Abstract
Purpose of Review
Liver transplantation (LT) remains the only way to cure patients with severe liver diseases. Important questions about neurological sequelae and quality of life after LT have emerged. In this review, we discuss the neurocognitive changes associated with LT and we conclude with recommendations in this regard for patients, caregivers, and physicians.
Recent Findings
Compared with other solid organ recipients, LT patients tend to have a higher incidence (up to 30%) of neurological complications post-LT. Even in absence of previous episodes of hepatic encephalopathy (HE), some patients display new onset of neurological symptoms post-LT, raising the concern about the role of other factors that may have a direct impact on cognitive function.
Summary
Different mechanisms have been postulated to explain these postoperative neurological symptoms. They include sequelae of HE, persistent impairment of cognitive function due to cirrhosis, or postoperative decompensation of an unknown or undiagnosed neurodegenerative disorder.
Keywords: Cognition, Brain function, Cognitive reserve, Cirrhosis, Transplant
Altered Cognition in Cirrhosis Before Transplant
Hepatic encephalopathy (HE) represents a spectrum of neurocognitive impairment in cirrhosis (SONIC) [1]. It constitutes of two major components: overt HE (OHE) and minimal HE (MHE) [2]. OHE can be diagnosed clinically, whereas MHE requires specialized testing. It has been estimated that OHE is present in 30–45% of patients with cirrhosis [3], compared with approximately 60–80% of patients who have evidence of MHE [4]. MHE can potentially progress to OHE and is associated with high mortality, poor quality of life, and a high risk of motor vehicle accidents [5]. HE continues to pose a major healthcare burden on patients, caregivers, and society [6•]. It has been reported that the healthcare costs for patients with HE are likely to increase over the coming years [7]. There are several clinical systems to diagnose the severity of HE [8–10]. However, most of them are subjective and not reproducible because of lack of objective criteria for the clinical diagnosis of HE [11]. The West-Haven criteria are the most widely used; however, they also lack objectivity through the entire spectrum of HE [12]. Other tests that can assist with diagnosing HE are clinical hepatic encephalopathy staging scale (CHESS) [13] and the Hepatic Encephalopathy Scoring Algorithm (HESA) [11] [14]. When making a clinical diagnosis of HE, it is important to exclude other etiologies that can lead to alteration in mental status, and to specifically investigate the cognitive function as well. Deterioration in mental status and in psychometric or neurophysiological function are the two levels of cognitive function that need to be acknowledged given the fact that clinical exam can diagnose mental status changes only.
Factors That Impair Cognitive Function
There are many factors that can precipitate HE in cirrhosis patients, which include infections, GI bleeding, electrolyte disorder, constipation, and diuretic overdose. Patients with alcohol-related cirrhosis exhibit more severe neurocognitive changes and more cortical lesions post-transjugular intrahepatic portosystemic shunt (TIPS) placement based on brain MRI compared with individuals with cirrhosis from other etiologies [15••]. Recent data suggest that patients with non-alcoholic steatohepatitis (NASH) are at risk for premature brain aging due to the presence of early cerebral atrophy [16]. Large studies have shown an association between hepatitis C virus (HCV) infection and increased risk of neurodegenerative disorders [17]. Global brain reserve is a term that describes a combination of structural changes (brain reserve) and the individual’s ability to endure alterations (cognitive reserve) [18]. Changes in global reserve have been shown to impact the progression of brain diseases such as dementia and multiple sclerosis [19, 20]. Cognitive reserve reflects the dynamic neuroadaptation that derives from socio-economic and educational factors. It has been shown to modulate the impact of brain disease or neurocognitive insults such as HE [21••]. In contrast to cognitive reserve, brain reserve is less adaptable and is dependent on disease etiology, severity, and progression [19]. The capacity of brain reserve was reported to be implicated in the lack of direct correlation between the clinical manifestations of brain diseases and the objective findings obtained by imaging or neurophysiological tests [22]. Variables such as education level [23], psychometrical intelligence [24], leisure time activities [25], and lifestyle [26] determine the brain’s ability to cope with damage. The expression of HE phenotype is determined by two opposing forces: cognitive reserve (resilience factor) and neuropsychiatric comorbidity (precipitating factor). It has been reported that patients with cirrhosis and higher cognitive reserve, regardless of degree of liver failure, have better quality of life [21••, 27]. The Barona Index is a validated IQ analysis which consists of age, sex, race, education, occupation, residence type (urban or rural), and region of residence (locations within the USA) [28]. It is an optimal method for testing pre-morbid intelligence that follows subject cognitive performance [29]. The Barona IQ is divided into the verbal IQ (VIQ), performance IQ (PIQ), and the full scale IQ (FSIQ). High cognitive reserve is defined as FSIQ ≥ 109 while lower than 109 is considered average cognitive reserve [30]. Cognitive Reserve Index questionnaire (CRIq) is another tool that produces CRI which reflects the amount of cognitive reserve based on educational level, work activities, and frequency of leisure time activities [31]. CRIq is different from the Barona Index because it measures long-life activities that modulate cognitive skills, and can be complimentary to the Barona Index in correlating between IQ and health-related quality of life in patients with cirrhosis [21••].
Changes in Brain Function After Transplant
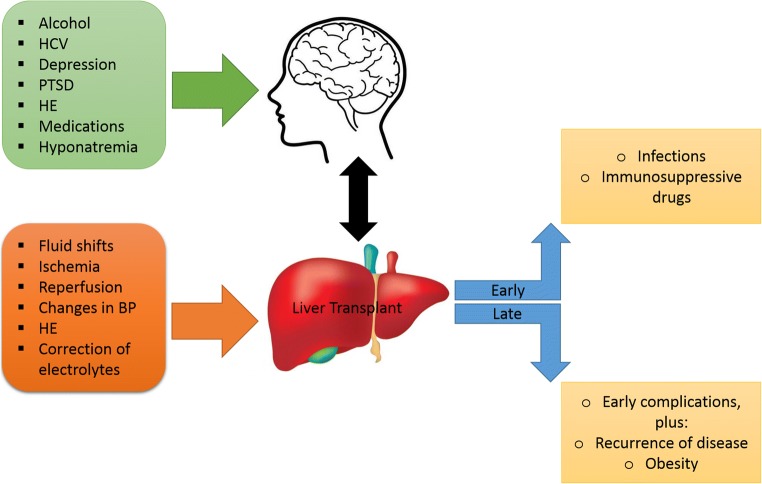
Liver transplantation (LT) remains the gold standard treatment for HE refractory to other treatments, but it carries risks. It has been shown that manifestations of HE are not completely fully reversible by transplantation as it may induce impairment of brain function [32]. The development of postoperative confusional syndrome is a problem of considerable magnitude due to the difficulty in finding the underlying etiology of alteration in mental status, and the likelihood of presence of multiple causes (Fig. 1). Patients with recurrent HE before transplantation and those with alcoholic liver disease (ALD) are at higher risk. There is a debate in the presence of HE sequelae after LT even if HE symptoms disappear [33–35]. This is because several data suggest that LT reverses the metabolic component of HE, but the structural component may persist [36••]. The hypotheses behind this assumption are as follows: hyperammonemia from decompensation due to urea cycle defects can lead to long-term neurological sequelae [34]; correlation between neurological impairment and history of previous episodes of HE [37, 38]. In contrast, some studies report that HE is totally reversible and that what is thought to be HE sequelae is a manifestation of competitive brain injuries such as from hyperammonemia [39]. Many LT patients have risk factors for neurocognitive changes such as age, alcoholism, and metabolic syndrome, regardless of previous bouts of HE [15••, 16]. An example of a causative factor for post-LT confusion is immunosuppressant drugs because they frequently have toxic effects especially if their blood levels are elevated. Diagnosis of adverse cerebral effects of drugs may be difficult. Infections (bacterial or viral) can result in confusion associated with fever, which requires a diligent workup. In general, altered mental status post-transplant must be approached from a broad clinical view since the etiology is usually multifactorial. The most frequent neurological complications of LT include seizures, stroke, central nervous system (CNS) infections, ICU-acquired weakness, and neurocognitive complications (due to decompensation of previous altered cerebral condition, general anesthesia, surgical procedure, or anticalcineurin toxicity). Factors that can indirectly lead to neurological complications include early graft function impairment, drugs, renal function impairment, sepsis, and altered ammonia clearance [40]. Some patients who have no history of cerebral impairment or previous evidence of HE can develop neurological impairment after LT. It has been suggested that possible causative factors include adverse effects of general anesthesia, the surgery itself, or postoperative ICU stay [41–45]. There are several causes of ICU admission that are implicated in long-term neurocognitive impairment such as sepsis or acute respiratory distress syndrome [41–43, 46]. Postoperative cognitive dysfunction has been recognized as a common problem that is independently associated with increased mortality outside the scope of LT [47]. Systemic inflammation has been hypothesized to be a pathophysiological mechanism explaining postoperative cognitive dysfunction or postoperative delirium [44]. The additional inflammation associated with the LT surgery worsens the pre-existing systemic inflammation in acute liver failure (ALF) and cirrhosis. Additional pre-LT risk factors that have been reported include duration of surgery, infections, and mechanical ventilation [48]. Evidence suggests that patients with cirrhosis tend to demonstrate long-lasting cognitive impairment after infection [49]. Other causes for cerebral impairment are the changes in cerebral blood flow and systemic hemodynamics.
Fig. 1.
Factors that affect the cognitive function before and after liver transplant
Long-Term and Short-Term Changes in Cognition
HE is presumed to be largely reversed after successful LT [50]. Data on long-term cognitive changes post-LT are variable (Table 1). Some studies have shown a continued improvement in cognitive function, while others have reported the contrary [10, 62•]. These neurocognitive deficits are assessed by extensive psychometric neurocognitive testing and have been confirmed by EEG, different MRI techniques, and positron emission tomography (PET) scanning [52, 62•]. MRI studies that use standard sequences, magnetic resonance spectroscopy (MRS), diffusion tensor imaging (DTI), and functional MRI are capable of showing changes in cerebral abnormalities before and after LT [51–53, 55]. Only a few studies have investigated the role of multi-modal brain MRI changes over a longer time period and reported conflicting results from longitudinal analyses of these MRI changes [32, 52, 65]. A study analyzed changes in magnetization transfer ratio (marker for brain edema) and MRS, and reported a progressive improvement in both markers between pre-LT, 1 month post-LT, and 1 year post-LT without continued improvement in cognition at 1 year post-LT [62•]. Another study showed improved extracellular cerebral edema and cognitive tests, but also possibly advanced white matter demyelination in the temporal lobe, 6 to 12 months after transplant [65]. It has been reported that there is a significant improvement in cognitive performance and health-related quality of life (HRQOL) at 6 months post-LT, accompanied by a significant improvement in white matter integrity and astrocytic consequences of hyperammonemia on multi-modal MRI [63]. Despite limited data from DTI, it appears that mean diffusivity (MD) decreases after LT, suggesting reversal of vasogenic edema with the improvement of liver function [36••, 59, 61]. It has been reported that MRS peaks can normalize as early as 3 months after LT [52]. Less is known about the results from functional MRI; however, improvement in cerebral function after LT was suggested [61, 66–70]. On the other hand, there is evidence reporting reduction in brain volume and a decrease in the MRS N-acetylaspartate (NAA)/creatinine (Cr) ratio which suggest the presence of structural brain changes and atrophy [32].
Table 1.
Studies on cognitive changes after LT
| References (authors, year) | Patient population | Time period pre-LT | Time period post-LT | Methods | Findings |
|---|---|---|---|---|---|
| Weissenborn et al. [51], 1995 | 50 cirrhotic patients | 3 months | 3 months | 1T MRI scanner, T1, T2 |
*Bilateral T1 hypersignals of the globus pallidus before LT; absent 3 months after LT *T2 hypersignals in the corticospinal tract (18% of the patients) *No correlation between the T1 signal intensity and liver function, neurological status, or grade of encephalopathy |
| Córdoba et al. [52], 2001 | 24 cirrhotic patients | 1.5T MRI scanner, magnetization transfer imaging, 1H-MRS |
*Bilateral T1 hypersignals of the globus pallidus still present at 1 month after LT but normalized at 1 year *MTR increase in frontal and parietal lobes |
||
| Rovira et al. [53], 2002 | 24 cirrhotic patients | 12 months | 1 month and 1 year | 1.5T MRI scanner, T2, Fast-FLAIR, magnetization transfer imaging | *White matter T2 hypersignals in the corticospinal tract decrease after LT |
| Rovira et al. [54], 2007 | 27 cirrhotic patients | 6 to 14 months | 6 to 14 months | 1.5T MRI scanner, T2, Fast-FLAIR |
*White matter (WM) T2 hypersignals decrease after LT *WM lesions were more common in cirrhosis of viral Etiology |
| García Martínez et al. [55], 2010 | 22 cirrhotic patients | 6–12 months and 6–9 years | 6–12 months and 6–9 years | 1.5T MRI scanner, T2, Fast-FLAIR |
*WM T2 lesions decrease, particularly in patients with previous bouts of HE before LT *Brain atrophy estimated at 8% decrease compared with before LT in short-term assessment and at 22% decrease in long-term assessment |
| Pegum et al. [56], 2011 | 92 abstinent patients with ALD | NS | 12 months | Psychometric tests (WAIS-R, WAIS-III, TMT-A and TMT-B, WMS, RCFT) | *Overall improvement in cognitive function occurs after liver transplantation in ALD |
| Garcia-Martinez et al. [32], 2011 | 52 cirrhotic patients | 2 months | 6–12 months | Psychometric tests (RAVLT, TMT-A, SDMT, GPT, COWAT, HVOT, JLO) | *The association of HE with cognitive function and brain volume suggests that having experienced HE before LT impairs the post-LT neurological outcome |
| Garcia-Martinez et al. [32], 2011 | 24 cirrhotic patients | NS | 6–12 months | 1.5T MRI scanner, 1H-MRS |
*Reduced brain volume in older patients, alcohol use, prior HE, and increased time from first episode of HE *Age-related decrease in brain volume was higher in patients with prior HE |
| Mattarozzi et al. [57], 2012 | 12 cirrhotic patients | NS | 7–10 years | Psychometric tests (VM, COAT, TMT-A, and TMT-B, SCT, digit span, Corsi test, VMT, RWIR, RWDR, brief story, PAL, supraspan learning, RCFRT, FAS, PC, Pcopy, PCopy, RCFT, DSST; Elithorn maze test) | *The improvements obtained in the first 2 years after LT remain stable during the 7 to 10 years thereafter, indicating long-term improvement in cognitive function after LT |
| Li et al. [58], 2013 | 25 cirrhotic patients | NS | 1 week | Psychometric tests (MMSE, VFT, DSTs, item memory, source memory (SM)) |
*Incidence of POCD in LT patients was greater than that reported in other surgical patients *There was increase in the serum biomarkers of dementia in the POCD patients |
| Ishihara at al. [59], 2013 | 12 cirrhotic patients | Just before LT | 6 months | Psychometric tests (MMSE, TMT-A and TMT-B, SCT, DSST, digit span, visual reproduction (plus delayed recall)) | *End-stage cirrhotic patients without clinical evidence of HE showed increased MD and decreased FA values in both frontal and temporal lobes. These parameters improved after LT, in line with cognitive function |
| Tryc et al. [60•], 2014 | 81 cirrhotic patients | 6 months | 6 and 12 months | Psychometric tests (PHES, ICT, CFF, RBANS) |
*1 year after LT, cognitive dysfunctions in LT patients are not residual symptoms but instead are new-onset cognitive disturbances *Cognitive deficits are linked to a decline in health-related quality of life |
| Lin et al. [61], 2014 | 28 cirrhotic patients 30 controls | NS | 6–12 months | Psychometric tests (WAIS-III, CASI, Wisconsin Card Sorting Test) |
*Improvement of the extracellular cerebral edema and of the demyelination of WM in MHE patients after LT *DTI may be useful for investigating the pathogenesis of MHE |
| Campagna et al. [62•], 2014 | 65 cirrhotic patients | NS | 3, 6, 9, and 12 months | Psychometric tests (TMT-A and TMT-B, digit span, VFT, DSST, memory with interference task at 10 s and at 30 s, immediate and delayed story recall memory) |
*Both neuropsychological and EEG performances had significantly improved 1 year after LT *Patients with a history of OHE showed greater improvements after LT than patients with a negative history, but their global cognitive function remained slightly worse; in contrast, EEGs normalized in both groups |
| Ahluwalia et al. [63], 2016 | 43 cirrhotic patients | 6 months | 6 months | 1.5T MRI scanner, T1, T2 | *No abnormalities on standard weighted sequences |
| Pflugrad et al. [64], 2017 | 85 patients who underwent LT | 12 months | 12 months | 3T MRI scanner, T1, T2 |
*Higher WM hypersignals in patients under CNI but no brain atrophy *Patients had more brain atrophy than controls *Patients under CNI had lower neuropsychological performance |
RBANS, repeatable battery for the assessment of neuropsychological status; WAIS-III, Wechsler Adult Intelligence Scale, Third Edition; CASI, Cognitive Ability Screening Instrument; VFT, verbal fluency test; TMT-A, Trail Making Test, A; TMT-B, Trail Making Test, B; DSTs, Digit Span Forward and Digit Span Backward tests of the Wechsler Adult Intelligence Scale; DSST, digit symbol substitution test; WAIS-R, Wechsler Adult Intelligence Scale, Revised; NS, not stated; ND, not done; RAVLT, Rey auditory verbal learning test; COWAT, Controlled Oral Word Association Test; JLO, Judgment of Line Orientation; SDMT, Symbol digit modalities test; RCFT, Rey–Osterrieth Complex Figure Recall Trial; GPT, Grooved Pegboard test; HVOT, Hooper Visual Organization Test; WMS, Wechler Memory Scale; SCT, Stroop color test; RBMT, Rivermead Behavioral Memory Test; SRT, simple reaction time; CRT, choice reaction time; NART, National Adult Reading Test; COAT, crossout a test; VM, visual matrices; VMT, Immediate Visual Memory Test; RWIR, Rey Auditory Verbal Learning Test immediate; RWDR, Rey Auditory Verbal Learning Test 15-min delay recall; PAL, paired associate learning; PC, Phrase Construction; FAS, word fluency; PCopy, Painting Copy; PCopyF, Painting Copy with Facilities; RCFC, Rey–Osterrieth Complex Figure Copy; WM, white matter; MHE, minimal hepatic encephalopathy; OHE, overt hepatic encephalopathy; POCD, postoperative cognitive dysfunction; ALD, alcoholic liver disease; CNI, calcineurin inhibitor
What Are the Expectations?
There are practice guidelines that have been published for the management of transplant candidates [71, 72]. HE alone is not considered an indication for LT unless associated with poor liver function. However, exceptions exist where cases may be candidates for LT even though liver status is good if there is severe compromise to the patient’s quality of life and lack of improvement despite maximal medical therapy. Due to the fact that neurocognitive impairment may occur or persist even after LT, it is highly recommended to evaluate for presence of shunts and consider embolization before or during transplantation [73]. Furthermore, during transplant evaluation, severe hyponatremia should be corrected slowly. Based on evidence from published date, LT is expected to improve HE but not neurodegenerative disorders. Therefore, it is important to distinguish HE from other causes of neurocognitive impairment such as Alzheimer’s disease and small-vessel cerebrovascular disease. Brain MRI and MRS should be performed, and the patient should be evaluated by a neuropsychology specialist and an expert in neurodegenerative diseases [74]. Patients and caregivers, especially in patients with prior HE and cognitive dysfunction, need to be counseled prior to listing about the expectations for recovery of brain function in the short-term and long-term course post-LT extensively. There are certain subgroups in which complete recovery may take decades, and in some subgroups, it may remain impaired. In some patients, there may even be new-onset cognitive dysfunction after LT. However, in most cases, these changes are manageable and do not interfere with daily function and independence post-LT. Regardless, pre-transplant counseling should involve a discussion regarding cognitive and functional expectations of the family and patients after liver transplant.
Compliance with Ethical Standards
Conflict of Interest
Dr. Albhaisi has no conflict of interest. Dr. Bajaj has served on Advisory boards for Norgine and Merz and his institution received research grants from Grifols and Valeant Pharmaceuticals.
Footnotes
This article is part of the Topical Collection on Frailty and Gerontology
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Somaya A. M. Albhaisi, Email: somaya.albhaisi@vcuhealth.org
Jasmohan S. Bajaj, Email: jasmohan.bajaj@vcuhealth.org
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Kappus MR, Bajaj JS. Covert hepatic encephalopathy: not as minimal as you might think. Clin Gastroenterol Hepatol. 2012;10:1208–1219. doi: 10.1016/j.cgh.2012.05.026. [DOI] [PubMed] [Google Scholar]
- 2.Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy--definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716–721. doi: 10.1053/jhep.2002.31250. [DOI] [PubMed] [Google Scholar]
- 3.Poordad FF. Review article: the burden of hepatic encephalopathy. Aliment Pharmacol Ther 2007;25 Suppl 1:3–9. [DOI] [PubMed]
- 4.Ortiz M, Jacas C, Córdoba J. Minimal hepatic encephalopathy: diagnosis, clinical significance and recommendations. J Hepatol. 2005;42 Suppl:S45–S53. [DOI] [PubMed]
- 5.Bajaj JS, Hafeezullah M, Franco J, Varma RR, Hoffmann RG, Knox JF, et al. Inhibitory control test for the diagnosis of minimal hepatic encephalopathy. Gastroenterology. 2008;135:1591–1600.e1. doi: 10.1053/j.gastro.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 6.Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, et al. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715–735. doi: 10.1002/hep.27210. [DOI] [PubMed] [Google Scholar]
- 7.Bajaj JS. Review article: the modern management of hepatic encephalopathy. Aliment Pharmacol Ther. 2010;31:537–547. doi: 10.1111/j.1365-2036.2009.04211.x. [DOI] [PubMed] [Google Scholar]
- 8.Parsons-Smith BG, Summerskill WH, Dawson AM, Sherlock S. The electroencephalograph in liver disease. Lancet. 1957;273:867–871. doi: 10.1016/s0140-6736(57)90005-3. [DOI] [PubMed] [Google Scholar]
- 9.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 10.Sotil EU, Gottstein J, Ayala E, Randolph C, Blei AT. Impact of preoperative overt hepatic encephalopathy on neurocognitive function after liver transplantation. Liver Transpl. 2009;15:184–192. doi: 10.1002/lt.21593. [DOI] [PubMed] [Google Scholar]
- 11.Hassanein T, Blei AT, Perry W, Hilsabeck R, Stange J, Larsen FS, Brown RS Jr, Caldwell S, McGuire B, Nevens F, Fontana R. Performance of the hepatic encephalopathy scoring algorithm in a clinical trial of patients with cirrhosis and severe hepatic encephalopathy. Am J Gastroenterol. 2009;104:1392–1400. doi: 10.1038/ajg.2009.160. [DOI] [PubMed] [Google Scholar]
- 12.Bajaj JS, Hafeezullah M, Zadvornova Y, Martin E, Schubert CM, Gibson DP, et al. The effect of fatigue on driving skills in patients with hepatic encephalopathy. Am J Gastroenterol. 2009;104:898–905. doi: 10.1038/ajg.2009.7. [DOI] [PubMed] [Google Scholar]
- 13.Ortiz M, Córdoba J, Doval E, Jacas C, Pujadas F, Esteban R, Guardia J. Development of a clinical hepatic encephalopathy staging scale. Aliment Pharmacol Ther. 2007;26:859–867. doi: 10.1111/j.1365-2036.2007.03394.x. [DOI] [PubMed] [Google Scholar]
- 14.Perry W, Hilsabeck RC, Hassanein TI. Cognitive dysfunction in chronic hepatitis C: a review. Dig Dis Sci. 2008;53:307–321. doi: 10.1007/s10620-007-9896-z. [DOI] [PubMed] [Google Scholar]
- 15.Ahluwalia V, Wade JB, Moeller FG, White MB, Unser AB, Gavis EA, et al. The etiology of cirrhosis is a strong determinant of brain reserve: a multimodal magnetic resonance imaging study. Liver Transpl. 2015;21:1123–1132. doi: 10.1002/lt.24163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weinstein G, Zelber-Sagi S, Preis SR, Beiser AS, DeCarli C, Speliotes EK, Satizabal CL, Vasan RS, Seshadri S. Association of nonalcoholic fatty liver disease with lower brain volume in healthy middle-aged adults in the Framingham study. JAMA Neurol. 2018;75:97–104. doi: 10.1001/jamaneurol.2017.3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wijarnpreecha K, Chesdachai S, Jaruvongvanich V, Ungprasert P. Hepatitis C virus infection and risk of Parkinson’s disease: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2018;30:9–13. doi: 10.1097/MEG.0000000000000991. [DOI] [PubMed] [Google Scholar]
- 18.Fratiglioni L, Wang H-X. Brain reserve hypothesis in dementia. J Alzheimers Dis. 2007;12:11–22. doi: 10.3233/jad-2007-12103. [DOI] [PubMed] [Google Scholar]
- 19.Stern Y. Cognitive reserve and Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20:S69–S74. doi: 10.1097/00002093-200607001-00010. [DOI] [PubMed] [Google Scholar]
- 20.Sumowski JF, Chiaravalloti N, Krch D, Paxton J, Deluca J. Education attenuates the negative impact of traumatic brain injury on cognitive status. Arch Phys Med Rehabil. 2013;94:2562–2564. doi: 10.1016/j.apmr.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 21.Patel AV, Wade JB, Thacker LR, Sterling RK, Siddiqui MS, Stravitz RT, et al. Cognitive reserve is a determinant of health-related quality of life in patients with cirrhosis, independent of covert hepatic encephalopathy and model for end-stage liver disease score. Clin Gastroenterol Hepatol. 2015;13:987–991. doi: 10.1016/j.cgh.2014.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katzman R, Aronson M, Fuld P, Kawas C, Brown T, Morgenstern H, Frishman W, Gidez L, Eder H, Ooi WL. Development of dementing illnesses in an 80-year-old volunteer cohort. Ann Neurol. 1989;25:317–324. doi: 10.1002/ana.410250402. [DOI] [PubMed] [Google Scholar]
- 23.Stern Y, Alexander GE, Prohovnik I, Mayeux R. Inverse relationship between education and parietotemporal perfusion deficit in Alzheimer’s disease. Ann Neurol. 1992;32:371–375. doi: 10.1002/ana.410320311. [DOI] [PubMed] [Google Scholar]
- 24.Alexander GE, Furey ML, Grady CL, Pietrini P, Brady DR, Mentis MJ, Schapiro MB. Association of premorbid intellectual function with cerebral metabolism in Alzheimer’s disease: implications for the cognitive reserve hypothesis. Am J Psychiatry. 1997;154:165–172. doi: 10.1176/ajp.154.2.165. [DOI] [PubMed] [Google Scholar]
- 25.pubmeddev, al VJ et. Leisure activities and the risk of dementia in the elderly. - PubMed - NCBI [Internet]. [cited 2020 Feb 11];Available from: https://www.ncbi.nlm.nih.gov/pubmed/12815136
- 26.Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R. Influence of education and occupation on the incidence of Alzheimer’s disease. JAMA. 1994;271:1004–1010. [PubMed] [Google Scholar]
- 27.Montagnese S, Cona G, Schiff S, Maresio G, Gatta A, Merkel C, Amodio P. The hunter and the pianist: two hepatic encephalopathy tales. J Clin Gastroenterol. 2011;45:563–566. doi: 10.1097/MCG.0b013e3181eab73e. [DOI] [PubMed] [Google Scholar]
- 28.Barona A, Reynolds CR, Chastain R. A demographically based index of premorbid intelligence for the WAIS—R. J Consult Clin Psychol. 1984;52:885–887. [Google Scholar]
- 29.Comparison of NART and Barona demographic equation premorbid IQ estimates in Alzheimer’s disease. - Abstract - Europe PMC [Internet]. [cited 2020 Feb 11];Available from: https://europepmc.org/article/med/9356900 [DOI] [PubMed]
- 30.Starkey NJ, Halliday T. Development of the New Zealand Adult Reading Test (NZART): preliminary findings. 2011.
- 31.Nucci M, Mapelli D, Mondini S. Cognitive Reserve Index questionnaire (CRIq): a new instrument for measuring cognitive reserve. Aging Clin Exp Res. 2012;24:218–226. doi: 10.3275/7800. [DOI] [PubMed] [Google Scholar]
- 32.Garcia-Martinez R, Rovira A, Alonso J, Jacas C, Simón-Talero M, Chavarria L, Vargas V, Córdoba J. Hepatic encephalopathy is associated with posttransplant cognitive function and brain volume. Liver Transpl. 2011;17:38–46. doi: 10.1002/lt.22197. [DOI] [PubMed] [Google Scholar]
- 33.Amodio P, Biancardi A, Montagnese S, Angeli P, Iannizzi P, Cillo U, D’Amico D, Gatta A. Neurological complications after orthotopic liver transplantation. Dig Liver Dis. 2007;39:740–747. doi: 10.1016/j.dld.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 34.Romero-Gómez M, Montagnese S, Jalan R. Hepatic encephalopathy in patients with acute decompensation of cirrhosis and acute-on-chronic liver failure. J Hepatol. 2015;62:437–447. doi: 10.1016/j.jhep.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 35.Weiss N, Jalan R, Thabut D. Understanding hepatic encephalopathy. Intensive Care Med. 2018;44:231–234. doi: 10.1007/s00134-017-4845-6. [DOI] [PubMed] [Google Scholar]
- 36.•• Chavarria L, Alonso J, García-Martínez R, Aymerich FX, Huerga E, Jacas C, et al. Biexponential analysis of diffusion-tensor imaging of the brain in patients with cirrhosis before and after liver transplantation. Am J Neuroradiol [Internet]. 2011 [cited 2020 Feb 13];Available from: http://www.ajnr.org/content/early/2011/06/23/ajnr.A2533. Study of MRI changes before and after liver transplant from a diffusion tensor imaging perspective. [DOI] [PMC free article] [PubMed]
- 37.Allampati S, Mullen KD. Does overt hepatic encephalopathy cause persistent cognitive defects even after successful liver transplantation? Liver Transpl. 2014;20:874–875. doi: 10.1002/lt.23938. [DOI] [PubMed] [Google Scholar]
- 38.You DD, Choi GS, Kim JM, Kwon CHD, Joh J-W, Lee S-K. Long-term outcomes for liver transplant recipients in terms of hepatic encephalopathy. Transplant Proc. 2017;49:1425–1429. doi: 10.1016/j.transproceed.2017.02.054. [DOI] [PubMed] [Google Scholar]
- 39.Bjerring PN, Bjerrum EJ, Larsen FS. Impaired cerebral microcirculation induced by ammonium chloride in rats is due to cortical adenosine release. J Hepatol. 2018;68:1137–1143. doi: 10.1016/j.jhep.2018.01.034. [DOI] [PubMed] [Google Scholar]
- 40.Weiss N, Thabut D. Neurological complications occurring after liver transplantation: role of risk factors, hepatic encephalopathy, and acute (on chronic) brain injury. Liver Transpl. 2019;25:469–487. doi: 10.1002/lt.25420. [DOI] [PubMed] [Google Scholar]
- 41.Herridge MS. Long-term outcomes after critical illness. Curr Opin Crit Care. 2002;8:331–336. doi: 10.1097/00075198-200208000-00010. [DOI] [PubMed] [Google Scholar]
- 42.Ely EW, Pandharipande PP. The evolving approach to brain dysfunction in critically ill patients. JAMA. 2016;315:1455–1456. doi: 10.1001/jama.2016.2708. [DOI] [PubMed] [Google Scholar]
- 43.Pandharipande PP, Girard TD, Jackson JC, Morandi A, Thompson JL, Pun BT, Brummel NE, Hughes CG, Vasilevskis EE, Shintani AK, Moons KG, Geevarghese SK, Canonico A, Hopkins RO, Bernard GR, Dittus RS, Ely EW, BRAIN-ICU Study Investigators Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369:1306–1316. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sonneville R, Verdonk F, Rauturier C, Klein IF, Wolff M, Annane D, et al. Understanding brain dysfunction in sepsis. Ann Intensive Care. 2013;3:15. doi: 10.1186/2110-5820-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Monk TG, Weldon BC, Garvan CW, Dede DE, van der Aa MT, Heilman KM, Gravenstein JS. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108:18–30. doi: 10.1097/01.anes.0000296071.19434.1e. [DOI] [PubMed] [Google Scholar]
- 46.Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz-Granados N, Cooper A, Guest CB, Mazer CD, Mehta S, Stewart TE, Kudlow P, Cook D, Slutsky AS, Cheung AM, Canadian Critical Care Trials Group Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 47.Newman S, Stygall J, Hirani S, Shaefi S, Maze M. Postoperative cognitive dysfunction after noncardiac surgery: a systematic review. Anesthesiology. 2007;106:572–590. doi: 10.1097/00000542-200703000-00023. [DOI] [PubMed] [Google Scholar]
- 48.Aceto P, Perilli V, Lai C, Ciocchetti P, Vitale F, Sollazzi L. Postoperative cognitive dysfunction after liver transplantation. Gen Hosp Psychiatry. 2015;37:109–115. doi: 10.1016/j.genhosppsych.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 49.Merli M, Lucidi C, Pentassuglio I, Giannelli V, Giusto M, Di Gregorio V, et al. Increased risk of cognitive impairment in cirrhotic patients with bacterial infections. J Hepatol. 2013;59:243–250. doi: 10.1016/j.jhep.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 50.Campagna F, Biancardi A, Cillo U, Gatta A, Amodio P. Neurocognitive-neurological complications of liver transplantation: a review. Metab Brain Dis. 2010;25:115–124. doi: 10.1007/s11011-010-9183-0. [DOI] [PubMed] [Google Scholar]
- 51.Weissenborn K, Ehrenheim C, Hori A, Kubicka S, Manns MP. Pallidal lesions in patients with liver cirrhosis: clinical and MRI evaluation. Metab Brain Dis. 1995;10:219–231. doi: 10.1007/BF02081027. [DOI] [PubMed] [Google Scholar]
- 52.Córdoba J, Alonso J, Rovira A, Jacas C, Sanpedro F, Castells L, Vargas V, Margarit C, Kulisewsky J, Esteban R, Guardia J. The development of low-grade cerebral edema in cirrhosis is supported by the evolution of (1)H-magnetic resonance abnormalities after liver transplantation. J Hepatol. 2001;35:598–604. doi: 10.1016/s0168-8278(01)00181-7. [DOI] [PubMed] [Google Scholar]
- 53.Rovira A, Córdoba J, Sanpedro F, Grivé E, Rovira-Gols A, Alonso J. Normalization of T2 signal abnormalities in hemispheric white matter with liver transplant. Neurology. 2002;59:335–341. doi: 10.1212/wnl.59.3.335. [DOI] [PubMed] [Google Scholar]
- 54.Rovira A, Mínguez B, Aymerich FX, Jacas C, Huerga E, Córdoba J, Alonso J. Decreased white matter lesion volume and improved cognitive function after liver transplantation. Hepatology. 2007;46:1485–1490. doi: 10.1002/hep.21911. [DOI] [PubMed] [Google Scholar]
- 55.García Martínez R, Rovira A, Alonso J, Aymerich FX, Huerga E, Jacas C, Simón-Talero M, Vargas V, Córdoba J. A long-term study of changes in the volume of brain ventricles and white matter lesions after successful liver transplantation. Transplantation. 2010;89:589–594. doi: 10.1097/TP.0b013e3181ca7bb3. [DOI] [PubMed] [Google Scholar]
- 56.Pegum N, Connor JP, Feeney GFX, Young RM. Neuropsychological functioning in patients with alcohol-related liver disease before and after liver transplantation. [Miscellaneous Article] Transplantation. 2011;92:1371–1377. doi: 10.1097/TP.0b013e3182375881. [DOI] [PubMed] [Google Scholar]
- 57.Mattarozzi K, Cretella L, Guarino M, Stracciari A. Minimal hepatic encephalopathy: follow-up 10 years after successful liver transplantation. Transplantation. 2012;93:639–643. doi: 10.1097/TP.0b013e318244f734. [DOI] [PubMed] [Google Scholar]
- 58.Li X, Wen D-X, Zhao Y-H, Hang Y-N, Mandell MS. Increase of beta-amyloid and C-reactive protein in liver transplant recipients with postoperative cognitive dysfunction. HBPD INT. 2013;12:370–376. doi: 10.1016/s1499-3872(13)60058-2. [DOI] [PubMed] [Google Scholar]
- 59.Ishihara T, Ito M, Niimi Y, Tsujimoto M, Senda J, Kawai Y, Watanabe H, Ishigami M, Ito T, Kamei H, Onishi Y, Nakamura T, Goto H, Naganawa S, Kiuchi T, Sobue G. Clinical and radiological impact of liver transplantation for brain in cirrhosis patients without hepatic encephalopathy. Clin Neurol Neurosurg. 2013;115:2341–2347. doi: 10.1016/j.clineuro.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 60.Tryc AB, Pflugrad H, Goldbecker A, Barg-Hock H, Strassburg CP, Hecker H, et al. New-onset cognitive dysfunction impairs the quality of life in patients after liver transplantation. Liver Transpl. 2014;20:807–814. doi: 10.1002/lt.23887. [DOI] [PubMed] [Google Scholar]
- 61.Lin W-C, Chou K-H, Chen C-L, Chen H-L, Lu C-H, Li S-H, et al. Longitudinal brain white matter alterations in minimal hepatic encephalopathy before and after liver transplantation. PLoS One. 2014;9:e105887. doi: 10.1371/journal.pone.0105887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Campagna F, Montagnese S, Schiff S, Biancardi A, Mapelli D, Angeli P, et al. Cognitive impairment and electroencephalographic alterations before and after liver transplantation: what is reversible? Liver Transpl. 2014;20:977–986. doi: 10.1002/lt.23909. [DOI] [PubMed] [Google Scholar]
- 63.Ahluwalia V, Wade JB, White MB, Gilles HS, Heuman DM, Fuchs M, Gavis EA, Fagan A, Tinsley F, Ganapathy D, Thacker LR, Sterling RK, Stravitz RT, Puri P, Sanyal AJ, Siddiqui MS, Matherly S, Luketic V, Steinberg J, Moeller FG, Bajaj JS. Liver transplantation significantly improves global functioning and cerebral processing. Liver Transpl. 2016;22:1379–1390. doi: 10.1002/lt.24498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pflugrad H, Tryc AB, Goldbecker A, Strassburg CP, Barg-Hock H, Klempnauer J, Weissenborn K. Hepatic encephalopathy before and neurological complications after liver transplantation have no impact on the employment status 1 year after transplantation. World J Hepatol. 2017;9:519–532. doi: 10.4254/wjh.v9.i10.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tranah TH, Vijay GKM, Ryan JM, Shawcross DL. Systemic inflammation and ammonia in hepatic encephalopathy. Metab Brain Dis. 2013;28:1–5. doi: 10.1007/s11011-012-9370-2. [DOI] [PubMed] [Google Scholar]
- 66.Lin W-C, Hsu T-W, Chen C-L, Lu C-H, Chen H-L, Cheng Y-F, Lin CP. Reestablishing brain networks in patients without overt hepatic encephalopathy after liver transplantation. J Cereb Blood Flow Metab. 2014;34:1877–1886. doi: 10.1038/jcbfm.2014.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cheng Y, Huang L, Zhang X, Zhong J, Ji Q, Xie S, Chen L, Zuo P, Zhang LJ, Shen W. Liver transplantation nearly normalizes brain spontaneous activity and cognitive function at 1 month: a resting-state functional MRI study. Metab Brain Dis. 2015;30:979–988. doi: 10.1007/s11011-015-9657-1. [DOI] [PubMed] [Google Scholar]
- 68.Zhang X-D, Cheng Y, Poon CS, Qi R, Xu Q, Chen H-J, et al. Long-and short-range functional connectivity density alteration in non-alcoholic cirrhotic patients one month after liver transplantation: a resting-state fMRI study. Brain Res. 1620;2015:177–187. doi: 10.1016/j.brainres.2015.04.046. [DOI] [PubMed] [Google Scholar]
- 69.Zhang G, Cheng Y, Shen W, Liu B, Huang L, Xie S. The short-term effect of liver transplantation on the low-frequency fluctuation of brain activity in cirrhotic patients with and without overt hepatic encephalopathy. Brain Imaging Behav. 2017;11:1849–1861. doi: 10.1007/s11682-016-9659-6. [DOI] [PubMed] [Google Scholar]
- 70.Cheng Y, Huang L-X, Zhang L, Ma M, Xie S-S, Ji Q, Zhang XD, Zhang GY, Zhang XN, Ni HY, Shen W. Longitudinal intrinsic brain activity changes in cirrhotic patients before and one month after liver transplantation. Korean J Radiol. 2017;18:370–377. doi: 10.3348/kjr.2017.18.2.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Martin P, DiMartini A, Feng S, Brown R, Fallon M. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology. 2014;59:1144–1165. doi: 10.1002/hep.26972. [DOI] [PubMed] [Google Scholar]
- 72.Lucey MR, Terrault N, Ojo L, Hay JE, Neuberger J, Blumberg E, Teperman LW. Long-term management of the successful adult liver transplant: 2012 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Liver Transpl. 2013;19:3–26. doi: 10.1002/lt.23566. [DOI] [PubMed] [Google Scholar]
- 73.Herrero JI, Bilbao JI, Diaz ML, Alegre F, Inarrairaegui M, Pardo F, Quiroga J. Hepatic encephalopathy after liver transplantation in a patient with a normally functioning graft: treatment with embolization of portosystemic collaterals. Liver Transpl. 2009;15:111–114. doi: 10.1002/lt.21552. [DOI] [PubMed] [Google Scholar]
- 74.Chavarria L, Alonso J, García-Martínez R, Simón-Talero M, Ventura-Cots M, Ramírez C, Torrens M, Vargas V, Rovira A, Córdoba J. Brain magnetic resonance spectroscopy in episodic hepatic encephalopathy. J Cereb Blood Flow Metab. 2013;33:272–277. doi: 10.1038/jcbfm.2012.173. [DOI] [PMC free article] [PubMed] [Google Scholar]