Abstract
Background
Studies assessing the effect of high dose tigecycline on severe infections are limited and remain controversial.
Objectives
To assess systematically the effectiveness and safety of high dose tigecycline in the treatment of severe infections.
Methods
Pubmed, Web of Science, Embase, MEDLINE, Cochrane Library and ClinicalTrials were searched up to February 20, 2019 for studies that compared the effectiveness and safety of high dose tigecycline with standard dose tigecycline or other non-tigecycline-containing regimens in the treatment of severe infections. Rates for all-cause mortality, clinical cure, microbiological eradication and adverse events were analysed.
Results
Ten studies with 593 patients were included. The results indicated that using high dose tigecycline resulted in better outcomes compared with controls with lower all-cause mortality (OR 0.44, 95% CI 0.30–0.66, p < 0.0001), higher clinical cure (OR 3.43, 95% CI 2.09–5.63, p < 0.00001), higher microbiological eradication (OR 2.25, 95% CI 1.44–3.50, p = 0.0003), and without increasing adverse events rates. Subgroup analysis showed that high dose tigecycline reduced all-cause mortality in nosocomial acquired pneumonia (OR 0.39, 95% CI 0.22–0.70, p = 0.002), bloodstream infections (OR 0.19, 95% CI 0.06–0.58, p = 0.004) and mixed infections (OR 0.20, 95% CI 0.07–0.59, p = 0.003), with no statistical differences in complicated intra-abdominal infections (OR 2.04, 95% CI 0.80–5.23, p = 0.14). In carbapenem-resistant pathogens, the microbiological eradication rate in those given high dose tigecycline did not differ from controls (OR 1.07, 95% CI 0.44–2.60, p = 0.87), although mortality was reduced (OR 0.20, 95% CI 0.09–0.45, p = 0.0001). The main limitation of the review is that most of the included studies are observational studies with small sample sizes and high risks of bias.
Conclusions
High dose tigecycline treatment is effective and safe for severe infections owing to its lower all-cause mortality, higher clinical cure, microbiological eradication and comparable adverse events. However, as a result of the high risks of bias of the included studies, well-designed randomised clinical trials are warranted to establish the effectiveness and safety of high dose tigecycline compared with standard dose tigecycline and other commonly used antibiotics.
Keywords: Carbapenem resistance, Gram-negative bacteria, Infectious disease, Multidrug resistance, Tigecycline
Key Summary Points
| Why carry out this study? |
| Resistance to carbapenems has been steadily increasing in many bacteria causing nosocomial infections. Therefore, antibiotics like tigecycline and colistin are considered as the last resort against some of those multidrug-resistant bacteria. |
| However, some studies have indicated that using the standard dose of tigecycline might result in worse clinical outcomes compared with other antibiotics. As a result, applying the higher dose of tigecycline has been a common clinical practice. Despite such widespread practice, studies assessing the effect of high dose tigecycline on severe infections are still limited and remain controversial. |
| What was learned from the study? |
| High dose tigecycline (200 mg loading dose, 100 mg q12h) had better outcomes (lower all-cause mortality, higher clinical cure and microbiology eradication rate) and comparable adverse events compared with standard dose tigecycline (100 mg loading dose, 50 mg q12h) and other antibiotics. |
| High dose tigecycline is recommended if a tigecycline-containing regimen is the clinical choice for severe infections, especially those with multidrug-resistant bacterial infections. |
| Well-designed randomised controlled trials with larger sample size are warranted to confirm the effectiveness and safety of high dose tigecycline in the treatment of severe infections. |
Introduction
Severe infections, especially those caused by multidrug-resistant (MDR) bacteria, are associated with increased mortality, length of hospital stay and cost [1–3]. MDR Gram-negative bacterial infections are responsible for more than 30% of hospital-acquired infections, with even higher rates in critically ill, cancer and immunosuppressed patients [4, 5]. Resistance to carbapenems, initially considered potent broad-spectrum antibiotics used to treat these infections [6], has increased significantly within the last decade because of the prevalence of carbapenemases among these pathogens [7–10]. Moreover, infections caused by extensively drug-resistant (XDR) or pandrug-resistant (PDR) organisms have emerged and spread all over the world [11–14]. Under such situations, older drugs like colistin, tigecycline, fosfomycin, clindamycin and cotrimoxazole are being deployed as the last resort in clinical practice for infections caused by MDR bacteria [15, 16].
Tigecycline was the first glycylcycline approved by the US Food and Drug Administration (FDA) to treat complicated skin and soft tissue infections (cSSTI), complicated intra-abdominal infections (cIAI), and community-acquired pneumonia [17–19]. Owing to its broad spectrum antibacterial activity, particularly against Gram-negative bacteria which are resistant to other antibiotics, it has been widely used off-label in ventilator-associated pneumonia (VAP), hospital-acquired pneumonia (HAP) and bloodstream infections (BSI) caused by MDR pathogens, especially carbapenem-resistant (CR) bacteria [20–22].
The efficacy of standard dose tigecycline (SDT) (100 mg initial dose, followed by 50 mg twice per day) in the clinic is controversial. Previous studies had indicated that tigecycline was not better than other antimicrobial agents and might be associated with increased mortality [23–25]. Pharmacokinetic and pharmacodynamic research suggested that this lack of efficacy may be due to its suboptimal concentrations in both serum and pulmonary epithelial lining fluid [26]. Therefore, a regimen of high dose tigecycline (HDT) (200 mg initial dose, followed by 100 mg twice per day) has been used in clinical practice. A systematic review in 2014 attempted to evaluate the effectiveness of HDT for the treatment of severe infections, but it could not draw conclusions regarding the efficacy of HDT because of limited clinical evidence [27]. With the accumulation of new studies, we aimed to reassess the effectiveness and safety of HDT for the treatment of severe infections.
Methods
Protocol and Guideline
The full protocol of the systematic review and meta-analysis was registered in PROSPERO (https://www.crd.york.ac.uk/prospero/) as CRD42019129283. The systematic review adhered to the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [28]. This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Literature Search
We performed an extensive search of PubMed, Web of Science, Embase, MEDLINE and the Cochrane Library using the terms “tigecycline”, “dose” and “dosage” up to February 20, 2019. In order to identify completed but unpublished or ongoing studies, ClinicalTrials.gov was also searched. The reference lists of identified reports were hand-searched for relevant studies. No language restrictions were applied.
Study Selection
The relevant studies were examined by two reviewers (L.Z. and L.P.) independently. Eligible studies compared the efficacy of HDT with SDT or other non-tigecycline antibiotic regimens in the treatment of severe infections regardless of pathogens. Single-arm studies, repetitive studies, case report, reviews, studies with limited or uncertain information and those using tigecycline in other dosage (i.e. not the defined high dose) were excluded, as were animal, pharmacokinetic/pharmocodynamic and in vitro studies. No restrictions were placed on the characteristics of participants, lengths of follow-up, antibiotics used in combination with HDT, and antibiotic regimens in the control group. Any disagreements were resolved through discussion with a third assessor (J.G.).
Data Extraction
Two reviewers working independently extracted the following information from each study: first author’s name and year of publication, study design, patient characteristics (age, infection sites and score of the severity of diseases), type of microorganism, concomitant antibiotics and antimicrobial agents used in the control groups, outcomes (all-cause mortality, clinical cure and microbiology eradication rate) and reported clinical adverse events.
Quality Assessment
The quality of the included non-randomised studies was evaluated using the modified Newcastle–Ottawa scale (NOS) [29]. Studies with NOS scores below 3 were considered as poor quality and excluded from this review. The risk of bias of included non-randomised studies was assessed using the ROBINS-I tool (Risk Of Bias In Non-randomised Studies of Interventions) [30]. The risk of bias of the single randomised controlled trial (RCT) included in this review was assessed with the Cochrane Collaboration’s tool for assessing the risk of bias [31].
Definitions and Outcomes
The outcome of primary interest of the review is all-cause mortality. Secondary outcomes include the clinical cure rate, microbiological eradication rate and adverse events (diarrhoea, nausea, vomiting, renal impact, hepatic injury and haematological injury). Clinical cure was defined as complete resolution or improvement from the symptoms and signs of infection. Microbiological eradication was defined as sterile culture or absence of the original pathogen in sequential culture after antibiotics treatment. As a result of the lack of standard definitions of adverse events, the criteria as reported in each study were used. HDT was defined as using tigecycline 100 mg twice per day after a 200 mg loading dose, whereas SDT was defined as using 50 mg twice per day after a 100 mg loading dose. HAP, VAP, BSI, cIAI and cSSTI were defined using criteria reported in each study. Mixed infection was defined as the presence of at least two types of infection in patients (i.e. patients diagnosed as cIAI, BSI and HAP were all included in one study).
Statistical Analysis
The review was performed using Review Manager 5.3 (Cochrane Collaboration, Oxford, UK). Statistical heterogeneity was assessed by the I2 test, and I2 > 50% was defined as substantial heterogeneity [32]. In the presence of substantial heterogeneity, a random-effects model was used. Otherwise, a fixed-effects model was calculated. Pooled odds ratios (OR) and 95% confidence intervals (CI) were calculated using the Mantel–Haenszel method. The sequential monitoring boundary and required information size (RIS) were constructed and calculated with the software Trial Sequential Analysis (http://www.ctu.dk/tsa/) [33]. Publication bias was evaluated with funnel plots and the Egger regression-based test implemented in Stata version 14 (StataCorp, College Station, Texas). A two-tailed p < 0.05 was considered statistically significant.
Results
Included Studies and Characteristics
Overall, 591 studies were identified from five databases, and one was identified through reference lists. After application of eligibility criteria, ten studies were included in the systematic review and meta-analysis (Fig. 1). Among the included studies, eight were retrospective observational studies [34–41], one was a prospective observational study [42], and one was an RCT [43]. A total of 593 patients were enrolled, with the majority (88.4%) being admitted to intensive care with severe infections (these patients had an Acute Physiology and Chronic Health Evaluation II [APACHE II] score more than 15).
Fig. 1.
Flow chart indicating the process of literature search and review for effectiveness and safety of high dose tigecycline for the treatment of severe infections based on eligible criteria
The main pathogens were CR-Gram-negative bacteria, especially CR-Klebsiella pneumoniae. The indications for using tigecycline were nosocomial pneumonia (HAP and VAP), BSI, cIAI and cSSTI. Seven studies [34, 36–38, 40–42] evaluated the sensitivity of pathogens to tigecycline, and the susceptibility rate ranged from 79.5% to 100%. The most commonly used antibiotics in the control group was SDT; only two studies [39, 43] assessed non-tigecycline treatments. The characteristics of the studies included in this review are shown in Table 1.
Table 1.
Characteristics of included studies in the systematic review and meta-analysis
| Reference | Study design, period, country | Population characteristics HDT/control (mean ± standard deviation) | Type of infection | Causative pathogens | Mortality assessed | Sample size (HDT/SDT) | Concomitant antibiotics in HDT group | Antibiotics used in control group | Sensitivity to TGC | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chen et al. [35] | SC, retrospective, 2013–2015, China |
NCU patients APACHE II 18.38 ± 4.73/19.59 ± 5.77, age 58.26 ± 17.5/64.59 ± 19.7 |
VAP | MDR-AB, MDR-KP, other GNB | 28 days | 69/54 | Cefoperazone–sulbactam/piperacillin–tazobactam/imipenem/meropenem | SDT + cefoperazone-sulbactam/piperacillin–tazobactam/imipenem/meropenem | NR | ||
| De Pascale et al. [36] |
SC, retrospective, 2009–2012, Italy |
ICU patients, SOFA 7.4 ± 2.7/7.8 ± 3.2, age 60.7 ± 12.5/64.5 ± 16.9 | VAP | GNB (AB, KP), CR (94%) | ICU | 33/30 | 87.9% used combination of antibiotics | SDT + 80% concomitant antibiotics | All MIC ≤ 2 mg/ml | ||
| Di Carlo et al. [42] | SC, prospective, 2011–2012, Italy | ICU patients, APACHE II 23.4 ± 1.7, age 56.6 ± 15 | BSI | CRKP | ICU | 12/18 | Colistin | SDT + colistin | 100% susceptible | ||
| Geng et al. [37] | SC, retrospective, 2014–2016, China | ICU patients, APACHE II 20.7 ± 9.4/20.2 ± 6.0, SOFA 4.7 ± 3.3/5.4 ± 2.9, age 65.1 ± 14.3/61.8 ± 13.9 | BSI | CRKP | In-hospital | 23/17 | Carbapenems or β-lactamase inhibitors or aminoglycosides | SDT + carbapenems or β-lactamase inhibitors or aminoglycosides | 79.5% susceptible | ||
| Ibrahim et al. [38] | SC, retrospective, 2013–2014, Egypt | ICU patients, SOFA 9.5, age 57.5 | cSSTI, cIAI, CAP | AB, KP, E. coli, Enterobacter, Enterococcus, Staphylococcus, Streptococcus | ICU | 35/33 | No | SDT | 100% susceptible | ||
| Maseda et al. [39] | MC, retrospective, 2012–2013, Spain | SICU patients, SOFA 7.0 ± 3.3/5.5 ± 3.7, age 65.7 ± 7.3/65.7 ± 16.3 | cIAI | Polymicrobial (Enterococcus as major) | 28 days | 54/67 | Piperacillin–tazobactam/antifungals | Carbapenems | NR | ||
| Moreno et al. [34] | SC, retrospective, 2009–2011, Spain | ICU patients, APACHE II 19.7 ± 8.2/21.8 ± 3.1, age 56.4 ± 15.8/51.5 ± 7.5 | Pneumonia (5), UTI (5), cIAI (3), BSI (2), meningitis (1) | CRKP | 30 days | 10/6 | Colistin/carbapenems/ciprofloxacin/piperacillin–tazobactam | SDT + colistin/carbapenems/ciprofloxacin/amikacin | 100% susceptible | ||
| Ramirez et al. [43] | MC, RCT, DB, 2008–2011, 75 sites | APACHE II, 74.3% ≤ 15/67.7% ≤ 15, age 61.5 ± 16.1/64.9 ± 15.3 | HAP (VAP 40.6%) | GNB, Staphylococcus aureus, Streptococcus spp. | 21 days | 35/34 | Ceftazidime and tobramycin or amikacin | Imipenem/cilastain + vancomycin and tobramycin or amikacin | NR | ||
| Vardakas et al. [40] | SC, retrospective, Greece |
ICU patients APACHE II 16.3 ± 7 age 65.8 ± 13.5 |
Bacteremia (22), LRTI (3), UTI (1), cIAI (3), cSSTI (3) | CRKP | In-hospital | 26/6 | Colistin/aminoglycoside/carbapenem | SDT + colistin/aminoglycoside/carbapenem | 96.8% (31) susceptible | ||
| Wu et al. [41] | SC, retrospective, 2013–2015, China |
RICU patients APACHE II 15–19 (IQR), SOFA 3.5 ± 1.1, age 74.6 ± 9.4 |
HAP (VAP) |
CR-GNB (CRAB, CRKP) |
ICU | 20/11 | Cefoperazone–sulbactam/piperacillin–tazobactam/carbapenem | SDT + cefoperazone–sulbactam/piperacillin–tazobactam/carbapenem | All susceptible except 1 AB intermediate | ||
SC single centre, MC multiple centres, NCU neurological care unit, RICU respiratory intensive care unit, ICU intensive care unit, APACHE II Acute Physiology and Chronic Health Evaluation II, SOFA Sequential Organ Failure Assessment, HAP hospital-acquired pneumonia, VAP ventilator-associated pneumonia, CAP community-acquired pneumonia, UTI urinary tract infection, cIAIc complicated intra-abdominal infections, BSI bloodstream infection, LRTI lower respiratory tract infection, cSSTIc complicated skin and soft tissue infections, MDR multidrug resistant, AB Acinetobacter baumannii, KP Klebsiella pneumoniae, CR carbapenem resistant, CRKP carbapenem-resistant Klebsiella pneumoniae, CRAB carbapenem-resistant Acinetobacter baumannii, GNB Gram-negative bacteria, NR not report, SDT standard dose tigecycline, HDT high dose tigecycline, MIC minimum inhibitory concentration, IQR interquartile range, TGC tigecycline
Assessment of Bias
Most of the included non-randomised studies had serious or critical risks of bias due to the nature of the design of observational studies (Table 2). When studies with critical risk or no information were excluded, the all-cause mortality in the HDT group was still lower than that in the control group without obvious heterogeneity (OR 0.32, 95% CI 0.20–0.50, I2 = 0%, p < 0.00001). The included RCT was assessed as unclear risk of bias because of unclear information in the selection bias domain, although other domains were at low risk.
Table 2.
Assessment of the risk of bias for included non-randomised studies
| Confounding | Selection bias | Classification bias of interventions | Deviations from intended interventions | Bias due to missing data | Measurement bias | Report bias | Overall | |
|---|---|---|---|---|---|---|---|---|
| Chen et al. [35] | Serious | Serious | Serious | Moderate | Low | Low | Moderate | Serious |
| De Pascale et al. [36] | Serious | Serious | Serious | NI | Low | Low | Moderate | Serious |
| Di Carlo et al. [42] | Serious | Serious | Serious | Moderate | Low | Low | Moderate | Serious |
| Geng et al. [37] | Serious | Serious | Serious | NI | Low | Low | Moderate | Serious |
| Ibrahim et al. [38] | Serious | Serious | Serious | NI | Low | Low | Moderate | Serious |
| Maseda et al. [39] | Critical | Critical | Serious | Serious | Low | Low | Moderate | Critical |
| Moreno et al. [34] | Serious | Serious | Serious | Serious | Low | Low | Moderate | Serious |
| Vardakas et al. [40] | NI | NI | Serious | NI | Low | Low | Moderate | NI |
| Wu et al. [41] | Serious | Serious | Serious | Serious | Low | Low | Moderate | Serious |
The funnel plot of all-cause mortality of the included studies is shown in Fig. 2. The Egger regression-based test gave p = 0.303, which means no obvious publication bias was detected.
Fig. 2.
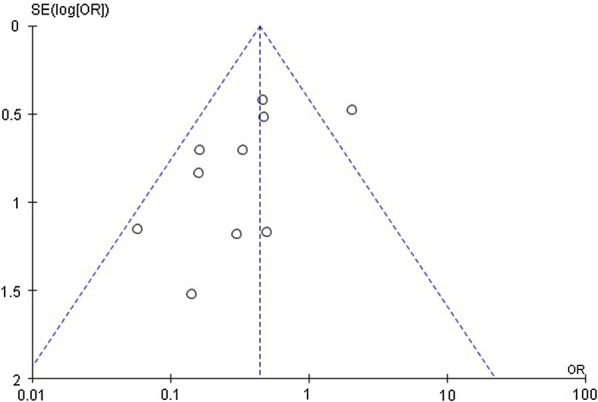
Funnel plot of all-cause mortality in high dose tigecycline (HDT) regimens compared with controls
All-Cause Mortality
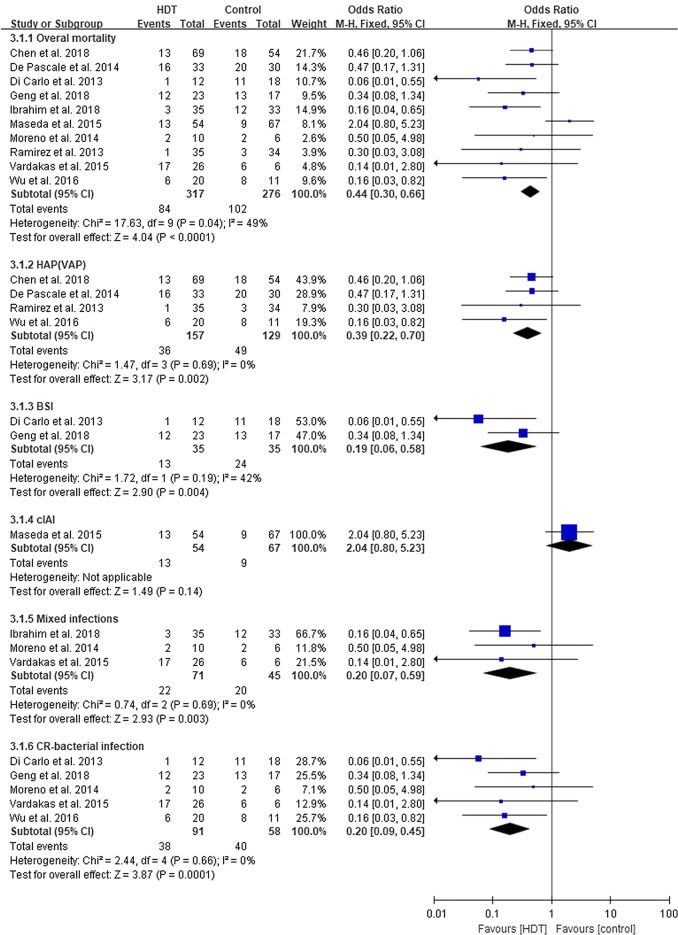
The pooled all-cause mortality was 31.4%. Compared with the control group, mortality in the HDT group was statistically lower (OR 0.44, 95% CI 0.30–0.66, I2 = 49%, p < 0.0001). Further analysis indicated that the major pathogens were Enterococcus faecium and Enterococcus faecalis in Maseda et al.’s study [39], whereas in other studies, the main pathogens were Gram-negative bacteria. When Maseda et al.’s study was excluded, statistical heterogeneity was eliminated (OR 0.30, 95% CI 0.19–0.48, I2 = 0%, p < 0.00001).
All subgroups (HAP (VAP) [35, 36, 41, 43], BSI [37, 42], and mixed infections [34, 38, 40]) except cIAI [39] showed a favourable outcome in the HDT group. In cIAI no statistical differences between HDT and control were seen (OR 2.04, 95% CI 0.80–5.23, p = 0.14). The impact of carbapenem resistance on mortality showed that HDT-containing regimens reduced mortality in CR-bacterial infections (OR 0.20, 95% CI 0.09–0.45, I2 = 0%, p = 0.0001) (Fig. 3) [34, 37, 40–42].
Fig. 3.
All-cause mortality of the high dose tigecycline (HDT) regimens compared with controls. HAP hospital-acquired pneumonia, VAP ventilator-associated pneumonia, BSI bloodstream infection, cIAI complicated intra-abdominal infections, CR carbapenem resistant
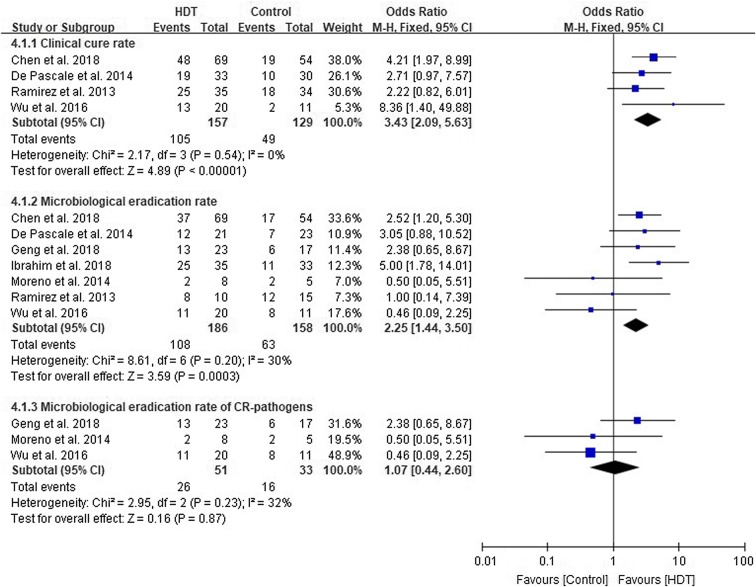
Clinical Cure and Microbiological Eradication Rate
Four studies [35, 36, 41, 43] with 286 patients evaluated the clinical cure rate. Patients given HDT had a higher clinical cure rate compared to controls (OR 3.43, 95% CI 2.09–5.63, I2 = 0%, p < 0.00001). In the seven studies [34–38, 41, 43] and 344 patients assessing the microbiological eradication rate, a pooled result favouring the HDT group was found (OR 2.25, 95% CI 1.44–3.50, I2 = 30%, p = 0.0003) (Fig. 4). However, the pooled result of the microbiological eradication rate did not reach statistical significance when bacteria were resistant to carbapenem (OR 1.07, 95% CI 0.44–2.60, I2 = 32%, p = 0.87) (Fig. 4) [34, 37, 41].
Fig. 4.
Clinical cure rate and microbiological eradication rate of high dose tigecycline (HDT) regimens compared with controls. CR carbapenem resistant
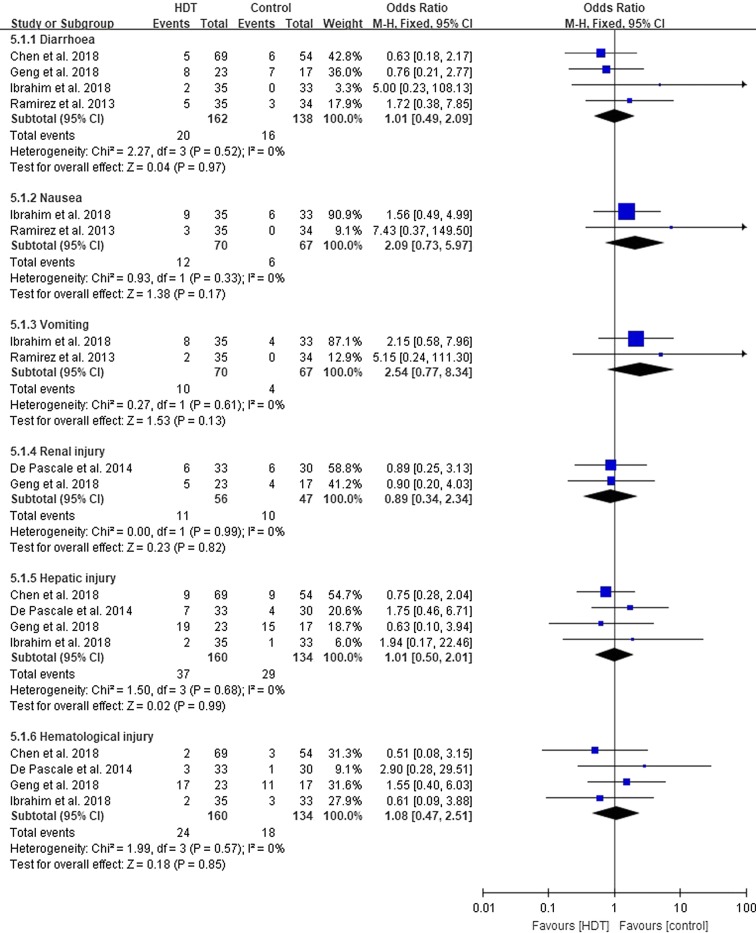
Adverse Events
Five studies [35–38, 43] documented 197 adverse events, including diarrhoea (n = 36), nausea (n = 18), vomiting (n = 14), renal injury (n = 21), hepatic injury (n = 66), and haematological injury (n = 42). There were no statistical differences in the distributions of adverse events in the two groups (Fig. 5).
Fig. 5.
Adverse events of the high dose tigecycline (HDT) regimens compared with controls
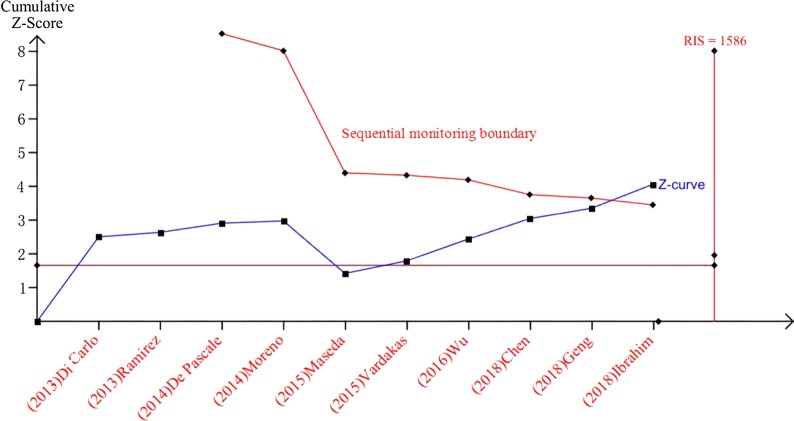
Reliability and Conclusiveness of the Primary Outcome
To determine the RIS for overall mortality, the control event rate was assumed to be 42% (calculated in this meta-analysis), the relative risk reduction was defined as 12% (estimated from this meta-analysis) with 80% power and a two-sided α error of 5%. At least 1586 patients were required to get a reliable treatment effect analysis. In this review, there were 593 patients enrolled for the analysis of all-cause mortality. Although the pooled sample size was less than the RIS, the cumulative curve (Z-curve) crossed the sequential monitoring boundary indicating that the result in our meta-analysis is reliable and conclusive (Fig. 6).
Fig. 6.
Cumulative meta-analysis assessing the effect of high dose tigecycline on all-cause mortality of severe infections. The sequential monitoring boundary, which assumes a 42% control event rate and a 12% relative risk reduction with 80% power and two-sided α of 5%, has been crossed, indicating that the cumulative evidence is conclusive
Discussion
Tigecycline is widely used for difficult-to-treat infections because of its broad spectrum of antimicrobial ability and low rate of resistance. Studies illustrated that tigecycline had good activity against MDR pathogens, included methicillin-resistant Staphylococcus aureus, Acinetobacter baumannii, Klebsiella pneumoniae, vancomycin-resistant Enterococcus, Clostridium difficile and other Enterobacteriaceae [6, 18, 44, 45]. However, studies evaluating the efficacy of SDT in the treatment of severe infections raised concern about its effectiveness. A meta-analysis of 14 RCTs of around 7400 patients showed that tigecycline treatment is no better than the control antibiotics [23]. Two other studies concluded that tigecycline increased mortality and adverse events [24, 46]. Ni et al. [47] reported that in terms of CR-Enterobacteriaceae infections, SDT had similar overall mortality, clinical response and microbiological eradication rates when compared with other antibiotics, but that the HDT group decreased the mortality rate compared with the SDT regimen (OR 0.08, 95% CI 0.013–0.080, p = 0.006).
In our meta-analysis, 10 studies with 593 patients indicated that treatment with HDT decreased overall mortality while improving both the clinical cure and microbiological eradication rates. Subgroup analysis of the type of infection illustrated that all subgroups except cIAI showed favourable results under HDT. In the cIAI group, the lack of effect of HDT could be explained by the severity of infection in patients enrolled in the HDT group compared to the control group (i.e. patients had significantly higher Sequential Organ Failure Assessment [SOFA] and Simplified Acute Physiology Score II [SAPS II], and a higher percentage of patients required mechanical ventilation, renal replacement therapy and presented septic shock) [39]. Since there are no other studies focusing on cIAI included in our meta-analysis, the true effect of HDT in cIAI cannot be concluded with certainty.
In the subgroup analysis of infections caused by CR-pathogens, the microbiological eradication rate did not show any statistical significance in the HDT group compared with SDT treatment. Epidemiological studies have shown that CR-Gram-negative bacteria usually present resistance against other antibiotics [8, 10, 48]. In our meta-analysis, all the included pathogens for CR-subgroup analysis were MDR K. pneumoniae and MDR A. baumannii, and the antibiotics used were those with high resistant rates in CR-K. pneumoniae and CR-A. baumannii. As tigecycline is a tetracycline-derived bacteriostatic agent, without the synergistic effect of other active bactericides, the clearance of those pathogens would be slow [18]. The combination of these reasons may explain the lack of difference in the microbiological eradication rate between HDT and the control group in the CR-subgroup.
Our analysis of the impact of carbapenem resistance on mortality showed that the HDT group experienced better outcomes. Previous studies reported that CR-infections had higher mortality rates because the proportion of inappropriate antibiotics therapy was higher [49–51]. In our meta-analysis, the patients in almost all studies (excepting Geng et al. [37]) showed around 100% susceptibility to tigecycline. Therefore, although the microbiological eradication rate did not show any statistical differences, the mortality rate is still lower in the HDT group of the CR-infections subgroup.
Previous studies reported higher rates of adverse events in the SDT group when compared with non-tigecycline regimens [24]. In our meta-analysis, eight out of ten studies used SDT as controls. The lack of any statistically significant differences in our results suggests that HDT is tolerable and as safe as SDT, although it has higher adverse events rate when compared with non-tigecycline regimens reported in one included study [43].
The adverse events analysis of our review failed to assess the rate of development of tigecycline resistance in the HDT and SDT group because of limited information of the included studies. However, the results in this review showed that the microbiological eradication rate was lower in the SDT group compared with the HDT group. The lower microbiological eradication rate suggests that there were more pathogens exposed to a suboptimal concentration of tigecycline and hence would possibly select for more antimicrobial resistant bacteria. A similar result was also found in a recently published review paper, which illustrated that the microbiological eradication rate was lower in the SDT group compared with other non-tigecycline antibiotics in the treatments of pneumonia caused by MDR A. baumannii [52]. Therefore, the selection of SDT-containing regimens as the clinical choice should be reconsidered because it might increase the probability of emergence of XDR or PDR pathogens. Nevertheless, the real effect of tigecycline dose on the selection of antimicrobial resistance should be further studied.
There are several limitations in our study. First, all the included studies are limited to observational studies of small sample sizes and one RCT. Although the accumulated sample size crossed the sequential monitoring boundary, the real efficacy of HDT would only be concluded through a well-designed, properly powered RCT because of the nature of unavoidable confounders and bias in observational studies. Second, apart from the only RCT and one observational study, all the other studies included in the review had the control group utilizing SDT regimens, which limited the conclusion to comparisons between HDT and SDT, rather than comparisons between HDT and other commonly used non-tigecycline antibiotics. Third, although the calculated heterogeneity values between studies are low, the variations of interventions (concomitant antibiotics, time to start therapy, inappropriate antibiotics therapy rate), outcomes measurement (time to assess mortality, definition of clinical response, time to evaluate microbiological eradication rate, etc.) may affect the interpretation of the results.
Conclusions
This systematic review and meta-analysis suggests that HDT treatment has better outcomes in the treatment of severe infections when compared with SDT and other non-tigecycline-containing regimens. The HDT regimen is associated with lower mortality rate, higher clinical cure and microbiological eradication rate, while having similar adverse events rates compared with controls. We recommend using HDT if a tigecycline-containing regimen is the clinical choice for severe infections, especially those infected with MDR bacteria. However, as a result of the high risks of bias of the included studies, well-designed, properly powered RCTs are warranted to confirm the effectiveness and safety of HDT compared with SDT and other commonly used antibiotics.
Acknowledgements
Funding
The study, including rapid service fees, was funded by Conch Hospital (Wuhu, Anhui, China) as part of the research project on multi-drug resistant bacteria (F20190301).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Design of Study and Conceptualization: LZ, BT, NF and EVV. Data Collection and Analysis: LZ, LP, JG and EVV. Original Draft Construction: LZ and LP. Draft Review and Scientific Revisions: BT, NF and EVV. Final draft approval: LZ, LP, JG, NF, EVV and BT.
Disclosures
Lei Zha, Lingling Pan, Jun Guo, Neil French, Elmer V Villanueva and Boris Tefsen have nothing to declare.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
All data generated or analysed during our study are included in this published article.
Footnotes
Enhanced Digital Features
To view enhanced digital features for this article go to 10.6084/m9.figshare.11591916.
References
- 1.Arthur C, Awa Marie CS, Bent HI, et al. Antimicrobial resistance: a priority for global health action. Bull World Health Organ. 2015;93(7):439. doi: 10.2471/BLT.15.158998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thabit AK, Crandon JL, Nicolau DP. Antimicrobial resistance: impact on clinical and economic outcomes and the need for new antimicrobials. Expert Opin Pharmacother. 2015;16(2):159–177. doi: 10.1517/14656566.2015.993381. [DOI] [PubMed] [Google Scholar]
- 3.Mauldin PD, Salgado CD, Hansen IS, Durup DT, Bosso JA. Attributable hospital cost and length of stay associated with health care-associated infections caused by antibiotic-resistant Gram-negative bacteria. Antimicrob Agents Chemother. 2010;54(1):109–115. doi: 10.1128/AAC.01041-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peleg AY, Hooper DC. Hospital-acquired infections due to Gram-negative bacteria. N Engl J Med. 2010;362(19):1804–1813. doi: 10.1056/NEJMra0904124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hawkey P. Multidrug-resistant Gram-negative bacteria: a product of globalization. J Hosp Infect. 2015;89(4):241–247. doi: 10.1016/j.jhin.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 6.Hawkey PM, Warren RE, Livermore DM, et al. Treatment of infections caused by multidrug-resistant Gram-negative bacteria: report of the British Society for Antimicrobial Chemotherapy/Healthcare Infection Society/British Infection Association Joint Working Party. J Antimicrob Chemother. 2018;73(suppl_3):iii2–iii78. doi: 10.1093/jac/dky027. [DOI] [PubMed] [Google Scholar]
- 7.Bedenić Branka, Plečko Vanda, Sardelić Sanda, Uzunović Selma, Godič Torkar Karmen. Carbapenemases in Gram-Negative Bacteria: Laboratory Detection and Clinical Significance. BioMed Research International. 2014;2014:1–3. doi: 10.1155/2014/841951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tal-Jasper R, Katz DE, Amrami N, et al. Clinical and epidemiological significance of carbapenem resistance in Acinetobacter baumannii infections. Antimicrob Agents Chemother. 2016;60(5):3127–3131. doi: 10.1128/AAC.02656-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munoz-Price LS, Poirel L, Bonomo RA, et al. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis. 2013;13(9):785–796. doi: 10.1016/S1473-3099(13)70190-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duin DV, Doi Y. The global epidemiology of carbapenemase-producing Enterobacteriaceae. Virulence. 2017;8(4):1–10. doi: 10.1080/21505594.2016.1222343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horcajada JP, Montero M, Oliver A, et al. Epidemiology and treatment of multidrug-resistant and extensively drug-resistant Pseudomonas aeruginosa infections. Clin Microbiol Rev. 2019;32(4):e00031–19. [DOI] [PMC free article] [PubMed]
- 12.Kaye KS, Pogue JM. Infections caused by resistant Gram-negative bacteria: epidemiology and management. Pharmacotherapy. 2015;35(10):949–962. doi: 10.1002/phar.1636. [DOI] [PubMed] [Google Scholar]
- 13.Nowak J, Zander E, Stefanik D, et al. High incidence of pandrug-resistant Acinetobacter baumannii isolates collected from patients with ventilator-associated pneumonia in Greece, Italy and Spain as part of the MagicBullet clinical trial. J Antimicrob Chemother. 2017;72(12):3277–3282. doi: 10.1093/jac/dkx322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pérez A, Gato E, Pérez-Llarena J, et al. High incidence of MDR and XDR Pseudomonas aeruginosa isolates obtained from patients with ventilator-associated pneumonia in Greece, Italy and Spain as part of the MagicBullet clinical trial. J Antimicrob Chemother. 2019;74(5):1244–1252. doi: 10.1093/jac/dkz030. [DOI] [PubMed] [Google Scholar]
- 15.Cassir N, Rolain J-M, Brouqui P. A new strategy to fight antimicrobial resistance: the revival of old antibiotics. Front Microbiol. 2014;5:551. doi: 10.3389/fmicb.2014.00551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Falagas ME, Kopterides P. Old antibiotics for infections in critically ill patients. Curr Opin Crit Care. 2007;13(5):592–597. doi: 10.1097/MCC.0b013e32827851d7. [DOI] [PubMed] [Google Scholar]
- 17.Kaewpoowat Q, Ostrosky-Zeichner L. Tigecycline: a critical safety review. Expert Opin Drug Saf. 2015;14(2):335–342. doi: 10.1517/14740338.2015.997206. [DOI] [PubMed] [Google Scholar]
- 18.Livermore DM. Tigecycline: what is it, and where should it be used? J Antimicrob Chemother. 2005;56(4):611–614. doi: 10.1093/jac/dki291. [DOI] [PubMed] [Google Scholar]
- 19.Pankey GA. Tigecycline. J Antimicrob Chemother. 2005;56(3):470–480. doi: 10.1093/jac/dki248. [DOI] [PubMed] [Google Scholar]
- 20.Kuti JL, Kim A, Cloutier DJ, Nicolau DP. Evaluation of plazomicin, tigecycline, and meropenem pharmacodynamic exposure against carbapenem-resistant Enterobacteriaceae in patients with bloodstream infection or hospital-acquired/ventilator-associated pneumonia from the CARE Study (ACHN-490-007) Infect Dis Ther. 2019;8(3):383–396. doi: 10.1007/s40121-019-0251-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J, Pan Y, Shen J, Xu Y. The efficacy and safety of tigecycline for the treatment of bloodstream infections: a systematic review and meta-analysis. Ann Clin Microbiol Antimicrob. 2017;16(1):24. doi: 10.1186/s12941-017-0199-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu L, Wang Y-L, Du S, Chen L, Long L-H, Wu Y. Efficacy and safety of tigecycline for patients with hospital-acquired pneumonia. Chemotherapy. 2016;61(6):323–330. doi: 10.1159/000445425. [DOI] [PubMed] [Google Scholar]
- 23.Tasina E, Haidich A-B, Kokkali S, Arvanitidou M. Efficacy and safety of tigecycline for the treatment of infectious diseases: a meta-analysis. Lancet Infect Dis. 2011;11(11):834–844. doi: 10.1016/S1473-3099(11)70177-3. [DOI] [PubMed] [Google Scholar]
- 24.Shen F, Han Q, Xie D, Fang M, Zeng H, Deng Y. Efficacy and safety of tigecycline for the treatment of severe infectious diseases: an updated meta-analysis of RCTs. Int J Infect Dis. 2015;39:25–33. doi: 10.1016/j.ijid.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 25.Prasad P, Sun J, Danner RL, Natanson C. Excess deaths associated with tigecycline after approval based on noninferiority trials. Clin Infect Dis. 2012;54(12):1699–1709. doi: 10.1093/cid/cis270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giamarellou H, Poulakou G. Pharmacokinetic and pharmacodynamic evaluation of tigecycline. Expert Opin Drug Metab Toxicol. 2011;7(11):1459–1470. doi: 10.1517/17425255.2011.623126. [DOI] [PubMed] [Google Scholar]
- 27.Falagas ME, Vardakas KZ, Tsiveriotis KP, Triarides NA, Tansarli GS. Effectiveness and safety of high-dose tigecycline-containing regimens for the treatment of severe bacterial infections. Int J Antimicrob Agents. 2014;44(1):1–7. doi: 10.1016/j.ijantimicag.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 28.Hutton Brian, Salanti Georgia, Caldwell Deborah M., Chaimani Anna, Schmid Christopher H., Cameron Chris, Ioannidis John P.A., Straus Sharon, Thorlund Kristian, Jansen Jeroen P., Mulrow Cynthia, Catalá-López Ferrán, Gøtzsche Peter C., Dickersin Kay, Boutron Isabelle, Altman Douglas G., Moher David. The PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-analyses of Health Care Interventions: Checklist and Explanations. Annals of Internal Medicine. 2015;162(11):777. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 29.Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: Ottawa Hospital Research Institute; 2011. [Google Scholar]
- 30.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Higgins TJP. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thorlund K, Anema A, Mills E. Interpreting meta-analysis according to the adequacy of sample size. An example using isoniazid chemoprophylaxis for tuberculosis in purified protein derivative negative HIV-infected individuals. Clin Epidemiol. 2010;2:57. doi: 10.2147/clep.s9242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balandin Moreno B, Fernandez Simon I, Pintado Garcia V, et al. Tigecycline therapy for infections due to carbapenemase-producing Klebsiella pneumoniae in critically ill patients. Scand J Infect Dis. 2014;46(3):175–180. doi: 10.3109/00365548.2013.861608. [DOI] [PubMed] [Google Scholar]
- 35.Chen Z, Shi X. Adverse events of high-dose tigecycline in the treatment of ventilator-associated pneumonia due to multidrug-resistant pathogens. Medicine. 2018;97(38):e12467. doi: 10.1097/MD.0000000000012467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Pascale G, Montini L, Pennisi M, et al. High dose tigecycline in critically ill patients with severe infections due to multidrug-resistant bacteria. Crit Care. 2014;18(3):R90. [DOI] [PMC free article] [PubMed]
- 37.Geng Ting-Ting, Xu Xin, Huang Man. High-dose tigecycline for the treatment of nosocomial carbapenem-resistant Klebsiella pneumoniae bloodstream infections. Medicine. 2018;97(8):e9961. doi: 10.1097/MD.0000000000009961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ibrahim MM, Abuelmatty AM, Mohamed GH, et al. Best tigecycline dosing for treatment of infections caused by multidrug-resistant pathogens in critically ill patients with different body weights. Drug Des Dev Ther. 2018;12:4171–4179. doi: 10.2147/DDDT.S181834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maseda E, Suárez-de-la-Rica A, Anillo V, et al. A practice-based observational study identifying factors associated with the use of high-dose tigecycline in the treatment of secondary peritonitis in severely ill patients. Rev Esp Quimioter. 2015;28(1):47–53. [PubMed] [Google Scholar]
- 40.Vardakas KZ, Matthaiou DK, Falagas ME, Antypa E, Koteli A, Antoniadou E. Tigecycline for carbapenem-resistant Klebsiella pneumoniae infections in the intensive care unit. Infect Dis. 2015;47(10):751–753. doi: 10.3109/23744235.2015.1049659. [DOI] [PubMed] [Google Scholar]
- 41.Wu Xiaomai, Zhu Yefei, Chen Qiuying, Gong Liuyang, Lin Jian, Lv Dongqing, Feng Jiaxi. Tigecycline Therapy for Nosocomial Pneumonia due to Carbapenem-Resistant Gram-Negative Bacteria in Critically Ill Patients Who Received Inappropriate Initial Antibiotic Treatment: A Retrospective Case Study. BioMed Research International. 2016;2016:1–7. doi: 10.1155/2016/8395268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Di Carlo P, Gulotta G, Casuccio A, et al. KPC-3 Klebsiella pneumoniae ST258 clone infection in postoperative abdominal surgery patients in an intensive care setting: analysis of a case series of 30 patients. BMC Anesthesiol. 2013;13(1):13. [DOI] [PMC free article] [PubMed]
- 43.Ramirez J, Dartois N, Gandjini H, Yan JL, Korth-Bradley J, McGovern PC. Randomized phase 2 trial to evaluate the clinical efficacy of two high-dosage tigecycline regimens versus imipenem-cilastatin for treatment of hospital-acquired pneumonia. Antimicrob Agents Chemother. 2013;57(4):1756–1762. doi: 10.1128/AAC.01232-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Draghi DC, Tench S, Dowzicky MJ, Sahm DF. Baseline in vitro activity of tigecycline among key bacterial pathogens exhibiting multidrug resistance. Chemotherapy. 2008;54(2):91–100. doi: 10.1159/000118660. [DOI] [PubMed] [Google Scholar]
- 45.Rizek C, Ferraz JR, van der Heijden IM, et al. In vitro activity of potential old and new drugs against multidrug-resistant Gram-negatives. J Infect Chemother. 2015;21(2):114–117. doi: 10.1016/j.jiac.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 46.Yahav D, Lador A, Paul M, Leibovici L. Efficacy and safety of tigecycline: a systematic review and meta-analysis. J Antimicrob Chemother. 2011;66(9):1963–1971. doi: 10.1093/jac/dkr242. [DOI] [PubMed] [Google Scholar]
- 47.Ni Wentao, Han Yuliang, Liu Jie, Wei Chuanqi, Zhao Jin, Cui Junchang, Wang Rui, Liu Youning. Tigecycline Treatment for Carbapenem-Resistant Enterobacteriaceae Infections. Medicine. 2016;95(11):e3126. doi: 10.1097/MD.0000000000003126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vardakas KZ, Matthaiou DK, Falagas ME, Antypa E, Koteli A, Antoniadou E. Characteristics, risk factors and outcomes of carbapenem-resistant Klebsiella pneumoniae infections in the intensive care unit. J Infect. 2015;70(6):592–599. doi: 10.1016/j.jinf.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 49.Siwakoti S, Subedi A, Sharma A, Baral R, Bhattarai NR, Khanal B. Incidence and outcomes of multidrug-resistant Gram-negative bacteria infections in intensive care unit from Nepal-a prospective cohort study. Antimicrob Resist Infect Control. 2018;7(1):114. doi: 10.1186/s13756-018-0404-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu Q, Li X, Li W, et al. Influence of carbapenem resistance on mortality of patients with Pseudomonas aeruginosa infection: a meta-analysis. Sci Rep. 2015;5:11715. doi: 10.1038/srep11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morata L, Cobos-Trigueros N, Martínez JA, et al. Influence of multidrug resistance and appropriate empirical therapy on the 30-day mortality rate of Pseudomonas aeruginosa bacteremia. Antimicrob Agents Chemother. 2012;56(9):4833–4837. doi: 10.1128/AAC.00750-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mei H, Yang T, Wang J, Wang R, Cai Y. Efficacy and safety of tigecycline in treatment of pneumonia caused by MDR Acinetobacter baumannii: a systematic review and meta-analysis. J Antimicrob Chemother. 2019;74(12):3423–3431. doi: 10.1093/jac/dkz337. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during our study are included in this published article.