Abstract
Natural killer (NK) cell deficiency (NKD) is a subset of primary immunodeficiency disorders (PID) in which an abnormality of NK cells represents a major immunological defect resulting in the patient’s clinical immunodeficiency. This is distinct from a much larger group of PIDs that include an NK cell abnormality as a minor component of the immunodeficiency. Patients with NKD most frequently have atypical consequences of herpesviral infections. There are now 6 genes that have been ascribed to causing NKD, some exclusively and others that also cause other known immunodeficiencies. This list has grown in recent years and as such the mechanistic and molecular clarity around what defines an NKD is an emerging and important field of research. Continued increased clarity will allow for more rational approaches to the patients themselves from a therapeutic standpoint. Having evaluated numerous individuals for NKD, I share my perspective on approaching the diagnosis and managing these patients.
Keywords: NK cells, natural killer cell deficiency, primary immunodeficiency
Introduction
It was humbling to have been asked to write an overview of management of NK cell deficiency (NKD), however I was unsure that there was anything to be said about this given the rarity of these patients and limited therapeutic options. When prompted to consider the topic however, I look back upon the cases that I have been or presently am personally engaged in as well as those that I have read about or discussed with colleagues and realize that there probably is some insight to be shared. If nothing more, it represents a point from which to move forward in the diagnosis and management of patients. As the diagnosis of NKD is becoming more established, in particular through a number of defined genetic underpinnings, what we need now is directed research and evaluation, retrospectively and prospectively, of how best to improve the health and well-being of patients diagnosed with NKD. As there is little in the way of specific research to call upon in guiding this topic from a management perspective, I have elected to write portions of this perspective in the first person. That said, I will do my best to call upon and assimilate relevant published anecdotal and communicated experience with regards to treatments and outcomes. I apologize in advance for those whose perspectives and ideas I have overlooked in assembling this brief.
At present, the most important part of proper management of NKD is establishing the correct diagnosis, which has been historically challenging. A main reason has been that diagnosis has in part depended upon the assessment of NK cell function using a biological assay to measure NK cell-mediated cytotoxicity. While an extremely informative assay, it is one that can be affected by a variety of conditions and physiological states, including severe illness, psychological stress, and depression amongst others. Furthermore, this represents only part of an NKD diagnosis and the identification of some genetic etiologies for NKD, as well as enhanced tools to assess NK cell phenotype and function have improved the ability to consider patients who might have the diagnosis and establish a reliable basis for their further investigation and care. Thus, in addition to presenting a perspective on the management of patients diagnosed with NKD, I will also provide a brief summary of how NKD is defined and how best to pursue the and/or confirm a diagnosis of NKD.
NKD-Directed Primer on NK Cell Biology and Function
By way of introduction, NK cells are categorized as lymphocytes of the innate immune system as a divergent branch of the innate lymphoid cell (ILC) lineage [1]. These cells are not vestigial and have been sustained through evolution for a variety of reasons [2]. They are postulated to serve relevant roles in reproductive success, surveillance of cancerous cells and control of viral infections [3–6]. This is supported by strong evidence accumulated in a wide variety of animal models and in select human contexts collected over the nearly 45 years since NK cells were named as such.
NK cells exist as approximately 10% of peripheral blood lymphocytes [7] as well as varying percentages of tissue-based lymphocyte populations in both lymphoid and non-lymphoid organs [8]. NK cells do not rearrange their germline DNA to gain specificity and inherently carry all relevant receptor sequences needed to exert function. Of note, this is despite the fact that under certain circumstances NK cells can fulfill the criteria of having immunological memory with antigen specific expansion, contraction and recall [9]. As far as we know, this occurs via a given set of otherwise invariant receptors. The receptors in NK cells capable of activation recognize a variety of signatures of disease or cell stress and exist in balance with a relatively large family of inhibitory receptors capable of repressing NK cell functions, most notably the Killer cell Immunoglobulin Receptor (KIR) family that is ligated by particular class-I MHC molecules [10, 11]. The identification of KIR receptors gave rise to the historical concept of “missing self” in which NK cells were believed to be called into action when a host cell had lost class-I MHC, an otherwise fundamental signature of cell health and function. “Missing self” represented a key theoretical concept in immunology for many years in that it was believed that T cells existed to recognize “altered self,” specifically MHC molecules presenting foreign peptides, and that NK cells were there to be engaged in circumstances where “self” was not present [12]. Critical examples where cells down-regulate or lose expression of class-I MHC (“missing self”) include when they become infected with particular viruses that are actively escaping cytotoxic T cell (CTL) responses, or in malignant cells that have lost growth control and similarly evaded CTL functions. The prime example of the former is the evasion mechanism used by many herpesviruses, some of which even have their own class-I MHC decoy molecules encoded in their genome to specifically try to inhibit and evade NK cells [13, 14]. Having downregulated or low levels of class-I MHC, however, is not sufficient for triggering NK cells as many cells that have inherently low levels, including erythrocytes, are not routine targets of NK cells. Thus, an activating receptor on an NK cell must also be engaged by a cognate ligand on a potential target cell to induce NK cell activity. There are many such receptors on NK cells, with varying strengths of signaling. Examples include the NK cell isoform of CD16, which is an Fc receptor that recognizes cells opsonized with IgG; and CD244, which recognizes CD48 that is expressed and upregulated on B cells infected with Epstein Barr Virus (EBV), and is also the target of viral evasion strategies [15]. It is important to view NK cell triggering as the integration of signals from often multiple activation receptors in excess of any inhibitory signaling: in other words, a balance favoring activation.
Once a threshold of NK cell receptors that are favoring activation is surpassed, one of a number of different functions can be accessed. These include cytokine production, contact-dependent co-stimulation, and cytotoxicity [3]. As for the ability to produce cytokines, NK cells are especially adept at contributing IFN-γ but are also capable of secreting others, including TNFα, IL-10, IL-5 and IL-13. With regards to co-stimulation, NK cells can express a variety of costimulatory receptors, including CD154 and CD137, to contribute to and facilitate other aspects of an immune response. Importantly, NK cell cytotoxicity is an end unto itself in being able to eradicate a susceptible target cell. Cytotoxicity is a well-orchestrated cellular process in which highly specialized organelles, called lytic granules, are organized and polarized towards a triggering diseased cell [16]. Once polarized, the contents of the lytic granules, which include the pore-forming molecule perforin and cell death-inducing granzymes, can be secreted through an interface known as the lytic immunological synapse leading to the death of the targeted cell. A surprisingly small number of these lytic granules are actually needed to destroy a targeted cell – on the order of two to four – thus defining NK cell cytotoxicity as a highly efficient and effective process [17]. Having NK cells that are disabled in cytotoxicity but fully functional in producing cytokines contributes to the primary immunodeficiency hemophagocytic lymphohistiocytosis (HLH) in a subset of the HLH syndromes [18]. In this subset of HLH, patient NK cells are capable of binding to virus-infected cells and producing abundant cytokines to promote inflammation, but they are not able to eradicate infected cells through cytotoxicity. Examples of this type of HLH include aberration of the cellular machinery needed for degranulation, such as Syntaxin 11 deficiency, as well as perforin deficiency, which renders lytic granules ineffective [19]. HLH that is not associated with impairment of NK cell cytotoxicity is typically caused by defective dampening of inflammation from dysfunction in regulatory components of the inflammasome and the like [20].
NK cells develop from hematopoietic precursors in the bone marrow and go through a number of peripheral and central stages of maturation to become the cytotoxicity-enabled mature NK cell characterized by the expression of CD56 (at low levels), KIR, and CD16 and high lytic granule content [8]. Over the past decades, a number of us in the field of PID and beyond have sought or identified patients that lack NK cells or their function. This has served the purpose of illustrating the how the defective biological pathways elucidated in PID impact human NK cells. One example of how PID has informed NK cell biology is Severe Combined Immunodeficiency (SCID) caused by aberration of the IL2RG gene that prevents appropriate expression of the IL-2 receptor common gamma chain (IL-2Rγ). Patients with IL2RG deficiency have SCID due to an inability to promote T cell development via IL-7 directed signaling through IL-2Rγ. While the absence of T cells is their major clinical problem, patients with IL2RG SCID also fail to produce NK cells. This supports in vitro and mouse models that demonstrate an absolute requirement for NK cell development upon IL-15 signaling, which requires IL-2Rγ to comprise the functional IL-15 receptor [21]. There are many other similar examples in which a PID includes an NK cell abnormality but other defective components of immunity lead to the primary clinical manifestation. This is the case in HLH where in addition to defective NK cell cytotoxicity, CD8+ CTL also lack the ability to kill, thus creating the severe clinical phenotype demonstrated by patients [19]. From an NK cell perspective, this larger group of diseases, including NK− SCID and HLH, are best referred to as PIDs with an NK cell abnormality. In this light, approximately 1 out of every 6 molecularly defined PID has some impact upon NK cells and these have been catalogued and discussed in several reviews [22–28]. While some of these PIDs may demonstrate a clinical contribution of having dysfunctional NK cells, this can be context specific and rather difficult to prove. Importantly, these diseases are not NKDs, illustrating the need for better understanding the specific role of NK cells in maintenance of human health. As such, true NKD represent powerful keys to answering the question of the role of NK cells in humans and defining the role that they play in human host defense.
What Is NKD
In order to be as clear as possible: NKD is a subset of PID in which the NK cell abnormality represents the major immunological defect resulting in the clinical immunodeficiency. NKD is a sub-subset of PIDs that include an impact upon NK cells – but in most of these (PIDs that include an impact upon NK cells, as opposed to NKD), the NK cell abnormality is not the majority immunological defect giving rise to clinical immunodeficiency. As delineated above an example of this broader subset of PIDs that include an impact upon NK cells is NK- SCID, where the lack of NK cells is potentially relevant but not the major immunological defect causing clinical immunodeficiency. Some NKDs also have abnormalities outside of the immune system and may also have subtle and sometimes poorly understood immune perturbations outside of the NK cell compartment. That said, to be an NKD, again the NK cell abnormality must represent the primary immunological defect presumably leading to the patient’s clinical immunodeficiency. It is useful to think of two broad categories of NKD what have been referred to as “classical” or “developmental” NKD (cNKD) and “functional” NKD (fNKD) [23, 26, 29]. These classifications distinguish NKD in which NK cells are either absent or very low in number from those where they are present in normal numbers but fail to function. In cNKD there is a gene defect that interferes with NK cell development, maturation or survival resulting in a population of NK cells in the peripheral blood that is undetectable, or unusually small. Given that absolute lymphocyte counts can be variable, the definition of cNKD we use is where NK cells constitute ≤ 1% of peripheral blood lymphocytes or where there is a clear missing developmentally relevant subset, such as CD56dim NK cells. This phenotype is frequently accompanied by evidence of NK cell immaturity or aberrant development as detected by high-resolution FACS phenotyping. Alternatively, there may be an unusual distribution of NK cell subsets within the peripheral blood population, indicating abnormal NK cell development or homeostasis. Importantly, however, even if NK cells are present in numbers that fall within normal ranges, the developmental defect conferred by cNKD can be assessed by NK cell developmental subset study and is accompanied by impaired functional maturation reflected by defective NK cell cytotoxicity. In fNKD, NK cells are present in the peripheral blood in normal numbers and with a normal phenotype (using standard clinical laboratory NK cell phenotyping), but the NK cells demonstrate consistently abnormal function. This latter category of NKD is more challenging to detect, as standard peripheral blood lymphocyte flow cytometry will yield a normal result for NK cells, but an NKD would need to be suspected and functional testing obtained. Thus, despite there being a normal number of peripheral blood NK cells an index of suspicion is particularly relevant in identifying fNKD patients (see diagnosis section).
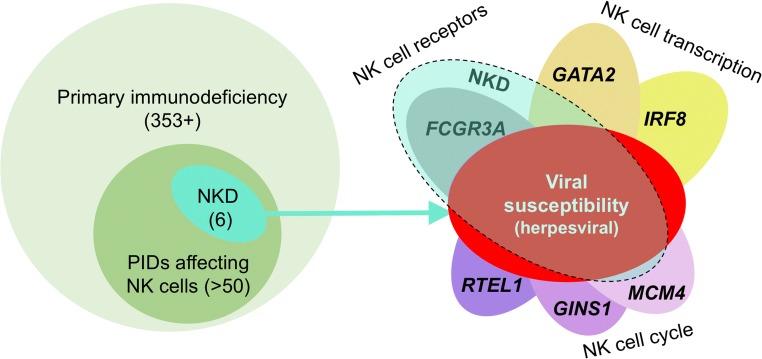
As of the time of writing this brief, there are 6 genes that have been published as at least in part causing NKD, which include 5 cNKD and 1 fNKD (Fig. 1). It is important to note and as will be discussed below there are examples of aberrations in most of these genes that cause immunodeficiencies that would not be categorized as NKD. Thus, for these genes NKD represents only a subset of what they can cause with regards to immunodeficiency. The exception is the particular FCGR3A variants, which have only been described to cause NKD as of the time of the writing of this perspective. There have also been a number of case reports in the literature describing convincing NKD cases that have not yet been associated with gene defects. Several abstracts have been published and presented at conferences that describe additional NKD genes, however these have yet to be published and subjected a full peer review process. I am aware of additional NKD gene candidates through patients we are investigating in our own program as well as candidate genes that have been shared with me by colleagues who are investigating patients in their own programs. While it is hard to say what the ultimate number of genes are that will be identified as causes of NKD, there are many that can be predicted based upon animal studies, in vitro studies and/or NK cell particular gene expression patterns found in public expression databases.
Fig. 1.
NKD is a subset of PID that affect NK cells, which are a subset of PID overall. Thus NKD is a sub-subset of PID in which the NK cell abnormality is the majority immunological defect contributing to the clinical immunodeficiency. From a genetic standpoint, of the 359 PIDs defined in the most recent IUIS classification document over 50 that have been genetically defined affect NK cells (left). Of these 50, six can be considered as causing NKD in which they share a majority NK cell defect and a common susceptibility to herpesviral infection. The genes identified as causing these NKDs are listed on the right and include defects affecting NK cell function, development or both. All but one of the genes causing NKD (FCGR3A) can also be associated with broader immunological defects and thus only a subset of patients having aberrations in these genes will have NKD. This is depicted in the image on the right by overlaying NKD (dashed teal ellipse) with the defects caused by the genes. Please note that in the case of IRF8, this represents only the diseases caused by biallelic damaging variants in the gene. This concept and schematic are modified from and build upon a previously published image [23]
The NKD genes that have been published in the peer-reviewed literature to date are GATA2 (AD) [30, 31], GINS1 (AR) [32], IRF8 (AR) [33], MCM4 (AR) [34, 35], and RTEL1 (AR) [36] for cNKD and FCGR3A (AR) for fNKD [37, 38]. In most cases these genes as causes for NKD were very surprising and all provide very interesting biological insights into NK cells, but that is beyond the scope of this brief and is summarized in recent review articles [39, 23, 26, 40]. In all but one of these, only some patients with aberrations in these genes have NKD while others will have a different clinical presentation/disorder. RTEL1 for example can cause Høyeraal-Hreidarsson Syndrome in the majority of patients described [36] and GATA2 that can cause a range of clinical presentations including myelodysplasia and Emberger Syndrome [31].
In the case of each of these genes, however, the original NKD case ultimately associated with them was fairly specific for NKD given the patient’s clinical history and the clinical laboratory assessments available at the time and in most cases has led to the identification of similar paradigms in other patients. The original GATA2 NKD patient had a very stable and well-studied NKD which characterized her immunodeficiency [41] and is in distinction from many, but not all GATA2 patients [30, 31]. The MCM4 cases had been long pursued with a founder effect family [42], and have been identified as possessing other abnormalities with regards to DNA stability although the contribution of these to immunodeficiency is not entirely clear [34]. In the RTEL1 NKD patient, the original case was fairly specific [43] although aberrations in this gene typically causes a distinct immunodeficiency and not NKD. That said, the corresponding author of the RTEL1 NKD case denoted that the patient had normal hemoglobin and platelets and “otherwise normal growth, development, and neurological progression as well as lack of bone marrow failure” (Amos Etzioni, Personal Communication, May 6 2016). In GINS1 deficiency there can be a deficiency of some ILC subsets (although the clinical relevance of this is unclear), and neutropenia [32], but the clinical immunodeficiency appears to be one of NK cells and was evident in the original case and investigations [44, 45].
For these genes causing cNKD, there are also likely to be only particular variants that will cause NKD. This is likely the case for IRF8 deficiency where the majority defect in a family originally reported with NKD many years ago [46] had a stable NKD over time, some notable differences from others with IRF8 variants and likely some particular impact of having biallelic mutations within the IRF-domain of the protein [33]. In fNKD the single identified gene, FCGR3A, has very specific genotype-phenotype connections to the clinical presentation of NKD, where this particular variant alters protein structure and interferes with costimulation provided by CD16 that is otherwise useful to NK cell cytotoxicity [37]. In the case of CD16 deficiency the original and subsequent case abnormalities [37, 38] have been quite specific to NK cell defense and share common clinical immunodeficiency phenotype components with the other NKD cases. Again, while these features are all relevant to conferring a diagnosis and to considering NKD in the context of many other actual and potential immunodeficiencies these particular topics are covered elsewhere and I would refer the reader to those sources [39, 26, 27, 23]. In all cases, further identification and investigation of patients with these gene aberrations will help define more specifically NKD or variations and relations to broader combined immunodeficiencies. For now, thinking of these genes in the context of NKD clinical immunodeficiency presentations is warranted and will undoubtedly prove helpful in attaining specific diagnoses and better understanding these conditions.
In all, however, a unifying clinical theme of susceptibility to herpesviruses as a clinical immunodeficiency is what links together the existing cases of NKD and the subsets of patients having NKD that are affected by variants in the NKD-associated genes. This may be subject to some ascertainment bias as many of us have been specifically seeking cases in this manner. I have been personally evaluating patients for NKD for a variety of reasons over the years, most notably including atypical manifestations of Herpesviruses. I have also evaluated patients, upon request, who have had incidental laboratory findings have been obtained, and ultimately the only unifying theme I have been able to identify where NK cells are truly deficient is that of where patients have clinical susceptibility herpesviruses and some warts. I am not presently aware of enough signal to attribute cancer susceptibility to NKD but remain concerned and would refer the reader to a recent overview [47].
What Is Not NKD
In the interest of best directing patient care, it is important to delineate true NKD from cases in which NK cell function or numbers may be transiently depressed or are stably impaired due to a PID that impacts NK cells. An individual having a low NK cell function test of one kind or another, or a low number of NK cells, does not in and of itself constitute an NKD and the diagnostic label should not be used broadly for patients who have an abnormal test result (see diagnosis section below). A patient with a known PID who also has abnormal NK cell numbers or functional tests should similarly not be labeled as having an NKD. As described in the preceding section this would be a PID that has an impact upon NK cells. For example, while NK cells in Wiskott-Aldrich Syndrome (WAS) are abnormal [48], it is incorrect to say that a patient has WAS with NKD. When a patient has a known PID that includes an NK cell abnormality it would be correct to refer to the disorder by the name of the PID only. There are some PIDs in which certain variants of a single gene more profoundly affect NK cell number or function more than others. In this case it would be appropriate to refer to the PID by name along with the qualifier and abnormal NK cell function/number such as NK− SCID. In these PIDs in which NK cells are affected, however, it is important to keep in mind that the NK cell defect can have an impact on the patient’s clinical immunodeficiency. While it is unlikely to be a majority contributor, these patients are at risk for deficiencies in some of the defenses that NK cells provide. For example, patients with WAS have a high incidence of herpesviral infection [49] that could represent a contribution from aberrant NK cell defenses. In all cases of PIDs including an NK cell defect, it is important in my opinion for clinicians to be attuned to characteristic infections potentially arising from inadequate NK cell defenses.
There are a number of other things that are not NKD. For one, and quite simply, a single abnormal NK cell function or enumeration test does constitute NKD. There are many reasons a single test can be abnormal and to be considered for NKD it is imperative to establish a stable defect over time. Both the number and function of NK cells are sensitive to overall physiology and critical illness, and other conditions such as those that affect the hypothalamic-pituitary-adrenal axis can have an effect. There have been numerous studies of physiologic or mental stress, or depression, leading to significant deviations in NK cell number or function. Similarly, there is a long list of medications that have been documented to affect NK cell number or function and others that can theoretically be predicted to have a similar effect. These topics are the focus of other publications in both the primary and review literature [50–53]. In light of all of this, it bears repeating that an abnormal test result should be stable over time to raise consideration of NKD and that these should be considered in the context of the known conditions and medications that can affect test results.
In using NK cell tests, it is also important that they be applied with some degree of index of clinical suspicion. While the false positive rate for the available tests is not known, like any non-genetic tests they account for a normal range across a healthy population. Applying them to individuals who are sick without a pretest probability enters into an unknown area and may require repeated testing to disprove that a patient has an NKD. In my own experience patients who have been exposed to HSV1 or HSV2 and have vesicular symptoms of recurrence within the dermatome of the initial infection do not generally have NKD. I believe that patients who have nearly continual recurrences, however, are worth considering. Patients with encephalitis caused by HSV also do not have NKD in my experience and it is now known that these cases result from a variety of defects that have little to do with NK cells and instead result from an impact on important components of CNS innate immunity [54].
Getting the NKD Diagnosis Right and the Index of Suspicion
The future of NKD as a primary immunodeficiency depends upon getting the diagnosis correct and accumulating valid experience in the overarching NKD field. Some of this will be accelerated by unbiased or targeted genetic diagnosis that will provide very specific answers. As an increasing number of NKD are defined by aberrations in single genes, currently available genetic tests have the ability to at least partially circumvent otherwise tedious and costly immunological evaluations. Several of the currently genetically defined NKD were discovered via whole exome sequencing [32, 33] and others have been detected via this approach [36, 30] and thus a clinical exome sequence should be able to cover most of the known present molecular NKD diagnoses. Many of the next generation sequencing gene panel tests for PID also include some of the known NKD genes and can be completed more quickly and at a lower cost. Both of these approaches have the advantage of including many of the PIDs that are known to have an NK cell component. As to which test to perform first, a next generation sequencing panel or whole exome sequence, this would at present depend upon your index of suspicion. If a patient seems to resemble a particular NKD for which the gene is present on a next gen panel then that would be a reasonable first approach. If not, then it would make sense to start with whole exome sequencing to be sure that the gene you suspect is covered. This is indeed a moving target, however, as the number of genes contained on PID sequencing panels are increasing regularly. Irrespective, there should be a relatively low threshold for considering a genetic test in the context of an evaluation for NKD. In our own center a suggestive clinical history and stably abnormal phenotypic or functional test is sufficient to order genetic tests for NKD, an approach supported by easy access to highly informative immunologic tests. The a priori pre-test probability of applying a genetic test in the context of a clinical history suggestive of an NK cell abnormality is presently unknown.
The diagnosis of NKD has long relied upon two sets of immunological tests. The first is that of NK cell percentage of total lymphocytes in the peripheral blood. The second is that of NK cell functions, most notably degranulation and cytotoxicity. In most evaluations of patients referred to me an NK cell enumeration was performed initially. This is typically because it is easier and often less expensive to obtain a flow cytometry lymphocyte panel and complete blood count to enumerate the NK cells amongst the other main lymphocyte subsets. This test is frequently obtained as part of an initial immunologic evaluation also resulting in the frequent incidental finding of low NK cells. In my experience having an NK cell population that is low in isolation from other lymphocyte populations and is ≤ 1% of total lymphocytes in peripheral blood is cause for concern and consistent with cNKD. In other words, the NK cell population will be underrepresented relative to other lymphocyte populations. If all lymphocyte populations are low, this is likely to be an immunodeficiency but not NKD. It is important to also ascertain the developmental state of the NK cells in suspected NKD using advanced phenotyping if available [55]. Specifically, there could be a normal percentage of NK cells but with drastic aberrations of maturity and even missing mature NK cell subsets. Per the above discussion it is also important to ensure that the reduction in NK cells is stable and not caused by some medication or intercurrent condition. The second set of tests are those that measure an NK cell function and those most commonly used in clinical laboratories are tests of degranulation performed by flow cytometry (CD107a upregulation) and cytotoxicity (target cell killing) performed by flow cytometry or 51Cr-release assay. While both functional tests are useful, it is important to appreciate that each they test different things and that there are cases where degranulation can be normal as reported by the clinical test, but cytotoxicity will be abnormal. These tests are the only ones that are generally accessible in clinical laboratories that will raise suspicion for a patient having fNKD. Thus, in the context of evaluating NKD it is important to obtain both sets of tests if one can. The availability and accessibility of these tests differs from center to center and in different parts of the world, so it is hard to recommend that one be performed before another. As discussed further below, the tests measure different things and both may be needed to ultimately define the NK cell abnormality in a given patient.
A classic example of discordance between the two tests in PID is perforin deficiency where there is an inability of NK cells to mediate target cell killing but CD107a positivity can be detected after activation. In NKD, the presence of sufficient numbers of immature NK cells (CD56bright) still capable of degranulation but inadequately prepared to mediate cytotoxicity can lead to a similar result, as is the case in biallelic IRF8 deficiency [33]. If NK cells are absent from peripheral blood then there will likely be no measurable NK cell cytotoxicity, and similarly when NK cells are low in percentage, they are likely to lead to low cytotoxic function when using a PBMC-based assay. That said, the degranulation of even an abnormally small number of NK cells can still be normal leading to a reportedly "normal" result. An exception to this could occur in the case of a phenotypically aberran NK cell population that obscures NK cell enumeration, as might be found in NK cell lymphocytosis and leukemia where there can be a loss of CD56 expression and is something to be aware of [56]. The reporting of cytotoxic function as a lytic unit is a valuable addition to some of the clinically available NK cell cytotoxicity assays. This calculation takes into consideration the number of NK cells in the peripheral blood and reflects the approximate killing capacity of individual cells. While not representing an actual analysis of individual cells, this can still be a helpful correlate in considering whether decreased function is attributable to decreased cell number or decreased functional capacity on a per cell basis (or both). In contrast, the results of degranulation functional testing are independent of NK cell number [57]. Since again it is possible to have a small number of NK cells that work perfectly normally there is a role for NK cell function testing in patients with low numbers, but full understanding of these patients may require research level interventions to be able to measure the cytotoxic function of individual NK cells. Finally, it is important to try and minimize the time from blood draw to performance of NK cell functional assays as the ex vivo presence of stress hormones and other related substances can disproportionally impact NK cell function [58]. Irrespective, when there is a clinical suspicion for NKD the repeated assessment of NK cell number and function is needed to demonstrate the stability of the defect and raise the suspicion of the diagnosis. Without a genetic diagnosis, I recommend 3 independent evaluations separated by one month and recommend obtaining both functional tests if possible to provide additional clarity. Given that there are a variety of tests available in the clinical laboratory environment and others described in research reports, I have attempted to summarize some of those that are more frequently used with regards to NKD patients (Table 1).
Table 1.
Methods for evaluating NK cells and their functions
| Method | Useful for cNKD | Useful for fNKD | Pro | Con |
|---|---|---|---|---|
| Standard lymphocyte subset analysis (flow cytometry) | Yes | No | Easily obtained, inexpensive | Many labs use combined reagents for CD56 and CD16 losing resolution for NK cell maturity |
| NK cell subset analysis (flow cytometry) | Yes | Yes | Can detect aberrations of NK cell development as well as CD16 deficiency using B73.1 anti-CD16 | Offered by very few clinical laboratories and age- and context-specific normal ranges are unclear |
| NK cell degranulation - CD107a upregulation (flow cytometry) | Some | Yes | Easily obtained surrogate for cytotoxic function. Not as affected by intercurrent illness. | Cells can degranulate or be very low in number and be defined as normal but still not kill. Not sensitive to aberrant development where NK cells can degranulate but not be armed as killers. |
| NK cell cytotoxicity – K562 target cell killing (51Cr-release or flow cytometry) | Yes | Yes | Gold-standard assay for NK cell function. Measures many steps in the process of NK cell lytic function | Sensitive to numerous factors including significant illness. Needs to be repeated to ensure stability. |
| Antibody dependent cell-mediated cytotoxicity – ADCC (51Cr-release or flow cytometry) | Yes | Yes | Useful alternative assessment of NK cell killing as well as distinct signaling pathways that NK cells use to access cytotoxicity. | Few laboratories perform. Rare examples of discordance from K562 killing (such as FCGR3A) |
| NK cell cytokine production (intracellular flow cytometry) | Some | Yes | Immature NK cells can be more effective cytokine producers. | Not clinically widely available. Not specifically diagnostic for any NKD at present. |
| Cytokine-induced NK cell function (cytotoxicity or flow cytometry) | Yes | Yes | Determines if NK cells possess ability to respond and can acquire function. | Not clinically widely available. Not specifically diagnostic for any NKD at present. |
| Research-level evaluations | Yes | Yes | Can be biologically quite insightful, such as in vitro NK cell development. | Only available through research protocols and require extensive controls and context |
From a clinical standpoint and to raise an index of suspicion it is important to understand what clinical problems patients with NKD typically have. This can be derived from what has been defined by genetically defined NKDs and also from the evolving experience of learning what is and what is not NKD. As a unifying theme, patients with NKD have atypical consequences with herpesviruses and/or problems with papilloma viruses. These features have consistently been reported for the known NKDs. In theory, NKD could present an increased risk for malignancy and there have been a few identified in individuals with NKD, particularly EBV-driven [59, 60] and HPV-driven [31] cancers and as such most reported appear to be virally-driven. An overview of these concerns has been reviewed and commented upon by others [47]. For now, the malignancy risk is more theoretical, and as NKD patients are collected and followed for prolonged periods more clarity will be obtained. In my experience, however, the highest yield for NKD is in patients that have had non-CNS atypical manifestations of a herpesvirus (or multiple herpesviruses). Many have also had some warts that can be a bit more difficult to treat and some have had molluscum. Again, atypical manifestations of herpesviral infection outside of the CNS is what I believe should raise concern.
A formal recounting of the clinical characteristics of the NKD cohort that we have collected over the years and in collaboration with many clinical immunologists is beyond the scope of this present perspective but is certainly planned. I am also particularly interested in which characteristics of patients help predict what ultimately was correctly diagnosed with NKD. This of course, requires a critical mass of diagnosed patients, which is perhaps not too far off. Some patients with NKD have presented at young ages but others surprisingly later on. I believe this is a feature of having a critical infectious exposure at a critical time, uncovering a vulnerability leading to a clinical manifestation of NKD.
Management and Treatment Concepts in NKD
Once a diagnosis of NKD is established, unfortunately there is far less in the way of specific guidance as to what to do clinically for the patient. A few things can be gleaned from existing case reports and genetically described cases of NKD, but there is no prospective evidence supporting any particular intervention. Thus, in considering how to manage and treat these patients one is left to their best judgment based upon the clinical history, active issues and ongoing concerns. Please note that while there are FDA-approved medications approved for treating the types of infections that NKD patients experience there are no specific indications for their use in NKD. Here I will also share some of my own experience with these patients, knowing that there is not enough at presence to represent any organized cumulative experience.
Upon establishing a diagnosis of NKD, I find it useful to determine whether a patient has experienced other herpesviral infections via immunoglobulin G (and M) titer testing, and if they have to obtain viral nucleic acid tests to ensure that they are not actively infected. If a patient is naïve to certain herpesviruses it does raise the consideration of prophylactic therapies, whereas if they are actively infected and experiencing clinical ramifications of that infection it suggests potential treatments.
With regards to prophylaxis, for patients who are naïve to herpesviruses, providing passive immunity via polyclonal immunoglobulin depending upon the clinical history and availability of active vaccination for the particular infection are an extreme but available option. Hyperimmune immunoglobulin, particularly for CMV, is probably best reserved for a difficult to treat infections in the context of NKD as directed by clinical circumstances, and not for prophylaxis. When there were only live viral vaccines available for Varicella Zoster Virus (VZV), I was always somewhat concerned about administering the vaccine to a patient with NKD in order to try and bolster adaptive immunity to VZV, but now would recommend utilizing recombinant zoster vaccine if possible (although the applicability of this vaccine in the young is questionable). I also recommend providing human papilloma virus vaccination (HPV) given the concern over susceptibility to HPV in NKD. Given the presumed cost-benefit, current likelihood of natural exposure and lack of other known vaccine-related illness in NKD patients, I also do recommend providing MMR if required by schedule. Providing prophylaxis with drugs, such as acyclovir is a topic worth considering. At present there is little to no experience, as most current patients have presented with these types of infections, perhaps owing at least to some degree to selection bias. That said, I recommend holding on any prophylaxis and instead being sensitive to any early signs of herpesviral infection or exposure.
The concepts related to treatment of an active infection in an NKD patient, however, are different than those I use for prophylaxis. If an NKD patient is actively infected with HSV or VZV, I treat with acyclovir, famciclovir or valacyclovir. If there is active CMV, I similarly treat with valganciclovir. If the infections are particularly severe or recalcitrant, I have used intravenous treatment, typically in collaboration with a specialist in infectious diseases. For patients with recurrent HSV, I have provided prophylaxis with one of these antivirals using a recommended suppression regimen. The need to continue the regimen is really only best determined by the recurrence of infection when the prophylaxis is stopped. As is known in the general HSV suppression literature, recurrences can be reduced after a period of prophylaxis. I have worked with patients, however, who have had recurrence of infection whenever prophylaxis is stopped. In my own practice continual treatment to prevent herpesviral clinical infections in patients having already had recurrent disease is a mainstay.
A final therapeutic concept in the treatment of patients with NKD are the use of immunostimulants that could either augment an antiviral response independently of NK cells or could potentially augment NK cell function in the context of NKD itself. Essentially any of the immunostimulants described to have function against viral infection have the potential to be helpful to NKD patients struggling with viral illness. These include injectable interferon (IFN)-α, IFN-γ and some of the topical innate immune stimulants used in the treatment of warts such as imiquimod and sinecatechins. In the face of a defective NK cell response the ability to stimulate other relevant antiviral defenses has the potential to be useful. I have used these as would be indicated by the particular infection or manifestation a patient is experiencing but do not have enough collected experience to make any recommendation, other than to remind physicians that they are available and theoretically useful. Both interferon (IFN)-α and IFN-γ, in addition to inducing antiviral responses, are potent stimulators of NK cells in vitro [61] and can stimulate NK cell function in vivo [62, 63]. Other cytokine therapeutics such as interleukin (IL)-2 are also known to increase NK cell cytotoxicity in vitro [64] and in vivo [65]. In our experience NK cells from some NKD patients do not respond in vitro to these cytokines, but in other instances there is a slight increase in NK cell cytotoxicity measured in vitro. It is hard to know if this could be relevant clinically if administered to patients to induce some NK cell function. IL-2 has been used in PID with defective NK cell function and has increased NK cell function ex vivo [66]. While there is not enough experience in NKD patients to suggest this option, if a patient’s NK cells mediate some cytotoxic function after IL-2 stimulation it is perhaps something to consider while being acutely aware of all of the potential adverse events from these types of systemic treatments.
There are in theory, curative options for NKD although this is formative at best. While I have personally never referred a patient with NKD for transplantation, there have been descriptions in the literature of patients who have successfully received hematopoietic stem cell transplantation [67]. Presumably severity of historical/presenting illness and concern for future outcome have represented a driver in these cases. The advent of adoptive virus specific T cells (VST), however, represent a newer opportunity to help eradicate virus by redirecting a patient’s or an unrelated individual’s T cells to the virus. While this is presently only in the realm of research and for those who have persistent and measurable viral replication, it represents a direction of interest [68]. I am aware of some application of VST in NKD but there is not enough experience to make any judgment of utility.
A therapeutic question that patients often raise regards the value of certain nutritional supplements and other commercially available preparations that claim to induce immunity or even NK cells. I do not have experience with these and cannot make any recommendations. I am also not aware of any evidence for their use in the context of these types of NKD or related PID conditions. I do encourage patients to maintain adequate nutrition and other evidence-supported general measures that promote immune function, but with the explanation that they are unlikely to specifically help with the patient’s NKD. I do recommend regular and recommended health screenings, especially as advised for the conservative surveillance of cancer screening given the mostly theoretical connection of reduced NK cell activities to cancer susceptibility (the association to which has been considered in detail elsewhere [47]).
Future Needs and Directions in NKD
NKD represents a PID diagnosis associated with a refined set of susceptibilities, as is increasingly common for PIDs. There are a number of genetic etiologies which are biologically insightful and can be useful in conferring a definitive diagnosis. The NKD field, however, is in its infancy as diagnoses themselves are just coming into focus and the broader context of some of the genetic associations is not yet clear. The proper identification of new patients and the ongoing collection of their data and experiences will help us, reduce ascertainment bias, identify true signals, and better understand who should be considered for NKD and what might be useful for their health and best outcomes. I do believe that coming years will provide further advances as there are both increasing numbers of patients in my experience, new potential genetic diagnoses as well as useful new insights into the underlying biology of NKD. This will undoubtedly lead to better approaches to considering and managing NKD both diagnostically and clinically.
Acknowledgments
I would like to thank Dr. Emily Mace who has been my close collaborator in advancing the understanding of NKD and who provided thoughtful feedback on this manuscript. My effort towards this topic was supported by NIH-NIAID R01AI120989. I would also like to thank my colleagues who have indulged me with the opportunity to think about challenging patients with them as well as those who have been collaborators in NK cell-oriented investigation. Finally, I would like to thank the patients and families who have participated in our research efforts to try and understand more about NK cells in PID and human immune defenses.
Compliance with Ethical Standards
Conflict of Interest
The author declares the following potential conflicts of interest: Royalty received for authorship of Up To Date (Wolters Kluwer Publishing) chapters on NK cell deficiency, and fees received in consultation to manufacturers of therapeutic immunoglobulin ADMA biologics (scientific advisory board membership) and Takeda.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lim AI, Di Santo JP. ILC-poiesis: ensuring tissue ILC differentiation at the right place and time. Eur J Immunol. 2019;49(1):11–18. doi: 10.1002/eji.201747294. [DOI] [PubMed] [Google Scholar]
- 2.Orange JS, Mace EM, French AR, Yokoyama WM, Fehniger TA, Cooper MA. Comment on: evidence of innate lymphoid cell redundancy in humans. Nat Immunol. 2018;19(8):788–789. doi: 10.1038/s41590-018-0164-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9(5):503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 4.Ali A, Gyurova IE, Waggoner SN. Mutually assured destruction: the cold war between viruses and natural killer cells. Curr Opin Virol. 2019;34:130–139. doi: 10.1016/j.coviro.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malmberg KJ, Carlsten M, Bjorklund A, Sohlberg E, Bryceson YT, Ljunggren HG. Natural killer cell-mediated immunosurveillance of human cancer. Semin Immunol. 2017;31:20–29. doi: 10.1016/j.smim.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Sojka DK, Yang L, Yokoyama WM. Uterine natural killer cells: to protect and to nurture. Birth Defects Res. 2018;110(20):1531–1538. doi: 10.1002/bdr2.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Angelo LS, Banerjee PP, Monaco-Shawver L, Rosen JB, Makedonas G, Forbes LR, Mace EM, Orange JS. Practical NK cell phenotyping and variability in healthy adults. Immunol Res. 2015;62(3):341–356. doi: 10.1007/s12026-015-8664-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freud AG, Mundy-Bosse BL, Yu J, Caligiuri MA. The broad spectrum of human natural killer cell diversity. Immunity. 2017;47(5):820–833. doi: 10.1016/j.immuni.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geary CD, Sun JC. Memory responses of natural killer cells. Semin Immunol. 2017;31:11–19. doi: 10.1016/j.smim.2017.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sivori S, Vacca P, Del Zotto G, Munari E, Mingari MC, Moretta L. Human NK cells: surface receptors, inhibitory checkpoints, and translational applications. Cell Mol Immunol. 2019;16(5):430–441. doi: 10.1038/s41423-019-0206-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rajalingam R. Diversity of killer cell immunoglobulin-like receptors and disease. Clin Lab Med. 2018;38(4):637–653. doi: 10.1016/j.cll.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Vitale M, Cantoni C, Della Chiesa M, Ferlazzo G, Carlomagno S, Pende D, Falco M, Pessino A, Muccio L, de Maria A, Marcenaro E, Moretta L, Sivori S. An historical overview: the discovery of how NK cells can kill enemies, recruit defense troops, and more. Front Immunol. 2019;10:1415. doi: 10.3389/fimmu.2019.01415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orange JS, Fassett MS, Koopman LA, Boyson JE, Strominger JL. Viral evasion of natural killer cells. Nat Immunol. 2002;3(11):1006–1012. doi: 10.1038/ni1102-1006. [DOI] [PubMed] [Google Scholar]
- 14.Jonjic S, Babic M, Polic B, Krmpotic A. Immune evasion of natural killer cells by viruses. Curr Opin Immunol. 2008;20(1):30–38. doi: 10.1016/j.coi.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinez-Vicente P, Farre D, Sanchez C, Alcami A, Engel P, Angulo A. Subversion of natural killer cell responses by a cytomegalovirus-encoded soluble CD48 decoy receptor. PLoS Pathog. 2019;15(4):e1007658. doi: 10.1371/journal.ppat.1007658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mace EM, Dongre P, Hsu HT, Sinha P, James AM, Mann SS, Forbes LR, Watkin LB, Orange JS. Cell biological steps and checkpoints in accessing NK cell cytotoxicity. Immunol Cell Biol. 2014;92(3):245–255. doi: 10.1038/icb.2013.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gwalani LA, Orange JS. Single degranulations in NK cells can mediate target cell killing. J Immunol. 2018;200(9):3231–3243. doi: 10.4049/jimmunol.1701500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orange JS. Formation and function of the lytic NK-cell immunological synapse. Nat Rev Immunol. 2008;8(9):713–725. doi: 10.1038/nri2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sepulveda FE, de Saint Basile G. Hemophagocytic syndrome: primary forms and predisposing conditions. Curr Opin Immunol. 2017;49:20–26. doi: 10.1016/j.coi.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Chinn IK, Eckstein OS, Peckham-Gregory EC, Goldberg BR, Forbes LR, Nicholas SK, Mace EM, Vogel TP, Abhyankar HA, Diaz MI, Heslop HE, Krance RA, Martinez CA, Nguyen TC, Bashir DA, Goldman JR, Stray-Pedersen A, Pedroza LA, Poli MC, Aldave-Becerra JC, McGhee S, al-Herz W, Chamdin A, Coban-Akdemir ZH, Jhangiani SN, Muzny DM, Cao TN, Hong DN, Gibbs RA, Lupski JR, Orange JS, McClain K, Allen CE. Genetic and mechanistic diversity in pediatric hemophagocytic lymphohistiocytosis. Blood. 2018;132(1):89–100. doi: 10.1182/blood-2017-11-814244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huntington ND. The unconventional expression of IL-15 and its role in NK cell homeostasis. Immunol Cell Biol. 2014;92(3):210–213. doi: 10.1038/icb.2014.1. [DOI] [PubMed] [Google Scholar]
- 22.Ham H, Billadeau DD. Human immunodeficiency syndromes affecting human natural killer cell cytolytic activity. Front Immunol. 2014;5:2. doi: 10.3389/fimmu.2014.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mace EM, Orange JS. Emerging insights into human health and NK cell biology from the study of NK cell deficiencies. Immunol Rev. 2019;287(1):202–225. doi: 10.1111/imr.12725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orange JS. Human natural killer cell deficiencies and susceptibility to infection. Microbes Infect. 2002;4(15):1545–1558. doi: 10.1016/S1286-4579(02)00038-2. [DOI] [PubMed] [Google Scholar]
- 25.Orange JS. NK cell deficiency syndromes. In: Rose B, (ed) UpToDate. Waltham 2018. https://www.uptodate.com/home/how-use-uptodate-presentation
- 26.Orange JS. Natural killer cell deficiency. J Allergy Clin Immunol. 2013;132(3):515–525. doi: 10.1016/j.jaci.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Voss M, Bryceson YT. Natural killer cell biology illuminated by primary immunodeficiency syndromes in humans. Clin Immunol. 2017;177:29–42. doi: 10.1016/j.clim.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 28.Mace EM. Requirements for human natural killer cell development informed by primary immunodeficiency. Curr Opin Allergy Clin Immunol. 2016;16(6):541–548. doi: 10.1097/ACI.0000000000000317. [DOI] [PubMed] [Google Scholar]
- 29.Orange JS. Natural killer cell deficiency. In: Sullivan KE, Stiehm ER, editors. Stiehm’s immune deficiencies. London: Elsevier/AP; 2014. pp. 765–772. [Google Scholar]
- 30.Mace EM, Hsu AP, Monaco-Shawver L, Makedonas G, Rosen JB, Dropulic L, Cohen JI, Frenkel EP, Bagwell JC, Sullivan JL, Biron CA, Spalding C, Zerbe CS, Uzel G, Holland SM, Orange JS. Mutations in GATA2 cause human NK cell deficiency with specific loss of the CD56(bright) subset. Blood. 2013;121(14):2669–2677. doi: 10.1182/blood-2012-09-453969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spinner MA, Sanchez LA, Hsu AP, Shaw PA, Zerbe CS, Calvo KR, Arthur DC, Gu W, Gould CM, Brewer CC, Cowen EW, Freeman AF, Olivier KN, Uzel G, Zelazny AM, Daub JR, Spalding CD, Claypool RJ, Giri NK, Alter BP, Mace EM, Orange JS, Cuellar-Rodriguez J, Hickstein DD, Holland SM. GATA2 deficiency: a protean disorder of hematopoiesis, lymphatics, and immunity. Blood. 2014;123(6):809–821. doi: 10.1182/blood-2013-07-515528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cottineau J, Kottemann MC, Lach FP, Kang YH, Vely F, Deenick EK, et al. Inherited GINS1 deficiency underlies growth retardation along with neutropenia and NK cell deficiency. J Clin Invest. 2017;127(5):1991–2006. doi: 10.1172/JCI90727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mace EM, Bigley V, Gunesch JT, Chinn IK, Angelo LS, Care MA, Maisuria S, Keller MD, Togi S, Watkin LB, LaRosa D, Jhangiani SN, Muzny DM, Stray-Pedersen A, Coban Akdemir Z, Smith JB, Hernández-Sanabria M, le DT, Hogg GD, Cao TN, Freud AG, Szymanski EP, Savic S, Collin M, Cant AJ, Gibbs RA, Holland SM, Caligiuri MA, Ozato K, Paust S, Doody GM, Lupski JR, Orange JS. Biallelic mutations in IRF8 impair human NK cell maturation and function. J Clin Invest. 2017;127(1):306–320. doi: 10.1172/JCI86276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gineau L, Cognet C, Kara N, Lach FP, Dunne J, Veturi U, Picard C, Trouillet C, Eidenschenk C, Aoufouchi S, Alcaïs A, Smith O, Geissmann F, Feighery C, Abel L, Smogorzewska A, Stillman B, Vivier E, Casanova JL, Jouanguy E. Partial MCM4 deficiency in patients with growth retardation, adrenal insufficiency, and natural killer cell deficiency. J Clin Invest. 2012;122(3):821–832. doi: 10.1172/JCI61014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hughes CR, Guasti L, Meimaridou E, Chuang CH, Schimenti JC, King PJ, Costigan C, Clark AJ, Metherell LA. MCM4 mutation causes adrenal failure, short stature, and natural killer cell deficiency in humans. J Clin Invest. 2012;122(3):814–820. doi: 10.1172/JCI60224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanna S, Beziat V, Jouanguy E, Casanova JL, Etzioni A. A homozygous mutation of RTEL1 in a child presenting with an apparently isolated natural killer cell deficiency. J Allergy Clin Immunol. 2015;136(4):1113–1114. doi: 10.1016/j.jaci.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 37.Grier JT, Forbes LR, Monaco-Shawver L, Oshinsky J, Atkinson TP, Moody C, Pandey R, Campbell KS, Orange JS. Human immunodeficiency-causing mutation defines CD16 in spontaneous NK cell cytotoxicity. J Clin Invest. 2012;122(10):3769–3780. doi: 10.1172/JCI64837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jawahar S, Moody C, Chan M, Finberg R, Geha R, Chatila T. Natural killer (NK) cell deficiency associated with an epitope-deficient Fc receptor type IIIA (CD16-II) Clin Exp Immunol. 1996;103(3):408–413. doi: 10.1111/j.1365-2249.1996.tb08295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jouanguy E, Gineau L, Cottineau J, Beziat V, Vivier E, Casanova JL. Inborn errors of the development of human natural killer cells. Curr Opin Allergy Clin Immunol. 2013;13(6):589–595. doi: 10.1097/ACI.0000000000000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mace EM, Orange JS. Genetic causes of human NK cell deficiency and their effect on NK cell subsets. Front Immunol. 2016;7:545. doi: 10.3389/fimmu.2016.00545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Biron CA, Byron KS, Sullivan JL. Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med. 1989;320(26):1731–1735. doi: 10.1056/NEJM198906293202605. [DOI] [PubMed] [Google Scholar]
- 42.Eidenschenk C, Dunne J, Jouanguy E, Fourlinnie C, Gineau L, Bacq D, et al. A novel primary immunodeficiency with specific natural-killer cell deficiency maps to the centromeric region of chromosome 8. Am J Hum Genet. 2006;78(4):721–727. doi: 10.1086/503269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Etzioni A, Eidenschenk C, Katz R, Beck R, Casanova JL, Pollack S. Fatal varicella associated with selective natural killer cell deficiency. J Pediatr. 2005;146(3):423–425. doi: 10.1016/j.jpeds.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 44.Bernard F, Picard C, Cormier-Daire V, Eidenschenk C, Pinto G, Bustamante JC, et al. A novel developmental and immunodeficiency syndrome associated with intrauterine growth retardation and a lack of natural killer cells. Pediatrics. 2004;113(1 Pt 1):136–141. doi: 10.1542/peds.113.1.136. [DOI] [PubMed] [Google Scholar]
- 45.Eidenschenk C, Jouanguy E, Alcais A, Mention JJ, Pasquier B, Fleckenstein IM, et al. Familial NK cell deficiency associated with impaired IL-2- and IL-15-dependent survival of lymphocytes. J Immunol. 2006;177(12):8835–8843. doi: 10.4049/jimmunol.177.12.8835. [DOI] [PubMed] [Google Scholar]
- 46.Fleisher G, Starr S, Koven N, Kamiya H, Douglas SD, Henle W. A non-x-linked syndrome with susceptibility to severe Epstein-Barr virus infections. J Pediatr. 1982;100(5):727–730. doi: 10.1016/s0022-3476(82)80572-6. [DOI] [PubMed] [Google Scholar]
- 47.Moon WY, Powis SJ. Does natural killer cell deficiency (NKD) increase the risk of cancer? NKD may increase the risk of some virus induced cancer. Front Immunol. 2019;10:1703. doi: 10.3389/fimmu.2019.01703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Orange JS, Ramesh N, Remold-O’Donnell E, Sasahara Y, Koopman L, Byrne M, et al. Wiskott-Aldrich syndrome protein is required for NK cell cytotoxicity and colocalizes with actin to NK cell-activating immunologic synapses. Proc Natl Acad Sci U S A. 2002;99(17):11351–11356. doi: 10.1073/pnas.162376099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sullivan KE, Mullen CA, Blaese RM, Winkelstein JA. A multiinstitutional survey of the Wiskott-Aldrich syndrome. J Pediatr. 1994;125(6 Pt 1):876–885. doi: 10.1016/s0022-3476(05)82002-5. [DOI] [PubMed] [Google Scholar]
- 50.Reiche EM, Nunes SO, Morimoto HK. Stress, depression, the immune system, and cancer. Lancet Oncol. 2004;5(10):617–625. doi: 10.1016/S1470-2045(04)01597-9. [DOI] [PubMed] [Google Scholar]
- 51.Zorrilla EP, Luborsky L, McKay JR, Rosenthal R, Houldin A, Tax A, et al. The relationship of depression and stressors to immunological assays: a meta-analytic review. Brain Behav Immun. 2001;15(3):199–226. doi: 10.1006/brbi.2000.0597. [DOI] [PubMed] [Google Scholar]
- 52.Goyos A, Fort M, Sharma A, Lebrec H. Current concepts in natural killer cell biology and application to drug safety assessments. Toxicol Sci. 2019. 10.1093/toxsci/kfz098. [DOI] [PubMed]
- 53.Cederbrant K, Marcusson-Stahl M, Condevaux F, Descotes J. NK-cell activity in immunotoxicity drug evaluation. Toxicology. 2003;185(3):241–250. doi: 10.1016/S0300-483X(02)00613-3. [DOI] [PubMed] [Google Scholar]
- 54.Alsweed A, Alsuhibani M, Casanova JL, Al-Hajjar S. Approach to recurrent herpes simplex encephalitis in children. Int J Pediatr Adolesc Med. 2018;5(2):35–38. doi: 10.1016/j.ijpam.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mahapatra S, Mace EM, Minard CG, Forbes LR, Vargas-Hernandez A, Duryea TK, Makedonas G, Banerjee PP, Shearer WT, Orange JS. High-resolution phenotyping identifies NK cell subsets that distinguish healthy children from adults. PLoS One. 2017;12(8):e0181134. doi: 10.1371/journal.pone.0181134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lamy T, Moignet A, Loughran TP., Jr LGL leukemia: from pathogenesis to treatment. Blood. 2017;129(9):1082–1094. doi: 10.1182/blood-2016-08-692590. [DOI] [PubMed] [Google Scholar]
- 57.Rubin TS, Zhang K, Gifford C, Lane A, Choo S, Bleesing JJ, Marsh RA. Perforin and CD107a testing is superior to NK cell function testing for screening patients for genetic HLH. Blood. 2017;129(22):2993–2999. doi: 10.1182/blood-2016-12-753830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gotlieb N, Rosenne E, Matzner P, Shaashua L, Sorski L, Ben-Eliyahu S. The misleading nature of in vitro and ex vivo findings in studying the impact of stress hormones on NK cell cytotoxicity. Brain Behav Immun. 2015;45:277–286. doi: 10.1016/j.bbi.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shaw RK, Issekutz AC, Fraser R, Schmit P, Morash B, Monaco-Shawver L, Orange JS, Fernandez CV. Bilateral adrenal EBV-associated smooth muscle tumors in a child with a natural killer cell deficiency. Blood. 2012;119(17):4009–4012. doi: 10.1182/blood-2011-10-385377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cohen JI. GATA2 Deficiency and Epstein-Barr virus disease. Front Immunol. 2017;8:1869. doi: 10.3389/fimmu.2017.01869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Santoli D, Trinchieri G, Koprowski H. Cell-mediated cytotoxicity against virus-infected target cells in humans. II. Interferon induction and activation of natural killer cells. J Immunol. 1978;121(2):532–538. [PubMed] [Google Scholar]
- 62.Bolay H, Karabudak R, Aybay C, Candemir H, Varli K, Imir T, Kansu E. Alpha interferon treatment in myasthenia gravis: effects on natural killer cell activity. J Neuroimmunol. 1998;82(2):109–115. doi: 10.1016/S0165-5728(97)00146-X. [DOI] [PubMed] [Google Scholar]
- 63.Evans A, Main E, Zier K, Ikegaki N, Tartaglione M, Kennett R, Lampson L. The effects of gamma interferon on the natural killer and tumor cells of children with neuroblastoma. A preliminary report. Cancer. 1989;64(7):1383–1387. doi: 10.1002/1097-0142(19891001)64:7<1383::AID-CNCR2820640702>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 64.Trinchieri G, Matsumoto-Kobayashi M, Clark SC, Seehra J, London L, Perussia B. Response of resting human peripheral blood natural killer cells to interleukin 2. J Exp Med. 1984;160(4):1147–1169. doi: 10.1084/jem.160.4.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Caligiuri MA, Murray C, Robertson MJ, Wang E, Cochran K, Cameron C, Schow P, Ross ME, Klumpp TR, Soiffer RJ. Selective modulation of human natural killer cells in vivo after prolonged infusion of low dose recombinant interleukin 2. J Clin Invest. 1993;91(1):123–132. doi: 10.1172/JCI116161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jyonouchi S, Gwafila B, Gwalani LA, Ahmad M, Moertel C, Holbert C, Kim JY, Kobrinsky N, Roy-Ghanta S, Orange JS. Phase I trial of low-dose interleukin 2 therapy in patients with Wiskott-Aldrich syndrome. Clin Immunol. 2017;179:47–53. doi: 10.1016/j.clim.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 67.Notarangelo LD, Mazzolari E. Natural killer cell deficiencies and severe varicella infection. J Pediatr. 2006;148(4):563–564. doi: 10.1016/j.jpeds.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 68.Naik S, Nicholas SK, Martinez CA, Leen AM, Hanley PJ, Gottschalk SM, et al. Adoptive immunotherapy for primary immunodeficiency disorders with virus-specific T lymphocytes. J Allergy Clin Immunol. 2016;137(5):1498–505 e1. doi: 10.1016/j.jaci.2015.12.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]