Abstract
Objective
Residual paravalvular regurgitation (PVR) has been associated to adverse outcomes after transcatheter aortic valve replacement (TAVR). This study sought to evaluate the impact of device landing zone (DLZ) calcification on residual PVR after TAVR with different next-generation transcatheter heart valves.
Methods
642 patients underwent TAVR with a SAPIEN 3 (S3; n=292), ACURATE neo (NEO; n=166), Evolut R (ER; n=132) or Lotus (n=52). Extent, location and asymmetry of DLZ calcification were assessed from contrast-enhanced CT imaging and correlated to PVR at discharge.
Results
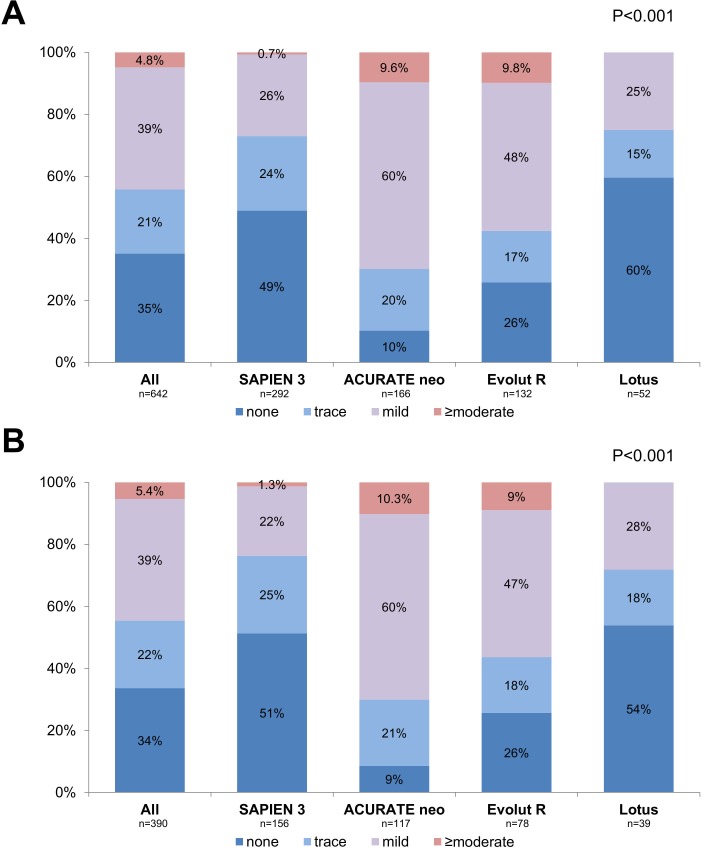
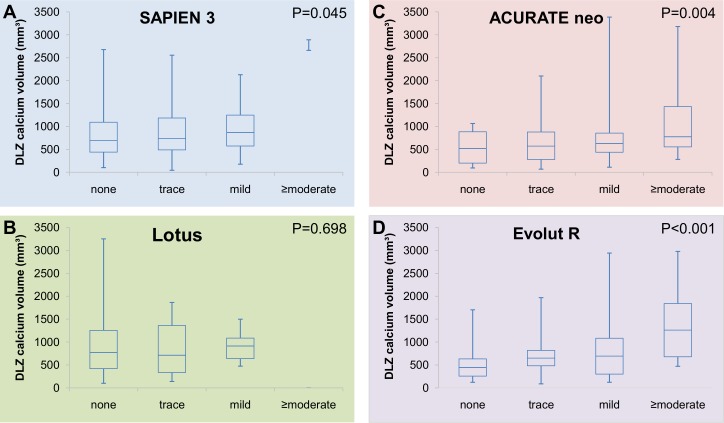
PVR was ≥moderate in 0.7% of S3 patients, 9.6% of NEO patients, 9.8% of ER patients and 0% of Lotus patients (p<0.001), and these differences remained after matching for total DLZ calcium volume. The amount of DLZ calcium was significantly related to the degree of PVR in patients treated with S3 (p=0.045), NEO (p=0.004) and ER (p<0.001), but not in Lotus patients (p=0.698). The incidence of PVR ≥moderate increased significantly over the tertiles of DLZ calcium volume (p=0.046). On multivariable analysis, calcification of the aortic valve cusps, LVOT calcification and the use of self-expanding transcatheter aortic valve implantation (TAVI) prostheses emerged as predictors of PVR.
Conclusions
The susceptibility to PVR depending on the amount of calcium was mainly observed in self-expanding TAVI prostheses. Thus, DLZ calcification is an important factor to be considered in prosthesis selection for each individual patient, keeping in mind the trade-off between PVR reduction, risk of new pacemaker implantation and unfavourable valve ha emodynamics.
Keywords: prosthetic heart valves, aortic valve disease, calcium
Key questions.
What is already known about this subject?
The extent of device landing zone (DLZ) calcification is an important risk factor for paravalvular regurgitation (PVR) after transcatheter aortic valve replacement (TAVR).
What does this study add?
The relation between DLZ calcium and PVR differed between different TAVR devices with significantly higher susceptibility for DLZ calcium and, consequently, higher rates of PVR after TAVR with self-expanding devices compared with balloon or mechanically expanding devices. Besides PVR, also the need for new permanent pacemaker implantation and valve haemodynamics differed significantly between the devices.
How might this impact on clinical practice?
Our data might help clinicians to consider the advantages and disadvantages of each prosthesis for an individualised prosthesis selection.
Introduction
Over the last decade, transcatheter aortic valve replacement (TAVR) has evolved to the standard of care for the treatment of severe aortic stenosis in patients at intermediate or high risk for conventional surgery and expansion to lower risk populations is on the rise.1–3 Paravalvular regurgitation (PVR) is a major limitation of TAVR, being linked to increased mortality.4 5 The rapid extension of interventional therapy has been facilitated by significant advancements in device technology resulting in lower rates of periprocedural complications.6 Device landing zone (DLZ) calcifications which impact sealing of the transcatheter heart valves (THVs) to the aortic valve cusps (AVCs) and left ventricular outflow tract (LVOT) have been identified as the main predictor of PVR in numerous studies with first-generation devices.7–12 With the introduction of next-generation TAVR devices with dedicated sealing mechanisms, the rate of relevant PVR decreased significantly, however to a variable extent with different prostheses due to differences in device design, implantation mechanism and radial force. The aim of this study was to determine the impact of extent and distribution of DLZ calcium on PVR with several next-generation valves in order to facilitate prosthesis selection since comparative data on different device systems of the current generation are lacking.
Methods
In total, 792 consecutive patients underwent TAVR for severe native aortic stenosis with various next-generation transcatheter valves at three centres. For CT analysis, 150 patients were excluded because of missing or low-quality CT (n=73) or transthoracic echocardiography (TTE) data (n=70), or conversion to conventional surgery (n=7). Six hundred forty-two patients with complete data were included into the study cohort. Prostheses used included the SAPIEN 3 (S3; Edwards Lifesciences, Irvine, CA, USA), ACURATE neo (NEO; Boston Scientific, Marlborough, MA, USA), Evolut R (ER; Medtronic, Minneapolis, MN, USA) and Lotus (Boston Scientific). Prosthesis selection was at the discretion of the operating physicians considering individual patient characteristics. The study was conducted in accordance with German and European regulations. Informed consent was waived by the ethics committee due to the retrospective nature of the analysis. Patients were considered not suitable for surgical aortic valve replacement by the local interdisciplinary heart-team. Pre-procedural routine work-up included echocardiography, coronary angiography and contrast-enhanced multi-slice computed tomography (MSCT). Valve sizing was based on the manufacturer’s recommendations. Primary endpoint was the degree of PVR at discharge. Secondary clinical and safety VARC-2 endpoints are reported on the overall population in order to reduce an inherent bias for certain complications in case of presenting only patients with both CT and TTE data.
MSCT data analysis
All MSCT data analysis was performed by two independent observers who were unaware of outcome measures (interobserver variability for total calcium volume: 47.8±123.6 mm³, intraclass correlation coefficient 0.98). Images were acquired on a dual source CT scanner (Siemens) with a slice thickness of 1 mm and 40 mL of intravenously administered Accupaque contrast agent. The aortic annulus was defined as a virtual basal plane at the nadirs of the valve cusps. The DLZ was defined as the composite of the AVC region (basal plane to the lower coronary ostium) and the LVOT (from basal plane 10 mm into the left ventricle). The calcium volume in the DLZ was measured by using an automated volume-scoring tool (3mensio Medical Imaging) with an empiric starting threshold of 500 Hounsfield units (HU). The threshold was subsequently adjusted manually (steps of 20 HU) in each patient depending on the patient’s individual density of luminal contrast medium for best discrimination between calcium and contrast medium. Both AVC and LVOT were subdivided into three regions of interest along the AVCs and analysed separately (online supplementary figure 1).
openhrt-2019-001164supp001.pdf (1.1MB, pdf)
Area oversizing was calculated using the formula THV (nominal area/annulus area–1)×100, and perimeter oversizing accordingly. Eccentricity of the aortic annulus was evaluated with an index calculated as 1–(minimum diameter/maximum diameter). Asymmetry of calcium distribution was calculated as maximum absolute difference in calcium volume between leaflet sectors for both AVC and LVOT.
Evaluation of residual PVR
Residual PVR was analysed by two observers based on pre-discharge TTE using both quantitative and qualitative parameters according to the VARC-2 recommendations.
Statistics
Categorical variables are reported as frequencies and percentages, continuous variables as mean±SD or median (IQR), as applicable. Differences between THV groups were evaluated using Fisher’s exact test for categorical variables and Kruskal-Wallis test for continuous variables. Kendall’s τ correlation coefficients were calculated for the comparison of patients with different degrees of PVR. An ordinal logistic regression model was fitted to estimate the impact of AVC calcification, LVOT calcification and valve type (reference: S3) on the degree of PVR. Asymmetry was not considered in the model as asymmetry and calcification are highly correlated (Pearson’s correlation coefficient r=0.613 for AVC calcium/asymmetry; r=0.934 for LVOT calcium/asymmetry). Case-matching was performed based on total DLZ calcium volume in a nearest neighbour fashion (4:3:2:1, 390 matched patients: S3: n=156; NEO: n=117; ER: n=78; Lotus: n=39) with an average SD tolerance of 6.6%±2.7%. Two-sided p values <0.05 were considered statistically significant. All analyses were performed using IBM SPSS Statistics V.22.
Results
Patient population and DLZ characteristics
A total of 642 patients undergoing TAVR for severe aortic stenosis with a next-generation transcatheter device, including the SAPIEN 3 (n=292), ACURATE neo (n=166), Evolut R (n=132) or Lotus (n=52), were analysed. Baseline and DLZ characteristics were significantly different between the different treatment groups (table 1). In the overall cohort, mean age was 82.0±5.8 years, 58.3% were female and patients had an intermediate to high risk for surgery (EuroSCORE I: 22.8%±15.9%; EuroSCORE II: 4.4%±4.0%). A transfemoral, transapical and transaortic approach was chosen in 93.9%, 5.0% and 1.1%of patients, respectively.
Table 1.
Patient characteristics
| All | SAPIEN 3 | ACURATE neo | Evolut R | Lotus | P value | |
| n=642 | n=292 | n=166 | n=132 | n=52 | ||
| Baseline and procedural characteristics | ||||||
| Age (years) | 82.0±5.8 | 81.2±6.4 | 82.9±4.8 | 83.1±5.5 | 81.4±5.7 | 0.01 |
| Female sex | 374 (58.3) | 127 (43.5) | 123 (74.1) | 96 (92.7) | 28 (53.8) | <0.001 |
| BMI (kg/m²) | 27.3±8.1 | 27.6±10.1 | 27.6±5.7 | 25.9±5.9 | 28.5±6.4 | 0.007 |
| BSA (m²) | 1.82±0.22 | 1.87±0.22 | 1.79±0.19 | 1.74±0.21 | 1.89±0.25 | <0.001 |
| Baseline creatinine (mg/dL) | 1.27±0.78 | 1.34±0.82 | 1.19±0.57 | 1.19±0.81 | 1.40±1.00 | 0.007 |
| EuroSCORE I (%) | 22.8±15.9 | 24.3±17.2 | 22.1±15.0 | 21.5±13.6 | 19.8±15.7 | 0.122 |
| EuroSCORE II (%) | 4.6±4.1 | 4.8±4.4 | 4.3±4.1 | 4.3±3.3 | 4.7±4.6 | 0.791 |
| Pre-existing pacemaker | 73 (11.4) | 36 (12.3) | 16 (9.7) | 14 (10.6) | 7 (13.5) | 0.795 |
| Predilation | 359 (56.5) | 128 (44.0) | 151 (92.6) | 33 (25.6) | 47 (90.4) | <0.001 |
| Postdilation | 82 (12.9) | 12 (4.1) | 41 (25.2) | 28 (21.7) | 1 (1.9) | <0.001 |
| Comorbidities | ||||||
| COPD | 110 (17.1) | 66 (22.6) | 21 (12.7) | 15 (11.4) | 8 (15.4) | 0.009 |
| Peripheral artery disease | 132 (20.6) | 73 (25.0) | 33 (19.9) | 17 (12.9) | 9 (17.3) | 0.032 |
| Diabetes | 200 (31.2) | 92 (31.5) | 54 (32.5) | 39 (29.5) | 15 (28.8) | 0.939 |
| Hypertension | 584 (91) | 258 (88.4) | 157 (94.6) | 121 (91.7) | 48 (92.3) | 0.16 |
| Coronary artery disease | 402 (62.2) | 174 (59.6) | 111 (66.9) | 83 (62.9) | 34 (65.4) | 0.464 |
| Previous cardiac surgery | 124 (19.3) | 82 (28.1) | 20 (12.0) | 20 (15.2) | 2 (3.8) | <0.001 |
| Atrial fibrillation | 262 (40.9) | 123 (42.1) | 63 (38.2) | 57 (43.2) | 19 (36.5) | 0.717 |
| Device landing zone characteristics | ||||||
| Area (mm²) | 479.9±102.8 | 510.5±104.2 | 454.9±98.9 | 449.4±94.1 | 464.7±80.3 | <0.001 |
| Perimeter (mm) | 79.1±8.1 | 81.6±8.3 | 77.1±7.8 | 76.8±7.2 | 77.5±6.6 | <0.001 |
| AVC calcium volume (mm³) | 616 (406–979) | 697 (425–1059) | 581 (359–834) | 590 (375–879) | 718 (414–1005) | 0.009 |
| NCC (mm³) | 273 (156–4449) | 286 (168–481) | 251 (150–378) | 253 (149–384) | 336 (177–514) | 0.048 |
| RCC (mm³) | 175 (96–305) | 197 (114–333) | 154 (87–244) | 165 (87–305) | 181 (86–337) | 0.007 |
| LCC (mm³) | 157 (89–276) | 172 (99–323) | 142 (73–261) | 142 (88–251) | 170 (88–251) | 0.028 |
| LVOT calcium volume (mm³) | 25 (4–114) | 28 (4–109) | 19 (2–98) | 33 (5–140) | 24 (2–158) | 0.488 |
| LVOTNC (mm³) | 6 (0–38) | 6 (0–36) | 4 (0–31) | 9 (0–44) | 7 (0–49) | 0.341 |
| LVOTRC (mm³) | 3 (0–8) | 0 (0–8) | 0 (0–8) | 1 (0–8) | 0 (0–6) | 0.811 |
| LVOTLC (mm³) | 4 (0–33) | 4 (0–34) | 3 (0–16) | 4 (0–48) | 6 (0–71) | 0.672 |
| Total DLZ calcium volume (mm³) | 683 (441–1073) | 740 (472–1175) | 626 (388–882) | 620 (411–983) | 819 (434–12 289) | 0.006 |
| AVC absolute asymmetry (mm³) | 199±156 | 213±162 | 171±127 | 185±133 | 247±226 | 0.07 |
| LVOT absolute asymmetry (mm³) | 56±92 | 52±82 | 51±93 | 71±115 | 63±80 | 0.456 |
Values are mean±SD, n (%) or median (IQR).
AVC, aortic valve cusp; BMI, body mass index; BSA, body surface area; COPD, chronic obstructive pulmonary disease; DLZ, device landing zone; LVOT, left ventricular outflow tract; NCC/RCC/LCC, non-/right/left coronary cusp.
Total DLZ calcium volume was 834±569 mm³ with significant differences between the different THV with highest average calcium volume in the Lotus group and lowest calcium volumes in the NEO group (p=0.006). Median calcium volume of the aortic valve cusps was 616 (406–979) mm³, with the non-coronary cusp being the most calcified region, followed by the right and left coronary cusp. Median LVOT calcium volume was 25 (4–114) mm³, being individually widely dispersed. The mean AVC maximum absolute asymmetry was 199±156 mm³, and LVOT absolute asymmetry was 56±92 mm³.
Residual PVR and clinical endpoints
The occurrence and degree of residual PVR differed significantly between the devices (figure 1A). PVR was ≥moderate in 0.7% of S3 patients, 9.6% of NEO patients, 9.8% of ER patients and 0.0% of Lotus patients, respectively (p<0.001). Due to significant differences in DLZ calcification between the different devices (being lower in self-expanding devices), patients were case-matched according to total DLZ calcium. After matching, total DLZ calcium was well balanced between the different groups (866±593 mm³; p=0.999). However, the degree of residual PVR remained significantly different between groups, with PVR ≥moderate being present in 1.3% of S3, 10.3% of NEO, 9.0% of ER and 0% of Lotus patients (p<0.001; figure 1B).
Figure 1.
Degree of residual paravalvular regurgitation. (A) Degree of paravalvular regurgitation (PVR) with different devices. (B) Degree of PVR with different devices after matching for total device landing zone calcium volume.
Haemodynamics were significantly different between the studied devices with significantly lower mean transvalvular gradients after TAVR with the self-expanding NEO or ER compared with S3 and Lotus (table 2). Permanent pacemaker implantation (PPI) rates (excluding patients with pre-existing pacemaker) were 11.3%, 10.4%, 21.8% and 37.7% in S3, NEO, ER and Lotus patients, respectively (p<0.001). Rates of 30-day mortality and stroke were numerically different without being statistically significant, whereas the unadjusted rates of major vascular complications and major or life-threatening bleeding were significantly different with highest event rates in the Lotus group (table 2).
Table 2.
Clinical endpoints
| SAPIEN 3 | ACURATE neo | Evolut R | Lotus | P value | |
| n=382 | n=189 | n=157 | n=63 | ||
| 30-day mortality | 15 (3.9) | 3 (1.6) | 7 (4.5) | 5 (7.9) | 0.105 |
| Major vascular complication | 15 (3.9) | 2 (1.1) | 4 (2.5) | 5 (7.9) | 0.042 |
| Major/life-threatening bleeding | 21 (5.5) | 4 (2.1) | 3 (1.9) | 6 (9.5) | 0.019 |
| Stroke | 8 (2.1) | 3 (1.6) | 5 (3.2) | 3 (4.8) | 0.397 |
| Early safety | 38 (9.9) | 7 (3.7) | 14 (8.9) | 12 (19.0) | 0.002 |
| New permanent pacemaker* | 36 (11.3) | 18 (10.4) | 31 (21.8) | 20 (37.7) | <0.001 |
| ΔPmean post TAVI (mm Hg) | 12.3±4.7 | 9.2±3.9 | 8.6±4.3 | 13.5±5.0 | <0.001 |
Values are n (%) or mean±SD.
*Patients with pre-existing pacemaker were excluded from these calculations.
TAVI, transcatheter aortic valve implantation.
DLZ calcification patterns and residual PVR
Higher DLZ calcium load was significantly related to the occurrence and degree of PVR in patients treated with the self-expanding NEO (p=0.004) and ER (p<0.001), but to a lesser extent after S3 implantation (p=0.045) and not related in Lotus valve patients (p=0.698 figure 2A–D).
Figure 2.
Device landing zone (DLZ) calcium volume and paravalvular regurgitation (PVR) with different devices. Relation between DLZ calcification and degree of PVR in patients treated with (A) SAPIEN 3; (B) Lotus; (C) ACURATE neo; (D) Evolut R.
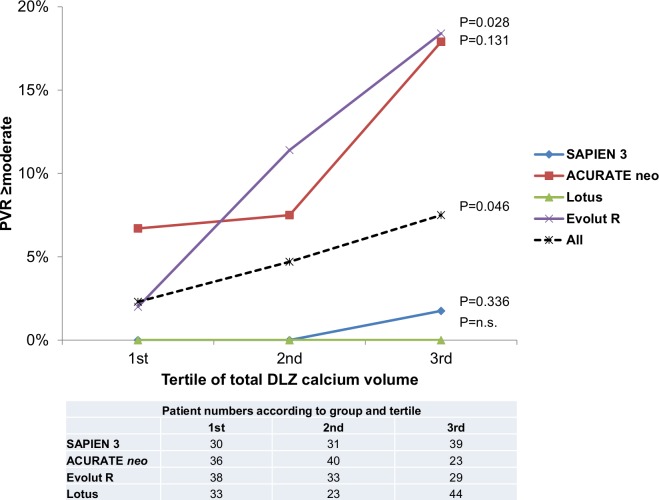
In the overall cohort, the rate of PVR ≥moderate increased significantly over the 3 tertiles of total DLZ calcium volume (2.3%, 4.7% and 7.5%, respectively). However, this increase was predominantly observed in NEO and ER patients, but not in S3 and Lotus patients (p=0.046; figure 3). Combined ROC-curve analysis was performed for NEO and ER patients to predict PVR ≥moderate (AUC 0.70; p<0.001). The risk of PVR ≥moderate was significantly higher above a DLZ calcium load of 1257.9 mm³ (8.6 vs 33.3%; OR 5.3, 95% CI 2.4 to 11.6; p<0.001).
Figure 3.
Paravalvular regurgitation (PVR) ≥moderate according to total calcium volume. The rate of patients with PVR ≥moderate increased significantly over the 3 tertiles of device landing zone (DLZ) calcium volume, and this increase was driven by increasing rates of PVR ≥moderate after self-expanding transcatheter aortic valve replacement.
Besides the susceptibility for PVR depending on total DLZ calcium volume, also the influence of calcium location and distribution as assessed by absolute sector asymmetry varied between the different devices. In NEO and ER patients, both AVC and LVOT calcium were predictive for the degree of PVR, whereas only AVC calcium load predicted PVR in S3 patients, but not LVOT calcification. Both AVC and LVOT asymmetry were related to the degree of PVR in NEO patients, but only LVOT asymmetry in ER patients. In patients treated with S3 and Lotus, asymmetry was not related to the degree of PVR. Annular eccentricity or prosthesis sizing in relation to the native annulus was not associated to the degree of PVR in any of the devices. The details of the different calcification patterns in relation to the degree of PVR are given in online supplementary table 1.
openhrt-2019-001164supp002.pdf (36.5KB, pdf)
An ordinal logistic regression model to predict the degree of PVR was fitted including valve type, AVC and LVOT calcification. Thereby, the use of NEO or ER (with the SAPIEN 3 as reference), AVC calcium and LVOT calcium emerged as independent predictors of PVR, whereas the use of Lotus did not (table 3).
Table 3.
Ordinal logistic regression analysis for the prediction of PVR
| OR | 95% CI | P value | |
| Valve type (SAPIEN 3 reference) | |||
| ACURATE neo | 7.2 | 5.0 to 10.5 | <0.001 |
| Evolut R | 3.8 | 2.5 to 5.7 | <0.001 |
| Lotus | 0.7 | 0.4 to 1.3 | 0.212 |
| AVC calcium | 1.005 | 1.002 to 1.009 | 0.004 |
| LVOT calcium | 1.014 | 1.003 to 1.026 | 0.013 |
ORs for calcium per 10 mm³ increase.
AVC, aortic valve cusp; LVOT, left ventricular outflow tract; PVR, paravalvular regurgitation.
Discussion
To our best knowledge, we present the currently broadest study addressing the impact of device landing zone calcification patterns on PVR after TAVR with a variety of different next-generation prostheses. The main findings of our study are (1) higher DLZ calcium volume and asymmetry as well as the use of a self-expanding device result in higher degrees of PVR; (2) the relation between DLZ calcium and PVR differed between the tested devices with significantly higher susceptibility for DLZ calcium and consequently, higher rates of PVR ≥moderate after TAVR with self-expanding devices; (3) despite PVR, also the need for new permanent pacemaker implantation, valve haemodynamics and short-term clinical outcome differed between the devices which may be taken into consideration in an individualised prosthesis selection.
Numerous studies have shown the negative impact of PVR ≥moderate on mortality after TAVR, some suggesting even mild PVR might have a negative impact on outcome.4 5 Several risk factors for PVR have been identified for first-generation valves, including DLZ calcification, valve type, undersizing and annulus eccentricity.5 7–12 Our study showed a significantly higher susceptibility to DLZ calcium of the self-expanding NEO and ER compared with the balloon-expandable S3 and the mechanically expandable Lotus resulting in significantly higher rates of PVR after self-expanding TAVR. The observed PVR rates of all valves are within the range of previously published studies.13–17 Notably, the degree of PVR seems unrelated to DLZ calcification patterns in Lotus treated patients, although the low patient number in this treatment group precludes any further conclusion. The difference in patients with PVR ≥moderate was most pronounced in the tertile of patients with highest DLZ calcium load, putting these patients at a highly increased risk for relevant PVR (figure 3). Conversely, the rate of PVR was acceptably low with all studied devices in the lower third of patients. Based on these data, any device might be used in patients with mild to moderate calcifications, whereas in patients with higher calcium load, a balloon-expandable or mechanically expandable THV might be advantageous to avoid PVR. This has to be counterweighed with the rare risk of aortic root or LVOT injury that has previously been associated with LVOT calcification in smaller studies.18 In our cohort, we did not see an association of LVOT calcification and aortic root complications (n=3). However, the incidence of aortic root injury decreased over the last years, most likely due to spreading of CT-based sizing strategies and growing operator experience.19 This may also be the reason that—in contrast to previous studies20—we did not see an association of undersizing or annulus eccentricity and PVR in our study.
Beyond the total extent of DLZ calcium, also the location and asymmetry of calcification affected the studied devices to a variable degree. Again, the self-expanding devices were more susceptible to PVR in asymmetrically calcified anatomies, whereas there was no influence of an asymmetric calcium distribution after balloon-expandable or mechanically expandable TAVR.
In line with previously published data, transvalvular gradients were most favourable after TAVR with the self-expanding devices and significantly higher after balloon-expandable and even more pronounced after mechanically expandable devices.21 Elevated transvalvular gradients and consecutively calculated parameters such as prosthesis–patient mismatch are well-known predictors of mortality, recurrence of heart failure symptoms, rehospitalisation and structural valve deterioration after surgical aortic valve replacement and TAVR.22–25
Besides PVR, the unadjusted rates of other important endpoints which may affect long-term outcomes were different. Namely, in line with other contemporary studies, the rate of new-onset conduction disturbance requiring PPI were 10.4% and 11.3% for NEO and S3 treated patients, but significantly higher after TAVR with ER or Lotus (21.8% and 37.7%), respectively.26–28 However, it has to be noted that in contrast to PVR, the reported secondary endpoints are more prone to be influenced by differences in baseline patient characteristics and should consequently be interpreted with caution.
Lower risk populations with longer inherent life expectancy and higher levels of activity may be more prone to the negative effects of long-term right ventricular pacing or unfavourable haemodynamics compared with the initially treated high-risk patients. The current rapid expansion of TAVR indications to such lower risk populations1 emphasises the need for a tailored prosthesis selection in each individual patient. Clinicians need to keep in mind the risk factors for certain complications, such as DLZ calcification patterns for PVR, as shown in this study, pre-existing conduction disturbances for new PPI, namely a pre-existing right bundle branch block, and for example, small annular dimensions for unfavourable haemodynamics. With current next-generation devices, there seems to be a trade-off between protection against PVR on one side, and the risk for new PPI or unfavourable haemodynamics on the other side that has to be considered in each individual patient and counterweighed along with the advantages and disadvantages of each valve system in order to achieve the best possible long-term results for our patients. None of the current studied devices seem to address all these issues in a favourable manner. Whether these limitations may be overcome by the further developed upcoming generation of TAVR devices, such as the S3 Ultra, the NEO AS, the Lotus Edge or the Evolut Pro, remains to be elucidated.
Study limitations
This study is a retrospective observational analysis with all inherent limitations. The individual valve choice in each patient was not randomised, but based on the best clinical knowledge of the operating heart-team at the time of procedure. Differences in DLZ calcium volume in the unadjusted dataset underline that the clinical decision on TAVR device was already based on the assumption that self-expanding TAVI prostheses are more susceptible to PVR. Thus, a certain confirmation bias cannot be fully excluded despite case-matching on DLZ calcium volume. Moreover, the reference standard for calcium quantification is the calculation of Agatston units using non–contrast-enhanced CT scans. In the present study, calcium was measured using a CT protocol based on routinely acquired contrast-enhanced CT images since a contrast-free sequence is not performed routinely at many centres to avoid increased radiation exposure. The used method of calcium quantification has been previously shown to correlate well with the reference standard (R=0.84, p<0.001) and to be superior to methods with unadjusted thresholds, although calcium may be underestimated in patients with high aortic attenuation.29 Finally, not all studied devices were commercially available during the complete study period. The Evolut Pro device which seems to be less susceptible to calcifications was not available during the study period and has therefore not been evaluated.
Conclusion
DLZ calcification predicted the degree of PVR after TAVR. The probability of PVR depending on the amount of calcium differed significantly with higher susceptibility in the self-expanding NEO and ER compared with the S3 or the Lotus. However, also permanent pacemaker implantation rates and transvalvular gradients differed significantly between the implanted devices. Thus, DLZ calcification is an important factor to be considered in prosthesis selection for each individual patient, keeping in mind the trade-off between PVR reduction, the occurrence of new-onset conduction disturbances requiring permanent pacemaker implantation and unfavourable valve haemodynamics.
Acknowledgments
We thank Anne Adams, M. Sc., from the Institute of Medical Statistics and Computational Biology, University of Cologne, for additional statistical advice.
Footnotes
Contributors: VM and TR designed the study. TF, FD, KM, AR, NS, MA and SS performed CT analysis. VM, FD, KM, MIK, KF and NS performed TTE analysis. VM, TF, FD, KM, AR, MIK, NS, MA, KF, EWK and SS contributed to clinical patient data acquisition. VM and KK performed the statistical analyses. VM, VR and TR interpreted the data. VM and TR wrote the main manuscript text. EWK, VR, TCWW, SB, NM and US contributed to important intellectual content and critically revised the manuscript. TR supervised the work. All authors read and approved the final manuscript.
We acknowledge support by the DFG Open Access Publication Funds of the Ruhr-Universität Bochum.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: FD received speakers honoraria from Boston Scientific. NS received travel compensation from Edwards and speakers honoraria and travel compensation from Boston Scientific. VR received speakers honoraria from Medtronic. TCWW and NM are proctors for Edwards. SB is a proctor for Edwards and received a research grant from Edwards. US is proctor for Edwards and Symetis SA and received research grants from Symetis SA and Edwards. TR is proctor for Boston Scientific and and received speakers honoraria from Edwards, Medtronic and Boston Scientific/Symetis.
Patient consent for publication: Not required.
Ethics approval: The study was approved by the Ethics Committee of the University Hospital of Cologne (ID 19-1032).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available from the first author on reasonable request.
References
- 1.Popma JJ, Deeb GM, Yakubov SJ, et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med 2019;380:1706–15. 10.1056/NEJMoa1816885 [DOI] [PubMed] [Google Scholar]
- 2.Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129:e650:2440–92. 10.1161/CIR.0000000000000029 [DOI] [PubMed] [Google Scholar]
- 3.Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J 2017;38:616–64. 10.1093/ejcts/ezx324 [DOI] [PubMed] [Google Scholar]
- 4.Kodali S, Pibarot P, Douglas PS, et al. Paravalvular regurgitation after transcatheter aortic valve replacement with the Edwards sapien valve in the PARTNER trial: characterizing patients and impact on outcomes. Eur Heart J 2015;36:449–56. 10.1093/eurheartj/ehu384 [DOI] [PubMed] [Google Scholar]
- 5.Athappan G, Patvardhan E, Tuzcu EM, et al. Incidence, predictors, and outcomes of aortic regurgitation after transcatheter aortic valve replacement: meta-analysis and systematic review of literature. J Am Coll Cardiol 2013;61:1585-95. 10.1016/j.jacc.2013.01.047 [DOI] [PubMed] [Google Scholar]
- 6.Athappan G, Gajulapalli RD, Tuzcu ME, et al. A systematic review on the safety of second-generation transcatheter aortic valves. EuroIntervention 2016;11:1034–43. 10.4244/EIJV11I9A211 [DOI] [PubMed] [Google Scholar]
- 7.Seiffert M, Fujita B, Avanesov M, et al. Device landing zone calcification and its impact on residual regurgitation after transcatheter aortic valve implantation with different devices. Eur Heart J Cardiovasc Imaging 2016;17:576–84. 10.1093/ehjci/jev174 [DOI] [PubMed] [Google Scholar]
- 8.Buellesfeld L, Stortecky S, Heg D, et al. Extent and distribution of calcification of both the aortic annulus and the left ventricular outflow tract predict aortic regurgitation after transcatheter aortic valve replacement. EuroIntervention 2014;10:732–8. 10.4244/EIJV10I6A126 [DOI] [PubMed] [Google Scholar]
- 9.Khalique OK, Hahn RT, Gada H, et al. Quantity and location of aortic valve complex calcification predicts severity and location of paravalvular regurgitation and frequency of post-dilation after balloon-expandable transcatheter aortic valve replacement. JACC Cardiovasc Interv 2014;7:885–94. 10.1016/j.jcin.2014.03.007 [DOI] [PubMed] [Google Scholar]
- 10.Ewe SH, Ng ACT, Schuijf JD, et al. Location and severity of aortic valve calcium and implications for aortic regurgitation after transcatheter aortic valve implantation. Am J Cardiol 2011;108:1470–7. 10.1016/j.amjcard.2011.07.007 [DOI] [PubMed] [Google Scholar]
- 11.Feuchtner G, Plank F, Bartel T, et al. Prediction of paravalvular regurgitation after transcatheter aortic valve implantation by computed tomography: value of aortic valve and annular calcification. Ann Thorac Surg 2013;96:1574–80. 10.1016/j.athoracsur.2013.06.049 [DOI] [PubMed] [Google Scholar]
- 12.Fonseca P, Figueiredo B, Almeida C, et al. Aortic valve calcium volume predicts paravalvular regurgitation and the need for balloon post-dilatation after transcatheter aortic valve implantation. J Interv Cardiol 2016;29:117–23. 10.1111/joic.12267 [DOI] [PubMed] [Google Scholar]
- 13.Asch FM, Vannan MA, Singh S, et al. Hemodynamic and echocardiographic comparison of the Lotus and CoreValve transcatheter aortic valves in patients with high and extreme surgical risk: an analysis from the Reprise III randomized controlled trial. Circulation 2018;137:2557–67. 10.1161/CIRCULATIONAHA.118.034129 [DOI] [PubMed] [Google Scholar]
- 14.Noble S, Stortecky S, Heg D, et al. Comparison of procedural and clinical outcomes with Evolut R versus Medtronic CoreValve: a Swiss TAVI registry analysis. EuroIntervention 2017;12:e2170–6. 10.4244/EIJ-D-16-00677 [DOI] [PubMed] [Google Scholar]
- 15.Enríquez-Rodríguez E, Amat-Santos IJ, Jiménez-Quevedo P, et al. Comparison of the hemodynamic performance of the balloon-expandable SAPIEN 3 versus self-expandable Evolut R transcatheter valve: a case-matched study. Rev Esp Cardiol 2018;71:735–42. 10.1016/j.rec.2017.10.025 [DOI] [PubMed] [Google Scholar]
- 16.Pibarot P, Hahn RT, Weissman NJ, et al. Association of paravalvular regurgitation with 1-year outcomes after transcatheter aortic valve replacement with the SAPIEN 3 valve. JAMA Cardiol 2017;2:1208. 10.1001/jamacardio.2017.3425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lanz J, Kim W-K, Walther T, et al. Safety and efficacy of a self-expanding versus a balloon-expandable bioprosthesis for transcatheter aortic valve replacement in patients with symptomatic severe aortic stenosis: a randomised non-inferiority trial. Lancet 2019;394:1619–28. 10.1016/S0140-6736(19)32220-2 [DOI] [PubMed] [Google Scholar]
- 18.Barbanti M, Yang T-H, Rodès Cabau J, et al. Anatomical and procedural features associated with aortic root rupture during balloon-expandable transcatheter aortic valve replacement. Circulation 2013;128:244–53. 10.1161/CIRCULATIONAHA.113.002947 [DOI] [PubMed] [Google Scholar]
- 19.Eggebrecht H, Vaquerizo B, Moris C, et al. Incidence and outcomes of emergent cardiac surgery during transfemoral transcatheter aortic valve implantation (TAVI): insights from the European Registry on Emergent Cardiac Surgery during TAVI (EuRECS-TAVI). Eur Heart J 2018;39:676–84. 10.1093/eurheartj/ehx713 [DOI] [PubMed] [Google Scholar]
- 20.Popma JJ, Reardon MJ, Khabbaz K, et al. Early clinical outcomes after transcatheter aortic valve replacement using a novel self-expanding bioprosthesis in patients with severe aortic stenosis who are suboptimal for surgery: results of the Evolut R U.S. Study. JACC Cardiovasc Interv 2017;10:268–75. 10.1016/j.jcin.2016.08.050 [DOI] [PubMed] [Google Scholar]
- 21.Mauri V, Kim WK, Abumayyaleh M, et al. Short-term outcome and hemodynamic performance of next-generation self-expanding versus balloon-expandable transcatheter aortic valves in patients with small aortic annulus: a multicenter propensity-matched comparison. Circ Cardiovasc Interv 2017;10. 10.1161/CIRCINTERVENTIONS.117.005013 [DOI] [PubMed] [Google Scholar]
- 22.Dayan V, Vignolo G, Soca G, et al. Predictors and outcomes of prosthesis-patient mismatch after aortic valve replacement. JACC Cardiovasc Imaging 2016;9:924–33. 10.1016/j.jcmg.2015.10.026 [DOI] [PubMed] [Google Scholar]
- 23.Pibarot P, Dumesnil JG, Lemieux M, et al. Impact of prosthesis–patient mismatch on hemodynamic and symptomatic status, morbidity and mortality after aortic valve replacement with a bioprosthetic heart valve. J Heart Valve Dis 1998;7:211-8. [PubMed] [Google Scholar]
- 24.Rodriguez-Gabella T, Voisine P, Puri R, et al. Aortic bioprosthetic valve durability: incidence, mechanisms, predictors, and management of surgical and transcatheter valve degeneration. J Am Coll Cardiol 2017;70:1013–28. 10.1016/j.jacc.2017.07.715 [DOI] [PubMed] [Google Scholar]
- 25.Herrmann HC, Daneshvar SA, Fonarow GC, et al. Prosthesis–patient mismatch in patients undergoing transcatheter aortic valve replacement. J Am Coll Cardiol 2018;72:2701–11. 10.1016/j.jacc.2018.09.001 [DOI] [PubMed] [Google Scholar]
- 26.Möllmann H, Hengstenberg C, Hilker M, et al. Real-world experience using the ACURATE neo prosthesis: 30-day outcomes of 1,000 patients enrolled in the SAVI TF registry. EuroIntervention 2018;13:e1764–70. 10.4244/EIJ-D-17-00628 [DOI] [PubMed] [Google Scholar]
- 27.Rampat R, Khawaja MZ, Hilling-Smith R, et al. Conduction abnormalities and permanent pacemaker implantation after transcatheter aortic valve replacement using the repositionable LOTUS device: the United Kingdom experience. JACC Cardiovasc Interv 2017;10:1247–53. 10.1016/j.jcin.2017.03.044 [DOI] [PubMed] [Google Scholar]
- 28.Reardon MJ, Van Mieghem NM, Popma JJ, et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med 2017;376:1321–31. 10.1056/NEJMoa1700456 [DOI] [PubMed] [Google Scholar]
- 29.Kim W-K, Renker M, Rolf A, et al. Accuracy of device landing zone calcium volume measurement with contrast-enhanced multidetector computed tomography. Int J Cardiol 2018;263:171–6. 10.1016/j.ijcard.2018.02.042 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
openhrt-2019-001164supp001.pdf (1.1MB, pdf)
openhrt-2019-001164supp002.pdf (36.5KB, pdf)