Kao K-T, Pedraza B-A, McClune A-C, et al. Microscopic colitis: a large retrospective analysis from a health maintenance organization experience. World J Gastroenterol 2009;15:3122–7. 10.3748/wjg.15.3122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bohr J, Tysk C, Eriksson S, et al. Collagenous colitis: a retrospective study of clinical presentation and treatment in 163 patients. Gut 1996;39:846–51. 10.1136/gut.39.6.846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nyhlin N, Wickbom A, Montgomery SM, et al. Long-term prognosis of clinical symptoms and health-related quality of life in microscopic colitis: a case-control study. Aliment Pharmacol Ther 2014;39:963–72. 10.1111/apt.12685 [DOI] [PubMed] [Google Scholar]
- 18. Olesen M, Eriksson S, Bohr J, et al. Lymphocytic colitis: a retrospective clinical study of 199 Swedish patients. Gut 2004;53:536–41. 10.1136/gut.2003.023440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pardi DS, Ramnath VR, Loftus EV, et al. Lymphocytic colitis: clinical features, treatment, and outcomes. Am J Gastroenterol 2002;97:2829–33. 10.1111/j.1572-0241.2002.07030.x [DOI] [PubMed] [Google Scholar]
- 20. Langner C, Aust D, Ensari A, et al. Histology of microscopic colitis-review with a practical approach for pathologists. Histopathology 2015;66:613–26. 10.1111/his.12592 [DOI] [PubMed] [Google Scholar]
- 21. Fraser AG, Warren BF, Chandrapala R, et al. Microscopic colitis: a clinical and pathological review. Scand J Gastroenterol 2002;37:1241–5. 10.1080/003655202761020489 [DOI] [PubMed] [Google Scholar]
- 22. Fernández-Bañares F, Casalots J, Salas A, et al. Paucicellular lymphocytic colitis: is it a minor form of lymphocytic colitis? A clinical pathological and immunological study. Am J Gastroenterol 2009;104:1189–98. 10.1038/ajg.2009.65 [DOI] [PubMed] [Google Scholar]
- 23. Bjørnbak C, Engel PJH, Nielsen PL, et al. Microscopic colitis: clinical findings, topography and persistence of histopathological subgroups. Aliment Pharmacol Ther 2011;34:1225–34. 10.1111/j.1365-2036.2011.04865.x [DOI] [PubMed] [Google Scholar]
- 24. Kane JS, Rotimi O, Ford AC, et al. Macroscopic findings, incidence and characteristics of microscopic colitis in a large cohort of patients from the United Kingdom. Scand J Gastroenterol 2017;52:988–94. 10.1080/00365521.2017.1334813 [DOI] [PubMed] [Google Scholar]
- 25. Rasmussen MA, Munck LK. Systematic review: are lymphocytic colitis and collagenous colitis two subtypes of the same disease - microscopic colitis? Aliment Pharmacol Ther 2012;36:79–90. 10.1111/j.1365-2036.2012.05166.x [DOI] [PubMed] [Google Scholar]
- 26. Pardi DS, Tremaine WJ, Carrasco-Labra A. American Gastroenterological Association Institute technical review on the medical management of microscopic colitis. Gastroenterology 2016;150:e11:247–74. 10.1053/j.gastro.2015.11.006 [DOI] [PubMed] [Google Scholar]
- 27. Abdo A, Raboud J, Freeman HJ, et al. Clinical and histological predictors of response to medical therapy in collagenous colitis. Am J Gastroenterol 2002;97:1164–8. 10.1111/j.1572-0241.2002.05688.x [DOI] [PubMed] [Google Scholar]
- 28. Fiehn A-MK, Bjørnbak C, Warnecke M, et al. Observer variability in the histopathologic diagnosis of microscopic colitis and subgroups. Hum Pathol 2013;44:2461–6. 10.1016/j.humpath.2013.06.004 [DOI] [PubMed] [Google Scholar]
- 29. Kane JS, Rotimi O, Everett SM, et al. Development and validation of a scoring system to identify patients with microscopic colitis. Clin Gastroenterol Hepatol 2015;13:1125–31. 10.1016/j.cgh.2014.12.035 [DOI] [PubMed] [Google Scholar]
- 30. Kane JS, Sood R, Law GR, et al. Validation and modification of a diagnostic scoring system to predict microscopic colitis. Scand J Gastroenterol 2016;51:1206–12. 10.1080/00365521.2016.1186221 [DOI] [PubMed] [Google Scholar]
- 31. Cotter TG, Binder M, Pardi DS. Validation of a scoring system to predict microscopic colitis in a cohort of patients with chronic diarrhea. Clin Gastroenterol Hepatol 2016;14:777–8. 10.1016/j.cgh.2015.12.015 [DOI] [PubMed] [Google Scholar]
- 32. Cotter TG, Binder M, Harper EP, et al. Optimization of a scoring system to predict microscopic colitis in a cohort of patients with chronic diarrhea. J Clin Gastroenterol 2017;51:228–34. 10.1097/MCG.0000000000000565 [DOI] [PubMed] [Google Scholar]
- 33. Townsend T, Campbell F, O’Toole P, et al. Microscopic colitis: diagnosis and management. Frontline Gastroenterol 2019;10:388–93. 10.1136/flgastro-2018-101040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pisani LF, Tontini GE, Marinoni B, et al. Biomarkers and microscopic colitis: an unmet need in clinical practice. Front. Med. 2017;4 10.3389/fmed.2017.00054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Marlicz W, Skonieczna-Żydecka K, Yung DE, et al. Endoscopic findings and colonic perforation in microscopic colitis: A systematic review. Dig Liver Dis 2017;49:1073–85. 10.1016/j.dld.2017.07.015 [DOI] [PubMed] [Google Scholar]
- 36. Abboud R, Pardi DS, Tremaine WJ, et al. Symptomatic overlap between microscopic colitis and irritable bowel syndrome: a prospective study. Inflamm Bowel Dis 2013;19:550–3. 10.1097/MIB.0b013e31827febfd [DOI] [PubMed] [Google Scholar]
- 37. Guagnozzi D, Arias Á, Lucendo AJ. Systematic review with meta-analysis: diagnostic overlap of microscopic colitis and functional bowel disorders. Aliment Pharmacol Ther 2016;43:851–62. 10.1111/apt.13573 [DOI] [PubMed] [Google Scholar]
- 38. Sperber AD, Dumitrascu D, Fukudo S, et al. The global prevalence of IBS in adults remains elusive due to the heterogeneity of studies: a Rome Foundation working team literature review. Gut 2017;66:1075–82. 10.1136/gutjnl-2015-311240 [DOI] [PubMed] [Google Scholar]
- 39. Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol 2012;10:e4:712–21. 10.1016/j.cgh.2012.02.029 [DOI] [PubMed] [Google Scholar]
- 40. Lacy B, Patel N. Rome criteria and a diagnostic approach to irritable bowel syndrome. Journal of Clinical Medicine 2017;6 10.3390/jcm6110099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hungin APS, Molloy-Bland M, Claes R, et al. Systematic review: the perceptions, diagnosis and management of irritable bowel syndrome in primary care--a Rome Foundation working team report. Aliment Pharmacol Ther 2014;40:1133–45. 10.1111/apt.12957 [DOI] [PubMed] [Google Scholar]
- 42. Schiller LR. Chronic diarrhea. Gastroenterology 2004;127:287–93. 10.1053/j.gastro.2004.05.028 [DOI] [PubMed] [Google Scholar]
- 43. Seifert B, Rubin G, de Wit N, et al. The management of common gastrointestinal disorders in general practice: a survey by the European Society for Primary Care Gastroenterology (ESPCG) in six European countries. Dig Liver Dis 2008;40:659–66. 10.1016/j.dld.2008.02.020 [DOI] [PubMed] [Google Scholar]
- 44. Schiller LR. Evaluation of chronic diarrhea and irritable bowel syndrome with diarrhea in adults in the era of precision medicine. Am J Gastroenterol 2018;113:660–9. 10.1038/s41395-018-0032-9 [DOI] [PubMed] [Google Scholar]
- 45. Münch A, Aust D, Bohr J, et al. Microscopic colitis: current status, present and future challenges: statements of the European Microscopic Colitis Group. J Crohns Colitis 2012;6:932–45. 10.1016/j.crohns.2012.05.014 [DOI] [PubMed] [Google Scholar]
- 46. Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: a clinical review. JAMA 2015;313:949–58. 10.1001/jama.2015.0954 [DOI] [PubMed] [Google Scholar]
- 47. Green PHR, Yang J, Cheng J, et al. An association between microscopic colitis and celiac disease. Clin Gastroenterol Hepatol 2009;7:1210–6. 10.1016/j.cgh.2009.07.011 [DOI] [PubMed] [Google Scholar]
- 48. National Institute for Health and Care Excellence The use of faecal calprotectin in primary care as a decision diagnostic for inflammatory bowel disease and irritable bowel syndrome. NICE, 2017. Available: https://wwwniceorguk/guidance/dg11/resources/endorsed-resource-the-use-of-faecal-calprotectin-in-primary-care-as-a-decision-diagnostic-for-inflammatory-bowel-disease-and-irritable-bowel-syndrome-4595859613 [Accessed 23 May 2019].
- 49. National Institute for Health and Care Excellence Faecal calprotectin diagnostic tests for inflammatory disease of the bowel [DG11]. NICE, 2017. Available: https://wwwniceorguk/guidance/dg11 [Accessed 23 May 2019].
- 50. Wildt S, Nordgaard-Lassen I, Bendtsen F, et al. Metabolic and inflammatory faecal markers in collagenous colitis. Eur J Gastroenterol Hepatol 2007;19:567–74. 10.1097/MEG.0b013e328058ed76 [DOI] [PubMed] [Google Scholar]
- 51. von Arnim U, Wex T, Ganzert C, et al. Fecal calprotectin: a marker for clinical differentiation of microscopic colitis and irritable bowel syndrome. Clin Exp Gastroenterol 2016;9:97–103. 10.2147/CEG.S97701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Labaere D, Smismans A, Van Olmen A, et al. Comparison of six different calprotectin assays for the assessment of inflammatory bowel disease. United European Gastroenterol J 2014;2:30–7. 10.1177/2050640613518201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Raju SA, Kurien M, Chew TS, et al. P294 Microscopic colitis: a missed opportunity to diagnose during colonoscopy. J Crohns Colitis 2019;13(Suppl 1):S246–S247. 10.1093/ecco-jcc/jjy222.418 [DOI] [Google Scholar]
- 54. Kidney E, Greenfield S, Berkman L, et al. Cancer suspicion in general practice, urgent referral, and time to diagnosis: a population-based GP survey nested within a feasibility study using information technology to flag-up patients with symptoms of colorectal cancer. BJGP Open 2017;1 10.3399/bjgpopen17X101109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Miehlke S, Madisch A, Kupcinskas L, et al. Budesonide is more effective than mesalamine or placebo in short-term treatment of collagenous colitis. Gastroenterology 2014;146:1222–30. 10.1053/j.gastro.2014.01.019 [DOI] [PubMed] [Google Scholar]
- 56. Miehlke S, Aust D, Mihaly E, et al. Efficacy and safety of budesonide, vs mesalazine or placebo, as induction therapy for lymphocytic colitis. Gastroenterology 2018;155:e3:1795–804. 10.1053/j.gastro.2018.08.042 [DOI] [PubMed] [Google Scholar]