Abstract
Acute hepatopancreatic necrosis disease (AHPND) or formerly known as early mortality syndrome (EMS) is an emerging disease that has caused significant economic losses to the aquaculture industry. The primary causative agent of AHPND is Vibrio parahaemolyticus, a Gram-negative rod-shaped bacterium that has gained plasmids encoding the fatal binary toxins Pir A/Pir B that cause rapid death of the infected shrimp. In this review, the current research studies and information about AHPND in shrimps have been presented. Molecular diagnostic tools and potential treatments regarding AHPND were also included. This review also includes relevant findings which may serve as guidelines that can help for further investigation and studies on AHPND or other shrimp diseases.
Keywords: Acute hepatopancreatic necrosis disease (AHPND), Pir A, Pir B, Diagnosis, Treatment
Introduction
Aquaculture industry has a big role in global economy. This provides job opportunities and revenue to business sectors. Its demand increases throughout the years and is very much dependent on human consumption. Among the different areas of aquaculture, crustacean industry has developed rapidly in the past years due to the increase market demands of crustaceans all over the world (Harlıoğlu and Farhadi 2017). Prawn and shrimp industries have been considered major revenue generators, and many aquaculturists focus on cultivating shrimps and prawns as primary protein sources for consumption (Lakshmi et al. 2013). Shrimp is considered one of the most important commodities that are being traded in terms of market value. Although there is an increased production of global farmed shrimp, leading producing countries, particularly Asia, have experienced a decline in production due to shrimp diseases (Zorriehzahra and Banaederakhshan 2015).
One of the most crucial problems in aquaculture is the occurrence of different diseases risking the health and production of aquatic animals. Marine animals literally swim in potential pathogens making them susceptible to viruses, bacteria, fungi, and even protozoans. General epidemic outbursts induced by viruses, such as Taura syndrome virus (TSV), infectious hypodermal and hematopoietic virus (IHHNV), white spot syndrome virus (WSSV), hepatopancreatic parvovirus (HPV), yellow head virus (YHV), and monodon baculovirus (MBV), brought about 15 billion US dollars of losses to shrimp farmers for the past 15 years with 80% of such loss attributed to Asia alone (Flegel 2012; Hong et al. 2016). The outbreak of diseases can significantly reduce the economic growth and cultivation of different aquatic species. Last 2017, a recent discovery of iridescent virus in China which caused high mortality and serious diseases in Litopenaeus vannamei has been verified and termed as shrimp hemocyte iridescent virus (SHIV) (Qiu et al. 2017). In addition to these lists, an infectious disease called acute hepatopancreatic necrosis disease (AHPND) has emerged with clinical signs of severe atrophy of the shrimp hepatopancreas and accompanied by unique histopathology at the acute stage of the infection (Tran et al. 2013).
AHPND is formerly known as early mortality syndrome (EMS) for it can cause sudden, mass mortalities of up to 100% in shrimps that can be observed within 30–35 days of stocking (de la Peña et al. 2015). This disease shows greater susceptibility in Penaeus monodon (Asian tiger shrimp) and L. vannamei (whiteleg shrimp) (Tran et al. 2013; Zorriehzahra and Banaederakhshan 2015). The involvement of these shrimp species leaves devastating economics losses that reached about billions of dollars annually since the first outbreak in China last 2009 and consecutively affected other southeast Asian countries like Vietnam (2010), Malaysia (2011), Thailand (2012), Philippines (2013), and Mexico (2013) (de la Peña et al. 2015; Nunan et al. 2014; Soto-Rodriguez et al. 2015; Tran et al. 2013). In order to prevent AHPND disease outbreak, several countries established import bans and strengthened the import rules and conditions (Kim and Kim 2015). In 2012, the Asia Pacific Emergency Regional Consultation held a meeting in Thailand in order to address the potential risks and severity of AHPND, and this was followed by a technical workshop for the following year in Vietnam (Devadas et al. 2019). In January 2016, AHPND was listed in the World Organization of Animal Health (OIE) so that information can easily be disseminated to its member countries to avert the disease outbreaks through updates or reports.
Vibrio parahaemolyticus, the causative agent of AHPND, is a Gram-negative rod-shaped bacterium that is ubiquitous in nature. AHPND-causing V. parahaemolyticus is detrimental to shrimps but not to humans. This bacterium produces a thermolabile hemolysin (TLH) diagnostic marker negative of both human toxigenic genes: thermostable direct hemolysin (TDH) and TDH-related hemolysin (TRH) (Wang et al. 2015). The pathogenic bacteria contain unique extrachromosomal plasmids that are not found in non-pathogenic strains (Xiao et al. 2017). There are two identified types of AHPND-causing V. parahaemolyticus bacteria based on geographical variations. Isolated V. parahaemolyticus in Mexico and Central USA showed a 4243-bp Tn3-like transposon insert at ORF4 which is not present on the Asian type (isolated from China, Vietnam, and Thailand) (Han et al. 2015d). The transposon-like insert is unrelated to AHPND and shows no difference on virulence between the two groups even if it is found on virulence plasmids (Tran et al. 2013). The unique extrachromosomal plasmid found in pathogenic Vibrio species encodes the genes for AHPND’s main virulence factors which are Pir A and Pir B toxins (Xiao et al. 2017). Pathogenic effects of AHPND include atrophication and production of lesions despite detection of low population of bacteria. A study by Lai et al. (2015) detected the presence of Pir A and Pir B toxins in protein lysates from the stomach, hepatopancreas, and hemolymph of diseased shrimps. Within 6 h, sloughing of shrimp hepatopancreas is evident and accompanied by a high concentration of Pir B. Bacteria were detected in the hepatopancreas 12 h post-infection (hpi) while 18 hpi for Pir A (Lai et al. 2015). The simultaneous occurrence of sloughing and presence of Pir B in the hepatopancreas provides evidence that Pir B is enough to cause AHPND infection in shrimps.
Shortly after the discovery of V. parahaemolyticus, metagenomics sequencing was done to provide insights on the disease mechanism in shrimps and develop diagnostic tools. It was also found that an AHPND-causing non-V. parahaemolyticus species V. owensii (Liu et al. 2015), V. campbellii (Ahn et al. 2017; Dong et al. 2017b; Han et al. 2017), and a strain close to V. harveyi (Kondo et al. 2015) possessed pVA1-like plasmids which implies that these toxin genes can be transferred to various species of Vibrio (Kondo et al. 2015). It can be hypothesized that these genes are flanked in the plasmid by mobile genetic elements which could be a transposase-coding sequence that may be potentially involved in horizontal gene transfer (Han et al. 2015d). The pVA1 plasmid contains two plasmid mobilization genes and a group of transfer genes for conjugation indicating that the plasmid may be self-transmissible (Lee et al. 2015). Bacterial clades with high degree of similarity at both phenotypic and genotypic levels have demonstrated gene transfer through recombination events, transposition, conjugation, and plasmid uptake during infection period. These processes explain the huge possibility of conversion from non-pathogenic to pathogenic strain and positively enhance the spread of AHPND (Restrepo et al. 2018). Furthermore, V. parahaemolyticus AHPND strains from Mexico (13-511/A1 and13-306D/4) were found to be carrying tetB gene coding tetracycline resistance gene (Han et al. 2015a), and V. campbellii (Vc3S01) from China was found to be carrying multiple antibiotic resistance genes (Dong et al. 2017a). This results lead to failure of disease control if traditional methods of disease management and eradication will be employed for AHPND.
Review papers published on 2015 and 2016 addressed different aspects of AHPND which covered the outbreak, causes, etiology, target species, gross clinical signs, histopathology, potential treatments, prevention, and different molecular and biological diagnoses of AHPND (Hong et al. 2016; Zorriehzahra and Banaederakhshan 2015). These reviews, however, lacked updated information on the diagnostic techniques necessary to hasten AHPND studies. This review paper aims to add relevant knowledge and findings by reporting the different strategies in diagnosing and potential treatments for AHPND. Additional information also includes recent findings on the disease which can further accelerate discovery of pathogenic mechanisms involved in AHPND and other shrimp disease studies.
Preliminary and confirmatory tests
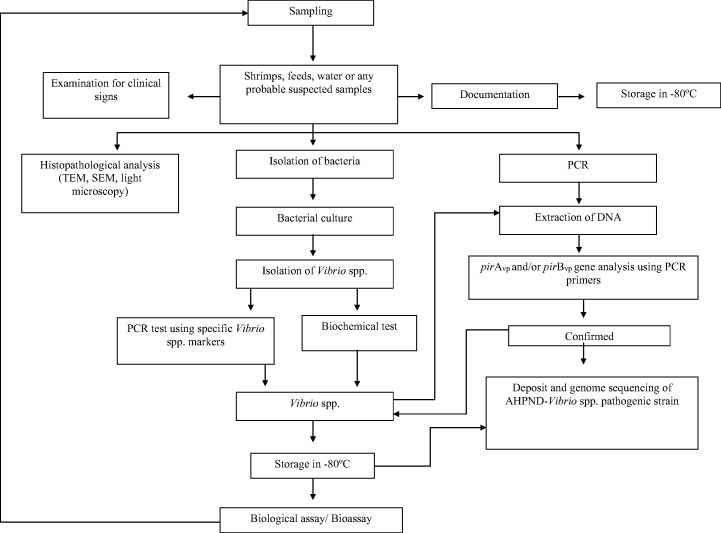
In aquaculture studies, diagnosis is very important to prevent further complications and outbreak of diseases. Preliminary steps involved in preventing the outbreak of a disease are the isolation and identification of the pathogen then establishing different diagnostic platforms and tools to identify the agent. Bioassays have been very helpful in identifying the virulence and pathogenicity of the disease (Thomas and Elkinton 2004). Koch’s postulates are often used as guides to identify the cause of the disease; therefore, an irrefutable link between the pathogen and the disease should be properly established (Cambau and Drancourt 2014). Figure 1 shows the process flow of diagnosing AHPND caused by Vibrio species.
Fig. 1.
Process flow diagram of detecting and handling of AHPND-infected samples
Many studies have been done which used challenge experiments to identify the pathogenicity and virulence of the strain being tested (Table 1) which was detected using polymerase chain reaction (PCR) and whole genome sequencing of the pathogen.
Table 1.
Challenge and detection methods used in AHPND
| Shrimp species | Method of infection | Detection method | Reference |
|---|---|---|---|
| SPF Peneus vannamei |
Reverse gavage Immersion |
Histopathology | Tran et al. (2013) |
| SPF Peneus vannamei |
Intramuscular injection Immersion |
Histopathology PCR |
Joshi et al. (2014) |
| SPF Peneus vannamei |
Per os Immersion |
Histopathology PCR |
Nunan et al. (2014) |
| Peneus vannamei | Immersion |
Histopathology PCR |
Soto-Rodriguez et al. (2015) |
| Peneus vannamei | Immersion |
Histopathology PCR |
Dabu et al. (2017) |
Clinical signs and histopathology
As a primary diagnostic method, clinical signs of AHPND are used to examine affected shrimps. These early diagnostic clinical signs must be based on the Network of Aquaculture Centres in Asia-Pacific (NACA) disease card and OIE fact sheet (Devadas et al. 2019). Many studies have shown that shrimps affected by AHPND characteristically display a pale and shrunken hepatopancreas (HP) and gastric and gut emptying. Some changes in its behavior such as sluggishness, swimming spirally, and reduced feeding were also observed (Zorriehzahra and Banaederakhshan 2015). These behavioral signs provide presumptive inferences on the disease while histological examinations are still the main method for confirming the infection in shrimps. The main and unique histological features of AHPND in early to middle stage of the disease are the sloughing and massive rounding of hepatopancreatic tubule epithelial cells with no detectable causative pathogen (Tran et al. 2013). These features are critical for diagnosis, and it is recommended that 10 or more shrimp specimens are to be collected and examined from any suspected pond to ensure that at least one specimen is at this stage of the disease. This is the reasonable approach since at the initial stages of AHPND, the disease is characterized by medial to distal dysfunction of B (blister-like), F (fibrillar), and R (resorptive) cells, prominent karyomegaly, and lack of mitotic activity in E cells (embryonic). The end stages which are described by tremendous secondary infections may be difficult to evaluate. For instance, the late stages of the disease are characterized by huge aggregation of hemocytes and emergence of melanized granulomas and accompanied by infection of many colonies of different bacteria in tubule lumens. These manifestations in infected shrimps cannot be easily distinguished from serious and other conventional infections brought by non-AHPND isolates of various bacterial species (Hong et al. 2016).
Suspected samples such as shrimps, sediments, or feeds must be treated for about 1–2 days with Davidson’s AFA fixative (Bell and Lightner 1988) for light microscopy examination and stained with hematoxylin and eosin (Lightner 1996). Besides light microscopy, transmission electron microscopy (TEM) and scanning electron microscopy (SEM) can be used to examine samples (Lightner 1996).
Polymerase chain reaction and loop-meditated isothermal amplification methods
The use of PCR as a diagnostic tool in detecting pathogens has been used for many pathological studies and can be utilized also in examining AHPND-suspected samples. PCR primers target specific DNA sequences present in samples to detect AHPND. This diagnostic tool can also identify pathogenic V. parahaemolyticus–causing AHPND gathered in several locations (Han et al. 2015b). The use of AHPND primer set 1 (AP1) and AHPND primer set 2 (AP2) which target the DNA sequences of AHPND was the first PCR diagnostic tool used in 2013 (Flegel and Lo 2014a). In 2014, Flegel and Lo (2014b) used three sets of AHPND primers (AP1, AP2, and AP3) but a false positive result from AP2 was found due to the plasmid mutation lacking the toxin gene. Among the three AHPND primer sets (AP1, AP2, and AP3), AP3 demonstrated the highest sensitivity and best specificity (Flegel 2014). In addition, TUMSAT-Vp3 primer was used by Tinwongger et al. (2014) targeting the AHPND DNA sequences. An improved nested PCR method (AP4) showed higher sensitivity and does not need pre-enrichment culture before extraction of DNA (Dangtip et al. 2015), but using AP4 can amplify Pir A and Pir B genes in V. parahaemolyticus and can only detect AHPND strains containing these two genes. Among the recent PCR methods, using AP3 primer which targets the Pir A V. parahaemolyticus is considered the most promising tool for detecting AHPND as it exhibited high sensitivity and specificity (Soto-Rodriguez et al. 2015). Thus, the use of AP3 primers which can detect Pir A gene and duplex PCR method to detect Pir A and Pir B genes are the most recommended confirmatory tests for AHPND detection (Table 2). Further comparative studies are still needed to verify the specificity and sensitivity of these methods.
Table 2.
PCR methods used to detect AHPND
| Type (method) | Primers (5′-3′) | Size | Reference |
|---|---|---|---|
| One step (AP1) |
AHPND Primer set 1 AP1F: CCTTGGGTGTGCTTAGAGGATG AP1R: GCAAACTATCGCGCAGAACACC |
700 bp | Flegel and Lo 2013 |
| One step (AP2) |
AHPND Primer set 2 AP2F: TCACCCGAATGCTCGCTTGTGG AP2R: CGTCGCTACTGTCTAGCTGAAG |
||
| One step (89F/R) |
89F: GTCGCTACTGTCTAGCTGAA 89R: ACGGCAAGACTTAGTGTACC |
470 bp | Nunan et al. (2014) |
| One step (TUMSAT-Vp3) |
TUMSAT-Vp3 F: TGTTGCATAATTTTTGTGCA TUMSAT-Vp3 R: TGTACAGAAACCACGACTA |
360 bp | Tinwongger et al. (2014) |
| One step (AP3) |
AP3-F: ATGAGTAACAATATAAAACATGAAAC AP3-R: GTGGTAATAGATTGTACAGAA |
333 bp | Sirikharin et al. (2014) |
| Two step (AP4) |
Step 1 AP4-F1: ATGAGTAACAATATAAAACATGAAAC (= AP3-F)a AP4-R1: ACGATTTCGACGTTCCCCAA |
1269 bp | Dangtip et al. (2015) |
|
Step 2 AP4-F2: TTGAGAATACGGGACGTGGG AP4-R2: GTTAGTCATGTGAGCACCTTC |
230 bp | ||
| One step (duplex) |
VpPirA-284F: TGACTATTCTCACGATTGGACTG VpPirA-284R: CACGACTAGCGCCATTGTTA |
284 bp | Han et al. (2015c) |
|
VpPirB-392F: TGATGAAGTGATGGGTGCTC VpPirB-392R: TGTAAGCGCCGTTTAACTCA |
392 bp | ||
| TaqMan qPCR |
VpPirA_F: TTGGACTGTCGAACCAAACG VpPirA_R: GCACCCCATTGGTATTGAATG Probe: AGACAGCAAACATACACCTATCATCCCGCA |
135 bp | Han et al. (2015c) |
aPrimer AP4-F1 from AP4 method is the same to primer AP3-F from the AP3 method
A Tn3-like transposon element around 4243 bp which is responsible for the secretion of Pir A- and Pir B-like binary toxins was found only in AHPND strains in Mexico and other countries in Central America but not in Southeast Asian strains isolated from Thailand, China, and Vietnam (Han et al. 2015b). Thus, Han et al. (2015b) developed a PCR method to detect these differences in these isolates. Moreover, a quantitative PCR method (qPCR) was developed by Han et al. (2015c) to quantify the virulence and detect AHPND with specificity and high sensitivity. Another reliable and convenient method for detection is loop-meditated isothermal amplification (LAMP) which generates results that can be easily interpreted suitable for detecting the early onset of AHPND (Kongrueng et al. 2015) (Table 3). Additionally, recent studies were able to develop an on-site detection method for V. parahaemolyticus AHPND (Arunrut et al. 2016; Koiwai et al. 2016; Kongrueng et al. 2015). Moreover, a method using PCR-DNA chromatography along with multiplex PCR was used to identify AHPND, hypodermal and hepatopancreatic necrosis infections, enterocytozoon hepatopenaei, and white spot disease concurrently (Koiwai et al. 2018).
Table 3.
LAMP methods for detecting AHPND
| Type (method) | Primers (5’-3’) | Size | Reference |
|---|---|---|---|
| LAMP-A3 Assay | FIB: GTATTGAATGGTAAGCTCCCCGGAAGTCGGTCGTAGTGT | 40bp | Kongrueng et al. (2015) |
| BIP: AATGGGGTGCGCCATTTATGAAGTTTCATCACGTTGTACC | 40bp | ||
| F3: GCAAACATACACCTATCATCC | 21bp | ||
| B3: GCATTATCAGGGCGTTGT | 18bp | ||
| LF: ACGTCCCGTATTCTCAATGTCT | 22bp | ||
| LB: GCTGGCGGCTGGAAAGT | 17bp | ||
| LAMP-AuNP Assay | F3-EMS: GTGCAATTTAATAGGAGAACATC | 23bp | Arunrut et al. (2016) |
| B3-EMS: GAATGGTAAGCTCCCCAC | 18bp | ||
| FIP-EMS: CGTTTGGTTCGACAGTCCAATTTTTATGAGTAACAATATAAAACATGA | 48bp | ||
| BIP-EMS: GAGGCGTCACAGAAGTAGACATTTTCCCGTATTCTCAATGTCTACAC | 47bp | ||
| LF-EMS: CGTGAGAATAGTCAGTT | 17bp | ||
| LB-EMS: ACATACACCTATCATCCCGGAAG | 23bp | ||
| Probe-Thiol-EMS: (SH)A10-ATCATCCCGGAAGTCGGTCG | 30bp | ||
| AHPND-LAMP Assay | F3: TGATAATGCATTCTATCATCAGC | 23bp | Koiwai et al. (2016) |
| B3: ATTTGAAAGACCAAATGAAACC | 22bp | ||
| FIP: | |||
| F1c: GTGAGCACCTTCTTAGTGGTAATA | 24bp | ||
| F2: GTTGTAATTAACAATGGCGCTAG | 23bp | ||
| BIP: | |||
| B1c: TGACGGAATTTAACCCTAACAATGC | 25bp | ||
| B2: GCTTTGAAAGCATAGTTAGGATC | 23bp |
Biological sensors/biosensors
Biosensor is another emerging diagnostic tool that can be used to detect pathogens. It produces a quantifiable signal that is directly proportional to the sample being analyze (pathogen, biological/cellular substance, or toxin) (Oluwaseun et al. 2018). The use of biosensors nowadays for diagnosing diseases has been growing fast. Its detection can be based on chemical responses, electrical signals, or optical signals generated through the interaction of protein or nucleic acid components. Biosensors are grouped and classified according to its transducing mechanism which can be grouped into optical (Pires et al. 2014), electrochemical (Ahmed et al. 2014), mechanical (Cheng et al. 2012), and electrical (Luo and Davis 2013). It can also be grouped according to the mechanism of the receptors used wherein it can be affinity or catalytic biosensors. Detection of disease-related proteins requires to be specific, sensitive, and cost-effective. Many biosensors have been developed for the detection of specific molecules, and each has its own specificity and component. A biosensor is an analytical device that corporates a sensing element and an electrical transducer to measure a biological event using electrical output. Biosensors include polymer supports in its setup and these supports are often solid type. The polymer can be hydrophilic or hydrophobic depending on what type of receptor is being immobilized on its surface. For instance, an electrochemical immunosensor using methylene blue immobilized graphene oxide was used for detecting WSSV in shrimp and crab samples (Natarajan et al. 2017). The advantage of this method is that it provides electrochemical immunosensing of WSSV from tissues. Another biosensor utilizing the electronic properties of DNA derived from DNA Schottky diodes was used to detect viruses and bacteria from different shrimp samples (Rizan et al. 2018). This method can be a basis for non-laboratory diagnosis and pathological studies. Until now, no biosensor has been established for AHPND detection. DNA-based biosensor shows a promising tool in diagnosing AHPND, but more studies are needed to develop immunosensing strategies for AHPND diagnosis.
Environmental DNA
Environmental DNA (eDNA) is an important tool in ecological studies which helps in biodiversity monitoring and detection of invasive species (Barnes et al. 2014; Goldberg et al. 2016). Since organisms interact with each other, DNA is shed and its detection and quantification in marine ecosystems are being used (Ficetola et al. 2008). eDNA is widely used since early detection of the pathogen can prevent further use of animal samples; however, this tool has not been developed for AHPND detection and further studies are required to establish this diagnostic tool in preventing AHPND disease outbreak.
Potential treatments and therapies
Phage therapy
The administration of antibiotics in aquaculture is the most commonly used method to treat outbreaks caused by Vibrio species; however, antibiotic resistance is a major concern along with the possible spread of the drug in the environment. The use of lytic phages termed as phage/bacteriophage therapy is a promising method for prevention and treatment of vibriosis in aquaculture (Kalatzis et al. 2018). The phage therapy was first introduced in Japan against Lactococcus garvieae (Nakai et al. 1999). Since then, it has been a great subject in the scientific community (Defoirdt et al. 2011; Oliveira et al. 2012; Richards 2014). Some studies have been conducted using phage to treat different causative agents of vibriosis (Table 4). To evaluate the effectiveness of this method, Lomelí-Ortega and Martínez-Díaz (2014) found that administration of lytic phages A3S and Vp1 6 h post-infection was found to be effective in reducing the mortality of L. vannamei caused by V. parahaemolyticus. Administration of lytic phages more than 6 hpi hindered the mortality and disease progression (Lomelí-Ortega and Martínez-Díaz 2014). This study however was done under gnotobiotic conditions, so further studies are needed to establish a model to examine the different external factors such as water quality and amount of organic matter on the phage therapy efficiency. Recently, a phage experimental therapy in P. vannamei showed significant protection and survival against V. parahaemolyticus AHPND challenge tests (Jun et al. 2018). Since AHPND disease progression has been rapidly occurring, correct timing or frequent administration of phage treatment may be the necessary approach for the treatment. Thus, more studies are needed to establish correct models of phage therapy treatment on AHPND disease progression and find the most optimum dosage and schedule for treatment.
Table 4.
Bacteriophage therapy against Vibrio spp.
| Animal species | Causative agent | Phage used | Reference |
|---|---|---|---|
| Litopenaeus vannamei | V. parahaemolyticus | Phages A3S and Vpms 1 | Lomelí-Ortega and Martínez-Díaz (2014) |
| Apostichopus japonicus | V. alginolyticus | Podoviridae (PVA1) and Myoviridae (PVA2) | Zhang et al. (2015) |
| Penaeus monodon | V. harveyi | Myoviridae (VHM1 and VHM2); Siphoviridae (VHS1) | Stalin and Srinivasan (2017) |
| Peneus vannamei | V. parahaemolyticus | Siphoviridae phage pVp-1 | Jun et al. (2018) |
Probiotics
Maintaining the biological balance among algae and bacteria in ponds and the gastrointestinal tract of shrimps is one of the ways to reduce the effects of AHPND infection (Aguilera-Rivera et al. 2014; Zorriehzahra and Banaederakhshan 2015). These microenvironments contribute in numerous important functions like direct and indirect immune response and digestion of nutrients (Harris 1993; Hooper et al. 2002; Li et al. 2008). The use of probiotics to inhibit certain bacterial infections in shrimps has been effective and has enhanced water quality for aquaculture purposes (Bernal et al. 2017; Kumar et al. 2013). Probiotic treatments such as the use of Lactobacillus casei, Rhodopseudomonas palustris, and Saccharomyces cerevisiae showed varying shrimp survival against AHPND with S. cerevisiae exhibiting the highest shrimp survival while maintaining the microbial flora in the shrimp gastrointestinal tract (Pinoargote et al. 2018). It is not very clear whether the actual probiotics or their natural products actually inhibit certain pathogenic microbes like Vibrio in aquaculture ponds. Purple non-sulfur bacteria (PNSB) are ubiquitous in the aquatic environment and are commonly found in shrimp farms where they have been shown to improve the growth of other marine organisms in culture (Chumpol et al. 2017a, b; Shapawi et al. 2012). Currently, there is still very little information of anti-Vibrio compounds produced by PNSB. Some bioactive compounds however have been documented and biosynthetically produced by Rhodobacter spihaeroides against V. fischeri and V. harveyi (Chandrasekaran and Ashok Kumar 2011). Lactic acid bacteria (LAB) have been reported to have immunomodulatory functions and have been introduced in L. vannamei (Chomwong et al. 2018). Lactobacillus plantarum strain SGLAB01 and the Lactococcus lactis strain SGLAB02 which were isolated from the gut of the shrimp displayed antimicrobial activity and provided protection in shrimps against AHPND infection (Chomwong et al. 2018). The above findings can be used to further improve the probiotic techniques and the use of L. plantarum and L. lactis as feed supplements which can provide protection against AHPND without compromising the host’s immune response. A recent study showed that probiotic bacteria CDA22 and CDM8 which were isolated from the hindgut of P. vannamei were found to exhibit a promising biocontrol agent against AHPND (Wang et al. 2018). For instance, CDA22 and CD8 can diminish the number of copies of Pir A gene responsible for the virulence of V. parahaemolyticus (Wang et al. 2018). When CDA22 and CD8 were combined, however, no protective effect was observed. Studies on the molecular mechanisms involved in competition or antagonism may be needed to provide a suitable explanation for these observations. Currently, very limited data are available on the synergistic effects of different probiotics in the protection of shrimps in aquaculture. Future research studies focusing on molecular analyses of metabolic pathways and functional genes can provide deeper understanding on the significance of diversity of microbial ecosystems in the environment and in the general health of marine animals. It is therefore vital to identify the microorganisms responsible for improving the aquaculture environment and health of the shrimps and analyze the connection among these microorganisms and their host.
Immune priming
Invertebrates, including shrimps, are believed to lack true lymphocytes and advanced humoral immune responses. They rely primarily on their innate or non-specific immunity as their primary and only protection against pathogens. Unlike vertebrates that have immune memory that easily recognizes specific pathogens by antigens and produces effector and memory cells through clonal expansion, the survival of non-adaptive invertebrates against microbial pathogens with generation times of just a few minutes was in question under the theory of modern evolution. Recently, studies about improved immunity of invertebrates after application of vaccine support possible existence of adaptive immunity (Lin et al. 2013; Netea et al. 2016).
Immune priming is a two-step vaccination technique wherein pathogens are introduced into the host’s system followed by a secondary vaccination or the infection of the same pathogen. In crustaceans, immune priming in L. vannamei resulted in a high phagocytic activity against WSSV after introduction of recombinant viral protein from WSSV within spores of Bacillus subtilis (Pope et al. 2011). Immunity of P. monodon against vibriosis using prior vaccination with formalin-killed Vibrio showed longer day post-delivery survival (Chou et al. 2011). Improved immunity against bacterial pathogens was also observed on both post-larvae and larger juveniles of L. vannamei. Larger juveniles exposed to formalin-inactivated V. harveyi have boosted natural antibacterial activity by selective increase in phagocytosis and percentage of hemocytes (Pope et al. 2011). In shrimps, higher resistance or tolerance to pathogen from which the antigen was originally derived has been exhibited, suggesting that protection by immune priming is specific only to the type and strain of pathogen used during primary vaccination. Immune specificity responds more readily to native or natural pathogens that commonly infect the host. Its lack of response to other pathogens presents a lower number of immune receptors as compared with jawed vertebrates with millions of antibodies. Immune priming is then considered to be neither universal nor specific but rather a selective immunity.
Further research studies are focusing on developing a vaccine against V. parahaemolyticus–causing AHPND. Since shrimps lack an adaptive immune system, a passive immunity could possibly protect shrimps from vibriosis by oral administration with specific egg yolk powders (IgY), and in vitro results showed that it can inhibit V. parahaemolyticus and V. harveyi (Gao et al. 2016a). Related studies have done this type of passive immunization which demonstrated that the specific IgY effectively inhibited the growth of V. parahaemolyticus and provided passive immunity to shrimps (Gao et al. 2016b; Hu et al. 2019). Recently, V. parahaemolyticus was attenuated by a gene knockout of LpxD gene responsible for the virulence of the bacteria and provided significant amount of protection in shrimps against challenge (Tsai et al. 2019). This method can be used to attenuate a virulent bacterium that may be employed as a live attenuated vaccine against V. parahaemolyticus. Since toxins A and B of V. parahaemolyticus can cause AHPND, monoclonal antibodies against toxins A and B were generated, and these monoclonal antibodies can be utilized to detect the toxins using dot blotting (Wangman et al. 2017). Although the generated monoclonal antibodies were proven effective in detecting toxins A and B and as a therapeutic agent, they are not yet proven effective as a treatment/therapeutic agent against AHPND.
As of now, there are no commercial shrimp vaccines available against AHPND. But many studies tried to utilize the V. parahaemolyticus antigens to develop a vaccine against this pathogenic bacterium (Peng et al. 2016, 2018). Some studies used anti-Pir A-like toxin IgY in feeds and exhibited passive immunization against AHPND (Nakamura et al. 2019). This method of adding anti-Pir A-IgY in feeds could be a potent prophylactic means against AHPND infection. The weakness or shortcoming that needs to be addressed is the vaccine development against V. parahaemolyticus. Vaccine progress against AHPND is a major issue since further studies to understand the underlying mechanisms and pathogenesis of the disease are needed to better understand the disease. Receptors, toxins, or genes responsible for the virulence of the disease combined with current knowledge and techniques currently available could make vaccine studies accelerate and further the AHPND studies.
Conclusions
Since the outbreak of AHPND in China back in 2009, control measures began and has been implemented to control and eradicate the disease. Many molecular diagnostic tools have been developed and implemented to reduce the effects of AHPND outbreak. Some of them have the potential to be used on-site to help the farmers diagnose AHPND, but the bigger issue remains on how do we prevent and treat AHPND. Several studies tackling on the development of therapeutic agents have the potential to control the disease and possibly prevent disease outbreaks. Since shrimps lack an adaptive immune response critical to combat infectious diseases like AHPND, further studies must be done to investigate the shrimp’s immune responses and the underlying mechanisms. These would be essential in developing vaccines and treatments against AHPND and other shrimp diseases. Concerning AHPND, the Pir A- and Pir B-like receptors need to be investigated further, as interaction mechanisms with the host organisms along with the pathogenesis could be proven useful for AHPND prevention studies. Understanding the underlying mechanism of the disease and pathogenicity is crucial in preventing and eradicating AHPND. All experimentations regarding AHPND must follow laboratory safety and biosecurity protocols in order to ensure the safety of its people and prevent the spread of infectious substances to the environment related to AHPND. Considering AHPND as a recent emerging disease, biosurveillance, treatment, diagnostics, and management of this disease are great dilemmas faced by many.
Funding information
This study was financially supported by the Research Center for Animal Biologics, from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education and the Ministry of Science and Technology (MOST 107-3017-F-020-002), Taiwan, R.O.C.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical statement
This article does not contain any studies with animals performed by any of the authors.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aguilera-Rivera D, Prieto-Davó A, Escalante K, Chávez C, Cuzon G, Gaxiola G. Probiotic effect of FLOC on Vibrios in the pacific white shrimp Litopenaeus vannamei. Aquaculture. 2014;424-425:215–219. doi: 10.1016/j.aquaculture.2014.01.008. [DOI] [Google Scholar]
- Ahmed A, Rushworth JV, Hirst NA, Millner PA. Biosensors for whole-cell bacterial detection. Clin Microbiol Rev. 2014;27:631–646. doi: 10.1128/cmr.00120-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn YS, Piamsomboon P, Tang KFJ, Han JE, Kim JH. Complete genome sequence of acute hepatopancreatic necrosis disease-causing Vibrio campbellii LA16-V1, isolated from Penaeus vannamei cultured in a Latin American country. Genome Announcements. 2017;5:e01011–e01017. doi: 10.1128/genomeA.01011-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arunrut N, Kampeera J, Sirithammajak S, Sanguanrut P, Proespraiwong P, Suebsing R, Kiatpathomchai W. Sensitive visual detection of AHPND bacteria using loop-mediated isothermal amplification combined with DNA-functionalized gold nanoparticles as probes. PLoS One. 2016;11:e0151769. doi: 10.1371/journal.pone.0151769. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Barnes MA, Turner CR, Jerde CL, Renshaw MA, Chadderton WL, Lodge DM. Environmental conditions influence edna persistence in aquatic systems. Environ Sci Technol. 2014;48:1819–1827. doi: 10.1021/es404734p. [DOI] [PubMed] [Google Scholar]
- Bell TA, Lightner DV (1988) A handbook of normal penaeid shrimp histology
- Bernal MG, Marrero RM, Campa-Córdova ÁI, Mazón-Suástegui JM. Probiotic effect of Streptomyces strains alone or in combination with Bacillus and Lactobacillus in juveniles of the white shrimp Litopenaeus vannamei. Aquac Int. 2017;25:927–939. doi: 10.1007/s10499-016-0085-y. [DOI] [Google Scholar]
- Cambau E, Drancourt M. Steps towards the discovery of Mycobacterium tuberculosis by Robert Koch, 1882. Clin Microbiol Infect. 2014;20:196–201. doi: 10.1111/1469-0691.12555. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran R, Ashok Kumar G (2011) Antagonistic activities of purple non-sulfur bacterial extracts against antibiotic resistant Vibrio sp
- Cheng CI, Chang Y-P, Chu Y-H. Biomolecular interactions and tools for their recognition: focus on the quartz crystal microbalance and its diverse surface chemistries and applications. Chem Soc Rev. 2012;41:1947–1971. doi: 10.1039/C1CS15168A. [DOI] [PubMed] [Google Scholar]
- Chomwong S, Charoensapsri W, Amparyup P, Tassanakajon A. Two host gut-derived lactic acid bacteria activate the proPO system and increase resistance to an AHPND-causing strain of Vibrio parahaemolyticus in the shrimp Litopenaeus vannamei. Dev Comp Immunol. 2018;89:54–65. doi: 10.1016/j.dci.2018.08.002. [DOI] [PubMed] [Google Scholar]
- Chou P-H, Chang H-S, Chen IT, Lee C-W, Hung H-Y, Han-Ching Wang KC. Penaeus monodon Dscam (PmDscam) has a highly diverse cytoplasmic tail and is the first membrane-bound shrimp Dscam to be reported. Fish Shellfish Immun. 2011;30:1109–1123. doi: 10.1016/j.fsi.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Chumpol S, Kantachote D, Nitoda T, Kanzaki H. The roles of probiotic purple nonsulfur bacteria to control water quality and prevent acute hepatopancreatic necrosis disease (AHPND) for enhancement growth with higher survival in white shrimp (Litopenaeus vannamei) during cultivation. Aquaculture. 2017;473:327–336. doi: 10.1016/j.aquaculture.2017.02.033. [DOI] [Google Scholar]
- Chumpol S, Kantachote D, Rattanachuay P, Vuddhakul V, Nitoda T, Kanzaki H. In vitro and in vivo selection of probiotic purple nonsulphur bacteria with an ability to inhibit shrimp pathogens: acute hepatopancreatic necrosis disease-causing Vibrio parahaemolyticus and other vibrios. Aquac Res. 2017;48:3182–3197. doi: 10.1111/are.13149. [DOI] [Google Scholar]
- Dabu IM, Lim JJ, Arabit PMT, Orense SJAB, Tabardillo JA, Jr, Corre VL, Jr, Maningas MBB. The first record of acute hepatopancreatic necrosis disease in the Philippines. Aquac Res. 2017;48(3):792–799. doi: 10.1111/are.12923. [DOI] [Google Scholar]
- Dangtip S, et al. AP4 method for two-tube nested PCR detection of AHPND isolates of Vibrio parahaemolyticus. Aquaculture Reports. 2015;2:158–162. doi: 10.1016/j.aqrep.2015.10.002. [DOI] [Google Scholar]
- de la Peña LD, et al. Acute hepatopancreatic necrosis disease (AHPND) outbreaks in Penaeus vannamei and P. monodon cultured in the Philippines. Dis Aquat Org. 2015;116:251–254. doi: 10.3354/dao02919. [DOI] [PubMed] [Google Scholar]
- Defoirdt T, Sorgeloos P, Bossier P. Alternatives to antibiotics for the control of bacterial disease in aquaculture. Curr Opin Microbiol. 2011;14:251–258. doi: 10.1016/j.mib.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Devadas S, Banerjee S, Yusoff FM, Bhassu S, Shariff M. Experimental methodologies and diagnostic procedures for acute hepatopancreatic necrosis disease (AHPND) Aquaculture. 2019;499:389–400. doi: 10.1016/j.aquaculture.2018.06.042. [DOI] [Google Scholar]
- Dong X et al (2017a) pirABvp-bearing Vibrio parahaemolyticus and Vibrio campbellii pathogens isolated from the same AHPND-affected pond possess highly similar pathogenic plasmids. Front Microbiol 8. 10.3389/fmicb.2017.01859 [DOI] [PMC free article] [PubMed]
- Dong X, Wang H, Xie G, Zou P, Guo C, Liang Y, Huang J. An isolate of Vibrio campbellii carrying the pir (VP) gene causes acute hepatopancreatic necrosis disease. Emerg Microbes Infect. 2017;6:e2–e2. doi: 10.1038/emi.2016.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ficetola GF, Miaud C, Pompanon F, Taberlet P. Species detection using environmental DNA from water samples. Biol Lett. 2008;4:423–425. doi: 10.1098/rsbl.2008.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegel T (2014) A game changer for the future development of aquaculture. In: 9th symposium on diseases in Asian aquaculture, Ho Chi Minh City, Vietnam
- Flegel T, Lo C (2013) Announcement regarding free release of primers for specific detection of bacterial isolates that cause acute hepatopancreatic necrosis disease (AHPND) Aquacult Asia 19:30
- Flegel T, Lo C. Announcement regarding free release of primers for specific detection of bacterial isolates that cause acute hepatopancreatic necrosis disease (AHPND) Aquacult Asia. 2014;19:30. [Google Scholar]
- Flegel T, Lo C (2014b) Interim primers for specific detection of bacterial isolates that cause acute hepatopancreatic necrosis disease (AHPND)
- Flegel TW. Historic emergence, impact and current status of shrimp pathogens in Asia. J Invertebr Pathol. 2012;110:166–173. doi: 10.1016/j.jip.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Gao Xiaojian, Zhang Xiaojun, Lin Li, Yao Dongrui, Sun Jingjing, Du Xuedi, Li Xiumei, Zhang Yue. Passive Immune-Protection of Litopenaeus vannamei against Vibrio harveyi and Vibrio parahaemolyticus Infections with Anti-Vibrio Egg Yolk (IgY)-Encapsulated Feed. International Journal of Molecular Sciences. 2016;17(5):723. doi: 10.3390/ijms17050723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Zhang X, Sun J, Du X, Li X, Zhang Y, Lin L. Passive protection effect of anti-Vibrio anguillarum IgY-encapsulated feed on half-smooth tongue sole (Cynoglossus semilaevi) against V. anguillarum. Fish Shellfish Immun. 2016;56:483–488. doi: 10.1016/j.fsi.2016.07.041. [DOI] [PubMed] [Google Scholar]
- Goldberg CS, et al. Critical considerations for the application of environmental DNA methods to detect aquatic species. Methods Ecol Evol. 2016;7:1299–1307. doi: 10.1111/2041-210x.12595. [DOI] [Google Scholar]
- Han JE, Mohney LL, Tang KFJ, Pantoja CR, Lightner DV. Plasmid mediated tetracycline resistance of Vibrio parahaemolyticus associated with acute hepatopancreatic necrosis disease (AHPND) in shrimps. Aquaculture Reports. 2015;2:17–21. doi: 10.1016/j.aqrep.2015.04.003. [DOI] [Google Scholar]
- Han JE, Tang KFJ, Aranguren LF, Piamsomboon P. Characterization and pathogenicity of acute hepatopancreatic necrosis disease natural mutants, pirABvp (−) V. parahaemolyticus, and pirABvp (+) V. campbellii strains. Aquaculture. 2017;470:84–90. doi: 10.1016/j.aquaculture.2016.12.022. [DOI] [Google Scholar]
- Han JE, Tang KFJ, Lightner DV. Genotyping of virulence plasmid from Vibrio parahaemolyticus isolates causing acute hepatopancreatic necrosis disease in shrimp. Dis Aquat Org. 2015;115:245–251. doi: 10.3354/dao02906. [DOI] [PubMed] [Google Scholar]
- Han JE, Tang KFJ, Pantoja CR, White BL, Lightner DV. qPCR assay for detecting and quantifying a virulence plasmid in acute hepatopancreatic necrosis disease (AHPND) due to pathogenic Vibrio parahaemolyticus. Aquaculture. 2015;442:12–15. doi: 10.1016/j.aquaculture.2015.02.024. [DOI] [Google Scholar]
- Han JE, Tang KFJ, Tran LH, Lightner DV. Photorhabdus insect-related (Pir) toxin-like genes in a plasmid of Vibrio parahaemolyticus, the causative agent of acute hepatopancreatic necrosis disease (AHPND) of shrimp. Dis Aquat Org. 2015;113:33–40. doi: 10.3354/dao02830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlıoğlu MM, Farhadi A. Feminization strategies in crustacean aquaculture. Aquac Int. 2017;25:1453–1468. doi: 10.1007/s10499-017-0128-z. [DOI] [Google Scholar]
- Harris JM. The presence, nature, and role of gut microflora in aquatic invertebrates: a synthesis. Microb Ecol. 1993;25:195–231. doi: 10.1007/bf00171889. [DOI] [PubMed] [Google Scholar]
- Hong X, Lu L, Xu D. Progress in research on acute hepatopancreatic necrosis disease (AHPND) Aquac Int. 2016;24:577–593. doi: 10.1007/s10499-015-9948-x. [DOI] [Google Scholar]
- Hooper LV, Midtvedt T, Gordon JI. How host-microbial interactions shape the nutrient environment of the mammalian intestine. Annu Rev Nutr. 2002;22:283–307. doi: 10.1146/annurev.nutr.22.011602.092259. [DOI] [PubMed] [Google Scholar]
- Hu B, Yang X, Guo E, Zhou P, Xu D, Qi Z, Deng L. The preparation and antibacterial effect of egg yolk immunoglobulin (IgY) against the outer membrane proteins of Vibrio parahaemolyticus. J Sci Food Agric. 2019;99:2565–2571. doi: 10.1002/jsfa.9470. [DOI] [PubMed] [Google Scholar]
- Joshi J, Srisala J, Truong VH, Chen IT, Nuangsaeng B, Suthienkul O, Lo CF, Flegel TW, Sritunyalucksana K, Thitamadee S. Variation in Vibrio parahaemolyticus isolates from a single Thai shrimp farm experiencing an outbreak of acute hepatopancreatic necrosis disease (AHPND) Aquaculture. 2014;428-429:297–302. doi: 10.1016/j.aquaculture.2014.03.030. [DOI] [Google Scholar]
- Jun JW, Han JE, Giri SS, Tang KFJ, Zhou X, Aranguren LF, Park SC. Phage application for the protection from acute hepatopancreatic necrosis disease (AHPND) in Penaeus vannamei. Indian J Microbiol. 2018;58(1):114–117. doi: 10.1007/s12088-017-0694-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalatzis PG, Castillo D, Katharios P, Middelboe M. Bacteriophage interactions with marine pathogenic Vibrios: implications for phage therapy. Antibiotics (Basel, Switzerland) 2018;7:15. doi: 10.3390/antibiotics7010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N-E, Kim D-H. Acute hepatopancreatic necrosis disease of shrimp and import health measures. J Fish Pathol. 2015;28:1–7. doi: 10.7847/jfp.2015.28.1.001. [DOI] [Google Scholar]
- Koiwai K, Kodera T, Thawonsuwan J, Kawase M, Kondo H, Hirono I. A rapid method for simultaneously diagnosing four shrimp diseases using PCR-DNA chromatography method. J Fish Dis. 2018;41:395–399. doi: 10.1111/jfd.12732. [DOI] [PubMed] [Google Scholar]
- Koiwai K, Tinwongger S, Nozaki R, Kondo H, Hirono I. Detection of acute hepatopancreatic necrosis disease strain of Vibrio parahaemolyticus using loop-mediated isothermal amplification. J Fish Dis. 2016;39:603–606. doi: 10.1111/jfd.12387. [DOI] [PubMed] [Google Scholar]
- Kondo H, Van PT, Dang LT, Hirono I. Draft genome sequence of non-Vibrio parahaemolyticus acute hepatopancreatic necrosis disease strain KC13.17.5, isolated from diseased shrimp in Vietnam. Genome announcements. 2015;3:e00978–e00915. doi: 10.1128/genomeA.00978-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongrueng J, Tansila N, Mitraparp-arthorn P, Nishibuchi M, Vora GJ, Vuddhakul V. LAMP assay to detect Vibrio parahaemolyticus causing acute hepatopancreatic necrosis disease in shrimp. Aquac Int. 2015;23:1179–1188. doi: 10.1007/s10499-014-9874-3. [DOI] [Google Scholar]
- Kumar RN, Raman RP, Jadhao SB, Brahmchari RK, Kumar K, Dash G. Effect of dietary supplementation of Bacillus licheniformis on gut microbiota, growth and immune response in giant freshwater prawn, Macrobrachium rosenbergii (de Man, 1879) Aquac Int. 2013;21:387–403. doi: 10.1007/s10499-012-9567-8. [DOI] [Google Scholar]
- Lai H-C, et al. Pathogenesis of acute hepatopancreatic necrosis disease (AHPND) in shrimp. Fish Shellfish Immun. 2015;47:1006–1014. doi: 10.1016/j.fsi.2015.11.008. [DOI] [PubMed] [Google Scholar]
- Lakshmi B, Viswanath B, Sai Gopal DVR. Probiotics as antiviral agents in shrimp aquaculture. J Pathog. 2013;2013:424123–424123. doi: 10.1155/2013/424123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C-T, et al. The opportunistic marine pathogen Vibrio parahaemolyticus becomes virulent by acquiring a plasmid that expresses a deadly toxin. Proc Natl Acad Sci. 2015;112:10798–10803. doi: 10.1073/pnas.1503129112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Wang B., Zhang M., Rantalainen M., Wang S., Zhou H., Zhang Y., Shen J., Pang X., Zhang M., Wei H., Chen Y., Lu H., Zuo J., Su M., Qiu Y., Jia W., Xiao C., Smith L. M., Yang S., Holmes E., Tang H., Zhao G., Nicholson J. K., Li L., Zhao L. Symbiotic gut microbes modulate human metabolic phenotypes. Proceedings of the National Academy of Sciences. 2008;105(6):2117–2122. doi: 10.1073/pnas.0712038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightner DV (1996) A handbook of shrimp pathology and diagnostic procedures for diseases of cultured penaeid shrimp
- Lin Y-C, Chen J-C, Morni WZW, Putra DF, Huang C-L, Li C-C, Hsieh J-F. Vaccination enhances early immune responses in white shrimp Litopenaeus vannamei after secondary exposure to Vibrio alginolyticus. PLoS One. 2013;8:e69722. doi: 10.1371/journal.pone.0069722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Xiao J, Xia X, Pan Y, Yan S, Wang Y. Draft genome sequence of Vibrio owensii strain SH-14, which causes shrimp acute hepatopancreatic necrosis disease. Genome Announcements. 2015;3:e01395–e01315. doi: 10.1128/genomeA.01395-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomelí-Ortega CO, Martínez-Díaz SF. Phage therapy against Vibrio parahaemolyticus infection in the whiteleg shrimp (Litopenaeus vannamei) larvae. Aquaculture. 2014;434:208–211. doi: 10.1016/j.aquaculture.2014.08.018. [DOI] [Google Scholar]
- Luo X, Davis JJ. Electrical biosensors and the label free detection of protein disease biomarkers. Chem Soc Rev. 2013;42:5944–5962. doi: 10.1039/C3CS60077G. [DOI] [PubMed] [Google Scholar]
- Nakai T, Sugimoto R, Park KH, Matsuoka S, Mori K, Nishioka T, Maruyama K. Protective effects of bacteriophage on experimental Lactococcus garvieae infection in yellowtail. Dis Aquat Org. 1999;37:33–41. doi: 10.3354/dao037033. [DOI] [PubMed] [Google Scholar]
- Nakamura R, Pedrosa-Gerasmio IR, Alenton RRR, Nozaki R, Kondo H, Hirono I. Anti-PirA-like toxin immunoglobulin (IgY) in feeds passively immunizes shrimp against acute hepatopancreatic necrosis disease. J Fish Dis. 2019;42:1125–1132. doi: 10.1111/jfd.13024. [DOI] [PubMed] [Google Scholar]
- Natarajan A, Devi KSS, Raja S, Senthil Kumar A. An elegant analysis of white spot syndrome virus using a graphene oxide/methylene blue based electrochemical immunosensor platform. Sci Rep. 2017;7:46169–46169. doi: 10.1038/srep46169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea MG, et al. Trained immunity: a program of innate immune memory in health and disease. Science. 2016;352:aaf1098. doi: 10.1126/science.aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunan L, Lightner D, Pantoja C, Gomez-Jimenez S. Detection of acute hepatopancreatic necrosis disease (AHPND) in Mexico. Dis Aquat Org. 2014;111:81–86. doi: 10.3354/dao02776. [DOI] [PubMed] [Google Scholar]
- Oliveira J, Castilho F, Cunha A, Pereira MJ. Bacteriophage therapy as a bacterial control strategy in aquaculture. Aquac Int. 2012;20:879–910. doi: 10.1007/s10499-012-9515-7. [DOI] [Google Scholar]
- Oluwaseun AC, Phazang P, Sarin NB (2018) Biosensors: a fast-growing technology for pathogen detection in agriculture and food sector. In: Biosensing technologies for the detection of pathogens-a prospective way for rapid analysis. IntechOpen,
- Peng B, Lin X-p, Wang S-n, Yang M-j, Peng X-x, Li H. Polyvalent protective immunogens identified from outer membrane proteins of Vibrio parahaemolyticus and their induced innate immune response. Fish Shellfish Immun. 2018;72:104–110. doi: 10.1016/j.fsi.2017.10.046. [DOI] [PubMed] [Google Scholar]
- Peng B, Ye J-z, Han Y, Zeng L, Zhang J-y, Li H. Identification of polyvalent protective immunogens from outer membrane proteins in Vibrio parahaemolyticus to protect fish against bacterial infection. Fish Shellfish Immun. 2016;54:204–210. doi: 10.1016/j.fsi.2016.04.012. [DOI] [PubMed] [Google Scholar]
- Pinoargote G, Flores G, Cooper K, Ravishankar S. Effects on survival and bacterial community composition of the aquaculture water and gastrointestinal tract of shrimp (Litopenaeus vannamei) exposed to probiotic treatments after an induced infection of acute hepatopancreatic necrosis disease. Aquac Res. 2018;49:3270–3288. doi: 10.1111/are.13791. [DOI] [Google Scholar]
- Pires NMM, Dong T, Hanke U, Hoivik N. Recent developments in optical detection technologies in lab-on-a-chip devices for biosensing applications. Sensors. 2014;14:15458–15479. doi: 10.3390/s140815458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope EC, Powell A, Roberts EC, Shields RJ, Wardle R, Rowley AF. Enhanced cellular immunity in shrimp (Litopenaeus vannamei) after ‘vaccination’. PLoS One. 2011;6:e20960. doi: 10.1371/journal.pone.0020960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu L, et al. Characterization of a new member of Iridoviridae, shrimp hemocyte iridescent virus (SHIV), found in white leg shrimp (Litopenaeus vannamei) Sci Rep. 2017;7:11834. doi: 10.1038/s41598-017-10738-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restrepo L, Bayot B, Arciniegas S, Bajaña L, Betancourt I, Panchana F, Reyes Muñoz A. PirVP genes causing AHPND identified in a new Vibrio species (Vibrio punensis) within the commensal Orientalis clade. Sci Rep. 2018;8:13080–13080. doi: 10.1038/s41598-018-30903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards GP. Bacteriophage remediation of bacterial pathogens in aquaculture: a review of the technology. Bacteriophage. 2014;4:e975540. doi: 10.4161/21597081.2014.975540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizan N, Yew CY, Niknam MR, Krishnasamy J, Bhassu S, Hong GZ, Devadas S, Din MSM, Tajuddin HA, Othman RY, Phang SM, Iwamoto M, Periasamy V. Electronic properties of synthetic shrimp pathogens-derived DNA Schottky diodes. Sci Rep. 2018;8:896. doi: 10.1038/s41598-017-18825-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapawi R, Ting TE, Al-Azad S. Inclusion of purple non sulfur bacterial biomass in formulated feed to promote growth, feed conversion ratio and survival of asian seabass Lates calcarifer juveniles. J Fish Aquat Sci. 2012;7:475–480. doi: 10.3923/jfas.2012.475.480. [DOI] [Google Scholar]
- Soto-Rodriguez SA, Gomez-Gil B, Lozano-Olvera R, Betancourt-Lozano M, Morales-Covarrubias MS. Field and experimental evidence of Vibrio parahaemolyticus as the causative agent of acute hepatopancreatic necrosis disease of cultured shrimp Litopenaeus vannamei in Northwestern Mexico. Appl Environ Microbiol. 2015;81:1689–1699. doi: 10.1128/aem.03610-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalin N, Srinivasan P. Efficacy of potential phage cocktails against Vibrio harveyi and closely related Vibrio species isolated from shrimp aquaculture environment in the south east coast of India. Vet Microbiol. 2017;207:83–96. doi: 10.1016/j.vetmic.2017.06.006. [DOI] [PubMed] [Google Scholar]
- Thomas SR, Elkinton JS. Pathogenicity and virulence. J Invertebr Pathol. 2004;85:146–151. doi: 10.1016/j.jip.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Tinwongger S, et al. Development of PCR diagnosis for shrimp acute hepatopancreatic necrosis disease (AHPND) strain of Vibrio parahaemolyticus. Fish Pathology. 2014;49:159–164. doi: 10.3147/jsfp.49.159. [DOI] [Google Scholar]
- Tran L, Nunan L, Redman RM, Mohney LL, Pantoja CR, Fitzsimmons K, Lightner DV. Determination of the infectious nature of the agent of acute hepatopancreatic necrosis syndrome affecting penaeid shrimp. Dis Aquat Org. 2013;105:45–55. doi: 10.3354/dao02621. [DOI] [PubMed] [Google Scholar]
- Tsai Ching-Yi, Santos Harvey M., Hu Shao-Yang, Sang Cheng Yu, Yanuaria Ciara Alyssa S., Lola Ernest Nicolo G., Tayo Lemmuel L., Nitura Karmella Marie A., Liu Chun Hung, Chuang Kuo Pin. LpxD gene knockout elicits protection to Litopenaeus vannamei, white shrimp, against Vibrio parahaemolyticus infection. Aquaculture International. 2019;27(5):1383–1393. doi: 10.1007/s10499-019-00398-y. [DOI] [Google Scholar]
- Wang H, Wang C, Tang Y, Sun B, Huang J, Song X. Pseudoalteromonas probiotics as potential biocontrol agents improve the survival of Penaeus vannamei challenged with acute hepatopancreatic necrosis disease (AHPND)-causing Vibrio parahaemolyticus. Aquaculture. 2018;494:30–36. doi: 10.1016/j.aquaculture.2018.05.020. [DOI] [Google Scholar]
- Wang R, Zhong Y, Gu X, Yuan J, Saeed AF, Wang S. The pathogenesis, detection, and prevention of Vibrio parahaemolyticus. Front Microbiol. 2015;6:6. doi: 10.3389/fmicb.2015.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangman P, et al. Development of monoclonal antibodies specific to ToxA and ToxB of Vibrio parahaemolyticus that cause acute hepatopancreatic necrosis disease (AHPND) Aquaculture. 2017;474:75–81. doi: 10.1016/j.aquaculture.2017.03.039. [DOI] [Google Scholar]
- Xiao J, et al. Shrimp AHPND-causing plasmids encoding the PirAB toxins as mediated by pirAB-Tn903 are prevalent in various Vibrio species. Sci Rep. 2017;7:42177–42177. doi: 10.1038/srep42177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Cao Z, Li Z, Wang L, Li H, Wu F, Xu Y. Effect of bacteriophages on Vibrio alginolyticus infection in the sea cucumber, Apostichopus japonicus (Selenka) J World Aquacult Soc. 2015;46(2):149–158. doi: 10.1111/jwas.12177. [DOI] [Google Scholar]
- Zorriehzahra M, Banaederakhshan R. Early mortality syndrome (EMS) as new emerging threat in shrimp industry. Adv Anim Vet Sci. 2015;3:64–72. doi: 10.14737/journal.aavs/2015/3.2s.64.72. [DOI] [Google Scholar]