Abstract
Schistosomiasis (bilharzia) is a neglected tropical disease caused by trematode worms of the genus Schistosoma. The transmission cycle involves human (or other mammalian) water contact with surface water contaminated by faeces or urine, as well as specific freshwater snails acting as intermediate hosts. The main disease-causing species are S. haematobium, S. mansoni and S. japonicum. According to the World Health Organisation, over 250 million people are infected worldwide, leading to considerable morbidity and the estimated loss of 1.9 million disability-adjusted life years (DALYs), a likely underestimated figure. Schistosomiasis is characterised by focal epidemiology and an over-dispersed population distribution, with higher infection rates in children. Complex immune mechanisms lead to the slow acquisition of immune resistance, but innate factors also play a part. Acute schistosomiasis, a feverish syndrome, is most evident in travellers following a primary infection. Chronic schistosomiasis affects mainly individuals with long-standing infections residing in poor rural areas. Immunopathological reactions against schistosome eggs trapped in host tissues lead to inflammatory and obstructive disease in the urinary system (S. haematobium) or intestinal disease, hepatosplenic inflammation and liver fibrosis (S. mansoni and S. japonicum). An effective drug—praziquantel—is available for treatment but, despite intensive efforts, no schistosomiasis vaccines have yet been accepted for public use. In this review, we briefly introduce the schistosome parasites and the immunopathogenic manifestations resulting from schistosomiasis. We then explore aspects of the immunology and host-parasite interplay in schistosome infections paying special attention to the current status of schistosomiasis vaccine development highlighting the advancement of a new controlled human challenge infection model for testing schistosomiasis vaccines.
Keywords: Schistosoma, Schistosomiasis, Urogenital schistosomiasis, Gastrointestinal/hepatosplenic schistosomiasis, Schistosomiasis vaccine, Controlled human infection model
Introduction
Schistosomiasis, also called bilharzia, is a neglected tropical disease of poverty that is widespread in the tropics and subtropics, impacting one billion people, with 250 million infected, in 74 countries [1–4]. The infection is caused by trematode blood flukes of the genus Schistosoma; these flatworms have a complex life cycle that involves, depending on species, aquatic or amphibious snails as intermediate hosts and mammalian definitive hosts (Fig. 1). The main clinically important species are as follows: S. japonicum, transmitted by the amphibious snail Oncomelania and resulting in intestinal and hepatosplenic schistosomiasis in the People’s Republic of China, the Philippines and Indonesia; S. haematobium, the most common species, transmitted by Bulinus snails and causing urogenital schistosomiasis in Africa and in some countries of the Arabian peninsula (it has also recently emerged on the French island of Corsica [5]); and S. mansoni which is transmitted by Biomphalaria snails and causes intestinal and hepatic disease in Africa, the Arabian peninsula and Latin America. S. guineensis and S. intercalatum (both endemic in Central and West Africa) and S. mekongi (restricted to a short stretch of the Mekong River in southern Lao People’s Democratic Republic and eastern Cambodia) are of local, regional importance [1–3]. Globally, over 250 million individuals are infected with Schistosoma spp. with some 780 million people at risk of infection [2–4]. The disease results in considerable human morbidity, even leading to mortality, notably in sub-Saharan Africa where more than 90% of all infections occur [4]. The Global Burden of Disease (GBD) study of 2016 estimated the global burden due to schistosomiasis at 1.9 million disability-adjusted life years (DALYs) [4], although reported DALY figures vary considerably with 70 million being the highest [4].
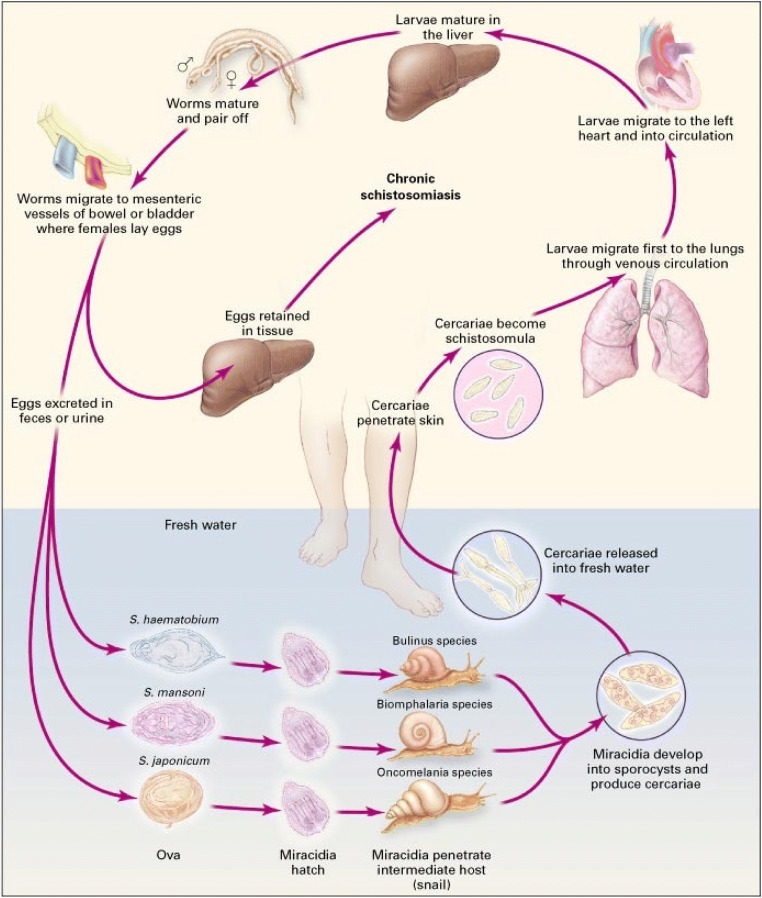
Fig. 1.
Life cycle of human schistosomes. Adult schistosome worms parasitise the blood vessels of humans and other vertebrates which act as definitive hosts, but their life cycle necessitates a phase of asexual multiplication and development within a freshwater snail. Infective larvae (cercariae) are periodically released from the snail, and these actively seek and penetrate the skin of the human definitive host; in zoonotic schistosomiasis (caused by S. japonicum), other mammals, especially bovines, can also be involved. Following penetration of the skin, the cercarial tails drop off in the skin and the parasites transform into schistosomula which enter the venous blood vessels and are transported to the lungs via the right heart, before reaching the left side of the heart to eventually enter the arterial circulation. The schistosomula migrate to, and live in, the mesenteric veins of the bowel (S. mansoni, S. japonicum) or the pelvic venous plexus (S. haematobium). There they mature into female or male adult worms with the females ending up in the male’s gynaecophoral canal and ultimately producing eggs over a period that in extreme cases may be as long as 20 years. The eggs are aimed at passing from the lumen of the blood vessels through the intestinal or bladder mucosa and are shed in the faeces (S. mansoni and S. japonicum) or urine (S. haematobium), but as many as 50% of them wind up in adjacent tissues or are flushed into the liver (mainly S. mansoni and S. japonicum) but can in rare cases find more remote organs. Eggs failing to be excreted from the host are trapped in the tissues inducing inflammatory responses and resulting in pathological lesions that are ultimately revealed as clinical disease. The life cycle is completed when the eggs hatch, releasing free-swimming ciliated miracidia which, in turn, locate and infect specific freshwater snails (Biomphalaria spp. for S. mansoni; Oncomelania spp. for S. japonicum, Bulinus spp. for S. haematobium). Cercariae are released after two generations of primary sporocysts and then daughter sporocysts within the snail. From Reference 1: Ross AG, Bartley PB, Sleigh AC, Olds GR, Li Y, Williams GM, McManus DP (2002) Schistosomiasis New Engl J Med 346: 1212–1220; Massachusetts Medical Society. Reprinted with permission.
A recently published review covered the main features of schistosome biology and the epidemiology, clinical features, pathogenesis, diagnosis, management and control of schistosomiasis [4]. Here we provide a more in-depth consideration of the pathophysiology and progression of clinical disease in schistosomiasis, the immunology and host-parasite interplay in schistosome infections and recent updates on progress in schistosomiasis vaccine development.
Immunopathogenesis and clinical disease
The main lesions in schistosome infection are due not to the adult parasites but to the eggs laid by the female worms [1–4]. Whereas many eggs reach the external environment to continue schistosome transmission, a large proportion is trapped in the liver, intestine or bladder of the definitive host. Here, they secrete various components including proteolytic enzymes that elicit eosinophilic inflammatory immune responses, including the formation of granulomas, which are progressively replaced by fibrotic deposits eventually resulting in urogenital disease (S. haematobium) or, alternatively, intestinal or advanced hepatosplenic schistosomiasis (S. mansoni and S. japonicum) [1–4].
There are three distinct stages of disease progression in schistosomiasis: acute, established active and late chronic [4]. Acute disease occurs mainly in travellers to areas endemic for schistosomiasis following a primary schistosome infection with the common presenting symptoms in S. mansoni– and S. japonicum–infected individuals being abdominal and muscle (myalgia) pain, fever, malaise and fatigue; haematuria (blood in the urine) is characteristic of individuals infected with S. haematobium [1–4]. Established active and late chronic schistosomiasis are found mainly in people with long-standing schistosome infection who live in poor rural areas. The clinical manifestations are inflammatory and obstructive and result from immunopathological reactions against schistosome eggs trapped in host tissues [1–4]. In S. japonicum and S. mansoni infections, we see intestinal disease by advanced hepatosplenic schistosomiasis, whereas in S. haematobium infection, the disease is urogenital mainly involving lesions of not only the bladder wall but also the reproductive system [1–4].
Morbidity is especially pronounced in high-intensity infections (i.e. those with a high worm burden) with large numbers of eggs released daily [1–4]. Importantly, morbidity/mortality associated with S. japonicum infections is exacerbated due to the substantially higher number of eggs, often released in packages, by this species [6]. The three phases of the disease mentioned above are dictated by the length of infection [4]. These differ with respect to symptoms and clinical consequences as well as to the rates of schistosome egg excretion in urine or faeces [4].
Immediate manifestations
The first clinical manifestation following infection is a maculopapular pruritic eruption, termed cercarial dermatitis, which may arise at the site of skin penetration by schistosome cercariae [7]. Cercarial dermatitis results from an innate immune response giving rise to hypersensitivity reactions to dead or dying parasites and can be observed with all human schistosome species. These skin reactions comprise discrete erythematous raised lesions varying in size (1–3 cm) which may develop within a few hours post-infection, although a rash may appear up to 7 days later in tourists or migrants moving into an area endemic for schistosomiasis for the first time. The resulting dermatitis is similar to, but less severe than swimmers’ itch, which is a response in persons when infected by schistosomes not adapted to humans (e.g. those adapted to birds) [1, 7].
Acute schistosomiasis
Following cercarial penetration and schistosomula maturation, the infection may proceed to the symptomatic acute schistosomiasis stage although the first clinical manifestations in non-immune subjects may be delayed by several weeks or even months following exposure [1, 7]. The symptom complex, known as Katayama fever or Katayama syndrome, is common in areas with high rates of schistosomiasis transmission and is an early clinical indicator of a first infection or a heavy reinfection with Schistosoma spp. cercariae. Acute schistosomiasis is the disease manifestation most likely to be underdiagnosed or misdiagnosed by physicians in non-endemic countries and remains, compared with the other clinical stages of schistosomiasis, poorly understood [7]. The syndrome is named after the Katayama District of Hiroshima prefecture in Japan where the first human case of S. japonicum was described in 1904 [8]. Patients commonly recall prior contact with natural freshwater bodies, such as streams or lakes between 2 weeks and 3 months earlier [7]. Common symptoms include generalised myalgia, right-upper-quadrant pain, bloody diarrhoea, headache, fever, eosinophilia, non-productive cough and patchy pulmonary infiltrates observed on chest radiography [3, 7]. These vary in severity depending on the infecting species but are thought to be due to systemic hypersensitivity reactions mediated by the immune system against antigens released by migrating schistosomula or, in most cases, eggs following their deposition in host tissues [3, 7]. Whereas the majority of patients recover spontaneously after 2–10 weeks, some develop a generalised rash and more serious and persistent disease with dyspnoea, abdominal pain, weight loss, hepatomegaly and diarrhoea [3, 7].
While Katayama fever caused by S. japonicum infections is commonly reported, it is rarely observed in relation to S. haematobium or S. mansoni in individuals from chronically exposed communities and may be the result of in-utero de-sensitisation and a lowering of immune responsiveness to schistosome antigens in infants born to infected mothers [4, 7]. An alternative explanation might be repeated exposure to schistosome cercarial antigens inducing CD4+ T cells that produce IL-10 in the skin leading to downregulation of the immune response [7]; however, it is also possible that cases from endemic areas simply remain undiagnosed. Katayama fever caused by S. japonicum infections not only is not restricted to a primary infection but also occurs in people living in areas with a history of previous infections, notably in the People’s Republic of China where such infections have been recorded in endemic communities exposed to flooding [6, 7]. Both repeated doses of 60 mg per kg body weight of the acylated quinoline-pyrazine compound, praziquantel (PZQ), and a prolonged daily course at 20 mg per kg body weight have been used as treatment [7]. The anti-malarial artemether (ART) (and other artemisinin derivatives, produced from leaves of the Chinese medicinal plant Artemisia annua) should kill all invading schistosomula if it is given every 2 weeks, thereby providing an avenue for preventing acute cases in high-risk groups such as tourists, fishermen and flood relief workers in areas endemic for schistosomiasis [6, 9].
Established active infection
In the majority of schistosomiasis cases, particularly in individuals residing in endemic areas, symptomatic, acute disease does not occur; instead, the disease develops directly into an established active infection characterised by the presence of mature adult worms, the production of eggs and their excretion in urine or faeces [4]. Live adults present in blood vessels do not generate an immune response with local inflammation, which is believed to be due to the worms’ ability to masquerade by acquiring host antigens or through molecular mimicry whereby the parasite antigens are hidden [3] and possibly also resulting from the continuous regeneration of the parasite tegumental external surface effectuated by unique somatic stem cells. Schistosome eggs, on the other hand, are unprotected, so all lesions in affected host organs and the resulting symptoms are entirely due to the inflammatory responses generated against them (Fig. 2). The eggs actively secrete soluble antigenic glycoproteins (that generate an inflammatory response mediated by CD4+ T-helper-2 lymphocytes), the function of which is to help facilitate their passage from the blood vessels, where they are released from the female worm, to the lumen of the urinary bladder or intestine, to eventually close the life cycle by reaching and infecting the intermediate snail host. However, these glycoproteins also induce the formation of granulomas (an assembly of inflammatory cells such as macrophages, lymphocytes, neutrophils and eosinophils) that amass around the eggs. The granulomas generally develop at the sites of maximal egg accumulation and, while killing the eggs, the end result is fibrotic lesions in the host tissues [1–4].
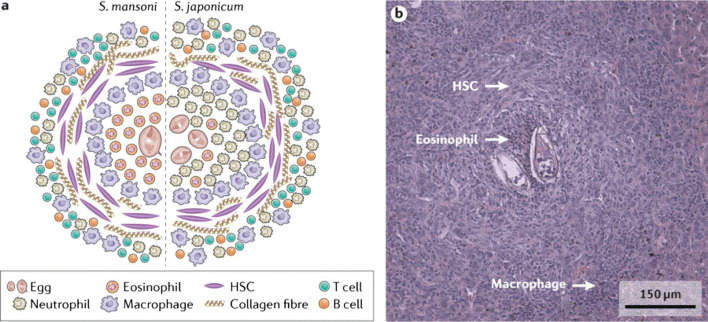
Fig. 2.
Features of schistosome-induced granuloma formation. Adult schistosome worm pairs residing in mesenteric veins produce eggs some of which become entrapped in the host’s liver (or other organs) tissue where they evoke a dominant CD4(+) TH2 immune response mediated by IL-4 and IL-13. This leads to the development of granulomas and fibrosis with hepatic stellate cells, macrophages, lymphocytes, neutrophils, and eosinophils, all identified as major cellular contributors to these events. a Major cellular populations located within and adjacent to the hepatic granuloma induced in either S. japonicum or S. mansoni infection. Whereas a dense population of eosinophils are present at the core of a S. mansoni-induced hepatic granuloma, the core in a S. japonicum infection is comprised chiefly of neutrophils. Chemokine-binding proteins secreted by the eggs of S. mansoni eggs bind neutrophil chemoattractant C-X-C-motif chemokine ligand 8 (CXCL8), thereby blocking the infiltration of neutrophils to the granuloma. In contrast, these proteins do not bind to eosinophil chemoattractant CC-chemokine ligand 11 (CCL11) and, therefore, do not inhibit the recruitment of eosinophils. b A granuloma in the liver of a S. mansoni-infected mouse with hepatic stellate cells (HSCs). Part a adapted with permission from Chuah, C., Jones, M. K., Burke, M. L., McManus, D. P. & Gobert, G. N. Cellular and chemokine-mediated regulation in schistosome-induced hepatic pathology. Trends Parasitol. 30, 141–150 (2014), Elsevier. Part b courtesy of A. M. O. Kildemoes, University of Copenhagen, Denmark
The organ-specific clinical symptoms in schistosomiasis often correlate positively with the intensity of infection (i.e. the number of eggs excreted) and are mediated by egg-induced inflammation followed by the granulomatous reaction [1–4]. Established active infections, characteristic of children in schistosomiasis-endemic areas, are partially reversible following killing of the adult worms through repeated treatment with PZQ [1] or ART + PZQ killing both young and adult worms [9]. As the disease becomes chronic, the elevated lymphocyte proliferative responses generated by the soluble egg antigens at this phase of infection progressively decrease [2, 4].
Chronic and advanced disease
Mature, patent schistosome infections, associated with chronic local inflammatory responses to schistosome eggs trapped in host tissues, may in urogenital infection (caused by S. haematobium) lead to obstructive disease in the urinary and reproductive system or, alternatively, intestinal disease, hepatosplenic inflammation and liver fibrosis in disease due to S. mansoni and S. japonicum [1, 2, 10]. Granulomas, which develop at the sites of maximal accumulation of eggs, destroy the eggs but result in fibrosis in host tissues. Granuloma formation and the local inflammatory response mediated by CD4+ T-helper-2 lymphocytes help in facilitating the passage of eggs into the lumen of the gut or urinary tract prior to their exit in faeces or urine [11–13]. However, the worm burden in most individuals exposed continuously to schistosome infection gradually declines over time as partial acquired immunity develops against new infections; concomitantly, due to natural death of parasites, there is a reduction in established worm numbers which results in smaller numbers of new eggs being lodged in host tissue or excreted [4, 10]. In addition, owing to immunological down-regulation, newly formed granulomas are smaller, while as eggs within them are killed, any earlier formed granulomas gradually resolve to be replaced by scarring (fibrous tissue), thereby contributing to a reduced severity of symptoms [4].
Urogenital schistosomiasis
Adult S. haematobium worms settle and reside in the small venules around the ureter and bladder of the urinary tract. Many eggs are trapped in the tissues, mainly the bladder wall where they eventually become calcified, while those that pass into the bladder lumen leave the body with the urine. In an established active infection, live egg clusters in the urogenital tissues are found surrounded by pronounced tissue eosinophilia and an intense inflammatory reaction [14, 15], while passage through the bladder wall is commonly accompanied by sloughing off of the epithelial surface followed by ulceration and bleeding. Intense egg-induced tissue inflammation results in thickening of the bladder wall and development of pseudopolyps and hypertrophic masses [16, 17]. Haematuria, appearing 10–12 weeks after infection, is the first sign of disease due to an established active S. haematobium infection and many children from endemic areas present with this symptom [18]. Along with dysuria, haematuria also occurs in the late disease phase. Late disease manifestations additionally include ureter and urethra obstruction, bladder calcification, secondary bacterial infections in the urinary tract, proteinuria (often nephrotic syndrome), hydronephrosis, renal colic and renal failure.
Epidemiological studies have found an association of chronic, urogenital schistosomiasis with squamous cell carcinoma of the bladder in Egypt and other parts of Africa. This is an important clinical complication of infection; indeed, S. haematobium has been classified as a carcinogenic infection by the International Agency for Research on Cancer [4, 19, 20]. The process of carcinogenesis is closely associated with tissue inflammation and well differentiated squamous cell carcinomas that metastasise locally. Inflammatory gene damage, β-glucuronidase and nitrosamines are possible factors contributing to the process of carcinogenesis although S. haematobium lesions may intensify the exposure of the bladder epithelium to mutagenic substrates such as tobacco [21].
In the genital system, parasite eggs are frequently deposited in the prostate and the seminal vesicles (in men) or in the cervix (in women), where characteristic cervical lesions are found with pronounced tissue inflammation with calcified (in the chronic stage) as well as live eggs in relatively new infections [4]. Infected females may present with other genital lesions (e.g. vulvar nodules), vaginal bleeding, fallopian tube damage and dyspareunia. Men with genital schistosomiasis have increased levels of inflammatory cytokines and increased numbers of leucocytes in semen [4]. In both men and women, S. haematobium infection has been correlated with increased risk of infection by, and transmission of, the human immunodeficiency virus (HIV) with attraction of inflammatory cells and possibly presence of the virus in semen. In women, intercourse can cause bleeding lesions on the friable mucosa of the cervix and the vagina [22].
Gastrointestinal and hepatosplenic schistosomiasis
Schistosome eggs retained in the gut wall induce inflammation, polyposis, ulceration, micro-abscess formation and hyperplasia [1, 3, 4]. Symptoms of gastrointestinal disease tend to be more pronounced in individuals with high infection intensities and include loss of appetite, diarrhoea (particularly in children) that may alternate with constipation, blood in stool (haematochezia) and colicky hypogastric pain or pain in the left iliac fossa [1, 2, 4]. Severe chronic intestinal disease may result in rectal or colonic stenosis. Colonic polyposis may manifest as a protein-losing enteropathy, whereas an inflammatory mass in the colon may even mimic cancer [1].
When eggs of S. japonicum and S. mansoni are flushed to the liver (which they commonly are), the granulomatous inflammatory response induces pre-sinusoidal inflammation and periportal (clay-pipe) stem fibrosis [1, 2, 4]; sustained heavy infection is frequently associated with this clinical picture, but it normally takes many years to develop [1, 2, 4]. Hepatomegaly occurs early in the evolution of chronic schistosomiasis, while late-stage periportal collagen deposits lead to the progressive obstruction of the blood flow, marked portal hypertension, which results in the development of alternative routes of the blood circulation (commonly causing varices inside the oesophagus, often leading to bleeding and vomiting of blood). Other common clinical signs are abdominal ascites and splenomegaly (enlargement and hardening of the spleen) [1, 2, 4, 21]. Periportal fibrosis, characteristic of schistosomiasis, can be seen on magnetic resonance imaging (MRI), computed tomography scan and ultrasonography [1, 21].
Other disease manifestations
The most severe clinical outcome associated with schistosome infection is probably neuroschistosomiasis, which results from the inflammatory response against eggs deposited in the brain or spinal cord following the aberrant migration of adult worms to these locations [23–25]. Common signs and symptoms of neuroschistosomiasis are radiculopathy, an increase in intracranial pressure, myelopathy and subsequent clinical sequelae [23–25]. Acute encephalitis of the cortex, subcortical white matter, basal ganglia or internal capsule is typical of S. japonicum infection, whereas myelopathy (acute transverse myelitis and subacute myeloradiculopathy) of the lumbosacral region is the most common neurological manifestation of S. mansoni or S. haematobium infection [23]. Cerebral complications include encephalopathy with headache, visual impairment, delirium, seizures, motor deficits and ataxia, whereas spinal symptoms include lumbar pain, lower limb radicular pain, muscle weakness, sensory loss and bladder dysfunction [23].
Schistosome-induced lesions in the heart and appendix have been documented and chronic infection with S. mansoni and (rarely) S. haematobium, but not S. japonicum, can lead to pulmonary schistosomiasis [26]. In this manifestation, eggs have been transported to the lung capillaries where they induce granuloma formation which may lead to fibrosis in the perialveolar area resulting in pulmonary hypertension and cor pulmonale (increased size of the right heart ventricle due to elevated pressure in the lung capillaries).
Immunology and host-parasite interplay in schistosome infections
Animal models
Experimental animal models provide invaluable information on the immunobiology of schistosomiasis, including the nature of the host innate and adaptive responses to schistosomes and the strategies implemented to manipulate these responses [27]. Indeed, much of our understanding of the human immune responses to schistosomes has been facilitated by the availability of murine experimental S. japonicum and S. mansoni infection models [28–30] which have provided invaluable help in the design of immunological studies in people with schistosomiasis. Briefly, these studies show that T cell-mediated immunity is pivotal in the development of acquired resistance to schistosomes in mice and that the processes of immune regulation and T cell regulation involved are complex. Whereas the T-helper (TH) response typically induced by these blood flukes is a pronounced TH2 one, it is the development of a balanced TH reaction that is critical in preventing disease progression since both TH1 and TH2 components, if one-sided and excessive, can lead to damaging pathology [27].
In recent years, the use of transgenic or gene knockout mice has considerably advanced our understanding of the immunopathological mechanisms and the process of granuloma formation that are important in both schistosomiasis and other fibrotic diseases [31]. However, whereas mouse models of schistosomiasis have proven useful in identifying factors involved in granuloma formation and in immunopathology, being naive hosts that do not live sufficiently long to develop protective immune responses after primary infection, they cannot be used for analysing immune correlates to reinfection resistance; consequently, much of what is known about these host–parasite interactions derives from human epidemiological studies considered in detail below.
Human studies
The interaction between schistosomes and the host immune system in the parasitised mammal is highly complex involving a number of discrete phases (characterised by penetrating cercariae, migrating schistosomula, adult worms, eggs excreted from the host or eggs entrapped in tissues). Immune responses against egg antigens are responsible, as indicated earlier, for the pathology in schistosomiasis, whereas responses towards cercariae, and possibly some of the other stages, notably schistosomula, are likely important for the development of resistance to re-infection. Protective immune responses in schistosomiasis do develop in people in Schistosoma-endemic areas but slowly (over a period of 10 to 15 years) [2, 4]. Accordingly, with adults usually resistant to reinfection following treatment, children less than 10 years old are highly susceptible which provides an explanation for the age-prevalence/age-intensity curves typically recorded for individuals in areas endemic for schistosomiasis [4].
Immune correlate studies in different geographical locations and a variety of epidemiological settings suggest that acquired anti-schistosome protective immunity after curative drug therapy is mediated (although not exclusively) by TH2 cell–associated responses characterised by eosinophilia and production of cytokines such as IL-4 and IL-5 as well as schistosome-specific IgE. High levels of IgG4 are also produced during infection, potentially blocking the protective effects of other immunoglobulins at a degree associated with susceptibility to infection [3]. Indeed, the age-dependent concomitant immunity against reinfection resulting from repeated natural adult worm death over time leads to establishment of protective immunity over several years and that this is more closely related to the IgE/IgG4 balance than to the absolute level of each isotype [2]. It has been hypothesised that the killing of adult worms, either after PZQ treatment or naturally, leads to release of antigens which may cross-react with larval antigens and stimulate protective IgE responses, implying that the more dead worms an infected individual has experienced the more intense the generated protective responses [32]. As schistosome worms generally live for 3–10 years, this hypothesis could provide an explanation, at least in part, why children, who have experienced few dying worms are susceptible to infection and accumulate more worms through continuous exposure, whereas adults who have experienced many dying worms develop protective immune responses; it may also explain why anti-schistosome immunity in human populations takes such a long time to develop [2, 4].
It is important to stress that the deployment of a vaccine for schistosomiasis dependent on IgE would likely be problematic and hampered by regulatory and safety issues since this class of antibodies is associated with allergic responses [3, 4, 33, 34]. Therefore, looking to the immune responses of chronically infected individuals, and even those who become refractory by producing IgE after PZQ drug treatment, should be approached with care [33]. Nevertheless, although vaccines do not currently figure prominently in the context of schistosomiasis control, it is imperative that research continues in this important area as genuine and lasting change of the disease spectrum in endemic settings can only be achieved by long-term protection involving vaccination [2, 34]. Indeed, the inability of PZQ to interfere prominently in transmission of the infection has led to a renewed interest in vaccine development.
Schistosomiasis vaccine development
Why is a vaccine needed?
Extensive efforts have been made in schistosomiasis control mainly as the result of population-based preventive chemotherapy delivered through mass drug administration (MDA), i.e. the periodic oral PZQ administration, targeting mainly school-aged children at risk without prior diagnosis [4]. Indeed, the global reduction in schistosomiasis morbidity resulting from this sustained PZQ treatment led to a strong emphasis (perhaps overly so) on chemotherapy as the exclusive method to achieve elimination of the disease [34]. However, this progress notwithstanding, schistosomiasis prevalence has remained largely unaffected as rapid reinfection quickly restores the prevailing levels of infection after each scheduled MDA programme [34]. Notable also is that PZQ is not 100% effective, and particularly, since it is given as a single dose in each treatment cycle, the untouched migrating schistosomula give rise to a new generation of adult worms 1–2 months after MDA [4]. Furthermore, due to the widespread use of the drug, the potential emergence of PZQ drug resistance is a continual concern, although there is, as yet, no direct definitive evidence that clinically relevant drug-resistant schistosomes have evolved in the field [4, 35].
In only a small number of schistosomiasis-endemic areas, notably the People’s Republic of China where elimination is now on the horizon, has a clear reduction in prevalence been reached. This effect can, however, be credited not only to the use of PZQ but also as much to effective snail control and other measures such as environmental modification and health education [34]. It must in this connection be mentioned that the snail species acting as intermediate hosts outside Southeast Asia are non-amphibious and, therefore, considerably more difficult to control [34]. There is therefore now an almost general acceptance that sustainable schistosomiasis prevention and control will require a multifaceted, integrated approach, a scenario that underscores support for the development and deployment of a vaccine as a key element in the line of attack making up for the short-term effect of chemotherapy [36]. Indeed, Science ranks a schistosomiasis-vaccine as one of the top 10 vaccines requiring urgent development based on feasibility and need [37]. Accordingly, the development and positioning of an effective vaccine within the spectrum of disease control is an option that would add a much needed long-term attribute to be applied in combination with MDA [34–36, 38]. Furthermore, the schistosomiasis vaccine does not need to be 100% effective as mathematical modelling provides support to the view that even a partially protective vaccine would contribute to reducing schistosome infections and interrupting endemic transmission [34, 36, 39, 40].
The sad reality that no commercial vaccine is available currently against any of the human schistosomes emphasises the need for continued efforts towards achieving this elusive goal. Researchers in the field have met with an uphill battle for many years, partly due to the scarce funding in face of the complicated immunology involved when the human host balances between resisting new infections and avoiding serious pathology when reacting against entrapped tissue eggs from prior infections. In addition, over the course of evolution, schistosomes have evolved intricate survival strategies enabling successful relocation between various, completely different environments to allow them access to the human host [41, 42]. In retrospect, one cannot avoid the sobering thought that it was unfortunate that the initially adequate allocation of funding for schistosomiasis vaccine development was not sustained. As donors dropped out, the dwindling levels of funding restricted the possibility of rapidly reaching the goal, making the development of an effective product even more challenging [34]. There is, however, some light at the end of the tunnel provided by the fact schistosomes do not replicate in their definitive hosts provides some respite in contrast to viral and bacterial infections. Thus, even a partially protective vaccine could contribute to reducing infection rates and interrupting transmission, a view indicated earlier supported by mathematical modelling [34, 36, 39, 40]. In addition, recent progress has shown some promise, not only due to the availability of novel adjuvants that can selectively manipulate the immune responses, but also because immunological research makes it possible to assess the specific responses each vaccine antigen elicits (and what it needs to elicit) through cell signalling studies [43]. It has been argued that immune responses directed against the adult schistosome worm might not be the major mechanism sought [44]. If this assumption is correct, the short interval between cercarial skin penetration and the presence of schistosomula in the lungs about 72 h later would be the time when the parasite should be the most vulnerable for immune attack [34]. This susceptibility is likely to at least partly spill over into the juvenile schistosome in the liver sinusoids after which time the parasite is already resistant to antibody-dependent cellular cytotoxicity (ADCC) controlled by complement [45].
Animal models and vaccine development
Animal experiments, particularly using the mouse challenge model, carried out over the past 50 years have provided a rich appreciation of Schistosoma spp.–host interactions [34]. However, although the protective immune responses to schistosome infection might be similar in humans and mice, important defensive mechanisms might differ, whereas human population studies in endemic areas cannot provide all the important information needed [34]. It has thus been advocated that it is useful to also carry out testing of schistosome vaccine antigen candidates in non-human primates, even if only to strengthen the safety aspect of the vaccination strategies. Furthermore, vaccines based on studies performed only in the mouse model could even have undesirable effects if taken prematurely to human clinical trials [46, 47]. It has even been speculated that schistosome vaccine/challenge trials undertaken in mice might be flawed, resulting in erroneous protective efficacy data [48]. Nevertheless, immunisation of mice with a single dose of irradiated cercariae [49], or schistosomula treated with artemether [50], induces high levels of protection [49–51]. Although it is doubtful that such approaches could be used clinically as they would likely carry too high a risk of adverse events, these pioneering findings motivated the search for the key antigens simulating these responses. For example, an early study of mice vaccinated with S. mansoni irradiated cercariae identified a group of five such molecules, i.e. triose phosphate isomerase (TPI), glutathione S-transferase (GST), heat shock protein 70 (HSP-70), paramyosin and a 23-kDa integral membrane protein [52]. The search for defined schistosomiasis vaccine candidates of this kind intensified thereafter, resulting in a large suite of putatively protective antigens [53]. Recent data on S. mansoni and S. haematobium vaccine candidates including their location, function, formulation and mode of delivery, as well as the adjuvants employed and the protective efficacy generated, are presented in Table 1. Similar data are available for S. japonicum [53].
Table 1.
Recent data on Schistosoma mansoni vaccine antigen candidates (modified from [53])
| Antigen | Location in adult worm | Identity/function | Immunisation strategy | Adjuvant | Host | Worm burden reduction (%) | Liver egg burden reduction (%) |
|---|---|---|---|---|---|---|---|
| Sm-p80 | Associated with tegument inner membrane | Calpain-neutral cysteine protease | Recombinant protein | Resiquimod | Mouse | 50 | 16 |
| Primed with pcDNA3 and boosted with recombinant protein | Resiquimod | Mouse | 49 | 30 | |||
| Primed and boosted with recombinant protein | Oligodeoxynucleotide | Mouse | 70 | 75 | |||
| Recombinant protein | Resiquimod | Baboon | 58 | – | |||
| DNA vaccine | – | Baboon | 38–46 | 32–28 | |||
| Fatty acid binding protein (FABP) (Sm14) | Whole body, cytosolic | Absorbs, transports and compartmentalises fatty acids from the host | Recombinant protein | – | Mouse | 67 | – |
| Tetraspanin protein 2 (Sm-TSP2) | Tegument apical membrane | Tetraspanin integral membrane protein | Recombinant protein | Freunds | Mouse | 57 | 64 |
| Recombinant protein | Alum/CpG | Mouse | 25 | 27 | |||
| Glutathione S-transferase (Sh28GST) | Whole body | Enzyme involved in fatty acid metabolism and prostaglandin D2 synthesis | Recombinant protein | Aluminium | Baboon | 0–80 | – |
| Sm29 | Tegument apical membrane | Unknown, but has a C-terminal domain | DNA vaccine with pUMVC3 plasmid | – | Mouse | 17–22 | – |
| Recombinant protein | Complete Freunds and incomplete Freunds | Mouse | 51 | – | |||
| Sm14 + Sm-29 | – | – | Multivalent recombinant proteins | Poly (I;C) | Mouse | 40 | 68 |
| Sm29 + Sm-TSP-2 | – | – | Multivalent DNA vaccine with pUMVC3 plasmid | – | Mouse | 24–32 | – |
| Multivalent Recombinant proteins | CpG-Alum | Mouse | 35 | – | |||
| Oesophageal gland secretion (Sm100.3) | Oesophagus | Digestive tract proteins (oesophageal) | Recombinant proteins | Freunds | Mouse | 25–32 | 33–44 |
| Cathepsin B1(SmCB1) (Combined with SG3PDHa + PRX-MAPb) | Gut (gastrodermis) | Gut protease (cysteine peptidase) | Recombinant proteins | Postulated to have inbuilt adjuvant properties | Mouse | 73 | 83 |
| S. mansoni cathepsin B (Sm-CB) | Gut (gastrodermis) | Gut protease (cysteine peptidase) | Recombinant proteins | CpG oligodeoxynucleotides | Mouse | 59 | 56 |
| Montanide ISA 750 VG | Mouse | 60 | 62 | ||||
| Schistosome cysteine proteinase, asparaginyl endopeptidase (SmAE) (Sm32) | Gut | Gut protease (Asparaginyl peptidase) | DNA vaccine | – | Mouse | No significant reduction | 37 |
| Lysosome-associated membrane glycoprotein (Sm-LAMP) | Gastrodermis | Processing of ingested blood | Recombinant protein | alum-CpG | Mouse | 16–25 | – |
| Dynein light chain proteins | Unknown | Evolutionarily conserved among different organisms | Recombinant protein | Alhydrogel | Mouse | ||
|
-DLC 12 -DLC 13 |
43 51 |
||||||
| S. mansoni Syntenin (SmSynt) | Intestinal tract | Scaffold supporting protein | Recombinant protein | Complete Freunds and incomplete Freunds | Mouse | 30–37 | – |
| Radiation-attenuated cercariae | – | – | UV-attenuated | – | Mouse | 43 | 73 |
| Antioxidants | |||||||
| -Cu/Zn cytosolic superoxide dismutase | – | – | DNA vaccine | – | Mouse | 44–60 | |
| -Signal peptide-containing superoxide dismutase | – | – | DNA vaccine | – | Mouse | 22–45 | |
| -Glutathione peroxidase enzymes | – | – | DNA vaccine | – |
Mouse Baboon |
23–55 17 |
|
aGlyceraldehyde 3-phosphate dehydrogenase
bPeroxiredoxin
Generally, the work followed a step-by-step approach that included identification of protective antigens, selection of the most promising ones, their molecular cloning, expression and purification followed by testing for vaccine efficacy in vaccinated and challenged mice [53]. In the next section, we consider the evaluation of the relatively few schistosomiasis vaccines that have advanced to clinical trials or have the potential to enter into clinical testing.
Human vaccine development
Three lead human schistosomiasis vaccines (Sh28GST, Sm-14 and Sm-TSP-2) are currently at differing phases of clinical development with a fourth (Sm-P80) to follow shortly.
Sh28GST
Recombinant 28-kDa glutathione S-transferase of S. haematobium (rSh28GST), presented with Alhydrogel (aluminium hydroxide) and designated as Bilhvax, was developed in partnership by Professor Andre Capron and his team at Inserm-Université Lille and Institut Pasteur de Lille in France together with scientists from Eurogentec, Liège, Belgium. The vaccine was shown to be safe and immunogenic, generating a TH2-type response in young healthy Caucasian male adults in a phase-1 randomised, clinical study; this encouraging outcome provided the impetus to continue trials with rSh28GST as a potential vaccine candidate against urogenital schistosomiasis (with a possibly useful degree of cross reaction against S. mansoni) [54]. Phase-2 clinical testing in Senegal showed that Bilhvax in combination with PZQ treatment was safe for infected adults and children (unpublished). A phase-3 trial (2009 to 2012) in 250 children (125 randomly assigned to receive the vaccine and 125 the placebo) aged 6–9 years in Senegal showed the Bilhvax vaccine to be immunogenic and well tolerated by all subjects investigated but, disappointingly, producing an insufficient level of protection, i.e. there was no significant delay in schistosomiasis recurrence between the vaccinated and the placebo groups as the primary endpoint [55]. Modifying the trial design and/or utilising an alternative adjuvant to provide a more balanced isotypic response, while at the same time reducing the number of vaccine administrations, may improve the efficacy of the rSh28GST vaccine and encourage further clinical testing.
Sm14
Dr. Miriam Tendler and her team in Rio de Janeiro, Brazil pioneered over many years the progression to clinical trials of a recombinant 14 kDa (rSm14) S. mansoni fatty acid-binding protein (FABP) vaccine candidate. The antigen was developed under a Brazilian platform led by the Oswaldo Cruz Foundation, the Health Ministry in Brazil. Like the S. mansoni homologue of Sh28GST (Sm28GST), Sm14 was a member of the original group of potential S. mansoni vaccine antigens, promoted by WHO/TDR in the late 1980s. The safety and immunogenicity of the rSm14 vaccine were evaluated in 2011 through an open, non-placebo-controlled, dose-standardised phase-1a trial, performed at a single site [56, 57]. Expressed in Pichia pastoris, the rSm14 vaccine was formulated with glucopyranosyl lipid A (GLA) adjuvant produced by the International Disease Research Institute (IDRI) (Seattle, WA, USA) in an oil-in-water emulsion (SE). Tested in 20 male volunteers from a non-endemic area for schistosomiasis in the state of Rio de Janeiro, Brazil, no serious adverse events were observed. Although the vaccine was shown to be immunogenic, no specific IgE response was generated, a major advantage of the product. A phase-1b clinical study, to evaluate the safety and immunogenicity of the rSm14 vaccine in 10 healthy female volunteers, was successfully concluded in 2012 [56], paving the way for a phase-2a clinical trial undertaken in 2015–2017 [58]. This trial was undertaken in 30 adult males living in a high-endemic area for both S. mansoni and S. haematobium in the Senegal River Basin where the safety and strong long-lasting immunogenicity of the rSm14 vaccine were confirmed. Based on these outcomes in adults, a phase-2b trial design and protocol were defined in 2018 involving 95 Senegalese school children, aged 7–11 years and living in the same area endemic for both schistosome species. Subsequent phase-2c and phase-2d (in Brazil) and phase-3 (in Senegal) clinical trials are planned.
Sm-TSP-2
Highly abundant at the outer-most tegumental membrane of the intra-mammalian stages of schistosomes, the tetraspanins constitute a group of proteins continually exposed to the host immune system [59]. Required for tegument biogenesis and integrity, Sm-TSP-1 and Sm-TSP-2 constitute the major S. mansoni tetraspanins [60, 61]. Furthermore, Sm-TSP-2 confers a high level of protection in the mouse vaccine model with the corresponding IgG antibodies correlating positively with protective immunity in naturally resistant people [62, 63]. As a result, Sm-TSP-2 was selected for further process development as a prime schistosomiasis vaccine candidate and is currently being advanced by the Sabin Vaccine Institute Product Development Partnership as a 9-kDa recombinant Sm-TSP-2/Alhydrogel® vaccine in combination with GLA-SE as adjuvant. The vaccine candidate can be readily scaled up in the Pichia pink (yeast) system, has undergone required toxicology studies and shown good pre-clinical results, while several clinical trials of the vaccine have been completed or are in progress in non-endemic and endemic communities [64]. These include an initial phase-1 study testing its safety and immunogenicity undertaken at the Baylor College of Medicine, Houston, TX, USA, a phase-1b trial underway in an endemic area of Brazil to assess the vaccine’s safety and immunogenicity in a group of healthy adults who may have previously been exposed to schistosomiasis, with further field trials in Uganda planned [64].
Sm-p80
A second S. mansoni surface protein, Sm-p80 (the large calcium-activated neutral cysteine protease subunit of calpain), expressed in all schistosome life cycle stages and likely to play critical role in the worm’s tegumental biogenesis and renewal [65], is another molecule rapidly moving towards clinical testing under the auspices of a consortium led by Dr. Afzal A Siddiqui, Texas Tech University Health Sciences Center at Lubbock, TX, USA. Sm-p80 has been tested for its vaccine efficacy using different vaccine formulations and approaches, including naked DNA, recombinant protein and prime/boost in three experimental animal models of infection and disease (mouse, hamster and baboon) and shown to generate significant protection against S. mansoni challenge infections in addition to cross-species protection against S. japonicum and S. haematobium [47, 66] Additionally, Sm-p80-specific IgE was not detected in infected populations from Africa and South America, thus potentially minimizing the risk of a hypersensitivity reaction following vaccination [66]. Furthermore, Sm-p80 was shown to have a therapeutic effect in vaccinated baboons through suppression of the numbers of established worms, reducing the retention of eggs in tissues and decreasing the number of eggs excreted by the host in faeces [67]. It is anticipated that the recombinant Sm-p80/GLA-SE vaccine, “SchistoShield” will soon move forward to phase-1 and phase-2 human clinical trials [66].
A transmission blocking vaccine for schistosomiasis japonica
Schistosomiasis control in the People’s Republic of China and the Philippines is complicated by the zoonotic nature of the disease with bovines (water buffalos and cattle) acting as major reservoir hosts for S. japonicum being responsible for up to 90% of environmental contamination of parasite eggs [68]. From the point of view of vaccine development, this permits a step-wise tactic that would start with the use of a “transmission-blocking” veterinary vaccine before moving on to a product aimed at human vaccination should that be necessary. In light of this, vaccination of animals, particularly bovines, is considered useful with respect to Asian schistosomiasis as it would assist in long-term prevention of human (and animal) infection, a concept supported by mathematical modelling [17, 39, 68, 69].
Vaccination can reduce egg excretion from buffalo and cattle, thereby interrupting transmission from bovines to snails, and its use would be particularly applicable in areas deemed unsuitable for the replacement of bovines by mechanised farming, one of the interventions featuring in the current integrated schistosomiasis control strategy in the People’s Republic of China [70]. Indeed, the implementation of an animal-based transmission-blocking vaccine as part of a package of integrated control measures sits well with the One Health concept synergizing human and animal health, a conclusion reached at two workshops on schistosomiasis elimination strategies and the potential role of a vaccine co-sponsored by the National Institute of Allergy and Infectious Diseases (NIAID) and the Bill & Melinda Gates Foundation [34, 71]. In the discussion at one of these workshops on the question of a clinical versus a veterinary vaccine, the latter was seen as the preferred target product profile (TPP) with respect to schistosomiasis japonica as the less rigorous safety concerns required would permit a more rapid implementation [34, 71]. Whereas this characteristic could potentially reduce the costs of deploying a schistosomiasis vaccine for use in livestock compared with one for human use, a comparative cost–benefit evaluation of applying the two different types of vaccine needs to be undertaken. A number of S. japonicum transmission-blocking vaccine candidates have been identified [33, 34, 53, 69, 71]) with Sj-GST26, Sj-GST28, Sj-97 (paramyosin) and Sj-TPI (triose-phosphate isomerase) the most tested (see Table 1).
The Sj-GSTs
Considerable early work showed the most repeatable host protective effect generated against the GSTs of all schistosome species was the significant inhibition of female worm fecundity resulting in decreased egg output and viability and, consequently, reduced transmission and a reduction in egg-induced pathology [72]; these important characteristics resulted in the clinical testing of the Bilhvax (rSh28GST) vaccine described earlier. Protective efficacy (the characteristic anti-fecundity effect) was reported in Chinese bovines (and sheep) vaccinated with S. japonicum 28 kDa GST (Sj-28GST) and in water buffaloes, cattle, sheep and pigs following vaccination with the 26 kDa GST isoform (Sj-26GST) [69]. Field testing showed the protective effect of Sj26GST against S. japonicum is, encouragingly, maintained in cattle and water buffaloes for at least 12 months post-vaccination [69] but, disappointingly, there have been no subsequent trials undertake, which is likely due to the considerable associated costs.
Sj-97
Paramyosin is a large (97-kDa) coiled coil myofibrillar protein found in the secretory glands of schistosome cercariae, in the muscles of adult worms and larvae, and on the surface tegument of lung stage schistosomula; it first caught interest as a vaccine candidate following encouraging results of vaccine/challenge experiments in mice targeting S. mansoni [69]. Then, early trials in sheep, pigs and water buffaloes with native or recombinant (Chinese strain) S. japonicum paramyosin (rSj-97) resulted in significant, albeit partial protection [69]. There was a pause in the progression of Sj-97 as a priority vaccine candidate in the early 2000s owing to its size, its poor expression, low protein yield and insolubility, the high cost associated of up-scaling production and the expense of completing large animal vaccine testing. However, a robust method for pilot-scale expression and purification of rSj-97 was later developed (2008) which signified a major advance on the road towards its pre-clinical assessment as a vaccine [69, 73]. Strengthened by the adjuvant Montanide ISA 206, rSj-97 proved safe, well tolerated and highly immunogenic in water buffaloes both in a highly endemic area for S. japonicum in the Philippines [69, 73] and in the People’s Republic of China [69, 73], thereby reinforcing its status as a promising anti-S. japonicum vaccine candidate. Phase-2 trials of rSj97/ISA206 in bovines and phase-1 trials in humans now need to be undertaken [73] and should be encouraged.
SjTPI
Triose-phosphate isomerase (TPI) is another lead anti-S. japonicum vaccine candidate; this compound is located on the surface membranes of newly transformed schistosomula and is present in most cells of adult schistosomes [71]. TPI has been tested in mice, pigs and bovines in various vaccine formulations [69, 71], such as with a plasmid-generated (Chinese strain of S. japonicum) DNA vaccine (SjCTPI- heat shock protein (hsp)-70), which induced very good protective efficacy against S. japonicum in Chinese water buffaloes when co-administered with IL-12 as adjuvant [74]. This vaccine was subsequently field-tested in bovines against natural S. japonicum challenge in Hunan Province in the People’s Republic of China in a double-blind, phase-3 cluster randomised controlled trial (RCT) [75]. The trial was designed to assess the impact on schistosomiasis transmission of a multi-component, integrated control strategy, including human treatment, mollusciciding against the intermediate snail host (Oncomelania hupensis) and the bovine vaccine. The regimen involved heterologous (DNA-protein) prime-boost delivery of the SjCTPI vaccine; bovines were primed with the SjTPI-hsp-70 DNA vaccine and booster vaccinated with the recombinant SjTPI protein using the VacSIM (vaccine self-assembling immune matrix) delivery method but without adjuvant [76, 77].
Although some of the outcomes of this large and complex trial were positive, the effect of the SjTPI vaccine in preventing human infection was inconclusive due mainly to the treatment, removal or sacrifice of bovines over the course of the trial by the Chinese authorities [78]. Indeed, most animals in the previously high schistosomiasis-endemic areas of Dongting and Poyang lakes have now been removed and replaced by mechanised tractors as a strategy that may provide the final element required for disease elimination from China [70, 78]. A similar RCT of schistosomiasis bovine vaccination using a multi-factorial design has been completed in the Philippines; it is anticipated the outcomes of the trial will be reported soon (Allen G. Ross, personal communication). As with the African schistosomes, the development of a vaccine for schistosomiasis japonica has proven highly challenging but the deployment of a transmission blocking vaccine, in tandem with other interventions (e.g. snail control plus improved water, sanitation and hygiene and health education), for the prevention of S. japonicum would be invaluable if the goal of elimination is to be reached.
Schistosomiasis vaccines: the way forward
Genomics, post-genomics and new vaccine antigen discovery
Despite the recent encouraging progress in schistosomiasis vaccine development, especially the clinical testing of lead molecules, the antigens currently used may still not provide the required level of immunological protective potency, so it is important to continue the search for novel target candidates. The application of new technologies [35] will be key in this endeavour, and there is no doubt that pivotal to many of the recent (and future) advances in antigen discovery are the availability of draft nuclear genomic sequences from S. japonicum and S. mansoni, published in 2009 [79, 80], and S. haematobium, which appeared 3 years later [81]. Improved genome assemblies are now available for S. mansoni [82], S. japonicum [83] and S. haematobium [85]. These seminal reports provide a wealth of invaluable data opening new avenues for the design and development of novel targeted anti-schistosomiasis interventions including vaccination, as well as new insights with regard to schistosome development, host-parasite interactions. Coupled with the availability of large Schistosoma transcriptomes and expanded genome databases [86], post-genomic technologies such as proteomics, glycomics, metabolomics (metabolic fingerprinting), proteome microarray profiling and exosomics that provide the necessary technology platforms for exploiting this treasure trove of information [35, 87–92], we are at a vantage point that promises rapid progress. As schistosomes are equipped with small RNA interference machinery, gene manipulation has already been used to target different life cycle stages of the three major schistosome species and this approach may guide the future development of novel intervention tools [89]. Indeed, deciphering important biological functions, such as those involved in nutrient uptake, the maintenance of tegumental integrity, immune modulation mechanisms, gene regulation, reproductive biology and fecundity, apoptosis and self-renewal, through the use of RNA interference (RNAi), can reveal important genes encoding proteins essential for parasite survival which can be targeted for immunological attack [87].
Although still in its infancy in relation to helminth parasites, the novel clustered regulatory interspaced short palindromic repeats (CRISPR) and CRISPR-associated protein (Cas9) genome editing tool is attracting considerable attention as a potent method to precisely target and then deactivate the genetic information a cell needs to produce a given protein [90]. The first (and, to date, only) application of this technology for the study of schistosomes is that reported by Ittiprasert et al. [90] who harnessed CRISPR/Cas9 to deactivate the gene that encodes S. mansoni egg omega-1 ribonuclease and create parasites that either do not produce this protein, or only very little of it. Mice infected with this kind of gene-edited eggs have been shown to produce markedly reduced granulomatous inflammation in the lungs and less pathology in general compared to those carrying non-edited parasites. This pivotal study provides a blueprint for editing other key schistosome genes in the future. Indeed, the application of this powerful, but affordable, technology may prove of value not only for targeting specific protein-encoding genes implicated in disease due to schistosomiasis but also for the identification of novel anti-schistosome vaccine (and drug) candidates.
A new paradigm—a controlled human challenge infection model for testing schistosomiasis vaccines
Introducing a paradigm shift, Meta Roestenberg and her team at the Leiden University Medical Centre in the Netherlands are pioneering the use of human skin explants showing that natural schistosome cercariae induce a predominantly regulatory immune response whereas radiation-attenuated parasites do not [92]. Along with this approach, accelerating schistosomiasis vaccine development, the Roestenberg research group is in the process of establishing an altogether novel approach—the S. mansoni controlled human infection (CHI-S) model [93]. Thanks to the use of skin explants, this road may now be open, not for morbidity manipulation or interference with parasite fecundity, but clearly for evaluation of how to stop cercarial skin penetration. There has been a recent resurgence in the popularity of CHI application, e.g. for the testing of vaccines against Helicobacter pylori, Norovirus and Plasmodium falciparum [94–96]. With respect to the most promising schistosomiasis vaccine candidates, the CHI-S model would indeed provide estimates of early protective efficacy to be used to guide further clinical development, thereby reducing the risk of downstream efficacy failure and, at the same time, advance understanding of the protective immunity generated by vaccination [93, 95].
The CHI-S work underway is especially noteworthy as the production of the schistosome cercariae required for challenge has to strictly comply with all regulatory requirements and good manufacturing practice (GMP) principles, a daunting, demanding and difficult endeavour. Safety issues are paramount in CHI trials, and to ensure safety of volunteers, the Leiden group conceptualised the propagation of single sex male schistosome cercariae that can infect human beings and mature to adult worms without mating so that egg-associated morbidity due to granuloma formation and fibrosis is circumvented [93]. The CHI-S model utilises the laboratory maintained lifecycle of S. mansoni, whereby individual Biomphalaria snails are carefully infected with a single miracidium which undergoes asexual reproduction in the molluscan host, producing after 5 weeks thousands of cercariae of a single clone, and hence single sex [93]. Following a series of quality control steps and determination of male or female sex by PCR, male cercariae are used for the controlled infection of volunteers which is performed by taping a chamber of water containing a predetermined number of male parasites onto the subject’s arm for an interval of 30 min [93]. Successful infections can be detected (usually after 6–12 weeks) and quantified by measuring circulating anodic antigen (CAA)—a protein which is secreted in large quantities by adult worms into the blood; this highly sensitive diagnostic assay was crucial for the model’s development, allowing for accurate quantification of worm loads despite the absence of eggs [97].
The CHI-S model would be suitable for testing new drugs and the currently available Sm14 and Sm-TSP-2 vaccines, although many more identified vaccine candidates [53] could be evaluated, either individually or in combination; molecules that predominantly generate anti-fecundity effects, such as the Sm-p80 vaccine, however, would unlikely be tested in this single sex infection model. The current CHI-S model from Leiden provides a blueprint for future development of infection models using female cercariae avoiding the morbidity otherwise following down the road. However, this will require evaluation from the full vaccine pipeline perspective.
The response to schistosomiasis and to candidate vaccines would likely differ markedly among people from endemic communities, notably in sub-Saharan Africa, where the vaccines are the most needed and where, compared to volunteers from Western countries, people are exposed to an abundance of potentially immunomodulating factors [3, 98]; in addition, prior schistosome exposure may display some immunological resistance noticeable in the CHI-S model [98]. Nevertheless, human epidemiological studies showing immune responses that correlate with clinical protection [4] indicate that challenge studies among volunteers from endemic settings, who would have naturally acquired immunity, have the potential to guide the development of the next generation of vaccines by allowing desirable immune responses to be identified and prioritised [98]. This means that implementation of the CHI-S model in an endemic setting, as was advanced at a stakeholders’ meeting held in November 2017 in Entebbe, Uganda, could not only provide critical additional information on correlates of protective immunity but also on immunogenicity, protective efficacy and safety of candidate vaccines [98]; considerable ethical and potential community-based hurdles might, however, have to be overcome. Furthermore, as earlier indicated, schistosome killing is mediated by antibodies, particularly IgE [1], so there is the ever present risk that a vaccine, even if effective, might result in allergic reactions related to TH2-induced IgE responses, especially among previously exposed endemic individuals, as occurred with the Na-ASP-2 hookworm protein [99] which immediately halted its clinical development.
The results of the first S. mansoni CHI trial in Leiden (equivalent to a phase-1 clinical trial) (https://clinicaltrials.gov/ct2/show/NCT02755324), testing safety and dose finding in Dutch volunteers, have not yet been reported but are awaited with anticipation. The successful establishment of such a model for testing novel vaccines (and drugs) against this devastating disease could be a game-changer.
Funding information
This work was supported by grants (grant numbers: APP1098244, APP1132975) and a Senior Principal Research Fellowship (APP1102926) to DPM from the National Health and Medical Research Council of Australia.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
The original version of this article was revised: The original version of this article inadvertently missed out to display the correct acknowledgement for Fig 2. The corrected figure legend of Fig. 2 is given below.
This article is a contribution to the special issue on: Immunopathology of unresolved tropical diseases - Guest Editor: Marcel Tanner
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
6/9/2020
The original version of this article inadvertently missed out to display the correct acknowledgement for Fig. 2. The corrected part of legend of Fig. 2 is given below.
References
- 1.Ross AG, Bartley PB, Sleigh AC, Olds GR, Li Y, Williams GM, McManus DP. Schistosomiasis. N Engl J Med. 2002;346:1212–1220. doi: 10.1056/NEJMra012396. [DOI] [PubMed] [Google Scholar]
- 2.Gryseels BC, Polman K, Clerinx J, Kestens L. Human schistosomiasis. Lancet. 2006;368:1106–1118. doi: 10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- 3.Colley DG, Bustinduy AL, Secor WE, King CH. Human schistosomiasis. Lancet. 2014;383:2253–2264. doi: 10.1016/S0140-6736(13)61949-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McManus DP, Dunne DW, Sacko M, Utzinger J, Vennervald BJ, Zhou XN. Schistosomiasis. Nat Rev Dis Primers. 2018;4:13. doi: 10.1038/s41572-018-0013-8. [DOI] [PubMed] [Google Scholar]
- 5.Boissier J, Grech-Angelini S, Webster BL, Allienne JF, Huyse T, Mas-Coma S, Toulza E, Barré-Cardi H, Rollinson D, Kincaid-Smith J, Oleaga A, Galinier R, Foata J, Rognon A, Berry A, Mouahid G, Henneron R, Moné H, Noel H, Mitta G. Outbreak of urogenital schistosomiasis in Corsica (France): an epidemiological case study. Lancet Infect Dis. 2016;16:971–979. doi: 10.1016/S1473-3099(16)00175-4. [DOI] [PubMed] [Google Scholar]
- 6.Ross AG, Sleigh AC, Li Y, Davis GM, Williams GM, Jiang Z, Feng Z, McManus DP. Schistosomiasis in the People’s Republic of China: prospects and challenges for the 21st century. Clin Microbiol Rev. 2001;14:270–295. doi: 10.1128/CMR.14.2.270-295.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ross AG, Vickers D, Olds GR, Shah SM, McManus DP. Katayama syndrome. Lancet Infect Dis. 2007;7:218–224. doi: 10.1016/S1473-3099(07)70053-1. [DOI] [PubMed] [Google Scholar]
- 8.Ishii A, Tsuji M, Tada I. History of Katayama disease: schistosomiasis japonica in Katayama district, Hiroshima, Japan. Parasitol Int. 2003;52:313–319. doi: 10.1016/S1383-5769(03)00046-1. [DOI] [PubMed] [Google Scholar]
- 9.Bergquist R, Elmorshedy H. Artemether and praziquantel: origin, mode of action, impact, and suggested application for effective control of human schistosomiasis. Trop Med Infect Dis. 2018;3:125. doi: 10.3390/tropicalmed3040125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilkins HA, Goll PH, de Marshall CTF, Moore PJ. Dynamics of Schistosoma haematobium infection in a Gambian community. III. Acquisition and loss of infection. Trans R Soc Trop Med Hyg. 1984;78:227–232. doi: 10.1016/0035-9203(84)90283-9. [DOI] [PubMed] [Google Scholar]
- 11.Doenhoff MJ. A role for granulomatous inflammation in the transmission of infectious disease: schistosomiasis and tuberculosis. Parasitology. 1997;115:S113–S125. doi: 10.1017/S0031182097001972. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz C, Fallon PG. Schistosoma “eggs-iting” the host: granuloma formation and egg excretion. Front Immunol. 2018;9:2492. doi: 10.3389/fimmu.2018.02492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costain AH, MacDonald AS, Smits HH. Schistosome egg migration: mechanisms, pathogenesis and host immune responses. Front Immunol. 2018;9:3042. doi: 10.3389/fimmu.2018.03042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Randrianasolo BS, Jourdan PM, Ravoniarimbinina P, Ramarokoto CE, Rakotomanana F, Ravaoalimalala VE, Gundersen SG, Feldmeier H, Vennervald BJ, van Lieshout L, Roald B, Leutscher P, Kjetland EF. Gynecological manifestations, histopathological findings, and schistosoma-specific polymerase chain reaction results among women with Schistosoma haematobium infection: a cross- sectional study in Madagascar. J Infect Dis. 2015;212:275–284. doi: 10.1093/infdis/jiv035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghoneim MA. Bilharziasis of the genitourinary tract. BJU Int. 2002;89:22–30. doi: 10.1046/j.1464-4096.2001.138.138.x. [DOI] [PubMed] [Google Scholar]
- 16.Burki A, Tanner M, Burnier E, Schweizer W, Meudt R, Degrémont A. Comparison of ultrasonography, intravenous pyelography and cystoscopy in detection of urinary tract lesions due to Schistosoma haematobium. Acta Trop. 1986;43:139–151. [PubMed] [Google Scholar]
- 17.Hatz C, Savioli L, Mayombana C, Dhunputh J, Kisumku UM, Tanner M. Measurement of schistosomiasis- related morbidity at community level in areas of different endemicity. Bull World Health Organ. 1990;68:777–787. [PMC free article] [PubMed] [Google Scholar]
- 18.Lengeler C, Utzinger J, Tanner M. Questionnaires for rapid screening of schistosomiasis in sub-Saharan Africa. Bull World Health Organ. 2002;80:235–242. [PMC free article] [PubMed] [Google Scholar]
- 19.Vennervald BJ, Polman K. Helminths and malignancy. Parasite Immunol. 2009;31:686–696. doi: 10.1111/j.1365-3024.2009.01163.x. [DOI] [PubMed] [Google Scholar]
- 20.Ishida K, Hsieh MH. Understanding urogenital schistosomiasis-related bladder cancer: an update. Front Med (Lausanne) 2018;5:223. doi: 10.3389/fmed.2018.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gray DJ, Ross AG, Li YS, McManus DP. Diagnosis and management of schistosomiasis. BMJ. 2011;342:d2651. doi: 10.1136/bmj.d2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Midzi N, Mduluza T, Mudenge B, Foldager L, Leutscher PDC. Decrease in seminal HIV-1 RNA load after praziquantel treatment of urogenital schistosomiasis coinfection in HIV-positive men - an observational study. Open Forum Infect Dis. 2017;4:ofx199. doi: 10.1093/ofid/ofx199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ross AG, McManus DP, Farrar J, Hunstman RJ, Gray DJ, Li YS. Neuroschistosomiasis. J Neurol. 2011;259:22–32. doi: 10.1007/s00415-011-6133-7. [DOI] [PubMed] [Google Scholar]
- 24.Ferrari TCA, Moreira PRR. Neuroschistosomiasis: clinical symptoms and pathogenesis. Lancet Neurol. 2011;10:853–864. doi: 10.1016/S1474-4422(11)70170-3. [DOI] [PubMed] [Google Scholar]
- 25.Vale TC, de Sousa-Pereira SR, Ribas JGR, Lambertucci JR. Neuroschistosomiasis mansoni: literature review and guidelines. Neurologist. 2012;18:333–342. doi: 10.1097/NRL.0b013e3182704d1e. [DOI] [PubMed] [Google Scholar]
- 26.Graham BB, Bandeira AP, Morrell NW, Butrous G, Tuder RM. Schistosomiasis-associated pulmonary hypertension: pulmonary vascular disease: the global perspective. Chest. 2010;137:20S–29S. doi: 10.1378/chest.10-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pearce EJ, MacDonald AS. The immunobiology of schistosomiasis. Nat Rev Immunol. 2002;2:499–511. doi: 10.1038/nri843. [DOI] [PubMed] [Google Scholar]
- 28.Barron L, Wynn TA. Macrophage activation governs schistosomiasis-induced inflammation and fibrosis. Eur J Immunol. 2011;41:2509–2514. doi: 10.1002/eji.201141869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen X, Yang X, Li Y, Zhu J, Zhou S, Xu Z, He L, Xue X, Zhang W, Dong X, Wu H, Li CJ, Hsu HT, Kong W, Liu F, Tripathi PB, Yu MS, Chang J, Zhou L, Su C. Follicular helper T cells promote liver pathology in mice during Schistosoma japonicum infection. PLoS Pathog. 2014;10:e1004097. doi: 10.1371/journal.ppat.1004097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tebeje BM, Harvie M, You H, Rivera V, McManus DP. T cell-mediated immunity in CBA mice during Schistosoma japonicum infection. Exp Parasitol. 2019;204:107725. doi: 10.1016/j.exppara.2019.107725. [DOI] [PubMed] [Google Scholar]
- 31.Cook PC, Owen H, Deaton AM, Borger JG, Brown SL, Clouaire T, Jones GR, Jones LH, Lundie RJ, Marley AK, Morrison VL, Phythian-Adams AT, Wachter E, Webb LM, Sutherland TE, Thomas GD, Grainger JR, Selfridge J, McKenzie AN, Allen JE, Fagerholm SC, Maizels RM, Ivens AC, Bird A, MacDonald AS. A dominant role for the methyl- CpG binding protein Mbd2 in controlling Th2 induction by dendritic cells. Nat Commun. 2015;6:6920. doi: 10.1038/ncomms7920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fitzsimmons CM, Jones FM, Pinot de Moira A, Protasio AV, Khalife J, Dickinson HA, Tukahebwa EM, Dunne DW. Progressive cross reactivity in responses: an explanation for the slow development of human immunity to schistosomiasis? Infect Immun. 2012;80:4264–4270. doi: 10.1128/IAI.00641-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McManus DP, Loukas A. Current status of vaccines for schistosomiasis. Clin Microbiol Rev. 2008;21:225–242. doi: 10.1128/CMR.00046-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bergquist R, McManus DP. Schistosomiasis vaccine development: the missing link. In: Jamieson BGM, editor. Schistosoma: biology, pathology, and control. Boca Raton: CRC Press; 2016. pp. 462–478. [Google Scholar]
- 35.Bergquist R, Utzinger J, McManus DP. Trick or treat: the role of vaccines in integrated schistosomiasis control. PLoS Negl Trop Dis. 2008;2:e244. doi: 10.1371/journal.pntd.0000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gray DJ, McManus DP, Li Y, Williams GM, Bergquist R, Ross AG. Schistosomiasis elimination: lessons from the past guide the future. Lancet Infect Dis. 2010;10:733–736. doi: 10.1016/S1473-3099(10)70099-2. [DOI] [PubMed] [Google Scholar]
- 37.Cohen J. Unfilled vials. Science. 2016;351(6268):16–19. doi: 10.1126/science.351.6268.16. [DOI] [PubMed] [Google Scholar]
- 38.Kura K, Truscott JE, Toor J, Anderson RM. Modelling the impact of a Schistosoma mansoni vaccine and mass drug administration to achieve morbidity control and transmission elimination. PLoS Negl Trop Dis. 2019;13:e0007349. doi: 10.1371/journal.pntd.0007349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams GM, Sleigh AC, Li Y, Feng Z, Davis GM, Chen H, Ross AG, Bergquist R, McManus DP. Mathematical modelling of schistosomiasis japonica: comparison of control strategies in the People’s Republic of China. Acta Trop. 2002;82:253–262. doi: 10.1016/S0001-706X(02)00017-7. [DOI] [PubMed] [Google Scholar]
- 40.Alsallaq RA, Gurarie D, Ndeffo Mbah M, Galvani A, King C. Quantitative assessment of the impact of partially protective anti-schistosomiasis vaccines. PLoS Negl Trop Dis. 2017;11:e0005544. doi: 10.1371/journal.pntd.0005544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jenkins SJ, Hewitson JP, Jenkins GR, Mountford AP. Modulation of the host’s immune response by schistosome larvae. Parasite Immunol. 2005;27:385–393. doi: 10.1111/j.1365-3024.2005.00789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walker AJ. Insights into the functional biology of schistosomes. Parasit Vectors. 2011;4:203. doi: 10.1186/1756-3305-4-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumar R, Mickael C, Kassa B, Gebreab L, Robinson JC, Koyanagi DE, Sanders L, Barthel L, Meadows C, Fox D, Irwin D, Li M, McKeon BA, Riddle S, Dale Brown R, Morgan LE, Evans CM, Hernandez-Saavedra D, Bandeira A, Maloney JP, Bull TM, Janssen WJ, Stenmark KR, Tuder RM, Graham BB. TGF-β activation by bone marrow-derived thrombospondin-1 causes Schistosoma- and hypoxia-induced pulmonary hypertension. Nat Commun. 2017;8:15494. doi: 10.1038/ncomms15494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pearce EJ, Hall BF, Sher A. Host-specific evasion of the alternative complement pathway by schistosomes correlates with the presence of a phospholipase C-sensitive surface molecule resembling human decay accelerating factor. J Immunol. 1990;144:2751–2756. [PubMed] [Google Scholar]
- 45.El Ridi R, Tallima H. Schistosoma mansoni ex vivo lung-stage larvae excretory-secretory antigens as vaccine candidates against schistosomiasis. Vaccine. 2009;27:666–673. doi: 10.1016/j.vaccine.2008.11.039. [DOI] [PubMed] [Google Scholar]
- 46.Lebens M, Sun JB, Czerkinsky C, Holmgren J. Current status and future prospects for a vaccine against schistosomiasis. Expert Rev Vaccines. 2004;3:315–328. doi: 10.1586/14760584.3.3.315. [DOI] [PubMed] [Google Scholar]
- 47.Siddiqui AA, Siddiqui BA, Ganley-Leal L. Schistosomiasis vaccines. Hum Vaccin. 2011;7:1192–1197. doi: 10.4161/hv.7.11.17017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilson RA, Li XH, Castro-Borges W. Do schistosome vaccine trials in mice have an intrinsic flaw that generates spurious protection data? Parasit Vectors. 2016;9:89. doi: 10.1186/s13071-016-1369-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mangold BL, Dean DA. The migration and survival of gamma-irradiated Schistosoma mansoni larvae and the duration of host-parasite contact in relation to the induction of resistance in mice. Parasitology. 1984;88(Pt 2):249–265. doi: 10.1017/S0031182000054512. [DOI] [PubMed] [Google Scholar]
- 50.Bergquist R, Utzinger J, Chollet J, Xiao SH, Weiss NA, Tanner M. Triggering of high-level resistance against Schistosoma mansoni reinfection by artemether in the mouse model. Am J Trop Med Hyg. 2004;71:774–777. doi: 10.4269/ajtmh.2004.71.774. [DOI] [PubMed] [Google Scholar]
- 51.Fukushige M, Mitchell KM, Bourke CD, Woolhouse ME, Mutapi F. A meta-analysis of experimental studies of attenuated Schistosoma mansoni vaccines in the mouse model. Front Immunol. 2015;6:85. doi: 10.3389/fimmu.2015.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Richter D, Reynolds SR, Harn DA. Candidate vaccine antigens that stimulate the cellular immune response of mice vaccinated with irradiated cercariae of Schistosoma mansoni. J Immunol. 1993;151:256–265. [PubMed] [Google Scholar]
- 53.Tebeje BM, Harvie M, You H, Loukas A, McManus DP. Schistosomiasis vaccines: where do we stand? Parasit Vectors. 2016;9:528. doi: 10.1186/s13071-016-1799-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Riveau G, Deplanque D, Remoué F, Schacht AM, Vodougnon H, Capron M, Thiry M, Martial J, Libersa C, Capron A. Safety and immunogenicity of rSh28GST antigen in humans: phase 1 randomized clinical study of a vaccine candidate against urinary schistosomiasis. PLoS Negl Trop Dis. 2012;6:e1704. doi: 10.1371/journal.pntd.0001704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Riveau G, Schacht AM, Dompnier JP, Deplanque D, Seck M, Waucquier N, Senghor S, Delcroix-Genete D, Hermann E, Idris-Khodja N, Levy-Marchal C, Capron M, Capron A. Safety and efficacy of the rSh28GST urinary schistosomiasis vaccine: a phase 3 randomized, controlled trial in Senegalese children. PLoS Negl Trop Dis. 2018;12:e0006968. doi: 10.1371/journal.pntd.0006968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tendler M, Almeida M, Simpson A. Development of the Brazilian anti schistosomiasis vaccine based on the recombinant fatty acid binding protein Sm14 plus GLA-SE adjuvant. Front Immunol. 2015;6:218. doi: 10.3389/fimmu.2015.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Santini-Oliveira M, Coler RN, Parra J, Veloso V, Jayashankar L, Pinto PM, Ciol MA, Bergquist R, Reed SG, Tendler M. Schistosomiasis vaccine candidate Sm14/GLA-SE: phase 1 safety and immunogenicity clinical trial in healthy, male adults. Vaccine. 2016;34:586–594. doi: 10.1016/j.vaccine.2015.10.027. [DOI] [PubMed] [Google Scholar]
- 58.Tendler M, Almeida MS, Vilar MM, Pinto PM, Limaverde-Sousa G. Current status of the Sm14/GLA-SE schistosomiasis vaccine: overcoming barriers and paradigms towards the first anti-parasitic human(itarian) vaccine. Trop Med Infect Dis. 2018;3:121. doi: 10.3390/tropicalmed3040121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Braschi S, Borges WC, Wilson RA. Proteomic analysis of the schistosome tegument and its surface membranes. Mem Inst Oswaldo Cruz. 2006;101(Suppl 1):205–212. doi: 10.1590/S0074-02762006000900032. [DOI] [PubMed] [Google Scholar]
- 60.Loukas A, Tran M, Pearson MS. Schistosome membrane proteins as vaccines. Int J Parasitol. 2007;37:257–263. doi: 10.1016/j.ijpara.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 61.Tran MH, Pearson MS, Bethony JM, Smyth DJ, Jones MK, Duke M, Don TA, McManus DP, Correa-Oliveira R, Loukas A. Tetraspanins on the surface of Schistosoma mansoni are protective antigens against schistosomiasis. Nat Med. 2006;200612:835–840. doi: 10.1038/nm1430. [DOI] [PubMed] [Google Scholar]
- 62.Pearson MS, Becker L, Driguez P, Young ND, Gaze S, Mendes T, Li XH, Doolan DL, Midzi N, Mduluza T, McManus DP, Wilson RA, Bethony JM, Nausch N, Mutapi F, Felgner PL, Loukas A. Of monkeys and men: immunomic profiling of sera from humans and non-human primates resistant to schistosomiasis reveals novel potential vaccine candidates. Front Immunol. 2015;6:213. doi: 10.3389/fimmu.2015.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hotez PJ, Bottazzi ME, Bethony J, Diemert DD. Advancing the development of a human schistosomiasis vaccine. Trends Parasitol. 2019;35:104–108. doi: 10.1016/j.pt.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 64.Keitel WA, Potter GE, Diemert D, Bethony J, El Sahly HM, Kennedy JK, Patel SM, Plieskatt JL, Jones W, Deye G, Bottazzi ME, Hotez PJ, Atmar RL. A phase 1 study of the safety, reactogenicity, and immunogenicity of a Schistosoma mansoni vaccine with or without glucopyranosyl lipid A aqueous formulation (GLA-AF) in healthy adults from a non-endemic area. Vaccine. 2019;37:6500–6509. doi: 10.1016/j.vaccine.2019.08.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Siddiqui AA, Zhou Y, Podesta RB, Karcz SR, Tognon CE, Strejan GH, Dekaban GA, Clarke MW. Characterization of Ca(2+)-dependent neutral protease (calpain) from human blood flukes, Schistosoma mansoni. Biochim Biophys Acta. 1993;1181:37–44. doi: 10.1016/0925-4439(93)90087-H. [DOI] [PubMed] [Google Scholar]
- 66.Zhang W, Molehin AJ, Rojo JU, Sudduth J, Ganapathy PK, Kim E, Siddiqui AJ, Freeborn J, Sennoune SR, May J, Lazarus S, Nguyen C, Redman WK, Ahmad G, Torben W, Karmakar S, Le L, Kottapalli KR, Kottapalli P, Wolf RF, Papin JF, Carey D, Gray SA, Bergthold JD, Damian RT, Mayer BT, Marks F, Reed SG, Carter D, Siddiqui AA. Sm-p80-based schistosomiasis vaccine: double-blind preclinical trial in baboons demonstrates comprehensive prophylactic and parasite transmission-blocking efficacy. Ann N Y Acad Sci. 2018;1425:38–51. doi: 10.1111/nyas.13942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Karmakar S, Zhang W, Ahmad G, Torben W, Alam MU, Le L, Damian RT, Wolf RF, White GL, Carey DW, Carter D, Reed SG, Siddiqui AA. Use of an Sm-p80-based therapeutic vaccine to kill established adult schistosome parasites in chronically infected baboons. J Infect Dis. 2014;209:1929–1940. doi: 10.1093/infdis/jiu031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.You H, Gobert GN, Cai P, Mou R, Nawaratna S, Fang G, Villinger F, McManus DP. Suppression of the insulin receptors in adult Schistosoma japonicum impacts on parasite growth and development: further evidence of vaccine potential. PLoS Negl Trop Dis. 2015;9:e0003730. doi: 10.1371/journal.pntd.0003730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.You H, Cai P, Tebeje B, Li YS, McManus DP. Schistosome vaccines for domestic animals. Trop Med Infect Dis. 2018;3:68. doi: 10.3390/tropicalmed3020068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang LD, Chen HG, Guo JG, Zeng XJ, Hong XL, Xiong JJ, Wu XH, Wang XH, Wang LY, Xia G, Hao Y, Chin DP, Zhou XN. A strategy to control transmission of Schistosoma japonicum in China. N Engl J Med. 2009;360:121–128. doi: 10.1056/NEJMoa0800135. [DOI] [PubMed] [Google Scholar]
- 71.You H, McManus DP. Vaccines and diagnostics for zoonotic schistosomiasis japonica. Parasitology. 2015;142:271–289. doi: 10.1017/S0031182014001310. [DOI] [PubMed] [Google Scholar]
- 72.Capron A. Schistosomiasis: forty years’ war on the worm. Parasitol Today. 1998;14:379–384. doi: 10.1016/S0169-4758(98)01322-2. [DOI] [PubMed] [Google Scholar]
- 73.Jiz MAL, II, Mingala CN, Lopez IFM, Chua M, Gabonada FG, Jr, Acosta LP, Wu H, Kurtis JD. A field trial of recombinant Schistosoma japonicum paramyosin as a potential vaccine in naturally-infected water buffaloes. Ann Parasitol. 2016;62:295–299. doi: 10.17420/ap6204.64. [DOI] [PubMed] [Google Scholar]
- 74.Da’dara AA, Li YS, Xiong T, Zhou J, Williams GM, McManus DP, Feng Z, Yu XL, Gray DJ, Harn DA. DNA-based vaccines protect against zoonotic schistosomiasis in water buffalo. Vaccine. 2008;26:3617–3625. doi: 10.1016/j.vaccine.2008.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gray DJ, Li YS, Williams GM, Zhao ZY, Harn DA, Li SM, Ren MY, Feng Z, Guo FY, Guo JG, Zhou J, Dong YL, Li Y, Ross AG, McManus DP. Multi-component integrated approach for the elimination of schistosomiasis in the Peoples’ Republic of China: design and baseline results of a 4-year cluster-randomised intervention trial. Int J Parasitol. 2014;44:659–668. doi: 10.1016/j.ijpara.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 76.Grenfell RF, Shollenberger LM, Samli EF, Harn DA. Vaccine self-assembling immune matrix is a new delivery platform that enhances immune response to recombinant HBsAg in mice. Clin Vaccine Immunol. 2015;22:336–343. doi: 10.1128/CVI.00714-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Da’Dara AA, Li C, Yu X, Zheng M, Zhou J, Shollenberger LM, Li YS, Harn DA. Prime-boost vaccine regimen for SjTPI and SjC23 schistosome vaccines, increases efficacy in water buffalo in a field trial in China. Front Immunol. 2019;20:10284. doi: 10.3389/fimmu.2019.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Williams GM, Li YS, Gray DJ, Zhao ZY, Harn DA, Shollenberger LM, Li SM, Yu X, Feng Z, Guo JG, Zhou J, Dong YL, Li Y, Guo B, Driguez P, Harvie M, You H, Ross AG, McManus DP. Field testing integrated interventions for schistosomiasis elimination in the People’s Republic of China: outcomes of a multifactorial cluster-randomized controlled trial. Front Immunol. 2019;10:645. doi: 10.3389/fimmu.2019.00645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schistosoma japonicum Genome Sequencing and Functional Analysis Consortium The Schistosoma japonicum genome reveals features of host-parasite interplay. Nature. 2009;460:345–351. doi: 10.1038/nature08140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Berriman M, Haas BJ, LoVerde PT, Wilson RA, Dillon GP, Cerqueira GC, Mashiyama ST, Al-Lazikani B, Andrade LF, Ashton PD, Aslett MA, Bartholomeu DC, Blandin G, Caffrey CR, Coghlan A, Coulson R, Day TA, Delcher A, DeMarco R, Djikeng A, Eyre T, Gamble JA, Ghedin E, Gu Y, Hertz-Fowler C, Hirai H, Hirai Y, Houston R, Ivens A, Johnston DA, Lacerda D, Macedo CD, McVeigh P, Ning Z, Oliveira G, Overington JP, Parkhill J, Pertea M, Pierce RJ, Protasio AV, Quail MA, Rajandream MA, Rogers J, Sajid M, Salzberg SL, Stanke M, Tivey AR, White O, Williams DL, Wortman J, Wu W, Zamanian M, Zerlotini A, Fraser-Liggett CM, Barrell BG, El-Sayed NM. The genome of the blood fluke Schistosoma mansoni. Nature. 2009;460:352–358. doi: 10.1038/nature08160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Young ND, Jex AR, Li B, Liu S, Yang L, Xiong Z, Li Y, Cantacessi C, Hall RS, Xu X, Chen F, Wu X, Zerlotini A, Oliveira G, Hofmann A, Zhang G, Fang X, Kang Y, Campbell BE, Loukas A, Ranganathan S, Rollinson D, Rinaldi G, Brindley PJ, Yang H, Wang J, Wang J, Gasser RB. Whole-genome sequence of Schistosoma haematobium. Nat Genet. 2012;44:221–225. doi: 10.1038/ng.1065. [DOI] [PubMed] [Google Scholar]
- 82.Protasio AV, Tsai IJ, Babbage A, Nichol S, Hunt M, Aslett MA, De Silva N, Velarde GS, Anderson TJ, Clark RC, Davidson C, Dillon GP, Holroyd NE, LoVerde PT, Lloyd C, McQuillan J, Oliveira G, Otto TD, Parker-Manuel SJ, Quail MA, Wilson RA, Zerlotini A, Dunne DW, Berriman M. A systematically improved high quality genome and transcriptome of the human blood fluke Schistosoma mansoni. PLoS Negl Trop Dis. 2012;6:e1455. doi: 10.1371/journal.pntd.0001455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Luo F, Yin M, Mo X, Sun C, Wu Q, Zhu B, Xiang M, Wang J, Wang Y, Li J, Zhang T, Xu B, Zheng H, Feng Z, Hu W. An improved genome assembly of the fluke Schistosoma japonicum. PLoS Negl Trop Dis. 2019;13:e0007612. doi: 10.1371/journal.pntd.0007612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stroehlein AJ, Korhonen PK, Chong TM, Lim YL, Chan KG, Webster B, Rollinson D, Brindley PJ, Gasser RB, Young ND (2019) High-quality Schistosoma haematobium genome achieved by single-molecule and long-range sequencing. Gigascience 8:giz108 [DOI] [PMC free article] [PubMed]
- 85.International Helminth Genomes Consortium Comparative genomics of the major parasitic worms. Nat Genet. 2018;51:163–174. doi: 10.1038/s41588-018-0262-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Driguez P, Doolan DL, Molina DM, Loukas A, Trieu A, Felgner PL, McManus DP. Protein microarrays for parasite antigen discovery. Methods Mol Biol. 2015;1201:221–233.87. doi: 10.1007/978-1-4939-1438-8_13. [DOI] [PubMed] [Google Scholar]
- 87.Cai P, Gobert GN, McManus DP. The Tao survivorship of schistosomes: implications for schistosomiasis control. Int J Parasitol. 2016;46:453–463. doi: 10.1016/j.ijpara.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 88.Driguez P, McManus DP, Gobert GN. Clinical implications of recent findings in schistosome proteomics. Expert Rev Proteomics. 2016;13:19–33. doi: 10.1586/14789450.2016.1116390. [DOI] [PubMed] [Google Scholar]
- 89.Liu R, Cheng WJ, Tang HB, Zhong QP, Ming ZP, Dong HF. Comparative metabonomic investigations of Schistosoma japonicum from SCID mice and BALB/c mice: clues to developmental abnormality of schistosome in the immunodeficient host. Front Microbiol. 2019;10:440. doi: 10.3389/fmicb.2019.00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ittiprasert W, Mann VH, Karinshak SE, Coghlan A, Rinaldi G, Sankaranarayanan G, Chaidee A, Tanno T, Kumkhaek C, Prangtaworn P, Mentink-Kane MJ, Cochran CJ, Driguez P, Holroyd N, Tracey A, Rodpai RH, Everts B, Hokke CH, Hoffmann KF, Berriman M, Brindley PJ. Programmed genome editing of the omega-1 ribonuclease of the blood fluke, Schistosoma mansoni. eLife. 2019;8:e41337. doi: 10.7554/eLife.41337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Smit CH, van Diepen A, Nguyen DL, Wuhrer M, Hoffmann KF, 1417 Deelder AM, Hokke CH (2015) Glycomic analysis of life stages of 1418 the human parasite Schistosoma mansoni reveals developmental 1419 expression profiles of functional and antigenic glycan motifs. Mol 1420 Cell Proteomics 14:1750–1769 [DOI] [PMC free article] [PubMed]
- 92.Winkel BMF, Dalenberg MR, de Korne CM, Feijt C, Langenberg MCC, Pelgrom L, Ganesh MS, Yazdanbakhsh M, Smits HH, de Jong EC, Everts B, van Leeuwen FWB, Hokke CH, Roestenberg M. Early induction of human regulatory dermal antigen presenting cells by skin-penetrating Schistosoma mansoni cercariae. Front Immunol. 2018;9:2510. doi: 10.3389/fimmu.2018.02510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Janse JJ, Langenberg MCC, Kos-Van Oosterhoud J, Ozir-Fazalalikhan A, Brienen EAT, Winkel BMF, Erkens MAA, van der Beek MT, van Lieshout L, Smits HH, Webster BL, Zandvliet ML, Verbeek R, Westra IM, Meij P, Visser LG, van Diepen A, Hokke CH, Yazdanbakhsh M, Roestenberg M. Establishing the production of male Schistosoma mansoni cercariae for a controlled human infection model. J Infect Dis. 2018;218:1142–1146. doi: 10.1093/infdis/jiy275. [DOI] [PubMed] [Google Scholar]
- 94.Darton TC, Blohmke CJ, Moorthy VS, Altmann DM, Hayden FG, Clutterbuck EA, Levine MM, Hill AV, Pollard AJ. Design, recruitment, and microbiological considerations in human challenge studies. Lancet Infect Dis. 2015;15:840–851. doi: 10.1016/S1473-3099(15)00068-7. [DOI] [PubMed] [Google Scholar]
- 95.Roestenberg M, Mo A, Kremsner PG, Yazdanbakhsh M. Controlled human infections: a report from the controlled human infection models workshop, Leiden University Medical Centre 4-6 May 2016. Vaccine. 2017;35:7070–7076. doi: 10.1016/j.vaccine.2017.10.092. [DOI] [PubMed] [Google Scholar]
- 96.Bachmann A, Bruske E, Krumkamp R, Turner L, Wichers JS, Petter M, Held J, Duffy MF, Sim BKL, Hoffman SL, Kremsner PG, Lell B, Lavstsen T, Frank M, Mordmüller B, Tannich E. Controlled human malaria infection with Plasmodium falciparum demonstrates impact of naturally acquired immunity on virulence gene expression. PLoS Pathog. 2019;15:e1007906. doi: 10.1371/journal.ppat.1007906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Corstjens PL, De Dood CJ, Kornelis D, Fat EM, Wilson RA, Kariuki TM, Nyakundi RK, Loverde PT, Abrams WR, Tanke HJ, Van Lieshout L, Deelder AM, Van Dam GJ. Tools for diagnosis, monitoring and screening of Schistosoma infections utilizing lateral-flow based assays and upconverting phosphor labels. Parasitology. 2014;141:1841–55. doi: 10.1017/S0031182014000626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Elliott AM, Roestenberg M, Wajja A, Opio C, Angumya F, Adriko M, Egesa M, Gitome S, Mfutso-Bengo J, Bejon P, Kapulu M, Seager Z, Lutalo T, Nazziwa WB, Muwumuza A, Yazdanbakhsh M, Kaleebu P, Kabatereine N, Tukahebwa E. Ethical and scientific considerations on the establishment of a controlled human infection model for schistosomiasis in Uganda: report of a stakeholders’ meeting held in Entebbe, Uganda. AAS Open Res. 2018;1:2. doi: 10.12688/aasopenres.12841.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Diemert DJ, Pinto AG, Freire J, Jariwala A, Santiago H, Hamilton RG, Periago MV, Loukas A, Tribolet L, Mulvenna J, Correa-Oliveira R, Hotez PJ, Bethony JM. Generalized urticaria induced by the Na-ASP-2 hookworm vaccine: implications for the development of vaccines against helminths. J Allergy Clin Immunol. 2012;130:169–176. doi: 10.1016/j.jaci.2012.04.027. [DOI] [PubMed] [Google Scholar]