Abstract
Objectives
Medicines reconciliation is an effective way of reducing errors at transitions of care. Much of the focus has been on medicines reconciliation at point of admission to hospital. Our objective was to evaluate medicines reconciliation after discharge from hospital by assessing the quality of information regarding medicines within discharge summaries and determining whether the information provided regarding medicines changes were acted on within 7 days of receiving the discharge information.
Methods
A retrospective collaborative evaluation of medicines-related discharge information by Clinical Commissioning Group (CCG) pharmacists using standardised data collection tools. Outcomes of interest included compliance with national minimum standards for medication-related information on discharge summaries, such as allergies, changes to medication regimen, minimum prescription standards, for example, dose, route, formulation and duration, and medicines reconciliation by the primary care team. Data were analysed centrally.
Results
43 CCGs covering each of the four National Health Service regions in England participated in the study and submitted data for 1454 patients and 10 038 prescribed medicines. The majority of medication details were stated in accordance with standards with the exception of indication (11.7% compliance), formulation (60.3% compliance) and instructions of ongoing use (72.5% compliance). Documentation about changes was poor: 1550/3164 (49%) newly started medicines, 186/477 (39%) dose changes and 420/738 (57%) stopped medicines had a reason documented. Changes were not acted on within 7 days of receiving the discharge information for 12.5% of patients.
Conclusions
Our evaluation revealed overall good compliance with discharge medication documentation standards, but a number of changes to medicines during hospitalisation were not fully communicated or documented on the discharge summary or actioned in the general practice after discharge.
Keywords: pharmacy management (organisation, financial); quality management; medical errors; clinical audit; dispensing forms
Introduction
Medicines reconciliation is recognised globally as a process that supports patient safety; however, the majority of the focus in developed health systems has been targeted on implementing medicines reconciliation at admission to hospital.1–4 Few studies have researched the practice at the point of discharge from secondary care (hospital care) into primary care (care provided while at home) despite a substantial body of evidence demonstrating that when patients move between care providers/interfaces (particularly from secondary care to primary care), the risk of miscommunication around changes to medicines is a significant problem.5–10
The focus of this paper is medicines reconciliation practices during the discharge of patients from secondary care to primary care in the UK setting. In the UK, a 2009 report11 by the health and social care regulator, the Care Quality Commission (CQC), stated that acute National Health Service (NHS) trusts (hospitals) need to improve the information they provide on changes to medication at discharge and made the following recommendation: ‘Ensure that contracts with acute trusts set out the requirements and quality markers for both the timeliness and content of discharge summaries. Information on diagnosis, changes to medication and the reason for them must be included. They should put in place contract variations to set this in place at the earliest opportunity, including incentives through the commissioning for higher quality and innovation (CQUIN) system and penalties for poor contract performance’. Prior to the CQC concerns, several national organisations and Royal Colleges12 13 had developed standards focusing on what (and how) medicines-related information should be communicated on the discharge summary/prescription when patients are transferred from secondary care to primary care. Following the CQC concerns in 2011, the UK Department of Health developed a toolkit to support NHS organisations to improve communication of medicines-related information during transfer of care.14 Despite these efforts before and after the CQC concerns, evidence suggests that communication of medicines-related information at discharge from hospital remains problematic.6 15 16 The landmark practice study5 discussed some of the difficulties that general practitioners (GPs) face when dealing with hospital discharge medications. For example, GPs highlighted the need for the wording of hospital correspondence to be clear and accurate with any medication changes clearly highlighted.
The objectives of this collaborative service evaluation led by the National Medicine Use and Safety Team (MUS) of the NHS England Specialist Pharmacy Service17 were to:
Assess the quality of information regarding medicines within discharge summaries provided by secondary care (acute, mental health and community services).
Determine whether GPs correctly acted on the information provided regarding medicines in the discharge summaries within 7 days of receiving the discharge information as per the National Institute for Health and Care Excellence (NICE) Medicines Optimisation Standard.4
Methods
The study was designed as an audit and retrospective review of discharge information by Clinical Commissioning Group (CCG) pharmacists using data collection guidance notes and tools developed by a steering group. The steering group composed of relevant stakeholders including pharmacists from primary care, secondary care and academia as well as a NICE medicines implementation consultant. CCGs are clinically led statutory NHS bodies responsible for the planning and commissioning of healthcare services for their local area. CCG pharmacists act as prescribing advisers and have knowledge of and access to GP systems and records.
In December 2015, all heads of medicines management/chief pharmacists in CCGs and Commissioning Support Units (organisations that provide services to CCGs that allow them to focus their clinical commissioning) across England were invited to participate in the study. On expression of interest, each CCG/CSU lead was emailed the necessary study tools (protocol, data collection form, hints and tips document and a collation of frequently asked questions),17 which had been piloted. During January 2016, the CCG pharmacists identified a list of patients in GP practices who had been discharged on medication from secondary care in the period October–December 2015. Using consecutive sampling methodology, every second patient on the list was selected until the required sample size of a minimum of 1 patient per 50 000 population per CCG was reached.
Outcomes of interest included compliance with national minimum standards10 13 for medication-related information on discharge summaries, such as allergies, changes to medication regimen as well as minimum prescription standards, that is, dose, route, formulation and duration. An area of high priority within the audit was to ascertain the quality of allergy status recording on discharge summaries/prescriptions. The standard set followed the recommendations made in the NICE CG 183 on drug allergy: diagnosis and management.18 The methodology required the CCG pharmacist to compare the allergy status on the GP system with the allergy documentation on the discharge summary/prescription and interpret whether the allergy status on the latter reflected those details kept in the GP electronic systems while being mindful that the patient may have developed new allergies during hospitalisation. Second, the CCG pharmacists were requested to reconcile medicines between the discharge summary and the preadmission medication list on the GP system and record any unintended discrepancies that they identified. Finally, they were also asked to document whether the GP had implemented any recommendations or changes from secondary care and any errors with potential for harm and to attempt to identify which member of the GP practice team undertook the medicines reconciliation.
Other information such as patient age and gender, route of admission to hospital (planned or unplanned), length of hospital stay, format of discharge prescription, whether there was evidence of a pharmacist review or sign off (clinical screening) for the discharge summary and length of time before the GP received the discharge summary was also collected.
An Excel spreadsheet was provided to aid data collection and submission by CCG pharmacists to the MUS team. Full details of methods and data collection tools are available on the NHS England Specialist Pharmacy Service website.17 MUS team collated all the datasets received and analysed the results centrally. Compliance to discharge summary documentation was calculated using the number of discharge summaries where the requisite information was present divided by the total number of discharge summaries expressed as a percentage. Compliance to medicine prescription standards was calculated using the number of medicines with the requisite information stated divided by the total number of medicines prescribed. Missing and ambiguous data were excluded from analysis and reported as appropriate. The sample size (n) stated throughout the results section reflects either the number of patient discharge summaries reviewed or the number of medicines prescribed. The data were analysed and formulated into a national report and presented to all the regional chief pharmacist groups in England. Each participating CCG was provided with a short report benchmarking their results against the national dataset.
As this was a service evaluation, NHS Research Ethics approval was not required.
Results
Forty-three CCGs covering each of the four NHS regions in England participated in the study representing approximately 20% of the CCGs in existence at the time. A total of 1454 patient discharge summaries and 10 038 prescribed medicines (mean of 6.9 medicines per patient) from 74 hospitals were reviewed. The median number of patients reviewed per CCG was 10 with a range of 3–404, with a significant (47%) proportion of the data returns from three CCGs only.
The median length of inpatient stay was 4 days, although two patients had a stay of over 100 days and one patient exceeded 200 days. The majority (78.6%) of patients audited were unplanned admissions. Generally, communication of the inpatient stay to the GP was timely with the arrival of the discharge summary on the same day as the discharge; however, there were some outliers with one discharge summary taking 38 days to arrive. Table 1 below shows the key demographic and pertinent indicators of the study sample.
Table 1.
Key demographic and pertinent indicators of the study sample
| Indicator | Value/result |
| Total number of patient discharge summaries audited | 1454 |
| Total number of medicines prescribed across all discharge summaries audited | 10 038 |
| Total number of participating CCGs | 43 |
| Total number of hospitals | 74 |
| Median age of patients audited (n=1419) | 72 years (range 0–102 years) |
| Gender of patients audited (n=1433) | Female=53%. Male=47%. |
| Median length of inpatient stay (n=1454) | 4 days (range 0–208 days) |
| Median length of time before GP† received the discharge summary/prescription (n=1434) | Same day as discharge (range 0–38 days) |
| Route of admission (n=1454) | Unplanned: 78.6%. Planned: 21.4%. |
| Format of discharge summaries (n=1454) | Electronic: 89%. Handwritten: 11%. |
CCG, Clinical Commissioning Group; GP, general practitioner.
Communication of changes to medication
A total of 1146 patients (79%) of the study sample had at least one new medicine started while an inpatient, 169 patients (11.6%) had five or more new medicines started and one patient had 13 new medicines started. Of the 3164 new medicines started across the study sample, only 49% had a reason documented on the discharge summary for why the medicine was being commenced.
Doses were changed of at least one medicine for 336 patients (23%) of the study sample during their inpatient stay. Twenty-five patients (1.72%) had three or more of their doses changed, and one patient had the doses of 10 medicines changed during their inpatient admission. Of the 477 medicines that were subjected to dose changes, only 39% had reason documented for the change.
At least one medicine was stopped in hospital for 388 patients (27%) of the study sample. Eighty-four patients (5.7%) had three or more medicines stopped, and one patient had 10 medicines stopped during their inpatient admission. Of the 738 medicines stopped across the study sample, only 57% had a reason documented for why the medicine was being stopped. Aside from them 738 medicines that were definitively stopped, the evaluation also identified 1565 preadmission medicines (mean of 1.1 medicine omission per discharge summary/prescription) that appeared to be inappropriately omitted from the discharge summary/prescription and presumably omitted for the duration of the inpatient stay.
Table 2 summarises the results regarding the communication and actions taken for medication changes.
Table 2.
Medication changes and communication at discharge for medicines that have been newly started, doses changed or stopped
| Newly started | Dose changed | Stopped | Unintentionally omitted | |
| Number of patients who had at least one medication change* | 1146 | 336 | 388 | 479 |
| Total number of medicines started, dose changed, stopped or unintentionally omitted | 3164 | 477 | 738 | 1565 |
| Number of medicines that had a reason documented for the medication change | 562 (49%) | 186 (39%) | 420 (57%) | 0 |
| Percentage of patients who had their medication changes actioned on the GP system | Yes (53%), no (13%) and no action required (34%). | Yes (65%), no (34.5%) and no action required (0.5%). | Yes (74.5%), no (21.7%) and data unavailable (3.6%). | Not assessed. |
| Percentage of patients who had their medication changes intentionally disregarded by the GP | Yes (16%), no (78.6%) and data unavailable (5.7%). | Yes (22.9%), no (76.5%) and data unavailable (0.6%). |
Yes (12.6%), no (83.8%) and data unavailable (3.6%). | Not assessed |
| Percentage of patients who had their medication changes actioned incorrectly by the GP | Yes (5.7%), no (78.8%) and data unavailable (1.1%). | Yes (8.6%), no (89.9%) and data unavailable (1.5%). | Yes (6.7%), no (89.7%) and data unavailable (3.6%). | Not assessed |
*Numbers exceed 1454 as patients may have had more than one medication change.
GP, general practitioner.
Processing of actions by primary care as required by the hospital discharge summary
For patients with a change in medication (started, stopped or doses changed) during the inpatient stay, in approximately 45% of cases the GP practice did action these changes within 7 days of receiving the discharge summary. In 42% of cases, although medication changes took place during the inpatient stay, there was no need for the GP to change anything on their prescribing system, for example, short courses of medicines. For the remaining 12.5% of patients, the changes were not acted on by the GP within 7 days of receiving the discharge summary.
In approximately half of the patients audited, the GP was clearly involved in reconciling the patient’s medication following discharge from hospital. In the remainder of the patients, other team members from within the GP surgery were identified as being the primary individual involved in reconciling the patient’s medication (table 3).
Table 3.
Medication reconciliation in primary care
| National audit results | |
| For medicines that were started/stopped or doses changed during the hospital inpatient stay, were the changes actioned by the GP within 7 days of the discharge being received? (n=1438) | Yes=655 (45.5%). No=180 (12.5%). No action required=603 (42%). |
| Who carried out the medicines reconciliation within the GP surgery for the discharge summaries received? (n=1441) | GP=742 (51.5%). No requirement to undertake medicines Reconciliation=217 (15.1%). Unable to identify=101 (7%). CCG/practice pharmacist=95 (6.6%). Medicines reconciliation not undertaken=82 (5.7%). Practice receptionist=80 (5.6%). Practice nurse=7 (0.49%). Practice manager=1 (0.07%). Other=116 (8.05%). |
Meeting the prescribing standards
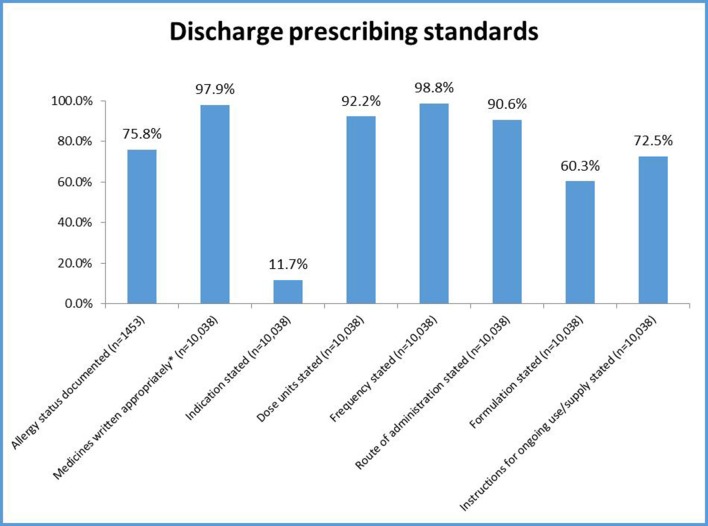
There was high compliance with many of the prescribing standards (figure 1), but information about allergies, indication, formulation of the drug and instructions for ongoing use was not always documented.
Figure 1.
Discharge summary demographic and information data compliance.* Medicines were considered to be written appropriately if written by generic name unless branded prescribing was warranted for example due to bioavailability issues or inhaler preparations where brand specificity is important.
Pharmacist clinical screening of the discharge summary
In approximately half (49%) of the discharge summaries audited there was clear evidence that they had been clinically reviewed (screened) by the secondary care pharmacist prior to being sent to the GP. 88% of discharge summaries that had been screened included the pharmacist’s name, but only 4% stated the contact details of the screening pharmacist.
Resolution of unintentional discrepancies
Although the study was not designed to measure the resolution of the unintentional discrepancies identified as part of the data collection, the CCG pharmacists qualitatively reported a number of follow-up actions that they undertook (see box 1). The key actions taken were contacting the secondary care prescriber or pharmacist, contacting the GP to ensure discrepancies were reviewed or contacting the patient and or carer to establish their medication use.
Box 1. Verbatim comments from CCG pharmacists regarding follow up actions for unintentional discrepancies.
‘GP to clarify new drugs which were not added to current PAM*’.
‘Had to contact carer to re-iterate if atorvastatin had been stopped by hosp[ital] as not listed on TTA†’.
‘At the time of discharge the dose of azithromycin had to be clarified with the Dr as the wrong dose (1 om[in the morning]) was on the discharge instead of the usual PAM‡ of 1 3x wkly [three times a week]’.
‘Checked with patient if they have enough supply for newly started anticoagulant drug until further sec[ondary] care clinic’.
‘GP to follow up dose that was not changed’.
‘GP - Dose of mouthwash altered from formulary default to that recommended by specialist unit’.
‘Potential for significant incident – SIRMS§ report filed’.
‘Illegible - had to phone eye clinic to check’.
‘GP to clarify new drugs which were not added to current medication list’.
*Preadmission medication list.
†To take away prescription.
§Serious incident risk system.
Discussion
The results of this national evaluation demonstrate that the communication of medicines-related information on the discharge summary/prescription from secondary care to primary care is problematic and requires improvement.
First, lower than expected compliance for minimum prescribing standards were surprising as these standards12 13 have been available since 2012. In theory, organisations had the opportunity to develop discharge templates to meet the national standards (particularly where electronic). The low rate for documenting the indication may reflect the challenges of hospital staff not always knowing the primary indication for established or chronic medicines, particularly if it has no bearing on the patient’s admission. Recording an erroneous or assumed indication in the absence of certainty has the potential to lead to confusion for the patient and GP.
Second, there was omission of established medicines throughout hospitalisation, at a mean rate of 1.1 medicines omitted per discharge summary. This suggests inadequate or a complete lack of medicines reconciliation being undertaken on admission to hospital and is comparable with another UK study of the quality of medicines reconciliation on hospital admission, which reported 0.97 omitted medicines.19
Third, only half of the discharge summaries had evidence of a clinical pharmacist review or screening. However, it cannot be assumed that the remaining half were not clinically screened by the pharmacist; potential reasons for absence of evidence include the design of the discharge summary template, which may not have included pharmacist screening details. For this reason, subanalysis to compare the influence of pharmacist screening was not performed.
Finally, reasons for changes to medication were only documented in approximately half the instances. Failure of secondary care prescribers to document details and rationale for medication changes, including initiation, on the discharge summary may be explained in part by the fact that a proportion of the prescribed medicines were for short courses or self-limiting conditions. For example, indications or durations of prescriptions for painkillers, laxatives and short antibiotic courses may be considered evident and the GP would not have been expected to continue these prescriptions.
Our findings are similar to two other UK studies with similar aims. Hammad et al 15 reported low compliance for the quality of medicines-related information contained within 3444 discharge summaries compared with the standards set out by UK National Prescribing Centre. Of note, only 48.9% of discharge summaries complied with standards around the communication of medication therapy changes (medicines initiated, discontinued or doses changed with a corresponding reason). Similarly, Grimes et al,6 in a study of 1245 discharge summaries reported that medication details documented at discharge from acute hospital care frequently contained prescription writing errors or failed to communicate information regarding changes to medication made while an inpatient. In their study, 21.5% of discharges failed to document that a medicine that the patient had been taking prior to admission had been stopped during the inpatient stay.6
A positive finding was that 89% of the discharge summaries were electronic and reached the GP on the same day. Despite this, for 12.5% of patients with medication changes that required action, this did not happen within 7 days of receiving the discharge summary. It is probable that appropriate actions were taken outside of the 7-day window. Once the discharge summary was received, various members of the primary care team were reported to have processed the medication-related actions. Both these findings require further study, as we did not analyse or explore the potential clinical consequences of delay in actioning the changes or ramifications of individuals other than the GP acting on the information contained in the discharge summary.
Although the study was not designed to identify any patient safety issues as part of the data collection, some of the CCG pharmacists undertaking the data collection reported interventions they undertook to ensure that the patient’s medication regime was safe and as intended posthospitalisation. These included correcting erroneous actions regarding the medication changes posthospitalisation and contacting the secondary care prescriber or pharmacist for clarity or confirmation of changes. This unexpected qualitative feedback highlights the need for clear and accurate discharge prescriptions, including contact details of secondary care staff.
Strengths and limitations
This is one of the largest studies undertaken in the UK encompassing 43 CCGs and 74 hospitals; however, there are limitations that need to be considered when interpreting the results. Many people were involved in data collection, which can introduce variability. This was recognised and minimised by developing a standard data collection tool that had drop-down menus and prompts, providing a hints and tips document and a clear data collection methodology. Nearly half of the data came from three CCGs and this may limit the generalisability of the findings. Even though patient safety issue were reported, the study was not designed to assess patient outcomes.
Practice and policy implications
The study highlights and focuses on a significant medication safety issue in the context of a national picture and at individual hospital and CCG levels. The results have been presented to chief pharmacist groups across England, and the following recommendations were made to improve safety at transitions of care:
CCGs and secondary care providers should collaborate to review the local hospital discharge template to ensure that it meets the needs of all involved, is in line with the standards set by the Royal Pharmaceutical Society10 and the Academy of Royal Colleges13 and supports transfer of medication related information.
CCGs to consider developing Commissioning for Quality and Innovation (CQUINs) to drive improving the quality of discharge communication by secondary care as previously recommended by the CQC.
Secondary care providers and hospital pharmacists should ensure that the medicines reconciliation process at admission is robust as this will affect the quality of medicines-related information contained in the discharge summary.
Shared access to records that allow health and medicines-related information to be kept up to date, for example, using the summary care record
GP practices should have clear processes in place on how information provided on discharge summaries/prescriptions is managed once received.
Consideration should be given to designating the responsibility of reconciling medicines posthospitalisation to the growing number of clinical pharmacists employed within GP practices.
The authors are aware that postpublication of the report in mid-2016 some CCGs have developed CQUINS (quality targets) to improve some of the issues identified in this study. The Northern Ireland Regulation and Quality Improvement Authority20 also sought permission to use and/or modify the tools within this study to conduct their regional audit of medicines reconciliation on discharge documentation.
Conclusions
Despite overall good compliance with standards of documentation for discharge summaries, our evaluation revealed issues with medicines reconciliation at transfers for care into and from hospital, with a number of changes to medicines during hospitalisation that were not fully communicated or documented on the discharge summary or actioned in the general practice after discharge.
What this paper adds.
What is already known on this subject
Medicines reconciliation rates on admission to hospital is a key performance indicator for the majority of National Health Service trusts in England.
Poor communication of medicines-related issues during transfer of care is a patient safety concern.
Evidence form primary care studies demonstrate that general practitioners (GPs) have concerns around the quality of information provided by secondary care around medication changes.
What this study adds
First England-wide evaluation of the quality of discharge information about medicines.
There continues to be poor communication to GPs particularly around documented reasons for changes to medication.
Some changes are documented incorrectly on the GP systems.
Acknowledgments
MUS would like to sincerely thank the members of the steering group for their time, support and expertise in helping to develop, pilot and validate the tools used in this study. MUS would like to sincerely thank the CCGs that participated in this collaborative evaluation for their time, expertise, feedback and willingness to support: Barnet, Brent, Central Manchester, City and Hackney, Coastal West Sussex, Cumbria, Doncaster, Ealing, East Sussex, Eastbourne, Fylde and Wyre, Haringey, Harrow, Hartlepool and Stockton, Hastings and Rother, Herefordshire, Hillingdon, Inner North West London, Isle of Wight, Islington, Kingston, Leeds South and East, Merton, Mid, Essex, Newcastle and Gateshead, North Tyneside, North West Surrey, Oxfordshire, Portsmouth, Salford, Sheffield, Slough, South Gloucestershire, South Reading, South Tees, Surrey Downs, Surrey Health, Waltham Forest, West Essex, Wigan, Windsor, Ascot and Maidenhead Wokingham.
Footnotes
Contributors: CS led the development of the project, coordinated its implementation and analysis and drafted the report. JH participated in the steering group, supported CS throughout the project and codrafted this article. YJ supported data analysis and reviewed and revised drafts of the paper.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. National Patient Safety Agency (2007) NICE/ NPSA, 2018. Guidance to improve medicines reconciliation at hospital admission. http://www.npsa.nhs.uk/corporate/news/guidance-to-improve-medicines-reconciliation/ (accessed Mar 2018).
- 2. The Joint Commission (2015) National Patient Safety. National patient safety goals effective accreditation programme. https://www.jointcommission.org/assets/1/6/2015_NPSG_HAP.pdf (accessed Mar 2018).
- 3. World health organisation’s high 5s medicines reconciliation project. http://www.safetyandquality.gov.au/our-work/medication-safety/medication-reconciliation/who-high-5s-medication-reconciliation-program/
- 4. National Institute for Health and Clinical Excellence (NICE), 2015. Medicines optimisation: the safe and effective use of medicines to enable the best possible outcomes https://www.nice.org.uk/guidance/ng5 (accessed Mar 2018). [PubMed]
- 5. Avery T, Barber N, Ghaleb M, et al. ; Investigating the prevalence and causes of prescribing errors in general practice: The PRACtICe Study. Nottingham: General Medical Council, 2011. [Google Scholar]
- 6. Grimes TC, Duggan CA, Delaney TP, et al. Medication details documented on hospital discharge: cross-sectional observational study of factors associated with medication non-reconciliation. Br J Clin Pharmacol 2011;71:449–57. 10.1111/j.1365-2125.2010.03834.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barber ND, Alldred DP, Raynor DK, et al. Care homes' use of medicines study: prevalence, causes and potential harm of medication errors in care homes for older people. Qual Saf Health Care 2009;18:341–6. 10.1136/qshc.2009.034231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Collins DJ, Nickless GD, Green CF. Medication histories: does anyone know what medicines a patient should be taking? Int J Pharm Pract 2004;12:173–8. 10.1211/0022357044454 [DOI] [Google Scholar]
- 9. Hippisley-Cox J, et al. The electronic patient record in primary care-regression or progression? A cross sectional study. BMJ 2003;326:1439–43. 10.1136/bmj.326.7404.1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Royal Pharmaceutical Society, 2012. Keeping patients safe when they transfer between care providers –getting the medicines right https://www.rpharms.com/Portals/0/RPS%20document%20library/Open%20access/Publications/Keeping%20patients%20safe%20transfer%20of%20care%20report.pdf (accessed in Mar 2018).
- 11. Care Quality Commission (CQC), 2009. Managing patients’ medicines after discharge from hospital http://webarchive.nationalarchives.gov.uk/20101122140156/http://www.cqc.org.uk/_db/_documents/Managing_patients_medicines_after_discharge_from_hospital.pdf (accessed Apr 2018).
- 12. National Prescribing Centre, 2008. Medicines reconciliation: a guide to implementation https://www.nicpld.org/courses/fp/assets/MM/NPCMedicinesRecGuideImplementation.pdf (accessed in Apr 2018).
- 13. Academy of Medical Royal Colleges, 2008. A Clinician’s Guide to Record Standards – Part 1: Why standardise the structure and content of medical records? https://www.rcoa.ac.uk/sites/default/files/FPM-clinicians-guide1.pdf (accessed Apr 2018).
- 14. Department of Health. The discharge Summary Tool Kit, Department of Health. 2011.
- 15. Hammad EA, Wright DJ, Walton C, et al. Adherence to UK national guidance for discharge information: an audit in primary care. Br J Clin Pharmacol 2014;78:1453–64. 10.1111/bcp.12463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dodds L, 2012. Improving safe and accurate transfer of medicines-related written discharge information: a pharmacy-led collaborative audit and service evaluation https://www.sps.nhs.uk/articles/improving-safe-and-accurate-transfer-of-medicines-related-written-discharge-information-a-pharmacy-led-collaborative-audit-and-service-evaluation/ (accessed Apr 2018).
- 17. Specialist Pharmacy Service. A collaborative audit on the quality of medication related information provided when transferring patients from secondary care to primary care and the subsequent medicines reconciliation in primary care report and tools. https://www.sps.nhs.uk/articles/a-collaborative-audit-on-the-quality-of-medication-related-information-provided-when-transferring-patients-from-secondary-care-to-primary-care-and-the-subsequent-medicines-reconciliation-in-primary-c/ (accessed Apr 2018).
- 18. National Institute for Health and Clinical Excellence (NICE), 2014. Clinical Guideline 183: Drug allergy: diagnosis and management. accessed Apr 2018 https://www.nice.org.uk/guidance/cg183. [PubMed]
- 19. Dodds L. Medicines Use and Safety, 2010. Results of a collaborative audit of pharmacy-led medicines reconciliation (mr) in 56 trusts across E & SE england. https://www.sps.nhs.uk/articles/results-of-a-collaborative-audit-of-pharmacy-led-medicines-reconciliation-mr-in-56-trusts-across-e-se-england/ (accessed April 2018).
- 20. Northern Ireland Regulation and Quality Improvement Authority. Regional audit of medicines reconciliation on the immediate discharge document. https://www.rqia.org.uk/RQIA/files/9d/9da8c378-696e-452a-b2e0-2bed505f53d5.pdf.