Abstract
Purpose
To assess the role of left ventricular overload and cumulated fluid balance in the development weaning-induced pulmonary edema (WIPO).
Methods
Ventilated patients in sinus rhythm with COPD and/or heart failure (ejection fraction ≤ 40%) were studied. Echocardiography was performed immediately before and during a 30-min spontaneous breathing trial (SBT) using a T-tube. Patients who failed were treated according to echocardiography results before undergoing a second SBT.
Results
Twelve of 59 patients failed SBT, all of them developing WIPO. Patients who succeeded SBT had lower body weight (− 2.5 kg [− 4.8; − 1] vs. + 0.75 kg [− 2.95; + 5.57]: p = 0.02) and cumulative fluid balance (− 2326 ml [− 3715; + 863] vs. + 143 ml [− 2654; + 4434]: p = 0.007) than those who developed WIPO. SBT-induced central hemodynamic changes were more pronounced in patients who developed WIPO, with higher E wave velocity (122 cm/s [92; 159] vs. 93 cm/s [74; 109]: p = 0.017) and E/A ratio (2.1 [1.2; 3.6] vs. 0.9 [0.8; 1.4]: p = 0.001), and shorter E wave deceleration time (85 ms [72; 125] vs. 147 ms [103; 175]: p = 0.004). After echocardiography-guided treatment, all patients who failed the first SBT were successfully extubated. Fluid balance was then negative (− 2224 ml [− 7056; + 100] vs. + 146 ml [− 2654; + 4434]: p = 0.005). Left ventricular filling pressures were lower (E/E′: 7.3 [5; 10.4] vs. 8.9 [5.9; 13.1]: p = 0.028); SBT-induced increase in E wave velocity (+ 10.6% [− 2.7/ + 18] vs. + 25.6% [+ 12.7/ + 49]: p = 0.037) and of mitral regurgitation area were significantly smaller.
Conclusion
In high-risk patients, WIPO appears related to overloaded left ventricle associated with excessive fluid balance. SBT-induced central hemodynamic changes monitored by CCE help in guiding therapy for successful weaning.
Electronic supplementary material
The online version of this article (10.1007/s00134-020-06061-y) contains supplementary material, which is available to authorized users.
Keywords: Ventilator weaning, Pulmonary edema, Water-electrolyte balance, Echocardiography, Echocardiography Doppler
Take-home message
| In high-risk patients, weaning-induced pulmonary edema is related to left ventricular overload associated with excessive fluid balance. Critical care echocardiography allows guiding treatment for successful weaning. |
Introduction
Since prolonged invasive mechanical ventilation is associated with increased morbidity and mortality, patients must be weaned from the ventilator as soon as possible [1]. Nevertheless, weaning failure rate ranges between 26 and 42% [2]. Spontaneous breathing trial (SBT) has been compared to a physical exercise [3]. The transition from positive to negative intrathoracic pressure alters unfavorably cardiac loading conditions, the net result being an abrupt increase in left ventricular (LV) filling pressures [4]. Accordingly, weaning pulmonary edema (WIPO) is a leading cause of weaning failure [5, 6], especially in high-risk patients with an underlying cardiopathy, chronic obstructive pulmonary disease (COPD), or obesity [7].
Negative passive leg raise reflecting the absence of fluid responsiveness [7, 8] and high B-type natriuretic peptide (BNP) [9–12] or NT pro-BNP [9, 13] levels have been shown to be associated with an increased risk of weaning failure. In addition, guidance of fluid management based on daily measurement of plasma BNP concentration significantly reduces fluid balance and shortens the duration of mechanical ventilation, especially in the presence of LV failure [14]. Taken together, these results underline the pivotal contribution of cumulated hydric balance and potentially associated volume overload in the development of WIPO [15].
This study aimed at assessing the ability of critical care echocardiography (CCE) [16] to accurately track SBT-induced central hemodynamic changes in patients at high risk of WIPO and its value for guiding therapy after a failed SBT due to WIPO.
Material and methods
This prospective, observational, single-center study was conducted in the Intensive Care Unit (ICU) of Limoges University Hospital over a 28-month period. The study was approved by the Ethics Committee of the Société de Réanimation de Langue Française which waived informed consent.
Patients
Patients were eligible if they fulfilled the following inclusion criteria: (1) invasive mechanical ventilation for more than 48 h; (2) medical history of COPD [17] and/or heart failure with a left ventricular ejection fraction (LVEF) ≤ 40%; (3) eligibility to perform a weaning trial, as previously described [2] (e-Appendix 1). Patients were not studied if they had at least one of the following criteria: non-sinus rhythm, severe mitral valve disease, mitral valve replacement or repair, tracheostomy, or inadequate imaging quality for Doppler assessment.
In each patient, the following data were recorded on ICU admission: patient’s characteristics, reason for ICU admission, medical history, SAPS II [18] and SOFA [19] scores. Data recorded at each weaning trial are detailed in e-Appendix 1. After extubation, patients were followed until ICU discharge to record the need for re-intubation and its reason, and vital status.
Weaning trials
Weaning trial was considered on a daily basis in a patient under pressure support [1, 2]. In eligible patients, a 30-min SBT on a T-tube was performed with the oxygen flow set to obtain a SpO2 ≥ 90% [2]. ST-segment was monitored continuously during SBT to detect potentially induced myocardial ischemia (Intellivue MP 70, Philips Healthcare, Germany).
SBT failure was clinically diagnosed on previously defined criteria [2, 6], and WIPO was confirmed using chest X-ray or lung ultrasound (e-Appendix 1). Patients were then reconnected to the ventilator, and the treatment was modified according to clinical presentation and CCE results [6, 20]: diuretics (LV overload reflected by increased E wave velocity and/or positive fluid balance), vasodilators including nitrates (hypertension, LV overload, ischemic cardiomyopathy reflected by LV regional wall motion abnormality) and/or angiotensin converting enzyme (ACE) inhibitor (hypertension, LV diastolic dysfunction with elevated filling pressure, worsened mitral regurgitation), or beta-blockers (hypertrophic cardiomyopathy, dynamic obstruction > 30 mmHg measured with continuous wave Doppler) [21]. Elevated LV filling pressure was identified in the presence of grade II or III diastolic dysfunction [22]. Patients were then reassessed daily and underwent a new SBT when weaning criteria were subsequently met. When SBT was clinically successful, the patient was extubated. Extubation failure was defined as the need for re-intubation within 48 h after extubation [2]. All cases of WIPO were independently confirmed off-line by two intensivists who separately reviewed the medical chart of patients who failed SBT, without having access to CCE data (e-Appendix 1, e-Table 1).
Transthoracic echocardiography
In all patients, CCE was systematically performed immediately before and at the end of SBT (after 30 min or earlier at the time of respiratory distress due to failed SBT, before reconnection to the ventilator). This sequence was repeated during subsequent weaning trials, if any. CCE was also systematically performed before re-intubation to determine the cardiac origin of extubation failure, or not. Parameters were measured in triplicate and averaged, off-line, on de-identified CCE images, by an independent and experienced investigator who had no access to medical charts (e-Appendix 1).
Statistical analysis
Considering that WIPO is deemed responsible for approximately 60% of weaning failures [7] and the large range of weaning failure rate reported in high-risk patients [2], we estimated that 61 patients should be studied to reach 20% of WIPO-induced SBT failure with a precision of 10% and an alpha risk of 5%. Quantitative variables were expressed as medians with their 25th and 75th percentiles and quantitative variables as percentages. No use of previous value or interpolation rule was used in the presence of missing data. Hemodynamic parameters were compared individually between baseline and the first SBT using paired samples Wilcoxon test. Patients were separated into two groups according to the success or failure of SBT, and data were compared between groups using a Mann–Whitney U test or a Chi-squared test. In the subset of patients who failed the first SBT but could be successfully extubated after the second weaning trial, individual parameters recorded at the time of the two SBTs were compared using a paired samples Wilcoxon test. Cutoff values of E wave velocity were identified to diagnose failed SBT due to WIPO, and alternatively to predict successful extubation, with a specificity > 90%. Statistical significance was defined as a p value < 0.05 (SPSS, Chicago, IL, USA).
Results
Study population
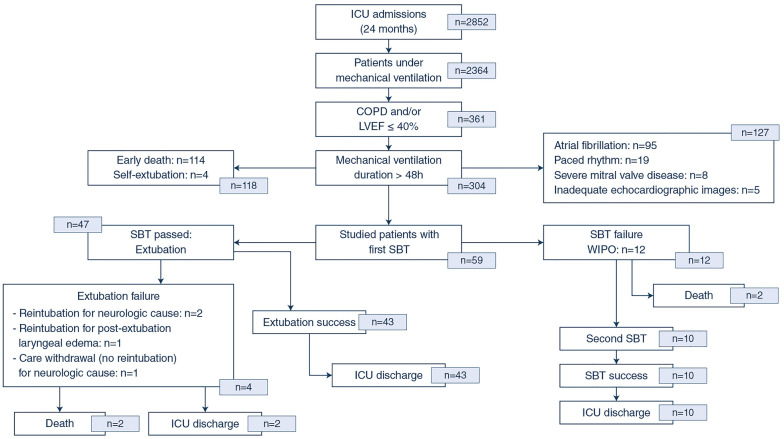
Of the 2852 patients admitted to our medical-surgical ICU during the study period, 2364 were mechanically ventilated (83%) and 361 had a COPD and/or heart failure (15%). Of them, 304 (84%) remained under invasive mechanical ventilation for more than 48 h but 114 died before ventilator weaning, 114 had a non-sinus rhythm, and 17 had exclusion criteria (Fig. 1). Finally, 59 patients were studied (39 men; median age 62 years [52–73]; SAPS2: 38.5 [30–53]; SOFA: 6 [4–9]). Forty-four patients (75%) had a documented heart failure, 21 patients (36%) had a COPD, 6 of them (10%) having both (Table 1). No significant ST-segment variations were recorded during SBTs.
Fig. 1.
Study flow chart. Abbreviations: ICU, intensive care unit; COPD, chronic obstructive pulmonary disease; LVEF, left ventricular ejection fraction; SBT, spontaneous breathing trial
Table 1.
Patients characteristics at inclusion according to the result of the first spontaneous breathing trial (SBT)
| Study population (n = 59) |
SBT success (n = 47) |
SBT failure (n = 12) |
P value | |
|---|---|---|---|---|
| Age, years | 62 (52–73) | 63 (52–73) | 58 (45–70) | 0.38 |
| Gender, M/F | 39/20 | 29/18 | 10/2 | 0.157 |
| Heart failure with LVEF ≤ 40% (%) | 44 (75) | 35 (74) | 9 (75) | 0.97 |
| COPD (%) | 20 (36) | 16 (34) | 4 (33) | 0.848 |
| Reason for ICU admission | ||||
| Acute pulmonary edema (%) | 13 (22) | 10 (21) | 3 (25) | 0.78 |
| Cardiogenic shock (%) | 7 (12) | 6 (13) | 1 (8) | 0.67 |
| Cardiorespiratory arrest (%) | 7 (12) | 6 (13) | 1 (8) | 0.78 |
| Exacerbation of COPD (%) | 6 (10) | 5 (11) | 1 (8) | 0.81 |
| Post cardiac surgery (%) | 9 (15) | 8 (17) | 1 (8) | 0.76 |
| Other (%) | 17 (29) | 12 (26) | 5 (41) | 0.47 |
| Medical history | ||||
| Ischemic cardiomyopathy (%) | 38 (53) | 31 (66) | 7 (58) | 0.95 |
| Dilated cardiomyopathy (%) | 11 (19) | 7 (28) | 4 (33) | 0.09 |
| Hypertrophic cardiomyopathy (%) | 6 (10) | 6 (13) | 0 (0) | 0.22 |
| Severe aortic valve disease (%) | 0 (0) | 0 (0) | 0 (0) | – |
| Severe pulmonary hypertension (%) | 0 (0) | 0 (0) | 0 (0) | – |
| Non severe valve disease (%) | 8 (17) | 6 (13) | 2 (17) | 0.62 |
| Obesity (%) | 13 (22) | 10 (21) | 3 (25) | 0.64 |
| Severity scores | ||||
| SAPS 2 | 38.5 (30–53) | 39 (30–53) | 35.5 (23.5–51) | 0.46 |
| SOFA | 6 (4–9) | 6 (4–9) | 6 (3–10) | 0.72 |
| Vital status | ||||
| ICU mortality (%) | 4 (7) | 2 (4) | 2 (17) | 0.13 |
| Therapy on inclusion | ||||
| Bronchodilators (%) | 9 (15) | 7 (15) | 2 (16) | 0.765 |
| Corticosteroids (%) | 8 (14) | 6 (13) | 2 (16) | 0.62 |
| Furosemide (%) | 25 (42) | 20 (43) | 5 (42) | 0.82 |
| Nitrates (%) | 1 (2) | 1 (2) | 0 (0) | 0.63 |
| Catecholamines (%) | 17 (29) | 13 (28) | 4 (33) | 0.54 |
| Respiratory parameters | ||||
| FiO2, % | 30 (30–40) | 30 (30–40) | 30 (30–37.5) | 0.695 |
| Pressure support level, cm H2O | 12 (10–14) | 12 (10–14) | 14 (11–15) | 0.234 |
| PEEP, cmH2O | 7 (4–10) | 6 (4–10) | 9 (7–10) | 0.128 |
| RR, breath/min | 19 (16–24) | 19 (16–22) | 20 (16–20) | 0.884 |
| Duration of mechanical ventilation, h | 110 (60–180) | 96 (54–171) | 127 (86–241) | 0.30 |
| SaO2, % | 98 (96–99) | 100 (96–100) | 97 (96–99) | 0.59 |
| PaO2, mmHg | 142 (81–234) | 99 (85–108) | 86 (85–120) | 0.51 |
| PaO2/FiO2 | 287 (235–351) | 287 (228–353) | 288 (241–340) | 0.91 |
| PaCO2, mmHg | 42 (33–60) | 36 (32–43) | 35 (32–40) | 0.55 |
| Hydric balance | ||||
| Weight difference (admission), kg | − 2.0 (− 4.2/ + 1.2) | − 2.5 (− 4.8/ + 1) | 0.75 (− 2.95/ + 5.6) | 0.02 |
| Fluid balance (admission), ml | − 2150 (− 3505/ + 1282) | − 2326 (− 3715/ + 863) | 146 (− 2654/ + 4434) | 0.047 |
Results are expressed as medians (with 25th and 75th percentiles in parentheses), or as numbers (percentages in parentheses)
COPD chronic obstructive pulmonary disease, SAPS Simplified Acute Physiology score, SOFA sepsis-related organ failure assessment, ICU intensive care unit, PEEP positive end-expiratory pressure, RR respiratory rate
First weaning trial
SBT was first performed after a median duration of mechanical ventilation of 96 h [54–171]. Forty-seven patients succeeded SBT and were subsequently extubated, whereas the remaining 12 patients (20%) developed WIPO and were maintained under mechanical ventilation. Four of the 47 extubated patients (8.5%) developed weaning failure within 48 h after the planned extubation, but none was of cardiac origin (Fig. 1).
When compared to positive-pressure ventilation, E wave maximal velocity significantly increased (95 cm/s [75–115] vs. 85 cm/s [61–100]: p < 0.001), E wave deceleration time shortened (128 ms [89–163] vs. 147 ms [109–206]: p < 0.001), E/E' ratio and peak velocity of tricuspid regurgitation increased during SBT (Table 2).
Table 2.
Hemodynamic parameters and echocardiographic findings obtained immediately before and during the first spontaneous breathing trial (SBT)
| General population (n = 59) |
SBT success (n = 47) |
SBT failure (n = 12) |
P value | |
|---|---|---|---|---|
| Before SBT | ||||
| Hemodynamic parameters | ||||
| HR, bpm | 94 (82–108) | 90 (80–103) | 96 (86–113) | 0.13 |
| sBP, mmHg | 138 (130–151) | 142 (130–153) | 131 (108–138) | 0.08 |
| dBP, mmHg | 79 (71–87)* | 78 (71–87) | 81 (69–88) | 0.65 |
| mBP, mmHg | 97 (87–107)* | 98 (88–107)* | 92 (82–110) | 0.61 |
| Echocardiography findings | ||||
| E, cm/s | 85 (61–100)* | 83 (62–91) | 101 (84–144) | 0.046 |
| A, cm/s | 85 (62–109) | 86 (23–172) | 58 (37–101) | 0.025 |
| E/A | 0.9 (0.8–1.4)* | 0.9 (0.7–1.1) | 1.7 (1.3–2.4) | 0.001 |
| DTE, ms | 147 (109–206)* | 173 (128–213) | 107 (94–129) | 0.002 |
| E′ lateral, cm/s | 9.9 (7.5–12.6) | 9.9 (7.5–12.4) | 9.8 (7–15.3) | 0.66 |
| E/E′ | 8.4 (5.7–11.3)* | 9.1 (5.6–11) | 9.2 (6.6–12.5) | 0.32 |
| MR/LA area | 0.18 (0.14–0.23) | 0.2 (0.16–0.24) | 0.15 (0.13–0.31) | 0.87 |
| LVEF, % | 35 (24–40) | 35 (26–40) | 24 (18–36) | 0.083 |
| LVOT VTI, cm | 16.1 (12.8–21) | 16.6 (13.4–20.9) | 14.6 (8.5–21.2) | 0.295 |
| TR peak velocity, m/s | 2.96 (2.66–3.38)* | 2.87 (2.53–3.05) | 3.39 (2.86–3.78) | 0.047 |
| During SBT | ||||
| Hemodynamic parameters | ||||
| HR, bpm | 102 (91–115) | 96 (86–113) | 131 (113–143) | < 0.0001 |
| sBP, mmHg | 146 (125–158) | 147 (125–157) | 140 (118–168) | 0.8 |
| dBP, mmHg | 80 (70–95)* | 80 (70–93) | 80 (65–98) | 0.98 |
| mBP, mmHg | 104 (90–113)* | 104 (93–113)* | 111 (72–113) | 0.95 |
| Echocardiography findings | ||||
| E, cm/s | 95 (75–115)* | 93 (74–109) | 122 (92–159) | 0.017 |
| A, cm/s | 83 (68–112) | 88 (75–114) | 60 (33–94) | 0.012 |
| E/A | 0.9 (0.8–1.8)* | 0.88 (0.8–1.3) | 2.1 (1.2–3.6) | 0.001 |
| DTE, ms | 128 (89–163)* | 147 (103–175) | 85 (72–125) | 0.004 |
| E′ lateral, cm/s | 10.6 (8–12.6) | 10 (8.1–12.3) | 11.4 (7–16.1) | 0.26 |
| E/ E′ | 9.5 (7.5–12.7)* | 9.6 (7.2–12.7) | 9.3 (7.8–16) | 0.84 |
| MR/LA area | 0.21 (0.15–0.3) | 0.21 (0.14–0.24) | 0.26 (0.17–0.4) | 0.57 |
| LVEF, % | 32 (22–42) | 33 (24–46) | 23 (13–33) | 0.033 |
| LVOT VTI, cm | 16.8 (12.8–20.7) | 17.5 (14.1–20.8) | 11.5 (7.7–19.2) | 0.088 |
| TR peak velocity, m/s | 3.18 (2.92–3.56)* | 3.01 (2.73–3.3) | 3.67 (3.15–4.15) | 0.12 |
HR heart rate, sBP systolic blood pressure, dBP diastolic blood pressure, mBP mean blood pressure, DTE deceleration time of mitral E wave, MR mitral regurgitation, LA left atrium, LVEF left ventricular ejection fraction, LVOT left ventricular outflow tract, VTI velocity–time integral, TR tricuspid regurgitation
*Significant difference between parameters measured under positive pressure ventilation and during the weaning trial
When compared to patients who failed SBT, patients who passed SBT and were extubated had a negative fluid balance during hospitalization (-2326 ml [− 3715/ + 863] vs. + 146 ml [− 2654/ + 4434]: p = 0.047) and loss of body weight relative to admission (− 2.5 kg [− 4.8/− 1.0] vs. + 0.75 kg [− 2.95/ + 5.6]: p = 0.02). No other significant difference was noted between clinical characteristics of both groups, except for a higher heart rate in weaning failure patients (Tables 1 and 2). Under positive pressure ventilation, patients who failed SBT exhibited a higher E wave maximal velocity (101 cm/s [84–144] vs. 83 cm/s [62–91]: p = 0.046), an increased E/A ratio (1.7 [1.3–2.4] vs. 0.9 [0.7–1.1]: p = 0.001), a shorter E wave deceleration time (107 ms [94–129] vs. 173 ms [128–213]: p = 0.002) and a higher tricuspid regurgitation peak velocity (3.39 m/s [2.86–3.78] vs. 2.87 m/s [2.53–3.05]: p = 0.047), while baseline LV ejection fraction tended to be lower (24% [18–36] vs. 35% [26–40]: p = 0.083). In contrast, no significant difference in the E' wave maximal velocity and E/E' ratio was noted (Table 2). During SBT, variations of Doppler parameters were more marked in patients who failed than in those who succeeded the weaning trial: higher increase in E wave maximal velocity (122 cm/s [92–159] vs. 93 cm/s [74–109]: p = 0.017) and of E/A ratio (2.1 [1.2–3.6] vs. 0.9 [0.8–1.4]: p = 0.001), and greater shortening of E wave deceleration time (85 ms [72–125] vs. 147 ms [103–175]: p = 0.004). Again, no significant difference was observed for the E' wave maximal velocity and E/E' ratio (Table 2).
Second weaning trial
Of the 12 patients who failed the first SBT due to WIPO, two died before subsequent weaning trial. All 10 remaining patients succeeded the second SBT and were successfully extubated without requiring re-intubation until ICU discharge (Fig. 1). Median body weight significantly decreased between both SBTs relative to admission (− 5.0 kg [− 9.5; − 1.7)] vs. + 1.6 kg [− 2.1; + 6.5]: p = 0.005) as did fluid balance (− 2224 ml [− 7056; + 100)] vs. + 146 ml [− 2654; + 4434]: p = 0.005).
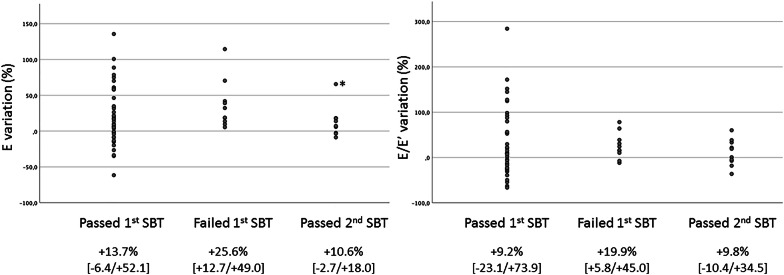
After the initial SBT failure, 9 of 10 patients received diuretics, 4 patients received ACE inhibitors, one patient received nitrates, and a single patient received beta-blocker for LV outflow tract dynamic obstruction associated with massive eccentric acute mitral regurgitation secondary to a systolic anterior motion of the mitral valve. Between both weaning trials, median duration of invasive mechanical ventilation reached 59 h [25.5–103]. Before the second SBT, E wave maximal velocity and E/E’ ratio were significantly lower than before the first SBT (84 cm/s [67–127] vs. 102 cm/s [85–149]: p = 0.017 and 7.3 [5.0–10.4] vs. 8.9 [5.9–13.1]: p = 0.028, respectively). During the second SBT, maximal E wave velocity, E/A and E/E′ ratios were lower than during the first weaning trial (100 cm/s [85–122] vs. 122 cm/s [93–168]: p = 0.009; 1.5 [0.9–2.3] vs. 2.5 [1.1–3.6]: p = 0.009; and 7.7 [6.2–9.9] vs. 9.3 [7.5–12.6]: p = 0.047, respectively), and tricuspid regurgitation peak velocity tended to be lower (2.90 [2.69–3.37] vs. 3.67 [3.00–4.18]: p = 0.068) (Table 3). In addition, functional mitral regurgitation jet area normalized by left atrial area significantly decreased during the second SBT (0.11 [0.06–0.29] vs. 0.26 [0.20–0.39]; p = 0.028) (Fig. 2). Beta-blocker dramatically reduced both the LV outflow tract obstruction and mitral regurgitation in the patient with hypertrophic cardiomyopathy. Finally, SBT-induced variation of E wave velocity significantly decreased during the second weaning attempt when compared to the initial failed SBT (+ 10.6% [− 2.7/ + 18.0] vs. + 25.6% [+ 12.7/ + 49.0]: p = 0.037) and was similar to that observed in patients who successfully passed the first weaning trial (+ 13.7% [− 6.4/ + 52.1]). Similar results were obtained for E/E’, the difference being not statistically significant (Fig. 3 and e-Table 2). Other Doppler indices were not significantly altered by SBT.
Table 3.
Hemodynamic parameters and echocardiography findings in the subset of patients who failed the first SBT but succeeded the second weaning trial
| 1st failed SBT (n = 10) |
2nd passed SBT (n = 10) |
P value | |
|---|---|---|---|
| Before weaning trials | |||
| Hemodynamic parameters | |||
| HR, bpm | 107 (92–126)* | 102 (91–114) | 0.308 |
| sBP, mmHg | 131 (108–142) | 144 (120–160) | 0.475 |
| dBP, mmHg | 82 (68–90) | 82 (77–91) | 0.477 |
| mBP, mmHg | 92 (82–111) | 102 (90–116) | 0.312 |
| Echocardiography parameters | |||
| E, cm/s | 102 (85–149) | 84 (67–127) | 0.017 |
| A, cm/s | 58 (41–110) | 62 (48–93) | 0.88 |
| E/A | 1.7 (1.2–2.7) | 1.3 (0.8–2.1) | 0.139 |
| DTE, ms | 103 (92–134) | 133 (103–184) | 0.139 |
| E′ lateral, cm/s | 11.1 (7.6–16.4) | 13.5 (8.8–15.4) | 0.646 |
| E/E′ | 8.9 (5.9–13.1) | 7.3 (5–10.4) | 0.028 |
| MR/LA area | 0.15 (0.14–0.2) | 0.11 (0.06–0.17) | 0.249 |
| LVEF, % | 30 (20–36) | 34 (27–38) | 0.161 |
| LVOT VTI, cm | 14.6 (9.3–22.6) | 15.0 (10.9–19) | 0.678 |
| TR peak velocity, m/s | 3.43 (2.69–4.52) | 3.2 (2.64–3.48) | 0.285 |
| During weaning trials | |||
| Hemodynamic parameters | |||
| HR, bpm | 131 (112–138) | 108 (99–111) | 0.011 |
| sBP, mmHg | 143 (125–168) | 133 (113–156) | 0.919 |
| dBP, mmHg | 88 (63–99) | 77 (70–78) | 0.308 |
| mBP, mmHg | 112 (72–115) | 94 (85–106) | 0.507 |
| Echocardiography parameters | |||
| E, cm/s | 122 (93–168) | 100 (85–122) | 0.009 |
| A, cm/s | 60 (34–103) | 67 (48–93) | 0.48 |
| E/A | 2.5 (1.1–3.6) | 1.5 (0.9–2.3) | 0.009 |
| DTE, ms | 81 (71–128) | 130 (97–133) | 0.093 |
| E′ lateral, cm/s | 12.5 (8.9–16.4) | 14.2 (8.5–15.6) | 0.878 |
| E/E′ | 9.3 (7.5–12.6) | 7.7 (6.2–9.9) | 0.047 |
| MR/LA area | 0.26 (0.2–0.39) | 0.11 (0.06–0.29) | 0.028 |
| LVEF, % | 26 (16–35) | 29 (26–36) | 0.401 |
| LVOT VTI, cm | 14 (8.9–20.1) | 15.5 (12.6–20) | 0.263 |
| TR peak velocity, m/s | 3.67 (3–4.18) | 2.90 (2.69–3.37) | 0.068 |
| Hydric balance | |||
| Weight difference (admission), kg | + 1.6 (− 2.1/ + 6.5) | − 5.0 (− 9.5/− 1.7) | 0.005 |
| Fluid balance (admission), ml | + 146 (− 2654/ + 4434) | − 2224 (− 7056/ + 100) | 0.005 |
HR heart rate, sBP systolic blood pressure, dBP diastolic blood pressure, mBP mean blood pressure, DTE deceleration time of mitral E wave, MR mitral regurgitation, LA left atrium, LVEF left ventricular ejection fraction, LVOT left ventricular outflow tract, VTI velocity–time integral, TR tricuspid regurgitation
*Significant difference between parameters measured under positive pressure ventilation and during the weaning trial
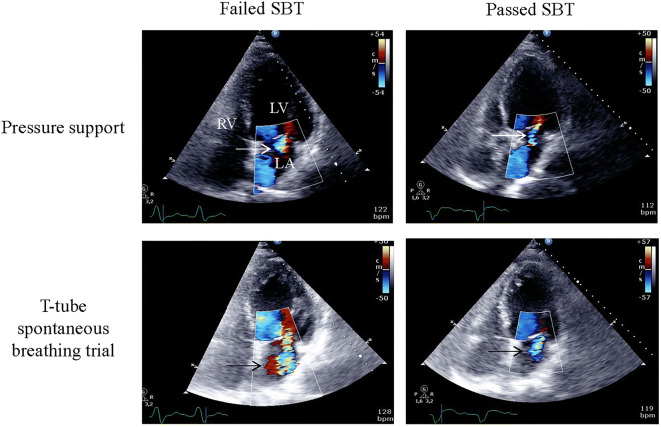
Fig. 2.
Variations of functional mitral regurgitation severity during spontaneous breathing trials (SBT) and effect of echocardiography-guided therapy. Central functional mitral regurgitation at baseline (upper left, arrow) significantly increased during the failed spontaneous breathing trial (lower left panel, arrow). After a treatment associating diuretics and angiotensin converting enzyme inhibitors, the central mitral regurgitation which remained trivial at baseline (upper right panel, arrow) failed to significantly worsen during the second weaning trial (lower right panel, arrow), and the patient was successfully extubated
Fig. 3.
Variations of E wave velocity and E/E′ induced by spontaneous breathing trials (SBT) in patients who succeed at the first attempt and in those who failed during the first weaning trial but then successfully passed the second SBT after an echocardiography-guided therapy. Results are expressed in percentages of baseline values (positive-pressure ventilation). *: despite a large SBT-induced increase in E wave velocity during the second (successful) weaning trial in this patient, the absolute value of E wave was markedly lower when compared to the first (failed) SBT: baseline 2 = 65 cm/s and SBT 2 = 103 cm/s vs. baseline 1 = 88 cm/s and SBT 1 = 122 cm/s
Proposed cutoff values
E velocity < 0.6 m/s both at baseline and during SBT had a 100% specificity and a 15% sensitivity to predict passed SBT. E velocity ≥ 0.6 m/s at baseline and E ≥ 1.2 m/s during SBT had a 91% specificity and 67% sensitivity to identify failed SBT due to WIPO.
Discussion
We showed in the present study that high-risk patients who failed SBT due to WIPO exhibited echocardiographic findings consistent with LV overload (i.e., increased preload and afterload) together with excessive cumulated fluid balance. Worsened or acutely developed mitral regurgitation appeared as a contributing factor to SBT failure. CCE-guided therapy helped attending physicians to successfully extubate patients who previously failed SBT due to WIPO, after a mean of 2.5 days.
The 27% SBT failure rate observed in the present study is in keeping with that commonly reported in a high-risk population [2]. As previously reported [23], our patients who developed WIPO had significantly higher maximal E wave velocity and E/A ratio, shorter E wave deceleration time, lower LVEF, and higher tricuspid regurgitation peak velocity under positive-pressure ventilation, when compared to their counterparts. This presumably reflects the incapacity of their cardiovascular system to sustain SBT-induced abrupt increase in cardiac work load [3–6]. LV was abruptly overloaded by SBT as reflected by a significant rise of maximal E wave velocity and E/A ratio, a significant shortening of E wave deceleration time [23, 24], and augmented tricuspid regurgitation peak velocity used as a surrogate of systolic pulmonary artery pressure [25]. Not surprisingly, these changes were even more pronounced in the subset of our patients who failed SBT due to WIPO. E/E′, a routinely used indicator of LV filling pressure, was in the lower range at baseline presumably because a large proportion of our high-risk patients received diuretics before SBT (median hydric balance: + 146 mL). In addition, E/E′ failed to significantly increase during SBT because E′ also increased presumably in response to sympathetic stimulation [26]. Maximal E wave velocity—which directly reflects the pressure gradient between the left atrium and the LV at early diastole—is the result of both LV loading conditions and relaxation properties [27]. Worsened functional mitral regurgitation during SBT may contribute to increase E wave velocity and subsequent weaning failure in response to augmented LV preload and afterload [13]. The increase in both E wave maximal velocity and E/A ratio associated with the shortening of E wave deceleration time concordantly reflect a significant rise of LV filling pressure [4], since these parameters are correlated to the pulmonary capillary pressure in patients with LV systolic dysfunction [22, 27, 28], a large proportion of our study population. In light of the absence of significant ST-segment variations during SBTs, this suggests that the abrupt rise of LV filling pressure induced by SBT was related to LV overload rather than deteriorated LV relaxation or compliance of ischemic origin [4, 6]. This pathophysiologic mechanism is also supported by the higher weight gain and cumulative fluid balance relative to ICU admission in patients who failed SBT [8–13]. Of note, with the exception of higher heart rate—a rather non-specific sign—no clinical parameter was significantly associated with SBT failure in our patients. This underlines the additional value of monitoring central hemodynamics using CCE during weaning trial in patients at high risk of WIPO [15, 29, 30].
In our high-risk patients who passed SBT, the absence of relevant central hemodynamic changes identified with CCE during the weaning trial confirmed the feasibility of extubation. This diagnostic strategy was successful since no extubation failure of cardiac origin was observed after tracheal tube withdrawal. In those who failed the first SBT, CCE was used to guide therapy according to a predefined therapeutic algorithm. Since a relevant increase in E wave velocity consistent with an overloaded LV was observed in most patients at the time of SBT failure, diuretics and vasodilators were predominantly used to reduce both LV preload and afterload [31, 32]. A beta-blocker was used in a patient with a dynamic LV outflow tract obstruction and severe acute mitral regurgitation related to a systolic anterior motion of the mitral valve [33]. After these therapeutic changes, all patients who initially failed the first SBT could be subsequently extubated successfully. Immediately before and during passed second SBT, maximal E wave velocity, E/A and E/E′ ratios, and tricuspid regurgitation peak velocity were lower than at the time of failed first weaning trial, whereas E wave deceleration time was prolonged. In our patients with relevant functional mitral insufficiency at baseline, the regurgitant volume reflected by the jet area significantly decreased between the two weaning trials. The resulting relative increase in E wave velocity induced by the weaning trial was significantly lower during the second (passed) SBT when compared to the first (failed) one. These changes suggest that CCE-guided therapy unloaded LV within a median delay of 2.5 days. The significant decrease in body weight and cumulated fluid balance relative to the first weaning trial suggest a link between LV overload and excessive fluid balance at the origin of WIPO. Volume overload has been associated with worse weaning outcome [34, 35].
The present results may not be reproducible using other types of weaning trial. Low-pressure support level without PEEP has been shown to induce less central hemodynamic variations than SBT using a T-piece [36]. In a recent meta-analysis, Sklar et al. [37] suggested that SBT using a T-piece best mimics changes in cardiac and respiratory loading conditions resulting from extubation. In our high-risk population, its use has presumably sensitized SBT to reveal WIPO. Since the precise cutoff value of LV filling pressure which would precipitate the development of WIPO is unknown for a given patient [4] and most Doppler indices are in the intermediate range at baseline [38, 39], the magnitude of SBT-induced variations should be precisely determined and interpreted in lights of clinical presentation, the patient being his own control. When compared to right-heart catheterization which is considered the reference for the diagnosis of WIPO [4], CCE is less invasive, rapid to implement, easy to use, and depicts certain mechanisms of WIPO which are not accessible to conventional monitoring systems, such as LV outflow tract obstruction as in one of our patient. In this specific clinical setting, right heart catheterization typically depicts elevated pulmonary artery occlusion pressure and low cardiac index which may erroneously lead to diuretics, vasodilators or even inotropes therapy, all of them exacerbating the hemodynamic burden [33].
This prospective study is limited by its strict eligibility criteria, excluding patients with non-sinus rhythm and selecting high-risk patients as reflected by the 100% prevalence of WIPO as a cause of SBT failure. Accordingly, the efficacy of CCE-based strategy of weaning needs to be confirmed in a broader population [40], using the herein proposed E wave cutoff values to guide volume depletion and vasodilators administration. The diagnosis of WIPO relied on both the clinical presentation and CCE findings, as advocated [6], rather than on invasive measurement of pulmonary artery occlusion pressure [4], and lung ultrasound was not systematically used. Nevertheless, this study is the first to establish a link between SBT-induced central hemodynamic changes, overloaded LV, positive fluid balance, and WIPO.
Conclusion
SBT failure due to WIPO appears related to overloaded LV, including the worsening or development of mitral regurgitation, associated with excessive cumulated fluid balance. SBT-induced central hemodynamic changes monitored by CCE helps guiding weaning of patients at high risk of WIPO.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions:
MG and PV included patients, analyzed the data and drafted the manuscript. PC, JBA, BE, ALF, TD and BF included patients and critically reviewed the manuscript. All authors read and approved the final version of the manuscript.
Funding
None.
Compliance with ethical standards
Conflict of interest
Authors has no conflict of interest to declare with respect to this work.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Girard TD, Alhazzani W, Kress JP, Ouellette DR, Schmidt GA, Truwit JD, et al. An Official American Thoracic Society/American College of Chest Physicians Clinical Practice Guideline: Liberation from Mechanical Ventilation in Critically Ill Adults. Rehabilitation Protocols, Ventilator Liberation Protocols, and Cuff Leak Tests. Am J Respir Crit Care Med. 2017;195:120–133. doi: 10.1164/rccm.201610-2075ST. [DOI] [PubMed] [Google Scholar]
- 2.Boles J-M, Bion J, Connors A, Herridge M, Marsh B, Melot C, Pearl R, Silverman H, Stanchina M, Vieillard-Baron A, Welte T. Weaning from mechanical ventilation. Eur Respir J. 2007;29:1033–1056. doi: 10.1183/09031936.00010206. [DOI] [PubMed] [Google Scholar]
- 3.Pinsky MR. Breathing as exercise: the cardiovascular response to weaning from mechanical ventilation. Intensive Care Med. 2000;26:1164–1166. doi: 10.1007/s001340000619. [DOI] [PubMed] [Google Scholar]
- 4.Lemaire F, Teboul JL, Cinotti L, Giotto G, Abrouk F, Steg G, Macquin-Mavier I, Zapol WM. Acute left ventricular dysfunction during unsuccessful weaning from mechanical ventilation. Anesthesiology. 1988;69:171–179. doi: 10.1097/00000542-198808000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Perren A, Brochard L. Managing the apparent and hidden difficulties of weaning from mechanical ventilation. Intensive Care Med. 2013;39:1885–1895. doi: 10.1007/s00134-013-3014-9. [DOI] [PubMed] [Google Scholar]
- 6.Teboul J-L, Monnet X, Richard C. Weaning failure of cardiac origin: recent advances. Crit Care. 2010;14:211. doi: 10.1186/cc8852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu J, Shen F, Teboul J-L, Anguel N, Beurton A, Bezaz N, Richard C, Monnet X. Cardiac dysfunction induced by weaning from mechanical ventilation: incidence, risk factors, and effects of fluid removal. Crit Care. 2016;20:369. doi: 10.1186/s13054-016-1533-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dres M, Teboul J-L, Anguel N, Guerin L, Richard C, Monnet X. Passive leg raising performed before a spontaneous breathing trial predicts weaning-induced cardiac dysfunction. Intensive Care Med. 2015;41:487–494. doi: 10.1007/s00134-015-3653-0. [DOI] [PubMed] [Google Scholar]
- 9.Zapata L, Vera P, Roglan A, Gich I, Ordonez-Llanos J, Betbesé AJ. B-type natriuretic peptides for prediction and diagnosis of weaning failure from cardiac origin. Intensive Care Med. 2011;37:477–485. doi: 10.1007/s00134-010-2101-4. [DOI] [PubMed] [Google Scholar]
- 10.Mekontso-Dessap A, de Prost N, Girou E, Braconnier F, Lemaire F, Brun-Buisson C, Brochard L. B-type natriuretic peptide and weaning from mechanical ventilation. Intensive Care Med. 2006;32:1529–1536. doi: 10.1007/s00134-006-0339-7. [DOI] [PubMed] [Google Scholar]
- 11.Chien J-Y, Lin M-S, Huang Y-CT, Chien Y-F, Yu C-J, Yang P-C. Changes in B-type natriuretic peptide improve weaning outcome predicted by spontaneous breathing trial. Crit Care Med. 2008;36:1421–1426. doi: 10.1097/CCM.0b013e31816f49ac. [DOI] [PubMed] [Google Scholar]
- 12.Dres M, Teboul J-L, Anguel N, Guerin L, Richard C, Monnet X. Extravascular lung water, B-type natriuretic peptide, and blood volume contraction enable diagnosis of weaning-induced pulmonary edema. Crit Care Med. 2014;42:1882–1889. doi: 10.1097/CCM.0000000000000295. [DOI] [PubMed] [Google Scholar]
- 13.Gerbaud E, Erickson M, Grenouillet-Delacre M, Beauvieux MC, Coste P, Durrieu-Jaïs C, Hilbert G, Castaing Y, Vargas F. Echocardiographic evaluation and N-terminal pro-brain natriuretic peptide measurement of patients hospitalized for heart failure during weaning from mechanical ventilation. Minerva Anestesiol. 2012;78:415–425. [PubMed] [Google Scholar]
- 14.Mekontso Dessap A, Roche-Campo F, Kouatchet A, Tomicic V, Beduneau G, Sonneville R, Cabello B, Jaber S, Azoulay E, Castanares-Zapatero D, Devaquet J, Lellouche F, Katsahian S, Brochard L. Natriuretic peptide-driven fluid management during ventilator weaning: a randomized controlled trial. Am J Respir Crit Care Med. 2012;186:1256–1263. doi: 10.1164/rccm.201205-0939OC. [DOI] [PubMed] [Google Scholar]
- 15.Vignon P. Cardiovascular failure and weaning. Ann Transl Med. 2018;6:354. doi: 10.21037/atm.2018.05.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayo PH, Beaulieu Y, Doelken P, Feller-Kopman D, Harrod C, Kaplan A, Oropello J, Vieillard-Baron A, Axler O, Lichtenstein D, Maury E, Slama M, Vignon P. American College of Chest Physicians/La Société de Réanimation de Langue Française statement on competence in critical care ultrasonography. Chest. 2009;135:1050–1060. doi: 10.1378/chest.08-2305. [DOI] [PubMed] [Google Scholar]
- 17.Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ, Nishimura M, Stockley RA, Sin DD, Rodriguez-Roisin R. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 18.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270:2957–2963. doi: 10.1001/jama.1993.03510240069035. [DOI] [PubMed] [Google Scholar]
- 19.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22:707–710. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 20.Routsi C, Stanopoulos I, Kokkoris S, Sideris A, Zakynthinos S. Weaning failure of cardiovascular origin: how to suspect, detect and treat-a review of the literature. Ann Intensive Care. 2019;9:6. doi: 10.1186/s13613-019-0481-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maron MS, Olivotto I, Zenovich AG, Link MS, Pandian NG, Kuvin JT, Nistri S, Cecchi F, Udelson JE, Maron BJ. Hypertrophic cardiomyopathy is predominantly a disease of left ventricular outflow tract obstruction. Circulation. 2006;114:2232–2239. doi: 10.1161/CIRCULATIONAHA.106.644682. [DOI] [PubMed] [Google Scholar]
- 22.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Alexandru Popescu B, Waggoner AD. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2016;17:1321–1360. doi: 10.1093/ehjci/jew082. [DOI] [PubMed] [Google Scholar]
- 23.Caille V, Amiel J-B, Charron C, Belliard G, Vieillard-Baron A, Vignon P. Echocardiography: a help in the weaning process. Crit Care. 2010;14:R120. doi: 10.1186/cc9076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ait-Oufella H, Tharaux P-L, Baudel J-L, Vandermeersch S, Meyer P, Tonnellier M, Dussaule JC, Guidet B, Offenstadt G, Maury E. Variation in natriuretic peptides and mitral flow indexes during successful ventilatory weaning: a preliminary study. Intensive Care Med. 2007;33:1183–1186. doi: 10.1007/s00134-007-0627-x. [DOI] [PubMed] [Google Scholar]
- 25.Vignon P. Assessment of pulmonary arterial pressure using critical care echocardiography: dealing with the Yin and the Yang? Crit Care Med. 2019;47:126–128. doi: 10.1097/CCM.0000000000003491. [DOI] [PubMed] [Google Scholar]
- 26.Nagueh SF, Sun H, Kopelen HA, Middleton KJ, Khoury DS. Hemodynamic determinants of the mitral annulus diastolic velocities by tissue Doppler. J Am Coll Cardiol. 2001;37:278–285. doi: 10.1016/S0735-1097(00)01056-1. [DOI] [PubMed] [Google Scholar]
- 27.Appleton CP, Hatle LK, Popp RL. Relation of transmitral flow velocity patterns to left ventricular diastolic function: new insights from a combined hemodynamic and Doppler echocardiographic study. J Am Coll Cardiol. 1988;12:426–440. doi: 10.1016/0735-1097(88)90416-0. [DOI] [PubMed] [Google Scholar]
- 28.Vanoverschelde JL, Raphael DA, Robert AR, Cosyns JR. Left ventricular filling in dilated cardiomyopathy: relation to functional class and hemodynamics. J Am Coll Cardiol. 1990;15:1288–1295. doi: 10.1016/S0735-1097(10)80016-6. [DOI] [PubMed] [Google Scholar]
- 29.Vignon P, Repessé X, Vieillard-Baron A, Maury E. Critical care ultrasonography in acute respiratory failure. Crit Care. 2016;20:228. doi: 10.1186/s13054-016-1400-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mayo P, Volpicelli G, Lerolle N, Schreiber A, Doelken P, Vieillard-Baron A. Ultrasonography evaluation during the weaning process: the heart, the diaphragm, the pleura and the lung. Intensive Care Med. 2016;42:1107–1117. doi: 10.1007/s00134-016-4245-3. [DOI] [PubMed] [Google Scholar]
- 31.Voors AA, de Jong RM. Treating diastolic heart failure. Heart Br Card Soc. 2008;94:971–972. doi: 10.1136/hrt.2008.144816. [DOI] [PubMed] [Google Scholar]
- 32.Elkayam U. Nitrates in the treatment of congestive heart failure. Am J Cardiol. 1996;77:41C–51C. doi: 10.1016/S0002-9149(96)00188-9. [DOI] [PubMed] [Google Scholar]
- 33.Adamopoulos C, Tsagourias M, Arvaniti K, Veroniki F, Matamis D. Weaning failure from mechanical ventilation due to hypertrophic obstructive cardiomyopathy. Intensive Care Med. 2005;31:734–737. doi: 10.1007/s00134-005-2616-2. [DOI] [PubMed] [Google Scholar]
- 34.Frutos-Vivar F, Ferguson ND, Esteban A, Epstein SK, Arabi Y, Apezteguía C, González M, Hill NS, Nava S, D'Empaire G, Anzueto A. Risk factors for extubation failure in patients following a successful spontaneous breathing trial. Chest. 2006;130:1664–1671. doi: 10.1378/chest.130.6.1664. [DOI] [PubMed] [Google Scholar]
- 35.National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, Connors AF, Jr, Hite RD, Harabin AL. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 36.Cabello B, Thille AW, Roche-Campo F, Brochard L, Gómez FJ, Mancebo J. Physiological comparison of three spontaneous breathing trials in difficult-to-wean patients. Intensive Care Med. 2010;36:1171–1179. doi: 10.1007/s00134-010-1870-0. [DOI] [PubMed] [Google Scholar]
- 37.Sklar MC, Burns K, Rittayamai N, Lanys A, Rauseo M, Chen L, Dres M, Chen GQ, Goligher EC, Adhikari NKJ, Brochard L, Friedrich JO. Effort to Breathe with Various Spontaneous Breathing Trial Techniques. A Physiologic Meta-analysis. Am J Respir Crit Care Med. 2017;195:1477–1485. doi: 10.1164/rccm.201607-1338OC. [DOI] [PubMed] [Google Scholar]
- 38.Moschietto S, Doyen D, Grech L, Dellamonica J, Hyvernat H, Bernardin G. Transthoracic Echocardiography with Doppler Tissue Imaging predicts weaning failure from mechanical ventilation: evolution of the left ventricle relaxation rate during a spontaneous breathing trial is the key factor in weaning outcome. Crit Care. 2012;16:R81. doi: 10.1186/cc11339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lamia B, Maizel J, Ochagavia A, Chemla D, Osman D, Richard C, Teboul JL. Echocardiographic diagnosis of pulmonary artery occlusion pressure elevation during weaning from mechanical ventilation. Crit Care Med. 2009;37:1696–1701. doi: 10.1097/CCM.0b013e31819f13d0. [DOI] [PubMed] [Google Scholar]
- 40.Al-Omari MA, Finstuen J, Appleton CP, Barnes ME, Tsang TSM. Echocardiographic assessment of left ventricular diastolic function and filling pressure in atrial fibrillation. Am J Cardiol. 2008;101:1759–1765. doi: 10.1016/j.amjcard.2008.02.067. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.