Abstract
Background
Despite the proven efficacy of vedolizumab (VDZ) for ulcerative colitis (UC) and Crohn’s disease (CD), suboptimal response is commonly encountered. However, data regarding the effectiveness of dose intensification (by interval shortening) to achieve response are limited.
Objectives
We evaluated the effectiveness of dose intensification at achieving response in patients with a previously suboptimal response to VDZ. Additionally, we aimed to identify predictors of response to this strategy.
Methods
We performed a retrospective cohort study of patients who underwent VDZ dose intensification for suboptimal response. Clinical disease activity was evaluated at the point of dose intensification (baseline) and at weeks 12 and 24. Response was defined as Harvey-Bradshaw Index (HBI) or Simple Clinical Colitis Activity Index (SCCAI) reduction of ≥3, and remission as HBI <5 or SCCAI <3.
Results
A total of 36 patients received dose intensification to 4-weekly infusions: 18 CD, 14 UC and 4 inflammatory bowel disease-unclassified (analysed in the UC group). Median SCCAI scores fell from 6 (range 0–11) at baseline to 4 (0–6, p=0.008) at week 24, while HBI scores did not change significantly (4 (0–27) and 3 (0–8), p=0.092). Overall median C reactive protein (CRP) fell from 6 mg/L (1–23) to 2 mg/L (1–17, p=0.011). Of 20 patients with clinically active disease at baseline, 10 (50%) responded, of whom 4 (20%) achieved remission at week 24. Univariate analysis demonstrated low baseline CRP (p=0.045) and response at week 12 (0.020) were associated with week 24 response.
Conclusions
Our findings demonstrate VDZ dose intensification to be effective at achieving clinical response in half of patients. Low baseline CRP and response at week 12 are potential predictors of week 24 response.
Keywords: vedolizumab, entyvio, dose intensification, ulcerative colitis, crohn’s disease, inflammatory bowel disease
Significance of this study.
What is already known on this topic
A recent systematic review and meta-analysis investigating rates of loss of response to vedolizumab demonstrated that this occurs in a significant proportion of patients.
Prior loss of response to a tumour necrosis factor antagonist and higher baseline C reactive protein (CRP) are important predictors of subsequent loss of response to vedolizumab.
Vedolizumab dose intensification (by interval shortening) has been shown to be an effective strategy to restore clinical response in approximately half of patients.
What this study adds
The findings of our study corroborate the previously demonstrated effectiveness of vedolizumab dose intensification at restoring clinical response in approximately half of patients.
We also observed a significant reduction in biochemical disease activity as demonstrated by CRP.
Low CRP at the point of dose intensification and response 12 weeks after were shown to be predictors of week 24 response.
How might it impact on clinical practice in the foreseeable future
Clinicians should consider vedolizumab dose intensification in cases of suboptimal response.
In patients who fail to respond to 12 weeks of dose-intensified treatment, vedolizumab discontinuation should be considered and other management strategies contemplated.
Introduction
Vedolizumab (VDZ) is a monoclonal antibody against alpha-4 beta-7 integrins which selectively inhibits leucocyte migration into the gut. It was approved in 2014 on the basis of trials that demonstrated efficacy in both ulcerative colitis (UC) (GEMINI 11) and Crohn’s disease (CD) (GEMINI 22). However, it is recognised that some patients fail to have any initial response to treatment (primary non-response, PNR), and others, who do have an initial good response, subsequently flare (loss of response, LOR). Rates of LOR vary between biological agents and appear, at least in part, to be related to immunogenicity, with more immunogenic agents (eg, infliximab) having higher rates of LOR than those with lower immunogenic potential (eg, VDZ).3 However, many cases of LOR cannot be explained by immunogenicity and may be related to other factors including factors related to drug exposure.4–6 As such, dose intensification (whether carried out empirically or guided by therapeutic drug monitoring, TDM) has been extensively studied and demonstrated to be an effective strategy to manage LOR to anti-tumour necrosis factor (anti-TNF) agents. As TDM for VDZ is not yet widely available and does not have a robust body of evidence on which to base interpretations, dose intensification is usually carried out empirically. This is achieved by shortening the interval between each 300 mg infusion from the standard 8 weeks (Q8) to 4 or 6 weeks (Q4 or Q6). However, there exist relatively limited data regarding the effectiveness of VDZ dose intensification in real-world cohorts. A recent systematic review7 identified just four published studies reporting the effectiveness of this strategy: two congress abstracts8 9 (one of which subsequently published in full10), one article11 and one letter to an editor.12 Between them these studies evaluated just over 100 patients in total, so further investigation appears necessary. Although the GEMINI long-term extension programme provided randomised controlled trial data regarding VDZ dose escalation,13 14 it is appreciated that patients included in trials are often significantly different from those treated in clinical practice.15
Due to the relative paucity of real-world evidence and the high cost of dose escalation, funding bodies are sometimes reluctant to approve this strategy. However, a novel inflammatory bowel disease (IBD) pathway16 devised in partnership between our centre and local clinical commissioning groups allowed the use of VDZ dose intensification in line with its licence and approval from the UK National Institute for Health and Care Excellence. Due to our early access to VDZ (November 2014), a relatively large cohort of patients receiving treatment and access to dose intensification, we carried out a retrospective cohort study with the aim of evaluating the effectiveness of VDZ dose intensification to achieve disease control in cases of suboptimal response.
Methods
We performed a retrospective cohort study by reviewing prospectively maintained clinical records for all patients commencing VDZ at Guy's and St Thomas' Hospital between November 2014 and October 2017. A total of 139 patients received at least one infusion of VDZ for CD, UC or IBD-unclassified (IBD-U) during this period. Of these, 36 (27%) had undergone dose intensification from Q8 to Q4. Dose intensification was carried out based on the opinion of the supervising clinician, after review in a multidisciplinary virtual biologics clinic. Reasons for dose intensification included ongoing or recurrent symptoms, or active inflammation based on objective measures (in the presence or absence of symptoms). Eighteen (50%) patients had CD, 14 (39%) UC and 4 (11%) IBD-U. Patients with IBD-U were included in the UC group for the purposes of analysis.
Demographic information as well as the following disease-related data were collected: disease distribution and behaviour (using the Montreal classification17), prior anti-TNF exposure, duration of VDZ treatment prior to intensification, reason for VDZ dose intensification, and concomitant use of immunomodulators and/or corticosteroids (table 1).
Table 1.
Baseline characteristics of patients undergoing vedolizumab dose intensification
| Characteristics | n=36 |
| Gender, male:female (%) | 26:10 (72:28) |
| IBD phenotype | |
| Ulcerative colitis/IBD-U | 18 (50%) |
| Left-sided | 11 |
| Extensive | 7 |
| Crohn’s disease | 18 (50%) |
| Ileal | 6 |
| Colonic | 4 |
| Ileocolonic | 8 |
| Median age, years (range) | 44 (17–70) |
| Concomitant immunomodulator use at baseline | 18 (50%) |
| Thiopurine | 17 |
| Methotrexate | 1 |
| Prior anti-TNF exposure | |
| Naïve | 5 (14%) |
| Exposed | 31 (86%) |
| Corticosteroid use at baseline | 6 (16%) |
| Median duration of vedolizumab treatment before dose intensification, months (range) | 7 (3–21) |
| Median duration of vedolizumab treatment after dose intensification, months (range) | 7 (2–25) |
IBD, inflammatory bowel disease; IBD-U, IBD-unclassified; TNF, tumour necrosis factor.
Our primary outcome was the clinical effectiveness of VDZ dose intensification. This was evaluated by assessing clinical disease activity data at the following predefined time points: baseline (defined as the infusion immediately prior to the first shortened interval) and at 12 and 24 weeks thereafter. At each time point, clinical disease activity was evaluated using the Harvey-Bradshaw Index (HBI)18 for CD and the Simple Clinical Colitis Activity Index (SCCAI)19 for UC. Outcomes were predefined as follows: clinical response was defined as a reduction of 3 or more in HBI or SCCAI, and clinical remission was defined as HBI less than 5 or SCCAI less than 3. These definitions were based on their previously demonstrated (partial) validity.20 21
Our secondary outcome was the effect of VDZ on biochemical disease activity measured by C reactive protein (CRP) concentrations at each study time point. In addition, we aimed to identify factors that could be used to predict response (or lack thereof) to dose intensification.
Continuous data are summarised as medians (and ranges). Paired SCCAI/HBI and CRP values were compared using Wilcoxon signed-rank test, and for unpaired continuous data Mann-Whitney test was used. Categorical variables were compared using Fisher’s exact test. Non-responder imputation analysis was used to deal with any patients who discontinued treatment prior to week 24. All analyses were carried out using GraphPad Prism V.8.0.1. The results of this study are reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for cohort studies.22
Results
Change in clinical disease activity
The median baseline HBI of the 18 patients with CD was 4 (0–27). It remained 4 (0–29, p=0.51 for n=18) at week 12 and had fallen to 3 (0–8, p=0.092 compared with baseline for n=15) at week 24 (figure 1 and online supplementary figure 1). The median baseline SCCAI of the 18 patients with UC was 6 (0–11). It had fallen to 5 (1–11, p=0.34 for n=18) at week 12 and further to 4 (0–6, p=0.008 compared with baseline for n=15) at week 24 (figure 2 and online supplementary figure 2).
Figure 1.
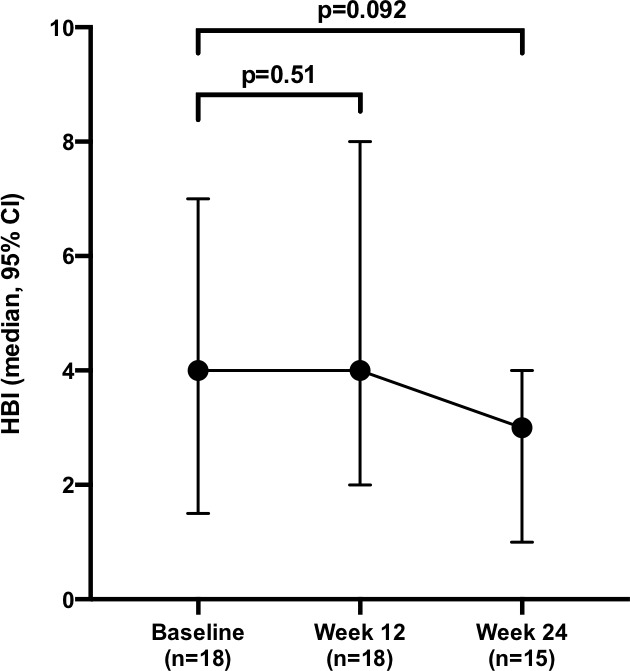
Change in median Harvey-Bradshaw Index (HBI) following dose intensification.
Figure 2.
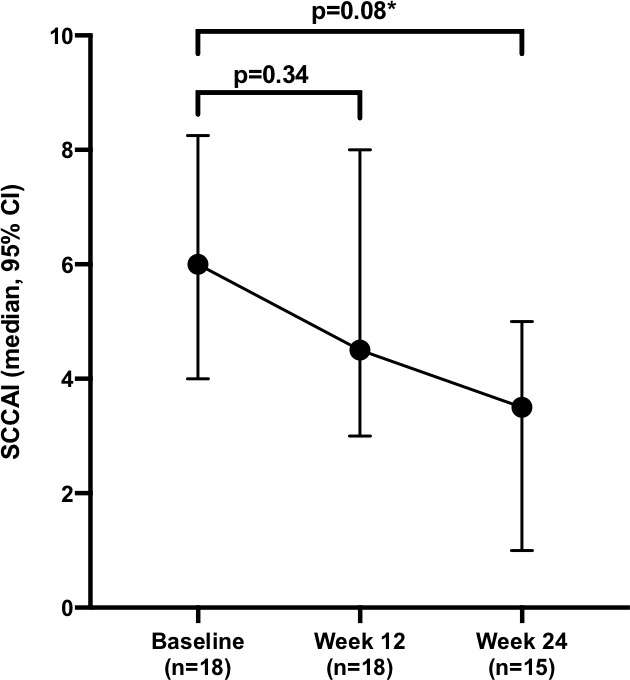
Change in median Simple Clinical Colitis Activity Index (SCCAI) following dose intensification. *denotes statistical significance (p<0.05).
flgastro-2019-101259supp001.pdf (171.5KB, pdf)
flgastro-2019-101259supp002.pdf (213.8KB, pdf)
Response and remission at week 12
At baseline 20 of 36 (56%; 6 CD and 14 UC) patients who underwent dose intensification had clinically active disease (HBI >5 or SCCAI >3). The remaining 16 (44%) were dose-intensified on the basis of active disease demonstrated on endoscopy or MRI. Of the 20 patients with active disease at baseline, 8 (40%) achieved a response at week 12, 2 (10%) of whom entered remission. Divided by IBD subtype, the response rates were 3 of 6 (50%) in CD and 6 of 14 (43%) in UC. Remission rates were 0 of 6 in CD and 2 of 14 (14%) in UC.
Response and remission at week 24
Of the 20 patients with clinically active disease at baseline, 4 patients had discontinued VDZ (either switched to a different biologic or experimental trial agent), and therefore did not have week 24 clinical disease evaluations recorded but were considered non-responders for the purposes of analysis. This resulted in a week 24 response rate of 10 of 20 (50%), of whom 4 (20%) achieved remission.
Of the 16 (12 CD and 4 UC) patients with inactive clinical disease at baseline, all remained in clinical remission at the end of weeks 12 and 24.
Predictors of week 24 response to dose intensification
Univariate analysis of potential factors that could be used to predict response is shown in table 2. Of the parameters investigated, only a low baseline CRP (p=0.045) and response at week 12 (p=0.020) were significantly associated with response at week 24.
Table 2.
Univariate analysis of potential predictors of response to dose intensification
| Week 24 responders n (%), median (range) |
Week 24 non-responders n (%), median (range) |
P value | |
| n | 10 | 10 | |
| Gender (male vs female) | 7 vs 3 (70 vs 30) | 7 vs 3 (70 vs 30) | >0.99 |
| IBD phenotype (UC vs CD) | 7 vs 3 (70 vs 30) | 8 vs 2 (80 vs 20) | >0.99 |
| Age, years | 40 (18–69) | 44 (24–63) | 0.73 |
| Concomitant immunomodulator (combotherapy vs monotherapy) | 4 vs 6 (40 vs 60) | 5 vs 5 (50 vs 50) | >0.99 |
| Prior anti-TNF experience (exposed vs naïve) | 9 vs 1 (90 vs 10) | 8 vs 2 (80 vs 20) | >0.99 |
| Duration on vedolizumab prior to dose intensification, months | 7 (3–23) | 7 (3–16) | 0.87 |
| CRP at baseline, mg/L | 5 (1–21) | 13 (1–23) | 0.045 |
| Week 12 response (response vs non-response) | 7 vs 3 (70 vs 30) | 1 vs 9 (10 vs 90) | 0.020 |
CD, Crohn’s disease; CRP, C reactive protein; IBD, inflammatory bowel disease; TNF, tumour necrosis factor; UC, ulcerative colitis.
Biochemical disease activity
Serum CRP data were available for all 36 patients at baseline and week 12, as well as all 30 patients who completed 24 weeks of dose-intensified VDZ (the remaining 6 patients had discontinued by this time point). The median baseline CRP was 6 mg/L (1-23), and this had fallen to 5 mg/L (1–46, p=0.42) at week 12 and further to 2 mg/L at week 24 (1–17, p=0.011 compared with baseline) (figure 3).
Figure 3.
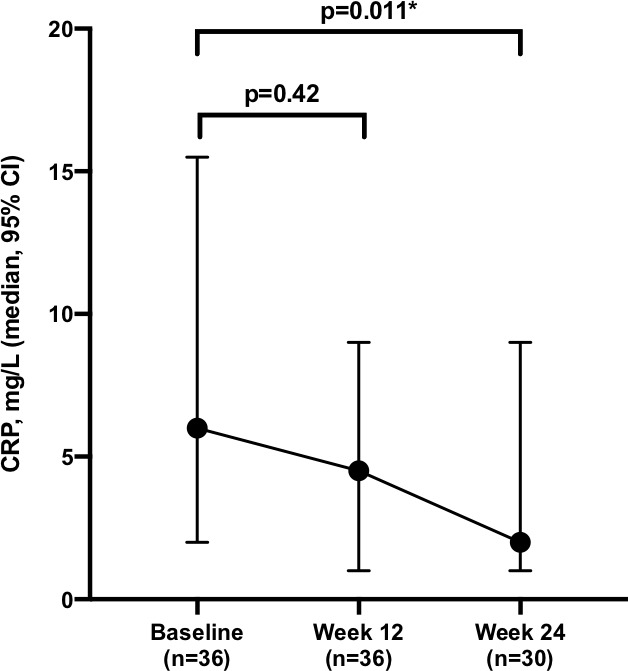
Change in median C reactive protein (CRP) following dose intensification. *denotes statistical significance (p<0.05).
Discussion
The benefits of VDZ include a favourable safety profile and tolerability, as well as its limited immunogenicity, which minimises the need for concomitant immunomodulation.23 In addition, the findings of ‘real world’ observational studies and long-term follow-up of randomised control trials also suggest high rates of treatment persistence24 and longevity of effect.13 14 However, by contrast a recent systematic review and meta-analysis identified that rates of LOR are far from negligible.7 By combining 10 eligible cohorts, comprising over 800 patients, the pooled incidence rates of LOR to VDZ were 47.9 per 100 person-years of follow-up among patients with CD and 39.8 per 100 person-years of follow-up among patients with UC. These findings highlight the need for studies designed to investigate both the mechanism(s) underlying LOR as well as potential strategies by which it could be overcome.
The results of our retrospective cohort study demonstrate that VDZ dose intensification, by interval shortening to 4-weekly infusions, is an effective strategy to recapture response. Among patients with clinically active disease at baseline, we observed a clinical response in 50% after 24 weeks of dose-intensified treatment. This figure is very much in keeping with a meta-analysis of four studies (two complete articles, one abstract and one letter to the editor), with a combined cohort of 111 patients, that demonstrated a random-effects pooled efficacy rate of 53.8%. We also observed a 20% remission rate at the same time point. Again, this finding is almost identical to the 6 of 33 (18%) remission rate reported by a multicentre US consortium of IBD investigators.10 Moreover, rates of week 28 response to dose intensification for CD and UC reported as part of the GEMINI long-term extension were also similar at 17 of 32 (54%) and 31 of 57 (53%), respectively.13 14 Studied as part of a meticulous clinical trial assessment schedule, these data offer the highest quality evidence available for the efficacy of dose escalation.
When evaluating absolute decrease in clinical disease indices, we observed a non-significant fall in median HBI from 4 to 3 over 24 weeks. However, the fall in SCCAI from 6 to 4 during the same time course was significant (p=0.008). These findings could indicate that dose intensification is perhaps more effective for UC than CD but is more likely due to the operating characteristics of the clinical disease activity indices involved. The low baseline HBI in several patients despite objective evidence of active disease is indicative of the fact that clinical disease activity indices used for CD (ie, HBI and Crohn's disease activity index (CDAI)) correlate less well with disease activity than those used for UC.25 26
As there is likely to be some degree of placebo effect in reported symptoms when halving the dosing interval (due to the perceived benefit of more frequent doses), we also evaluated the changes in an objective biomarker of disease activity, CRP. Among the overall cohort, we observed a significant fall over the course of 24 weeks, from a median of 6 mg/L at baseline to 2 mg/L, which is within the normal range for CRP (p=0.011). This demonstrates that, in addition to improving symptoms of active disease, VDZ dose intensification appears an effective strategy to reduce intestinal inflammatory activity.
In addition, we identified factors that may predict response to dose intensification. This is particularly important given the relatively high cost of VDZ, which is clearly amplified by dose intensification and restricts access to this option for some patients. We observed that patients with lower baseline CRP were significantly more likely to achieve a response at week 24 than those with higher values (median 13 vs 5 mg/L, p=0.045). This finding may help clinicians to determine which patients are more suitable candidates for intensification (ie, perhaps those with less severe disease), and therefore lead to a more judicious use of this strategy. We also identified that week 12 response was also a significant predictor (p=0.020) of ongoing response at week 24, with only 10% of patients who failed to respond by week 12 going on to achieve response at the later time point. This may also aid clinical decision-making by potentially limiting the time spent on a high-cost, dose-intensified regimen if this approach is ultimately likely to fail. If no response is seen after 12 weeks (three infusions with a 4-weekly interval), then clinicians could pursue an alternative strategy with clear cost-saving implications. Although other studies have identified predictors of LOR (eg, longer disease duration and previous LOR to anti-TNF10) and described the role of TDM in predicting likelihood of recapture on intensification,11 we believe the clinical predictors described here to be novel and clinically relevant. However, these predictors require external validation in larger data sets before they can be confidently used to guide clinical practice.
There is a great deal of interest in how TDM could be used to guide VDZ dosing. Studies have suggested that a week 6 VDZ trough level is a potential predictor of mucosal healing and the need for dose intensification.27 28 However, while uncertainty remains regarding exact thresholds and access to VDZ TDM remains limited, it appears likely that most decisions regarding changes in treatment regimen will be made empirically.
Our study has several limitations. Most notable are its retrospective design and the subjective nature of the clinical disease activity scores employed. In addition, the total number of patients included is relatively small (n=36) and reduces further when considering only those with clinically active disease at baseline (n=20). The size of our cohort is, however, broadly in keeping with other published experiences of VDZ dose intensification.9–12 Moreover, the findings described here closely reflect those described elsewhere, adding to their credibility. Another limitation is that patients were not divided by their initial response to VDZ (PNR or LOR), but instead were evaluated as a single group under the umbrella term ‘suboptimal response’. We were therefore unable to assess whether initial response (or lack thereof) influences subsequent response to dose intensification. We used a non-responder imputation for our response/remission analysis as it provides a conservative estimate of treatment effect. However, it is probable that the analysis of absolute disease scores was biased in favour of showing an effect in view of patients (most likely those with more active/less responsive disease) discontinuing before week 24. Finally, for patients who underwent dose intensification for active disease seen on endoscopy or MRI, there were often no paired data available for these investigations within the 24-week study period. We were, therefore, unable to comment on objective evidence of improvement on dose intensification. Despite these limitations we believe these results are relevant, reliable and generalisable. Indeed, this type of observational effectiveness research is becoming increasingly recognised as significant and necessary.29 Nonetheless, readers should be aware of the inherent methodological deficiencies associated with effectiveness research. These types of studies are commonly subject to a number of possible biases and confounders (eg, selection, attrition and misclassification).29 In an attempt to remedy some of these shortcomings, we followed the STROBE recommendations22 (see online supplementary material for STROBE checklist).
flgastro-2019-101259supp003.pdf (46.5KB, pdf)
In conclusion, our findings are consistent with the relatively limited data already available regarding VDZ dose intensification and show this strategy to be effective at recapturing clinical response in approximately half of patients. We also observed a significant fall in CRP after 24 weeks of intensified treatment. In addition, we identified lower baseline CRP and response at week 12 as potential predictors of week 24 response. These findings are consistent with other similar studies but require external validation in larger data sets.
Footnotes
Contributors: MAS, SB, MSM and PMI were responsible for the original concept and planning of the study. MAS, SB, MSM, SH, AGT and GC were responsible for data collection and analysis. MAS, SB and MSM drafted the manuscript, which IK, SR, JM, SHCA, JS and PMI critically reviewed and revised.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: MAS: served as a speaker, a consultant and/or an advisory board member for Janssen, Takeda, MSD and Falk. MSM: served as a speaker for Takeda. SH: served as a speaker for Janssen. PMI: served as a speaker, a consultant and/or an advisory board member for AbbVie, Warner Chilcott, Ferring, Falk Pharma, Takeda, MSD, Johnson & Johnson, Shire, Vifor Pharma, Pharmacosmos, Topivert, Genentech, Hospira and Samsung Bioepis, and has received research funding from MSD and Takeda.
Patient consent for publication: Not required.
Ethics approval: The Health Research Authority (HRA) does not consider postmarketing surveillance and research, and therefore recommend that NHS Research Ethics Committee (REC) approval was not necessary for this study.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request.
References
- 1. Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013;369:699–710. [DOI] [PubMed] [Google Scholar]
- 2. Sandborn WJ, Feagan BG, Rutgeerts P, et al. Vedolizumab as induction and maintenance therapy for Crohn's disease. N Engl J Med 2013;369:711–21. [DOI] [PubMed] [Google Scholar]
- 3. Vermeire S, Gils A, Accossato P, et al. Immunogenicity of biologics in inflammatory bowel disease. Therap Adv Gastroenterol 2018;11:1756283X1775035 10.1177/1756283X17750355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Klotz U, Teml A, Schwab M. Clinical pharmacokinetics and use of infliximab. Clin Pharmacokinet 2007;46:645–60. 10.2165/00003088-200746080-00002 [DOI] [PubMed] [Google Scholar]
- 5. Ordas I, Mould DR, Feagan BG, et al. Anti-Tnf monoclonal antibodies in inflammatory bowel disease: pharmacokinetics-based dosing paradigms. Clin Pharmacol Ther 2012;91:635–46. [DOI] [PubMed] [Google Scholar]
- 6. Rosen MJ, Minar P, Vinks AA. Review article: applying pharmacokinetics to optimise dosing of anti-TNF biologics in acute severe ulcerative colitis. Aliment Pharmacol Ther 2015;41:1094–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Peyrin-Biroulet L, Danese S, Argollo M, et al. Loss of Response to Vedolizumab and Ability of Dose Intensification to Restore Response in Patients With Crohn’s Disease or Ulcerative Colitis: A Systematic Review and Meta-analysis. Clinical Gastroenterology and Hepatology 2019;17:838–46. 10.1016/j.cgh.2018.06.026 [DOI] [PubMed] [Google Scholar]
- 8. Shmidt E, Winters A, Katta L, et al. P-040 assessing risk factors predicting loss of response to Vedolizumab in ulcerative colitis and Crohn's disease: outcomes from the victory Consortium. Inflammatory Bowel Diseases 2017;23:S18. [Google Scholar]
- 9. Shivashankar R, Mendoza Ladd AH, Grace R, et al. Effect of Vedolizumab dose escalation on Recapturing response in patients with inflammatory bowel disease. Gastroenterology 2017;152:S77 10.1016/S0016-5085(17)30608-X [DOI] [Google Scholar]
- 10. Shmidt E, Kochhar G, Hartke J, et al. Predictors and management of loss of response to Vedolizumab in inflammatory bowel disease. Inflamm Bowel Dis 2018;24:2461–7. 10.1093/ibd/izy171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Williet N, Boschetti G, Fovet M, et al. Association between low Trough levels of Vedolizumab during induction therapy for inflammatory bowel diseases and need for additional doses within 6 months. Clin Gastroenterol Hepatol 2017;15:1750–7. 10.1016/j.cgh.2016.11.023 [DOI] [PubMed] [Google Scholar]
- 12. Gouynou C, Pouillon L, Rousseau H, et al. Early changes in the pharmacokinetic profile of vedolizumab-treated patients with IBD may predict response after dose optimisation. Gut 2019;68:178–9. 10.1136/gutjnl-2017-315766 [DOI] [PubMed] [Google Scholar]
- 13. Loftus EV, Colombel J-F, Feagan BG, et al. Long-Term efficacy of Vedolizumab for ulcerative colitis. Journal of Crohn's and Colitis 2016;9:jjw177–11. 10.1093/ecco-jcc/jjw177 [DOI] [PubMed] [Google Scholar]
- 14. Vermeire S, Loftus EV, Sands BE, et al. Long-term Efficacy of Vedolizumab for Crohn’s Disease. Journal of Crohn's and Colitis 2016;11:412–24. [DOI] [PubMed] [Google Scholar]
- 15. Ha C, Ullman TA, Siegel CA, et al. Patients enrolled in randomized controlled trials do not represent the inflammatory bowel disease patient population. Clin Gastroenterol Hepatol 2012;10:1002–7. 10.1016/j.cgh.2012.02.004 [DOI] [PubMed] [Google Scholar]
- 16. South East London Area Prescribing Committee Primary & Secondary Care Inflammatory Bowel Disease Pathway May 2017. A partnership between NHS organisations in South East London: Bexley, Bromley, Greenwich, Lambeth, Lewisham and Southwark Clinical Commissioning Groups (CCGs) and GSTFT/KCH /SLAM/Oxleas NHS Foundation Trusts/Lewisham & Greenwich NHS Trust, 2017. [Google Scholar]
- 17. Satsangi J, Silverberg MS, Vermeire S, et al. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut 2006;55:749–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harvey RF, Bradshaw JM. A simple index of Crohn's-disease activity. Lancet 1980;1:514. [DOI] [PubMed] [Google Scholar]
- 19. Walmsley RS, Ayres RC, Pounder RE, et al. A simple clinical colitis activity index. Gut 1998;43:29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Higgins PD, Schwartz M, Mapili J, et al. Patient defined dichotomous end points for remission and clinical improvement in ulcerative colitis. Gut 2005;54:782–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vermeire S, Schreiber S, Sandborn WJ, et al. Correlation between the Crohn's disease activity and Harvey-Bradshaw indices in assessing Crohn's disease severity. Clin Gastroenterol Hepatol 2010;8:357–63. [DOI] [PubMed] [Google Scholar]
- 22. von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453–7. [DOI] [PubMed] [Google Scholar]
- 23. Rosario M, Dirks NL, Milch C, et al. A review of the clinical pharmacokinetics, pharmacodynamics, and immunogenicity of Vedolizumab. Clinical pharmacokinetics 2017;56:1287–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vivio EE, Kanuri N, Gilbertsen JJ, et al. Vedolizumab effectiveness and safety over the first year of use in an IBD clinical practice. Journal of Crohn's & colitis 2016;10:402–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Modigliani R, Mary J-Y, Simon J-F, et al. Clinical, biological, and endoscopic picture of attacks of Crohn's disease: evolution on prednisolone. Gastroenterology 1990;98:811–8. [DOI] [PubMed] [Google Scholar]
- 26. Restellini S, Chao C-Y, Martel M, et al. Clinical parameters correlate with endoscopic activity of ulcerative Colitis—a systematic review. Clinical Gastroenterology and Hepatology. [DOI] [PubMed] [Google Scholar]
- 27. Williet N, Boschetti G, Fovet M, et al. Association between low Trough levels of Vedolizumab during induction therapy for inflammatory bowel diseases and need for additional doses within 6 months. Clin Gastroenterol Hepatol 2017;15:1750–7. [DOI] [PubMed] [Google Scholar]
- 28. Yacoub W, Williet N, Pouillon L, et al. Early vedolizumab Trough levels predict mucosal healing in inflammatory bowel disease: a multicentre prospective observational study. Aliment Pharmacol Ther 2018;47:906–12. [DOI] [PubMed] [Google Scholar]
- 29. Salleron J, Danese S, D'Agay L, et al. Effectiveness research in inflammatory bowel disease: a necessity and a methodological challenge. J Crohns Colitis 2016;10:1096–102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
flgastro-2019-101259supp001.pdf (171.5KB, pdf)
flgastro-2019-101259supp002.pdf (213.8KB, pdf)
flgastro-2019-101259supp003.pdf (46.5KB, pdf)


