Abstract
Purpose
Cystic echinococcosis (CE) caused by Echinococcus granulosus sensu lato is a widespread zoonotic disease of global concern. In Nigeria, the exact picture/status of CE is unclear, as most of the states are largely uninvestigated. Yet, as with every parasitic zoonosis, the first step towards planning a comprehensive management and control programme involves assessment of available national/regional prevalence data, host range, and risk factors at play in the transmission dynamics.
Methods
Published articles on echinococcosis were searched on PubMed and Africa Journal Online (AJOL) databases. Inclusion criteria were based on studies reporting prevalence of echinococcosis in animals and humans (including case reports) from 1970 to 2018.
Results
In this study, we evaluated and summarized cystic echinococcosis reports in Nigeria and found that post 1970–80s, studies on cystic echinococcosis have remained sparse regardless of the high prevalence recorded in the early years of CE investigation. In addition, information on the genetic population structure and the role of wildlife in CE transmission is still lacking.
Conclusions
This study appraises the prevalence and distribution of CE in Nigeria and identified areas where surveillance and control efforts should be focused and intensified.
Keywords: Cystic echinococcosis, Prevalence, Risk factors, Nigeria
Introduction
In spite of the growing knowledge on the genetic population structure, host range and the complex taxonomic challenges, cystic echinococcosis (CE) remains a major neglected topical zoonotic disease across the world and more so in Nigeria. Caused by species of the genus Echinococcus, E. granulosus sensu stricto is responsible for the majority of CE cases, resulting in serious public health concerns [30] and losses reaching over $2 billion annually [83, 84]. CE is a chronic infection both in animals and humans and can take years for the infection to be noticed. Detection of CE infection is common during postmortem examination of animals and often incidental in humans. CE is sometimes asymptomatic due to its progressive nature except when cysts rupture to release antigenic material that causes reaction or active cysts located in certain anatomical regions (e.g., eyes and joints) exert pressure on surrounding tissues resulting in pain or discomfort (for further details on clinical manifestations, see [18, 43, 64]. Within the E. granulosus sensu lato group, mitochondrial genome studies showed considerable levels of variation [16, 17] which led to the adoption of the G1–10 nomenclature to accommodate the diversity. The grouping broadly consists of E. granulosus s.s. (G1 and G3), E. equinus (G4), E. ortleppi (G5), E. canadensis (G6–10), and E. felidis [47]. This variation has been found to be relevant for host specificity and infectivity, biochemical and morphological differences, development of diagnostic tools, drug production, vaccine development, and ultimately, management and control schemes [20, 78]. Meanwhile, there are pending controversies regarding the taxonomy of E. canadensis group and some authors have suggested categorization of genotypes G6/7 as E. intermedius, and genotypes G8 and G10 as E. canadensis [45, 47, 52].
Cystic echinococcosis is a common presentation in Africa as it is a major public health concern particularly in Northern and Eastern African countries. CE also results in huge economic losses due to the condemnation of infected animal organs slaughtered for food, and in severe cases could cause growth retardation and poor meat and milk outputs impacting negatively on the overall livestock value chain [39] with a potential annual financial loss of up to $100,000 [13, 34, 39, 65].
Nigeria is a West African country with a population of over 180 million people. It is divided into 36 states and a Federal Capital Territory (Abuja) and further grouped into six geopolitical zones (North-East, North-Central, North-West, South-East, South-South, and South-West) based on ethnicity and common history/ancestry. Owing to its favorable climate, it supports a large biodiversity and thus endemic for a number of parasitic zoonoses including echinococcosis. Historically, the earliest record of echinococcosis dates back to investigation in 1958 by the Nigerian Ministry of Health of 82 animals from the then Eastern region [31]. Thereafter, hydatid cysts were supposedly recovered from slaughtered cattle at the municipal abattoir in present day Kano state in 1961 with incomplete description of the encountered cysts as well as the number of infected animals and species responsible for the infection [32]. Between 1970 and 1990, CE investigations were frequently carried out in Nigeria (mainly in northern zones). Hitherto, epidemiological surveys have remained sparse since the 1990s, making the status of CE in Nigeria difficult to describe [5, 29]. So far, the information on CE for Nigeria has largely relied on data from 1970 to 2000 which could underestimate the disease’s current impact and importance considering likely changes that might have occurred overtime on possible sundry risk factors as well as the absence of active control programs. Therefore, to plan toward management and control, we presented a systematic review of CE studies in Nigeria from inception to date, identified research gaps, and highlighted the need to address potential bottlenecks to disease eradication.
Data Collection and Selection
Published articles on echinococcosis were searched on PubMed and Africa Journal Online (AJOL) databases in April/May 2018, and repeated in June/July 2018, using “echinococc* OR hydatid* AND Nigeria” alone or in combination as search terms. In addition, specific searches with the name of each state were also performed. Inclusion criteria were based on the studies reporting prevalence of echinococcosis in animals and humans (including case reports) from 1970 to 2018.
Prevalence of Cystic Echinococcosis in Nigeria
Prevalence in Animals
In Nigeria, dogs are the only definitive host known to be involved in maintaining the transmission of echinococcosis. The role of wild canids is currently unknown. The first record of echinococcosis in dogs was reported in 1979 from a population of stray dogs in northern Nigeria [24, 25]. Since then, infection rates in dogs have been found to vary across the country with certain factors contributing to the observed variation (Table 1). In another study, where prevalence among dogs from various backgrounds was investigated, significantly higher infection rates were found in hunting dogs than in companion dogs. Factors contributing to this disparity reportedly include feeding and management practices by dog owners as well as age of the dogs as infection was higher in the older than younger dogs [5]. On prevalence across the country, lower prevalence was commonly reported in the North [23–25] than in the South (Table 1) such that in a study, 85% prevalence was found in a community in Niger Delta [9]. In this region, dogs are slaughtered for food [33, 40, 76]; therefore, there is high demand for dog meat and consequently increase in dog rearing activities constituting a risk to an increase in disease prevalence and distribution. Also, disparity in dog density between the North and South could provide a possible explanation to the differences in prevalence between both regions [40, 56, 76]. Nonetheless, more studies are required to understand the distribution of CE across zones.
Table 1.
Cystic echinococcosis prevalence in animals in Nigeria (1970–2018)
| Zone | State | Host | Sample size | No infected | Prevalence (%) | Host status | Detection method | References |
|---|---|---|---|---|---|---|---|---|
| North-Central | Plateau | Pigs | 170 | 0 | 0 | Slaughtered | PM/microscopy | [26] |
| Plateau | Sheep | 293 | 4 | 1.4 | Slaughtered | PM/microscopy | [26] | |
| Plateau | Goats | 360 | 0 | 0 | Slaughtered | PM/microscopy | [26] | |
| Plateau | Cattle | 811 | 0 | 0 | Slaughtered | PM/microscopy | [26] | |
| Plateau | Dogs | 74 | 0 | 0 | Stray | PM/microscopy | [23] | |
| North-East | Yobe | Cattle | NA | NA | 0.4 | Slaughtered | PM/microscopy | [73] |
| Yobe | Camel | NA | NA | 6.3 | Slaughtered | PM/microscopy | [73] | |
| Yobe | Sheep | 29,120 | 20 | 0.07 | Slaughtered | PM/microscopy | [74] | |
| Yobe | Goats | 87,253 | 9 | 0.01 | Slaughtered | PM/microscopy | [74] | |
| Yobe | Camel | 404 | 7 | 1.73 | Slaughtered | PM/microscopy | [42] | |
| North-West | Kaduna | Dogs | 330 | 4 | 1.2 | Sub urban | NA | [24] |
| Kaduna | Sheep | NA | NA | 7.1 | Slaughtered | PM/microscopy | [24] | |
| Kaduna | Goats | NA | NA | 18.4 | Slaughtered | PM/microscopy | [24] | |
| Kaduna | Cattle | NA | NA | 1.5 | Slaughtered | PM/microscopy | [24] | |
| Kaduna | Camel | NA | NA | 70.9 | Slaughtered | PM/microscopy | [24] | |
| Kaduna | Pigs | NA | NA | 5 | Slaughtered | PM/microscopy | [24] | |
| Kaduna | Dogs | 180 | 1 | 0.6 | Stray | PM/microscopy | [25] | |
| Kano | Dogs | 145 | 9 | 6.21 | Stray | PM/microscopy | [29] | |
| Kano | Goats | 1260 | 334 | 26.5 | Slaughtered | PM/microscopy | [29] | |
| Kano | Sheep | 1800 | 205 | 11.4 | Slaughtered | PM/microscopy | [29] | |
| Kano | Camels | 3580 | 1987 | 55.5 | Slaughtered | PM/microscopy | [29] | |
| Kano | Cattle | 4844 | 712 | 14.7 | Slaughtered | PM/microscopy | [29] | |
| Kaduna | Camels | 18 | 9 | 50 | Slaughtered | PM/microscopy | [26] | |
| Kaduna | Pigs | 147 | 0 | 0 | Slaughtered | PM/microscopy | [26] | |
| Kaduna | Goats | 885 | 7 | 0.79 | Slaughtered | PM/microscopy | [26] | |
| Kaduna | Sheep | 910 | 2 | 0.21 | Slaughtered | PM/microscopy | [26] | |
| Kano | Goats | 1260 | 334 | 26.5 | Slaughtered | PM/microscopy | [26] | |
| Kaduna | Cattle | 1515 | 0 | 0 | Slaughtered | PM/microscopy | [26] | |
| Kano | Sheep | 1800 | 206 | 11.4 | Slaughtered | PM/microscopy | [26] | |
| Kano | Camels | 3580 | 1987 | 55.5 | Slaughtered | PM/microscopy | [26] | |
| Kano | Cattle | 4844 | 713 | 14.7 | Slaughtered | PM/microscopy | [26] | |
| Kano | Dogs | 145 | 9 | 6.2 | Stray | PM/microscopy | [23] | |
| Kaduna | Dogs | 330 | 4 | 1.2 | Stray | PM/microscopy | [23] | |
| Kano | Goats | 130 | 31 | 23.8 | Slaughtered | ELISA/WB | [46] | |
| Kano | Sheep | 138 | 50 | 36.2 | Slaughtered | ELISA/WB | [46] | |
| Sokoto | Camels | 3545 | 318 | 8.97 | Slaughtered | PM/microscopy | [48] | |
| Sokoto | Goats | 14,134 | 4 | 0.03 | Slaughtered | PM/microscopy | [48] | |
| Sokoto | Sheep | 16,345 | 23 | 0.14 | Slaughtered | PM/microscopy | [48] | |
| Sokoto | Cattle | 46,223 | 34 | 0.07 | Slaughtered | PM/microscopy | [48] | |
| Sokoto | Camels | 200 | 84 | 42 | Slaughtered | PM/microscopy | [49] | |
| Sokoto | Camels | 189 | 112 | 59.3 | Slaughtered | ELISA | [58] | |
| Sokoto | Cattle | 285 | 69 | 24.3 | Slaughtered | ELISA | [58] | |
| Sokoto | Cattle | 285 | 5 | 1.8 | Slaughtered | PM/microscopy | [59] | |
| Sokoto | Camel | 189 | 84 | 44.4 | Slaughtered | PM/microscopy | [59] | |
| Sokoto | Sheep | 186 | 0 | 0 | Slaughtered | ELISA, PM/microscopy | [67] | |
| Former Northern region | Northern region | Sheep | 458,603 | 422 | 0.09 | Slaughtered | PM/microscopy | [27] |
| Northern region | Goats | 1,417,096 | 614 | 0.04 | Slaughtered | PM/microscopy | [27] | |
| Northern region | Pigs | 23,830 | 39 | 0.16 | Slaughtered | PM/microscopy | [27] | |
| Northern region | Cattle | 1,815,792 | 1767 | 0.1 | Slaughtered | PM/microscopy | [27] | |
| Northern region | Camel | 49,220 | 659 | 1.34 | Slaughtered | PM/microscopy | [27] | |
| South-East | Anambra | Dogs | 182 | 8 | 4.4 | Rural dogs | PM/microscopy | [57] |
| Anambra | Cattle | 551 | 0 | 0 | Slaughtered | PM/microscopy | [61] | |
| Anambra | Pigs | 2126 | 0 | 0 | Slaughtered | PM/microscopy | [61] | |
| Anambra | Goats | 3830 | 0 | 0 | Slaughtered | PM/microscopy | [61] | |
| Anambra | Pigs | 31,005 | 1 | 0.003 | Slaughtered | PM/microscopy | [61] | |
| Anambra | Cattle | 373,242 | 7 | 0.002 | Slaughtered | PM/microscopy | [61] | |
| Anambra | Goats | 476,249 | 249 | 0.05 | Slaughtered | PM/microscopy | [61] | |
| South-South | Rivers | Dogs | 60 | 51 | 85 | Urban dogs | Microscopy | [9] |
| Rivers | Cattle | 320 | 101 | 31.6 | Slaughtered | PM/microscopy | [10] | |
| Rivers | Goats | 320 | 135 | 42.2 | Slaughtered | PM/microscopy | [10] | |
| Rivers | Pigs | 320 | 179 | 55.9 | Slaughtered | PM/microscopy | [10] | |
| Rivers | Sheep | 320 | 78 | 24.4 | Slaughtered | PM/microscopy | [10] | |
| Cross River | Dogs | 254 | 2 | 0.78 | Urban dogs | Microscopy | [77] | |
| South-West | Oyo | Dogs | 155 | 15 | 9.68 | Hunting/Companion | ELISA | [4] |
| South-West States | Dogs | 273 | 34 | 12.45 | Hunting/Companion | ELISA | [5] | |
| Oyo | Dogs | 102 | 49 | 48 | Owned dogs | Microscopy | [8] | |
| Oyo | Sheep/goats | 215 | 60 | 28 | Slaughtered | PM/microscopy | [8] |
PM postmortem examination, WB Western Blot, ELISA enzyme-linked immunosorbent assay
In Nigeria, besides meeting human needs, livestock rearing is occupational especially in the North and partly in the South where pastoralists often migrate in search for greener pasture and water for their herds. Over the years, while livestock population has risen significantly with current estimates of 19 million cattle, 37 million sheep, 65 million goats, 6 million pigs, 1 million donkeys, and 277,000 camels [53], the impact of echinococcosis on livestock production has remained unevaluated. For instance, between 1960 and 1980, when cattle population was around 4–12 million [15, 36], cystic echinococcosis prevalence was estimated at 1.5–14% in cattle [24, 29]. With the current estimates of cattle population and that of CE prevalence in cattle (0.07–24.3%) [48, 58, 59, 73] coupled with lack of control measures, the impact on cattle production may have risen.
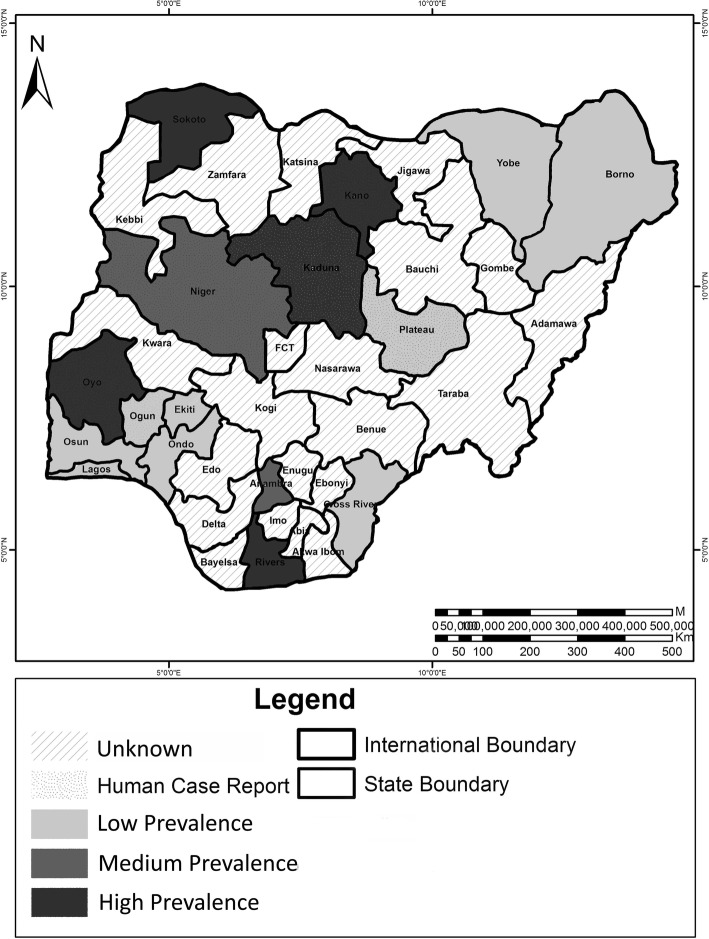
In the 1980s, CE prevalence in sheep and goats was reported to be highest in the Niger Delta region as they were 24.4% and 42.2%, respectively [10], while in Kano state, prevalence was 7.1–11.4% and 18.4–26.4%, respectively [20, 36]. Recent serological reports have also confirmed high levels of antibodies against Echinococcus spp. in sheep and goats slaughtered in Kano (Table 1). Conversely, other states like Kaduna, Sokoto, Yobe, Borno, and Plateau experience lower prevalence (Table 1), while a number of states are yet to be investigated (Fig. 1).
Fig. 1.
Distribution of cystic echinococcosis in Nigeria
Among all livestock forms, higher prevalence is frequently encountered in camels than in other hosts. In the North, between 55.5 and 70.9% have been reported in some states during the 1970–1980s [25, 29], while recent surveys within the last decade put CE prevalence between 8.97 and 59.3% in camels being higher than other concurrently examined livestock [48, 49, 58, 59]. In few northern states, infection in camels could be very low as reported in Maiduguri, Borno state where a prevalence of 1.73% (mainly fertile cysts) was found among 404 camels examined postmortem [42]. To date, this variation in infection rates among livestock across the country specifically in states where surveys have been conducted (Fig. 1, Table 1) has been reported to be largely influenced by different animal husbandry practices, conditions of slaughter slabs, and distribution or abundance of free range hosts among other factors [5, 10, 61]. Another uninvestigated factor in Nigeria probably accounting for the observed prevalence variation could be host susceptibility and/or specificity to species within E. granulosus sensu lato complex.
Prevalence in Humans
In humans, information on cystic echinococcosis is rather scarce. Although where available, low prevalence is commonly reported and has been attributed to the use of less sensitive diagnostic tools [28] or the fact that CE is not considered during routine medical examination. Till now, human CE has been investigated in few states and are mostly retrospective studies involving assessment of hospital records [28, 61] (Table 2). One of such earliest surveys conducted in three northern states (Plateau, Kano, and Kaduna) identified only one case out of 620,695 examined records [28]. Nonetheless, Echinococcus cyst develops progressively and detection in humans is most times incidental with infection being rather asymptomatic except during unusual presentation involving certain organs, in which case the resultant discomfort may warrant diagnosis. Despite that, misdiagnosis and consequently mistreatment are challenges often faced as a result of poor knowledge of the nature of the disease [35]. While human cases of CE remain a rarity, another report was a case of an 18-year-old female from southeastern Nigeria diagnosed of a fertile unilocular pulmonary cyst, presumed to be E. granulosus [6, 7]. This report prompted subsequent examination of hospital records of the University of Nigeria Teaching Hospital, in Enugu State (which also served Anambra State at the time) with preference to surgery cases from January 1977 to December 1986; however, findings from this investigation revealed no documented case. On further examination of rural health centers and some rural- and urban-located hospitals, it was observed that medical personnel in these institutions lacked requisite knowledge of the disease which might have resulted in misdiagnoses [61]. However, in recent times, two cases have been reported and include a musculoskeletal involvement with HIV coinfection in a patient admitted to The University of Jos Teaching Hospital [63], and an orbital echinococcosis manifested in protrusion of the eye and poor vision in The University College Hospital, Ibadan [35]. In the latter case, the patient had an existing record of being managed for 22 years in the teaching hospital with clinical history of painless protrusion of an eye and was treated for non-specific orbital inflammatory disease until further histopathological examination confirmed the presence of a cyst with a laminated and a germinal layer.
Table 2.
Human cystic echinococcosis survey and case reports in Nigeria (1970–2018)
| State | Sample size | Prevalence (%) | Target population | Detection method | References |
|---|---|---|---|---|---|
| Kano | 189,861 | 0.000005 | Hospital patients | Surgery (retrospective) | [28] |
| Plateau | 151,007 | 0 | Hospital patients | Surgery (retrospective) | [28] |
| Kaduna | 279,827 | 0 | Hospital patients | Surgery (retrospective) | [28] |
| Oyo | Case report | – | Hospital patient | CT scan, HIS | [35] |
| Anambra | Case report | – | NA | n/a | [7] |
| Plateau | Case report | – | HIV positive | n/a | [63] |
| Niger, Ogun | 176 | 0.53 | Hospital patients | CFT | [70] |
CFT complement fixation text, CT computed tomography, HIS Histopathology, n/a not available
Therefore, it is plausible that most human cases of CE presented in a number of hospitals could similarly be misdiagnosed and consequently mistreated. This problem of misdiagnosis remains a serious challenge in identifying CE cases in Nigeria. So far, the only population-based survey was carried out using 176 blood sera from hospital patients in Minna and Abeokuta in Niger and Ogun states, respectively, and mean result showed a 0.53% CE seroprevalence [70]. Although human CE in Nigeria is usually rare, a similar situation exists in many eastern and northern African countries where human prevalence usually ranged from 0 to 2% and is yet considered a disease of major public health concern [12, 44, 50, 68]. Regardless, the need for differential diagnosis of suspected cases remains invaluable in appraising the level of human CE infection across zones in Nigeria.
Risk Factors
In endemic regions across the world, infection is associated with resource poor settings, pastoral lifestyle, certain pasture types as well as socio-economic and behavioral practices [19, 55, 79, 80]. Other factors include sources of drinking water, changes in environmental conditions favouring egg survival, transboundary animal movement and livestock trade, high proportion/presence of stray dogs, frequency of dog-man contacts, poor abattoir conditions and poor disposal of waste from slaughterhouses [2, 11, 19, 55, 60, 62]. In Nigeria, besides these factors [5, 35], others such as age of livestock have been labeled to be largely responsible for higher infection rate in camels than in other livestock. For example, camels are often slaughtered after 8–10 years of age, leading to increased exposure and subsequent risk of acquiring the infection [59]. Recently, differences in ambient temperatures across the country were suggested as a possible factor that could cause prevalence to vary as some states experience temperatures as high as 45 °C resulting in desiccation of eggs [42]. Furthermore, since no study has been dedicated to identifying local factors responsible for human and livestock CE in Nigeria, the need for such studies to identify local transmission patterns cannot be overstated.
Bottleneck of Cystic Echinococcosis Research in Nigeria
Over the years, global echinococcosis research has recorded huge progress, including the discovery of new species and expanding knowledge on genotypes and haplotype variation, complete genome sequences, development of sensitive detection techniques, and the EG95 vaccine [1, 37, 51, 54, 75, 81, 85]. In spite of these feats, a lot of questions remain unanswered on the status of CE in Nigeria. For example, of the available studies on echinococcosis, over 60% were conducted between 1970 and 1990, and less than 10% in the last decade. Another challenge is in the choice of diagnosis/detection tools used, such as microscopy, postmortem examination, immunodiagnosis, and molecular techniques. While the challenge of microscopy is in its inappropriateness for investigating intermediate hosts, age of livestock, early infection, and variation in cyst development in intermediate hosts [38, 41, 71] may fraught the effort of postmortem approach (the gold standard). Only recently, surveys have sparingly employed ELISA [5, 46, 58, 67], sonography, and histopathology techniques [35, 63]. Following WHO publication of Standardized classification of ultrasound images of CE, the use of sonography in combination with differential diagnosis has been advocated as a useful tool in detecting different presentation of CE infection in humans especially among population at risk [82]. With serology, the inability of available immunodiagnostic tools (ELISA) to discriminate between strains/genotypes or the problem of specificity/sensitivity or cross reactivity remains a major reason why serology is still debatable [22, 66, 72]. Although some antigens and recombinant proteins have shown potential for diagnosis, standardization and understanding of their performance level is greatly needed (see [69] for details on diagnosis of CE).
On the other hand, PCR-based techniques are regarded to be highly useful for genotyping [17, 21], however, have not been applied in Nigeria till date; hence, the consequent lack of information regarding the genotypes and genetic population structure unlike in northern and eastern African countries where genotypes and their genetic variation have been largely investigated [3, 14]. Though no molecular data exists on the circulating E. granulosus genotypes, it will be apt to find the common E. granulosus s.s., and E. canadensis (G6) considering the involvement of intermediate hosts like sheep, goats, cattle, and camel coupled with the high species/haplotype diversity reported in the sub-Saharan African region [30]. Further, the interface between wildlife and domestic CE transmission remains largely uninvestigated.
Conclusion
The available data clearly emphasize the public health implication of CE, especially in regions where cases (both human and animals) have been reported and factors enhancing transmission are present. With the lack of information on two-third of the states and absence of data on the circulating genotypes, it is pertinent to state that CE in Nigeria is highly under-investigated and thus neglected regardless of its high zoonotic potential and the public health outcry raised in the early years of investigation. Thus, it is important to evolve and embark on a comprehensive animal and human survey across the country to make data available especially on the genetic population structure, local risk factors enhancing transmission, and the role of wildlife in CE transmission as a critical step to evaluating areas where control efforts should be prioritized. In addition, awareness of the nature of the disease and trainings on the diagnoses should be provided for medical personnel in hospitals to differentially diagnose and identify CE on presentation. Finally, we recommend participation in initiatives like the German–African consortium on cystic echinococcosis: Cystic Echinococcosis in sub-Saharan Africa Research Initiative, CESSARi (https://gdri-ehede.univ-fcomte.fr/spip.php?article23&lang=en), a collaborative platform and capacity building forum aimed at effectively and efficiently investigating CE epidemiology, economic impact and genetic diversity toward disease eradication.
Acknowledgements
This study was supported by Central Public-interest Scientific Institution Basal Research Fund (1610312017001; 1610312016012), National Key Basic Research Program (973 Program) of China (2015CB150300), National Key Research and Development Plan (2017YFD0501301), and NBCITS, MOA (CARS-38), while John Asekhaen Ohiolei’s Ph.D. program was funded by the Chinese Government Scholarship Programme of the Peoples Republic of China.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Abbasi I, Branzburg A, Campos-Ponce M, Abdel Hafez SK, Raoul F, Craig PS, Hamburger J. Copro-diagnosis of Echinococcus granulosus infection in dogs by amplification of a newly identified repeated DNA sequence. Am J Trop Med Hyg. 2003;69:324–330. doi: 10.1016/j.bmcl.2005.08.062. [DOI] [PubMed] [Google Scholar]
- 2.Addy F, Alakonya A, Wamae N, Magambo J, Mbae C, Mulinge E, Zeyhle E, Wassermann M, Kern P, Romig T. Prevalence and diversity of cystic echinococcosis in livestock in Maasailand, Kenya. Parasitol Res. 2012;111:2289–2294. doi: 10.1007/s00436-012-3082-8. [DOI] [PubMed] [Google Scholar]
- 3.Addy F, Wassermann M, Kagendo D, Ebi D, Zeyhle E, Elmahdi IE, Umhang G, Casulli A, Harandi MF, Aschenborn O, Kern P, Mackenstedt U, Romig T. Genetic differentiation of the G6/7 cluster of Echinococcus canadensis based on mitochondrial marker genes. Int J Parasitol. 2017;47:923–931. doi: 10.1016/j.ijpara.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Adediran OA, Kolapo TU. Canine echinococcosis in hunting and companion dogs in Oyo State, Nigeria: the public health significance. Acta Parasitol Globalis. 2014;5:59–64. doi: 10.5829/idosi.apg.2014.5.1.8328. [DOI] [Google Scholar]
- 5.Adediran OA, Kolapo TU, Uwalaka EC. Echinococcus granulosus prevalence in dogs in Southwest Nigeria. J Parasitol Res. 2014;2014:124358. doi: 10.1155/2014/124358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Afonja AO, Sofowora EO, Kolawole TM, Junaid TA, Fatunmbi BO. Hydatid disease of the lung in Nigeria: case report. J Trop Med Hyg. 1972;75:224–226. doi: 10.1016/0002-9610(85)90439-8. [DOI] [PubMed] [Google Scholar]
- 7.Amene P, Jacob A, Swarup A. Hydatid lung disease in a Nigerian female. Niger Med Pract. 1984;7:173–175. [Google Scholar]
- 8.Anyanwale FO, Dipeolu OO, Esuruoso GC. Incidence of Echinococcus infection in Dogs, Sheep and goats slaughtered in Ibadan, Nigeria. Int J Zoonoses. 1982;9:65–67. doi: 10.1111/j.1751-0813.1982.tb00690.x. [DOI] [PubMed] [Google Scholar]
- 9.Arene FO. Prevalence of toxocariasis and echinococcosis among dogs in the Niger Delta. J Trop Med Hyg. 1984;87:207–209. doi: 10.1177/146642408410400636. [DOI] [PubMed] [Google Scholar]
- 10.Arene FO. Prevalence of hydatid cysts in domestic livestock in the Niger Delta. Trop Anim Health Prod. 1985;17:3–5. doi: 10.1007/bf02356125. [DOI] [PubMed] [Google Scholar]
- 11.Assefa H, Mulate B, Nazir S, Alemayehu A. Cystic echinococcosis amongst small ruminants and humans in central Ethiopia. Onderstepoort J Vet Res. 2015;82:E1–E7. doi: 10.4102/ojvr.v82i1.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barclay S, Joseph J, Timothy F, Mounir L, Robert N, Brooker S, Sidhu P, Kolaczinski J. Cystic echinococcosis in Mundari tribe-members of South Sudan. Pathogens Glob Health. 2013;107:293–298. doi: 10.1179/2047773213y.0000000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bekele J, Butako B. Occurrence and financial loss assessment of cystic echinococcosis (hydatidosis) in cattle slaughtered at Wolayita Sodo municipal abattoir, Southern Ethiopia. Trop Anim Health Prod. 2011;43:221–228. doi: 10.1007/s11250-010-9680-5. [DOI] [PubMed] [Google Scholar]
- 14.Boufana B, Lett W, Lahmar S, Griffiths A, Jenkins DJ, Buishi I, et al. Canine echinococcosis: genetic diversity of Echinococcus granulosus sensu stricto (s.s.) from definitive hosts. J Helminthol. 2015;89:689–698. doi: 10.1017/s0022149x15000395. [DOI] [PubMed] [Google Scholar]
- 15.Bourn D, Milligan K. The dynamics of cattle distribution in the Nigeria Subhumid Zone. Report to ILCA’s Subhumid Programme. Kaduna: International Livestock Center for Africa, Subhumid Programme; 1983. [Google Scholar]
- 16.Bowles J, Blair D, Mcmanus DP. Genetic variants within the genus Echinococcus identified by mitochondrial DNA sequencing. Mol Biochem Parasitol. 1992;54:165–174. doi: 10.1016/0166-6851(92)90109-W. [DOI] [PubMed] [Google Scholar]
- 17.Bowles J, Mcmanus DP. Rapid discrimination of Echinococcus species and strains using a polymerase chain reaction-based RFLP method. Mol Biochem Parasitol. 1993;57:231–239. doi: 10.1016/0166-6851(93)90199-8. [DOI] [PubMed] [Google Scholar]
- 18.Budke CM, Carabin H, Ndimubanzi PC, Nguyen H, Rainwater E, Dickey M, et al. A systematic review of the literature on cystic echinococcosis frequency worldwide and its associated clinical manifestations. Am J Trop Med Hyg. 2013;88:1011–1027. doi: 10.4269/ajtmh.12-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Budke CM, Qiu JM, Craig PS, Torgerson PR. Modeling the transmission of Echinococcus granulosus and Echinococcus multilocularis in dogs for a high endemic region of the Tibetan plateau. Int J Parasitol. 2005;35:163–170. doi: 10.1016/j.ijpara.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Carmena D, Cardona GA. Echinococcosis in wild carnivorous species: epidemiology, genotypic diversity, and implications for veterinary public health. Vet Parasitol. 2014;202:69–94. doi: 10.1016/j.vetpar.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 21.Chaâbane-Banaoues R, Oudni-Mrad M, Mrad S, Amani H, Mezhoud H, Babba H. A novel PCR-RFLP assay for molecular characterization of Echinococcus granulosus sensu lato and closely related species in developing countries. Parasitol Res. 2016;115:3817–3824. doi: 10.1007/s00436-016-5143-x. [DOI] [PubMed] [Google Scholar]
- 22.Craig PS, Rickard MD. Studies on the specific immunodiagnosis of larval cestode infections of cattle and sheep using antigens purified by affinity chromatography in any enzyme-linked immunosorbent assay (ELISA) Int J Parasitol. 1981;11:441–449. doi: 10.1016/0020-7519(81)90062-x. [DOI] [PubMed] [Google Scholar]
- 23.Dada BJ. Taeniasis, cysticercosis and echinococcosis/hydatidosis in Nigeria: IV–prevalence of Echinococcus granulosus infection in stray dogs. J Helminthol. 1980;54:299–301. doi: 10.1017/s0022149x00006805. [DOI] [PubMed] [Google Scholar]
- 24.Dada BJ, Adegboye DS, Mohammed AN. The epidemiology of Echinococcus infection in Kaduna State, Nigeria. Vet Records. 1979;104:312–313. doi: 10.1136/vr.104.14.312. [DOI] [PubMed] [Google Scholar]
- 25.Dada BJ, Adegboye DS, Mohammed AN. A survey of gastro intestinal helminth parasites of stray dogs in Zaria, Nigeria. Vet Records. 1979;104:145–146. doi: 10.1136/vr.104.7.145. [DOI] [PubMed] [Google Scholar]
- 26.Dada BJO. Taeniasis, cysticercosis and echinococcosis/hydatidosis in Nigeria: III — prevalence of bovine and porcine cysticercosis, and hydatid cyst infection based on joint examination of slaughtered food animals. J Helminthol. 1980;54:293–297. doi: 10.1017/s0022149x00006799. [DOI] [PubMed] [Google Scholar]
- 27.Dada BJO. Taeniasis, cysticercosis and echinococcosis/hydatidosis in Nigeria: II—prevalence of bovine and porcine cysticercosis, and hydatid disease in slaughtered food animals based on retrospective analysis of abattoir records. J Helminthol. 1980;54:287–291. doi: 10.1017/s0022149x00006787. [DOI] [PubMed] [Google Scholar]
- 28.Dada BJO. Taeniasis, cysticercosis and echinococcosis/hydatidosis in Nigeria: I—prevalence of human taeniasis, cysticercosis and hydatidosis based on a retrospective analysis of hospital records. J Helminthol. 1980;54:281–286. doi: 10.1017/s0022149x00006775. [DOI] [PubMed] [Google Scholar]
- 29.Dada BJO, Adegboye DS, Mohammed AN. The epidemiology of Echinococcus infection in Kano State, Nigeria. Ann Trop Med Parasitol. 1980;74:515–517. doi: 10.1080/00034983.1980.11687378. [DOI] [PubMed] [Google Scholar]
- 30.Deplazes P, Rinaldi L, Alvarez Rojas CA, Torgerson PR, Harandi MF, Romig T, et al. Global distribution of alveolar and cystic echinococcosis. Adv Parasitol. 2017;95:315–493. doi: 10.1016/bs.apar.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Division of Medical Sciences (1962) Tropical Health: a report on a study of needs and resources. Publication 996, National Academy of Sciences-National Research Council. Washington DC, USA
- 32.Duncan M. A case of hydatid disease in Northern Nigeria. West Afr Med J. 1961;10(63):2. [PubMed] [Google Scholar]
- 33.Ekanem EE, Eyong KI, Philip-Ephraim EE, Eyong ME, Adams EB, Asindi AA. Stray dog trade fuelled by dog meat consumption as a risk factor for rabies infection in Calabar, southern Nigeria. Afr Health Sci. 2013;13:1170–1173. doi: 10.4314/ahs.v13i4.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ernest E, Nonga HE, Kassuku AA, Kazwala RR. Hydatidosis of slaughtered animals in Ngorongoro district of Arusha region, Tanzania. Trop Anim Health Prod. 2009;41:1179–1185. doi: 10.1007/s11250-008-9298-z. [DOI] [PubMed] [Google Scholar]
- 35.Fasina O, Ogun OG. Hydatid cyst of the orbit in a young Nigerian female: a case report. Ghana Med J. 2017;51:204–206. doi: 10.4314/gmj.v51i4.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Food and Agriculture Organization (FAO) (2017) FAOSTAT: statistics division. production/live animals Nigeria. http://www.fao.org/faostat/en/#data/QA/visualize. Accessed 2018
- 37.Gauci C, Heath D, Chow C, Lightowlers MW. Hydatid disease: vaccinology and development of the EG95 recombinant vaccine. Expert Rev Vaccines. 2005;4:103–112. doi: 10.1586/14760584.4.1.103. [DOI] [PubMed] [Google Scholar]
- 38.Gemmell MA, Lawson JR, Roberts MG. Population dynamics in echinococcosis and cysticercosis: biological parameters of Echinococcus granulosus in dogs and sheep. Parasitology. 1986;92:599–620. doi: 10.1017/S0031182000065483. [DOI] [PubMed] [Google Scholar]
- 39.Getaw A, Beyene D, Ayana D, Megersa B, Abunna F. Hydatidosis: prevalence and its economic importance in ruminants slaughtered at Adama municipal abattoir, Central Oromia, Ethiopia. Acta Trop. 2010;113:221–225. doi: 10.1016/j.actatropica.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 40.Hambolu SE, Dzikwi AA, Kwaga JKP, Kazeem HM, Umoh JU, Hambolu DA. Dog ecology and population studies in Lagos State, Nigeria. Glob J Health Sci. 2014;6:209–220. doi: 10.5539/gjhs.v6n2p209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heath DD. The life cycle of Echinococcus granulosus. In: Brown RW, Salisbury JR, White WE, editors. Recent advances in hydatid disease. Victoria: Hamilton Medical and Veterinary Association; 1973. [Google Scholar]
- 42.Igwenagu E, Onyiche ET, Saidu AM, Chahari AM, Waziri A, Kayeri BK. Prevalence of hydatidosis and fertility of hydatid cyst in slaughtered camels in Maiduguri, Nigeria. Ife J Sci. 2018;20:299–303. doi: 10.4314/ijs.v20i2.10. [DOI] [Google Scholar]
- 43.Kern P, Menezes da Silva A, Akhan O, Müllhaupt B, Vizcaychipi KA, Budke C, et al. The Echinococcoses: diagnosis, clinical management and burden of disease. Adv Parasitol. 2017;96:259–369. doi: 10.1016/bs.apar.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 44.Klungsoyr P, Courtright P, Hendrikson TH. Hydatid disease in the Hamar of Ethiopia: a public health problem for women. Trans R Soc Trop Med Hyg. 1993;87:254–255. doi: 10.1016/0035-9203(93)90114-6. [DOI] [PubMed] [Google Scholar]
- 45.Laurimäe T, Kinkar L, Moks E, Romig T, Omer RA, Casulli A, et al. Molecular phylogeny based on six nuclear genes suggests that Echinococcus granulosus sensu lato genotypes G6/G7 and G8/G10 can be regarded as two distinct species. Parasitology. 2018;145:1929–1937. doi: 10.1017/s0031182018000719. [DOI] [PubMed] [Google Scholar]
- 46.Luka S, Ajogi I, Nock I, Kudi C, Umoh J. Evaluation of enzyme-linked immunosorbent assay (ELISA) and Western Blotting for the immunodiagnosis of hydatid diseases in Sheep and Goats. Internet J Vet Med. 2008;5:1–7. [Google Scholar]
- 47.Lymbery AJ. Phylogenetic pattern, evolutionary processes and species delimitation in the genus Echinococcus. Adv Parasitol. 2017;95:111–145. doi: 10.1016/bs.apar.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 48.Magaji AA, Oboegbulem SI, Daneji AI, Garba HS, Salihu MD, Junaidu AU, et al. Incidence of Hydatid cyst disease in food animals slaughtered at Sokoto Central Abattoir, Sokoto State, Nigeria. Vet World. 2011;4:197–200. doi: 10.5455/vetworld.2011.197-200. [DOI] [Google Scholar]
- 49.Magaji AA, Onwuegbunam CU, Sonfada ML, Salihu MD. Prevalence of hydatidosis in camels slaughtered in Sokoto Central Abattoir, Sokoto, Nigeria. Bull Anim Health Prod Afr. 2012;60:219–224. [Google Scholar]
- 50.Magambo JK, Hall C, Zeyle E, Wachira TM. Prevalence of human hydatid disease in southern Sudan. Afr J Health Sci. 1996;3:154–156. [PubMed] [Google Scholar]
- 51.Morel N, Lassabe G, Elola S, Bondad M, Herrera S, Marí C, et al. A monoclonal antibody-based Copro-ELISA kit for canine echinococcosis to support the PAHO effort for hydatid disease control in South America. PLoS Negl Trop Dis. 2013;7:e1967. doi: 10.1371/journal.pntd.0001967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakao M, Lavikainen A, Hoberg E. Is Echinococcus intermedius a valid species? Trends Parasitol. 2015;31:342–343. doi: 10.1016/j.pt.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 53.National Agriculture Sample Survey, Nigeria (2010) National Bureau of Statistics. Public access dataset. http://nigerianstat.gov.ng/download/66. Accessed 18 Feb 2015
- 54.Ni X, Mcmanus DP, Yan H, Yang J, Lou Z, Li H, et al. Loop-mediated isothermal amplification (LAMP) assay for the identification of Echinococcus multilocularis infections in canine definitive hosts. Parasite Vectors. 2014;7:254. doi: 10.1186/1756-3305-7-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Njoroge EM, Mbithi PMF, Gathuma JM, Wachira TM, Gathura PB, Magambo JK, et al. A study of cystic echinococcosis in slaughter animals in three selected areas of northern Turkana, Kenya. Vet Parasitol. 2002;104:85–91. doi: 10.1016/S0304-4017(01)00614-8. [DOI] [PubMed] [Google Scholar]
- 56.Oboegbulem SI, Nwakonobi IE. Population density and ecology of dogs in Nigeria: a pilot study. Revue Scientifique Et Technique De Loie. 1989;8:733–745. doi: 10.20506/rst.8.3.426. [DOI] [PubMed] [Google Scholar]
- 57.Okolo MI. Prevalence and public health implications of Echinococcus granulosus in rural dogs in Eastern Nigeria. Int J Zoonoses. 1986;13:19–24. doi: 10.1111/j.1751-0813.1986.tb02948.x. [DOI] [PubMed] [Google Scholar]
- 58.Okolugbo B, Luka S, Ndams I. Enzyme-linked immunosorbent assay (ELISA) in the serodiagnosis of hydatidosis in camels (Camelus dromedarius) and cattle in Sokoto, Northern Nigeria. Internet J Infect Dis. 2014;13:1–6. [Google Scholar]
- 59.Okolugbo BC, Luka SA, Ndams IS. Hydatidosis of camels and cattle slaughtered in Sokoto State, Northern Nigeria. Food Sci Qual Manag. 2013;21:40–46. [Google Scholar]
- 60.Omadang L, Chamai M, Othieno E, Okwi A, Inangolet FO, Ejobi F, Oba P, Ocaido M. Knowledge, attitudes and practices towards cystic echinococcosis in livestock among selected pastoral and agro-pastoral communities in Uganda. Trop Anim Health Prod. 2018;50:11–17. doi: 10.1007/s11250-017-1394-5. [DOI] [PubMed] [Google Scholar]
- 61.Onah DN, Chiejina SN, Emehelu CO. Epidemiology of echinococcosis/hydatidosis in Anambra State, Nigeria. Ann Trop Med Parasitol. 1989;83:387–393. doi: 10.1080/00034983.1989.11812362. [DOI] [PubMed] [Google Scholar]
- 62.Othieno E, Okwi AL, Mupere E, Zeyhle E, Oba P, Chamai M, et al. Risk factors associated with cystic echinococcosis in humans in selected pastoral and agro-pastoral areas of Uganda. Int J One Health. 2017;3:1–6. doi: 10.14202/IJOH.2017.1-6. [DOI] [Google Scholar]
- 63.Ozoilo KN, Iya D, Kidmas AT, Uwumarogie O, Hassan S. Anterior abdominal wall hydatid cyst; an unusual presentation. Niger J Med. 2007;16:181–182. doi: 10.4314/njm.v16i2.37261. [DOI] [PubMed] [Google Scholar]
- 64.Pawlowski ZS, Eckert J, Vuitton DA, Ammann RW, Kern P, Craig PS, et al. Echinococosis in Humans: clinical aspects, diagnosis, and treatment. In: Eckert J, Gemmell MA, Meslin FX, Pawlowski ZS, et al., editors. Manual on Echinococcosis in humans and animals: a public health problem of global concern. Paris: World Health Organization for Animal Health and World Health Organization (OIE/WHO); 2001. pp. 20–72. [Google Scholar]
- 65.Regassa F, Molla A, Bekele J. Study on the prevalence of cystic hydatidosis and its economic significance in cattle slaughtered at Hawassa Municipal abattoir, Ethiopia. Trop Anim Health Prod. 2010;42:977–984. doi: 10.1007/s11250-009-9517-2. [DOI] [PubMed] [Google Scholar]
- 66.Sangaran A, Bino Sundar ST, Latha BR. Antigen based detection of cystic echinococcosis in buffaloes using ELISA and Dot-EIA. J Parasitic Dis. 2016;41:128–130. doi: 10.1007/s12639-016-0762-9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 67.Saulawa MA, Magaji AA, Faleke OO, Mohammed AA, Junaidu AU, Musawa AI, et al. Serodiagnosis of hydatidosis in sheep slaughtered at Sokoto abattoir, Sokoto state, Nigeria. Sokoto J Vet Sci. 2011;9:20–23. [Google Scholar]
- 68.Shambesh MA, Craig PS, Macpherson CN, Rogan MT, Gusbi AM, Echtuish EF. An extensive ultrasound and serologic study to investigate the prevalence of human cystic echinococcosis in northern Libya. Am J Trop Med Hyg. 1999;60:462–468. doi: 10.0000/pmid10466978. [DOI] [PubMed] [Google Scholar]
- 69.Siles-Lucas M, Casulli A, Conraths FJ, Müller N. Laboratory diagnosis of Echinococcus spp. in human patients and infected animals. Adv Parasitol. 2017;96:159–257. doi: 10.1016/bs.apar.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 70.Sixl W, Rosegger H, Schneeweiss H, Withalm H, Schuhmann G. Serological investigations in Nigeria for anthropozoonoses in human sera: brucellosis, echinococcosis, toxoplasmosis, chlamydial diseases, listeriosis, rickettsiosis (Coxiella burnetti and Rickettsia conori) J Hyg Epidemiol Microbiol Immunol. 1987;31:493–495. [PubMed] [Google Scholar]
- 71.Slais J. Experimental infection on sheep and pigs with Echinococcus granulosus (Batsch, 1786), and the origin of pouching in hydatid cysts. Acta Veterinaria Academiae Scientiarum Hungaricae. 1981;28:375–387. doi: 10.2754/avb197948010095. [DOI] [PubMed] [Google Scholar]
- 72.Tamarozzi F, Covini I, Mariconti M, Narra R, Tinelli C, De Silvestri A, et al. Comparison of the diagnostic accuracy of three rapid tests for the serodiagnosis of hepatic cystic Echinococcosis in humans. PLoS Negl Trop Dis. 2016;10(2):e0004444. doi: 10.1371/journal.pntd.0004444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tijjani AO, Musa HI, Atsanda NN. Prevalence of hydatid disease in cattle and camel slaughtered at Damaturu Abattoir, Nigeria. Sahel J Vet Sci. 2010;9:33–36. [Google Scholar]
- 74.Tijjani AO, Musa HI, Atsanda NN, Mamman B. Prevalence of hydatidosis in sheep and goats slaughtered at Damaturu abattoir, Yobe state, Nigeria. Niger Vet J. 2010;31:71–75. [Google Scholar]
- 75.Tsai IJ, Zarowiecki M, Holroyd N, Garciarrubio A, Sánchez-Flores A, et al. The genomes of four tapeworm species reveal adaptations to parasitism. Nature. 2013;496:57–63. doi: 10.1038/nature12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Udoidung NI, Adams EG, Ekpo EJ, Opara KNA. Survey on intestinal nematodes of dogs in Uyo, Akwa Ibom State, Nigeria. J Parasitol Vector Biol. 2018;10:39–44. doi: 10.5897/jpvb2017.0301. [DOI] [Google Scholar]
- 77.Ugochukwu EI, Ejimadu KN. Studies on the prevalence of gastro-intestinal helminths of dogs in Calabar, Nigeria. Int J Zoonoses. 1985;12:214–218. doi: 10.2754/avb198554030217. [DOI] [PubMed] [Google Scholar]
- 78.Wahlers K, Menezes CN, Wong ML, Zeyhle E, Ahmed ME, Ocaido M, et al. Cystic echinococcosis in sub-Saharan Africa. Lancet Infect Dis. 2012;12:871–880. doi: 10.1016/s1473-3099(12)70155-x. [DOI] [PubMed] [Google Scholar]
- 79.Wang Q, Huang Y, Huang L, Yu W, He W, Zhong B, et al. Review of risk factors for human echinococcosis prevalence on the Qinghai-Tibet Plateau, China: a prospective for control options. Infecti Dis Poverty. 2014;3:3. doi: 10.1186/2049-9957-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang Q, Vuitton DA, Xiao Y, Budke CM, Campos-Ponce M, Schantz PM, et al. Pasture types and Echinococcus multilocularis, Tibetan communities. Emerg Infect Dis. 2006;12:1008–1010. doi: 10.3201/eid1206.041229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wassermann M, Woldeyes D, Gerbi BM, Ebi D, Zeyhle E, Mackenstedt U, et al. A novel zoonotic genotype related to Echinococcus granulosus sensu stricto from southern Ethiopia. Int J Parasitol. 2016;46:663–668. doi: 10.1016/j.ijpara.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 82.WHO Informal Working Group International classification of ultrasound images in cystic echinococcosis for application in clinical and field epidemiological settings. Acta Trop. 2003;85:253–261. doi: 10.1016/s0001-706x(02)00223-1. [DOI] [PubMed] [Google Scholar]
- 83.World Health Organization (WHO) (2013) Sustaining the drive to overcome the global impact of neglected tropical diseases: second WHO Report on Neglected Diseases. World Health Organization
- 84.World Health Organization (WHO) (2015) Echinococcosis. Fact Sheet No. 377. World Health Organization
- 85.Zheng H, Zhang W, Zhang L, Zhang Z, Li J, Lu G, et al. The genome of the hydatid tapeworm Echinococcus granulosus. Nat Genet. 2013;45:1168–1175. doi: 10.1038/ng.2757. [DOI] [PubMed] [Google Scholar]