Abstract
Objective
To identify if cerebral perfusion pressure (CPP) can be non-invasively estimated by either of two methods calculated using transcranial Doppler ultrasound (TCD) parameters.
Design
Retrospective review of previously prospectively gathered data.
Setting
Pediatric intensive care unit in a tertiary care referral hospital.
Patients
Twenty-three children with severe traumatic brain injury (TBI) and invasive intracranial pressure (ICP) monitoring in place.
Interventions
TCD evaluation of the middle cerebral arteries was performed daily. CPP at the time of the TCD examination was recorded. For method 1, estimated cerebral perfusion pressure (CPPe) was calculated as: CPPe = MAP × (diastolic flow (Vd)/mean flow (Vm)) + 14. For method 2, critical closing pressure (CrCP) was identified as the intercept point on the x-axis of the linear regression line of blood pressure and flow velocity parameters. CrCP/CPPe was then calculated as MAP-CrCP.
Measurements and main results
One hundred eight paired measurements were available. Using patient averaged data, correlation between CPP and CPPe was significant (r = 0.78, p = < 0.001). However, on Bland-Altman plots, bias was 3.7 mmHg with 95% limits of agreement of − 17 to + 25 for CPPe. Using patient averaged data, correlation between CPP and CrCP/CPPe was significant (r = 0.59, p = < 0.001), but again bias was high at 11 mmHg with wide 95% limits of agreement of − 15 to + 38 mmHg.
Conclusions
CPPe and CrCP/CPPe do not have clinical value to estimate the absolute CPP in pediatric patients with TBI.
Keywords: Cerebral perfusion pressure, Transcranial Doppler ultrasound, Ultrasound, Head injury, Non-invasive monitoring, Traumatic brain injury
Cerebral perfusion pressure (CPP) is the driving pressure gradient which produces blood flow in the cerebral circulation. Thus, CPP is calculated as the difference between the mean cerebral arterial blood pressure and the effective downstream pressure or the critical closing pressure (CrCP) (CrCP = tissue pressure + cerebral vascular tone + mean cerebral venous pressure). As it is difficult to measure these parameters necessary to calculate CrCP, the current gold standard to determine CPP clinically is to subtract the invasively monitored intracranial pressure (ICP) from the mean systemic arterial pressure (MAP) (CPP = MAP − ICP).
In critical illness, systemic hypotension or intracranial hypertension may lead to a reduction in CPP and result in secondary neurologic insult. Additionally, when cerebral autoregulation is impaired or absent, as occurs in many disease states, the risk of these secondary insults increases as cerebral blood flow becomes linearly correlated with CPP [1–7]. As such, monitoring and optimizing CPP as a targeted therapy has shown promise to improve outcomes in children with critical illness [8–13]. Management guidelines for children with severe traumatic brain injury (TBI) recommend clinicians consider, as an option, maintaining minimum age-specific CPP goals [12]. In 317 children with TBI, a significant increase in mortality risk was noted when CPP fell below the desired level (relative risk of death (RR) = 8.1 (95% CI 3.58, 18.31)) [8]. In childhood bacterial meningitis, CPP-directed therapy has been shown to reduce overall mortality by 18% (RR = 2.1; 95% CI, 1.09–4.04; p = 0.02) and neurodisability by 33% (RR = 0.47; 95% CI, 0.27–0.83; p = 0.004) when compared with ICP directed therapy alone [10].
However, placement of an ICP monitor that allows for the calculation of CPP does not always occur in the clinical care of these children. Neurosurgical expertise necessary to place invasive monitors is often not available in rural or in resource-limited settings. Additionally, in some centers, there remains a lack of confidence that ICP/CPP-directed therapy benefits patients and children are managed with alternative therapeutic approaches [14–16]. Furthermore, while the risk is minimal, it is an invasive surgical technique occasionally associated with complications including infection and hemorrhage [17]. In a cohort of 4667 children with severe TBI, only 55% of patients had an ICP monitor placed [18]. Younger children may be even less likely to receive invasive ICP monitors. In another study of 238 children under age 24 months with severe TBI, only 17% had a monitor placed [19]. Outside the management of TBI, use of an ICP monitor in children may be even rarer. Odetola et al. evaluated > 1000 children requiring mechanical ventilation during treatment for severe bacterial meningitis, and found placement of an ICP monitor occurred in only 7% of cases [20]. Other examples where CPP monitoring may be helpful but in whom invasive monitoring is not routinely performed or is contraindicated include children with coagulopathy in the setting of sepsis or liver failure, or while undergoing support with extracorporeal membrane oxygenation [21–24].
Identifying a non-invasive, simple means by which CPP can accurately and reliably be quantified and monitored in children may be a helpful adjunct to care when treating clinicians cannot or do not place an invasive monitor but have an ongoing desire to evaluate cerebral perfusion. Transcranial Doppler ultrasound (TCD) is a portable, repeatable, non-invasive means that measures cerebral flow velocities (CBFVs) in the cerebral vasculature. In adult patients, TCD-derived parameters have successfully been used to calculate an estimated CPP by several different mathematical approaches [25–29]. Two that have been described require only systemic blood pressure monitoring and basic non-continuous TCD data [27, 30–32]. One method described by Czosnyka et al. calculates an estimated CPP (CPPe) as MAP × (diastolic flow (Vd)/mean flow (Vm)) + 14 [27]. In the second method, cerebral CrCP can be determined by plotting the dynamic pressure-flow relationship between systemic blood pressures and TCD flow velocities and determining the x-axis intercept of the linear regression line [30–32]. Estimated CPP by this method (CrCP/CPPe) is then calculated as MAP − CrCP [30–32]. There is a paucity of literature evaluating the use of either of these methods to non-invasively estimate CPP in pediatric patients. We therefore performed the current study to test the hypothesis that CPPe and CrCP/CPPe derived according to these methods would have good agreement with invasively monitored CPP in children.
Materials and methods
Study population
We performed a retrospective review of data previously gathered from 2011 to 2015 for a prospective, observational study in a tertiary care pediatric intensive care unit. This study was approved by the Institution Review Board. Children 1 day to 17 years of age admitted with a diagnosis of severe TBI (post-resuscitation Glasgow Coma Scale (GCS) score ≤ 8)) managed with an invasive ICP monitor (intraparenchymal monitor, Camino Integra Neurosciences, Plainsboro, NJ, USA) and radial arterial line were included. Patients with non-traumatic etiologies of admission were excluded. Demographic data including age, gender, GCS score, and mechanism of injury were recorded for all participants.
General management protocol
Patients were treated following the Society of Critical Care Medicine Guidelines [12]. Patients with surgical lesions underwent resection of the lesion and were left with a primary decompressive craniotomy. Secondary decompressive craniotomy for refractory intracranial hypertension was not performed in our institution during the time period the study was undertaken. All patients received sedation and anxiolysis with infusions of fentanyl and versed. Elevation in ICP (≥ 20 mmHg) despite adequate sedation was treated with osmolar therapy followed by neuromuscular blockade. CPP goals (CPP > 40 mmHg for children < 1 year of age, 50–60 mmHg for children < 12 years of age, and > 60 mmHg for adolescents) were set by the treatment team and were maintained using fluid boluses to a central venous pressure > 10 cmH20 followed by a vasoactive infusion.
Transcranial Doppler ultrasonography
TCD was performed at the participant’s bedside by one of two sonographers using a 2-MHz pulsed probe and commercially available ultrasonography unit (Sonara Digital TCD, CareFusion, Middleton, WI). The quality of the data obtained by TCD is highly influenced by operator-dependent factors such as skill and experience. Prior to the study beginning, sonographers were tested on standardized patients until a coefficient of variation < 10% for each study measurement was demonstrated. Middle cerebral arteries (MCAs) were insonated at 2-mm intervals using methods previously described, and CBFVs including the Vs, Vd, and Vm were recorded [25, 26]. All participants underwent the initial TCD within 24 h of injury and then daily thereafter through death or hospital day 8, whichever came first. Continuous TCD monitoring was not available at the time of this study.
Data capture and calculations
Arterial and intracranial pressure transducers were calibrated at the level of the skull. Outputs from the pressure monitors and the TCD unit (maximal frequency envelope) were connected to an analog-to-digital converter that was fitted into a laptop computer. Data were sampled (sampling frequency 50 Hz), digitized (12 bits), processed, and stored on the computer using software designed in-house for this purpose. Time-averaged (mean) values of pressures were calculated using time integration of waveforms for 5-s intervals. Time-averaged Vs, Vd, and Vm were calculated after spectral filtration to reduce the influence of noise and averaged within 5-s periods. Digital Fourier analysis was used to correlate corresponding values of arterial blood pressure and CBFV. CPPe was then calculated as CPPe = MAP × Vd/Vm + 14 [27]. CrCP was determined as the intercept point of a regression line between arterial systolic and diastolic pressures plotted along the x-axis and the systolic (Vs) and Vd flow velocities plotted along the y-axis [28–30]. CrCP/CPPe was then calculated as CrCP/CPPe = MAP − CrCP.
Statistical analysis
Descriptive values were expressed as frequencies for dichotomous variables and as mean ± standard deviation (SD) or median (interquartile range) for continuous variables. To assess the performance of the proposed methods, the correlation between CPP and the CPPe and CrCP/CPPe were evaluated using the Spearman correlation coefficient (r, with the level of significance set at 0.05). In addition, a generalized estimating equation was used to account for inter- and intraindividual differences between studies using original observations. Bland-Altman plots were constructed to study agreement between simultaneous invasively measured CPP and TCD derived CPPe (both CPPe and CrCP/CPPe). Two-by-two contingency tables were performed to determine sensitivity, specificity, positive predictive value, and negative predictive value. Statistical significance was assumed with a p ≤ 0.05. Statistical analysis was performed using Prism 5 (GraphPad Software, San Diego, CA).
Results
A total of 23 children met inclusion criteria and had complete records that allowed for retrospective calculation of CPPe and CrCP/CPPe. One hundred eight paired measurements were available for analysis. Demographic and clinical data for participants are available in Table 1.
Table 1.
Clinical and laboratory data of patients (n = 23)
| Characteristic | Result |
|---|---|
| Age in months (mean ± SD) | 96 ± 60 |
| Male (%) | 18 (78%) |
| Mechanism of injury (%) | |
|
Fall Motor vehicle accident Pedestrian vs auto Abusive head trauma Other |
2 (9%) 10 (43%) 6 (26%) 3 (13%) 2 (9%) |
| Glasgow Coma Score (median/IQR) | 5 (3, 7) |
| PaCO2 (mmHg) at time of TCD | 39 ± 6 |
|
ICP (mmHg, median/IQR) at time of TCD Range CPP (mmHg, median/IQR) at time of TCD Range |
10 (8, 15) 2–60 68 (60, 79) 44–94 |
| Decompressive craniotomy (%) | 8 (35%) |
| In-hospital mortality (%) | 3 (13%) |
SD standard deviation, vs versus, PaCO2 partial pressure carbon dioxide, ICP intracranial pressure, CPP cerebral perfusion pressure, TCD transcranial Doppler ultrasound, mmHg millimeters mercury
CPP and CPPe
Using patient averaged data, correlation between CPP and CPPe was significant (r = 0.78, p ≤ 0.001) (Fig. 1). However, when evaluating agreement between CPP and CPPe with a Bland-Altman plot, the bias or average discrepancy for all measurements was 3.7 mmHg with 95% limits of agreement of − 17 and + 25 mmHg (Fig. 1). When evaluating agreement over time, average discrepancy was best on post-injury day 1 and worsened over time (Table 2). The wide limits of agreement were relatively unchanged by day (Table 2). Fifty-three percent of all CPPe measurements were ≥ 10 mmHg below or above the invasively calculated CPP.
Fig. 1.
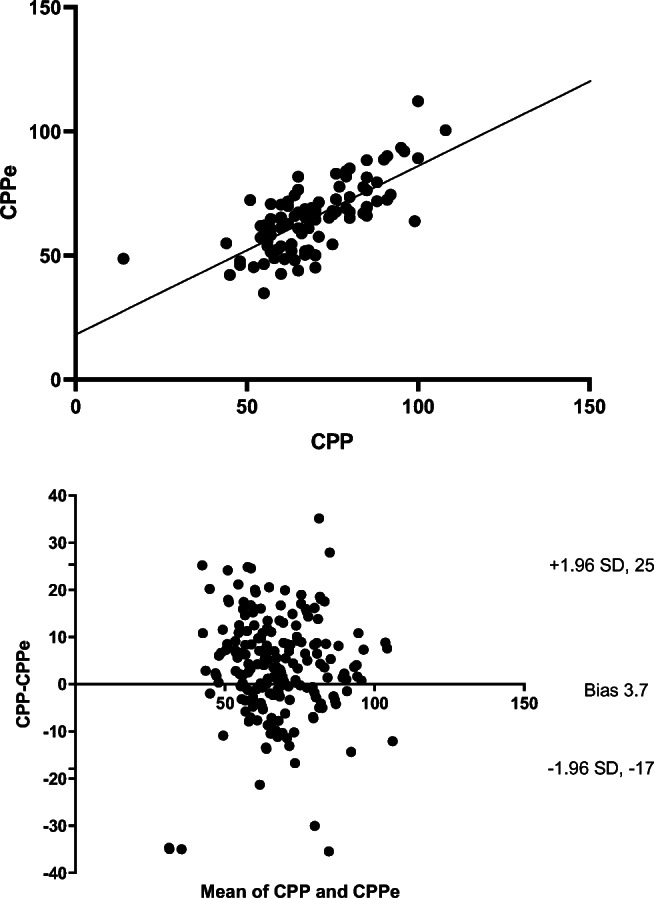
Using patient averaged data, correlation between cerebral perfusion pressure (CPP) and non-invasively estimated cerebral perfusion pressure (CPPe = (mean arterial blood pressure × diastolic flow velocity/mean flow velocity) + 14) was significant (r = 0.78, p ≤ 0.001). The Bland-Altman plot of agreement between the two revealed bias of 3.7 mmHg with 95% limits of agreement − 17, + 25 mmHg
Table 2.
Agreement between invasively calculated cerebral perfusion pressure (CPP) and non-invasively estimated cerebral perfusion pressure (CPPe) by day. Results represent bias and 95% lower and upper limits of agreement (in mmHg) by day
| Day | Bias | − 1.96 SD | + 1.96 SD |
|---|---|---|---|
| 1 | 1.8 | − 19 | 23 |
| 2 | 3.2 | − 14 | 21 |
| 3 | 4.7 | − 12 | 22 |
| 4 | 4 | − 16 | 24 |
| 5 | 4.2 | − 10 | 19 |
| 6 | 7.7 | − 16 | 32 |
| 7 | 6.4 | − 13 | 26 |
| 8 | 8.9 | − 7 | 25 |
mmHg millimeters mercury, SD standard deviation
CPP and CrCP/CPPe
Using patient-averaged data, correlation between CPP and CrCP/CPPe was significant (r = 0.59, p = <0.001) (Fig. 2). However, when evaluating the agreement between CPP and CrCP/CPPe with a Bland-Altman plot, the bias was 11 mmHg and 95% limits of agreement were − 15, + 38 mmHg (Fig. 2). Average discrepancy was high, and limits of agreement were wide on all days (Table 3). Forty-three percent of CrCP/CPPe measurements were ≥ 10 mmHg below or above the invasively calculated CPP.
Fig. 2.
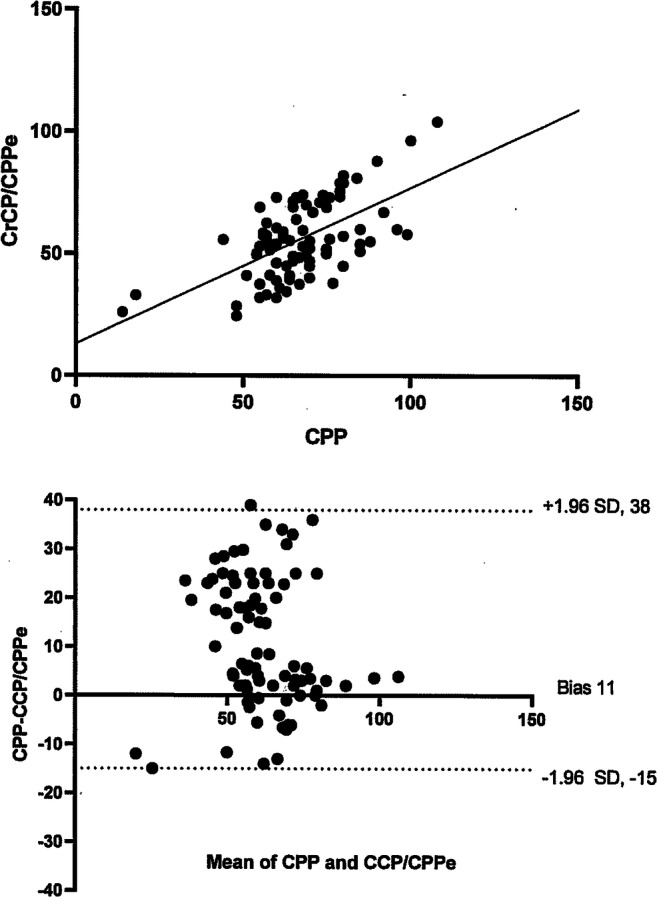
Using patient averaged data, correlation between cerebral perfusion pressure (CPP) and non-invasively estimated CPPe using TCD-derived critical closing pressure (CrCP = intercept point of the regression line between arterial systolic and diastolic pressures and systolic and diastolic flow velocities; CPPe in this scenario = MAP-CrCP) was significant (r = 0.59, p = < 0.001). The Bland-Altman plot of agreement between the two revealed bias of 11 mmHg with 95% limits of agreement − 15, + 38 mmHg
Table 3.
Agreement between invasively calculated cerebral perfusion pressure (CPP) and non-invasively estimated cerebral perfusion pressure based on critical closing pressure (CrCP/CPPe) by day. Results represent bias and 95% lower and upper limits of agreement (in mmHg) by day
| Day | Bias | − 1.96 SD | + 1.96 SD |
|---|---|---|---|
| 1 | 13.4 | − 16 | 43 |
| 2 | 14.2 | − 19 | 48 |
| 3 | 16.7 | − 12 | 44 |
| 4 | 15.9 | − 16 | 42 |
| 5 | 16.9 | − 14 | 39 |
| 6 | 16 | − 17 | 41 |
| 7 | 17.2 | − 18 | 42 |
| 8 | 16.6 | − 16 | 43 |
mmHg millimeters mercury, SD standard deviation
Estimated CPP as a screening tool for low CPP
There were no TCD examinations performed during episodes of CPP < 40 mmHg available for analysis. There were 14 episodes of CPP < 50 mmHg when the age specific goal was > 50 mmHg (mean CPP 44 ± 2 mmHg) and 22 episodes of CPP < 60 mmHg when the age specific goal was > 60 mmHg (mean CPP 51 ± 3 mmHg). Sensitivity, specificity, positive predictive value, and negative predictive value of both CPPe and CrCP/CPPe to detect reduced CPP below treatment goal values are in Table 4.
Table 4.
Performance of non-invasively estimated CPP (both CPPe and CrCP/CPPe) to detect episodes of reduced cerebral perfusion pressure (CPP) below clinical goal (n = 14 for CPP < 50 mmHg, n = 22 for CPP < 60 mmHg)
| CPPe < 50 mmHg | CrCP/CPPe < 50 mmHg | CPPe < 60 mmHg | CrCP/CPPe < 60 mmHg | |
|---|---|---|---|---|
| Sensitivity | 100 | 66 | 73 | 67 |
| Specificity | 91 | 53 | 78 | 63 |
| Positive predictive value | 27 | 8 | 60 | 48 |
| Negative predictive value | 100 | 96 | 90 | 100 |
CPPe estimated cerebral perfusion pressure, CrCP/CPPe critical closing pressure derived cerebral perfusion pressure
Confounders
Given the potential changes to intracranial compliance following surgical intervention, the agreement between CPP and CPPe as well as CPP and CrCP/CPPe was assessed in children who underwent a primary decompressive craniectomy (n = 8) separately than in those who did not (n = 15). The Bland-Altman plot testing agreement between CPP and CPPe in children with decompressive craniotomy found bias was 5 mmHg with 95% limits of agreement ranging from − 56 to 45 mmHg. In children without decompressive craniotomy, bias was 2 mmHg and 95% limits of agreement were − 28 to 32 mmHg. The Bland-Altman plot evaluating agreement between CPP and CrCP/CPPe in children with and without decompressive craniotomy revealed a bias of 15 mmHg with 95% limits of agreement of − 22 to 49 mmHg and bias of 13 mmHg with 95% limits of agreement of − 12 to 45 mmHg, respectively.
Bland-Altman plots were also performed to evaluate the effect of partial pressure of carbon dioxide (PaCO2) on the agreement between CPP and CPPe. At PaCO2 values of 30–35 mmHg (n = 28), bias was − 0.15 mmHg and 95% limits of agreement were − 27 to 27 mmHg. At PaCO2 values of 35–40 mmHg (n = 37), bias was 2.5 mmHg with 95% limits of agreement − 18 to 23 mmHg. At PaCO2 values of 40–45 mmHg (n = 30), bias was 5.9 mmHg with limits of agreement at − 17 and 29 mmHg. At PaCO2 > 45 mmHg (n = 13), bias was 6.1 mmHg with 95% limits of agreement at − 17 and 26 mmHg. Values for agreement between CPP and CrCP/CPPe were similar at different PaCO2 ranges in terms of limits of agreement (data not shown).
Minor observations
Three patients had repeated, real-time TCD recordings before, during, and after physiologic derangements that allowed for the calculation of serial CrCPs. In one patient, ICP spontaneously increased from 12 to 24 mmHg. MAP changed minimally from 84 to 86 mmHg. Pre-ICP spike, CrCP was calculated as 19 mmHg and then increased to 39 mmHg during the ICP spike. CrCP/CPPe was thus 65 mmHg at baseline and decreased to 47 mmHg during the ICP increase. Five milliliters (ml)/kg of 3% hypertonic saline were given and ICP decreased to 13 mmHg and MAP increased to 90 mmHg. CrCP downtrended to 18.8 mmHg (Fig. 3). CrCP/CPPe thus increased to 71.2 mmHg [33, 34]. During the ICP spike, end-tidal CO2 monitoring did not significantly change and there was no clear increase in cerebral metabolism (patient was undergoing sedation and neuromuscular blockade and electroencephalogram did not reveal seizures). Another patient had similar pathologic derangements with similar alterations to calculated CrCP and CrCP/CPPe during ICP spikes. A third patient experienced refractory intracranial hypertension with an ICP of 45 mmHg. The patient was given 1 g/kg of mannitol, 5 ml/kg of hypertonic saline, and mechanical ventilator rate was increased to result in an end tidal CO2 reduction from 35 to 30 mmHg. Despite these interventions, ICP increased further to 52 mmHg. CrCP was 60 mmHg when the ICP was 45 mmHg and increased further to 72.9 mmHg as ICP increased (Fig. 4). During this time, the MAP did not significantly change (112 to 113 mmHg) so the CrCP/CPPe decreased from 52 to 40 mmHg.
Fig. 3.
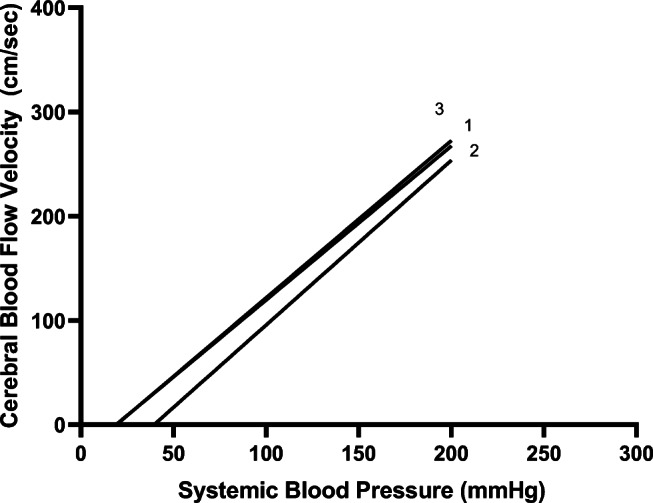
Linear regression analysis of pressure-flow relationships before [1], during [2], and after [3] use of hypertonic saline to manage intracranial pressure spike. The critical closing pressure (CrCP) is represented by the intercept of the regression line with the x-axis. CrCP for 1 = 19, CrCP for 2 = 39, and CrCP for 3 = 18.8. CrCP likely increased due to ICP increases given that other contributory physiologic variables that contribute to CrCP were unchanged
Fig. 4.
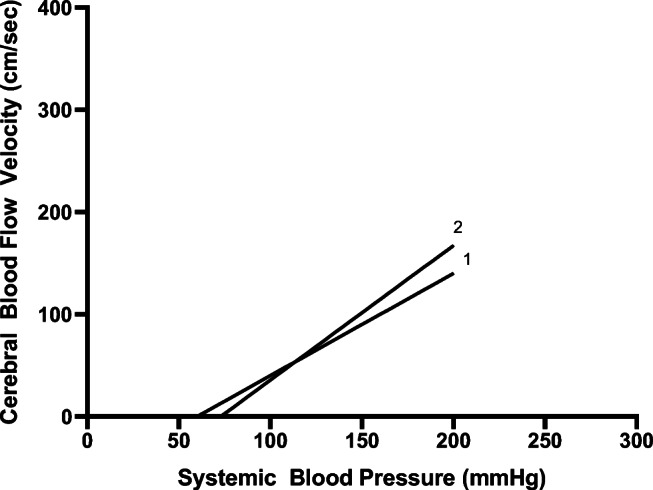
Linear regression analysis of pressure-flow relationships during [1], and after [2] use of mannitol, hypertonic saline, and hyperventilation to manage intracranial hypertension. The critical closing pressure (CrCP) is represented by the intercept of the regression line with the x-axis. CrCP for 1 = 60, CrCP for 2 = 72.9. CrCP high due to increased ICP. Increasing CrCP could be partially caused by increased cerebrovascular resistance from reducing partial pressure of carbon dioxide, but a lack of reduction in CrCP is consistent with an ICP that continued to increase despite treatment
Discussion
In critically ill children with a variety of primary diagnoses, maintenance of an age-appropriate CPP is suggested as a treatment option to ensure necessary substrate delivery and avoid secondary neurologic insult. However, direct ICP monitoring that allows for calculation of CPP is not always feasible given lack of resources or clinical contraindications. Thus, finding a non-invasive alternative method by which to assess and monitor CPP is desirable and may aid in the management of these children.
Mathematical models using combinations of systemic blood pressure measurements and TCD-derived flow velocities have been suggested to have clinical utility to non-invasively estimate CPP in adult patients [27–29, 32–39]. Given the paucity of literature on this topic in pediatric patients, we performed the current study to evaluate the utility of two methods to non-invasively estimate CPP in children with severe TBI. Our findings include the following: (1) CPPe had adequate average discrepancy from the invasively measured CPP, but had wide limits of agreement at − 17 to + 25 mmHg; (2) CrCP/CPPe had high average discrepancy from the invasively measured CPP and had wide limits of agreement at − 15 to 38 mmHg; (3) the ability of either method to predict CPP < 50 mmHg or < 60 mmHg was insufficient for clinical use; and (4) in a very small number of children, changes to calculated CrCP and CrCP/CPPe over time appropriately reflected underlying pathophysiologic alterations to ICP and CPP and the response of these values to interventions.
Using the first method, we evaluated, Czosnyka et al. determined the correlation between CPP and CPPe was r = 0.73, p < .0001 with an overall error < 15 mmHg in 84% of the examinations [27]. Schmidt et al. found, using the same formula, that the absolute difference between CPP and CPPe was < 10 mmHg in 89% of measurements with a 95% confidence range for prediction of the actual CPP no wider than ± 12 mmHg [28]. Using the second method we evaluated, CrCP/CPPe had good correlation with CPP in 70 adult patients with TBI (r = 0.92) [31]. These authors promoted the use of both of these methods to non-invasively estimate CPP in adult patients based on a good correlation between the invasively measured and estimated CPP. In fact, correlation of CPP and CPPe from our cohort was also reasonably good (r = 0.78). However, since correlation studies the relationship between one variable and another but not the differences between them, it may not be the most appropriate method to assess comparability between a gold standard and new method.
A Bland-Altman plot compares the agreement of two measurement techniques by plotting the difference of two paired measurements against the mean of two measurements. Results then quantify the mean difference between the two methods and give 95% limits of agreement. In adult studies of TBI, the agreement between invasively and non-invasively calculated CPP on Bland-Altman plots by either of these methods is generally wide [31, 32, 35]. These results likely explain why, despite the technique and technology now having been available for more than 20 years, the approach has not routinely been adopted into clinical practice for adults.
In a recently published paper using continuous TCD recordings to non-invasively estimate CPP in children who had suffered TBI, CPPe overestimated CPP by 19.61 mmHg with wide 95% CI of ± 40 mmHg on Bland-Altman analysis [36]. Our results are similar to this study in that we also identified wide limits of agreement for both the CPPe and CrCP/CPPe methods (> − 15 to > + 25). Thus, the clinical utility of either method to non-invasively estimate the absolute value of CPP at a single time point in children with TBI also appears to be limited.
Some work has previously been done suggesting that TCD parameters may have a role in non-invasively estimating CPP < 50 mmHg in children with severe TBI [40]. Figaji et al. reported that the TCD-derived PI was 0.95 ± 0.17 when CPP was ≤ 50 mmHg and 0.78 ± 0.20 when CPP was ≥ 50 mmHg. However, sensitivity and specificity of the PI to determine a CPP below 50 mmHg was not done due to an inadequate number of paired measurements when CPP was low. In our study, in a limited number of episodes (n = 14), CPPe was noted to have a sensitivity of 100%, specificity of 91%, and a positive predictive value of 27% to predict CPP < 50 mmHg. The low positive predictive value we report is likely due to the relatively low prevalence of cerebral hypoperfusion < 50 mmHg in this cohort of children. Larger studies can be considered to evaluate if these values of sensitivity and specificity for any of these methods hold with increased measured episodes of cerebral hypoperfusion < 50 mmHg. However, the significant reduction in sensitivity and specificity of this method in a larger number (n = 22) of subjects at a higher target CPP (< 60 mmHg) seen in our study suggests that they likely will not. Identifying a non-invasive means by which to determine cerebral hypoperfusion is thus still necessary.
The study revealed modestly improved agreement between the estimated and invasively measured CPP in children who had not undergone decompressive craniotomy compared with those that had. As ICP rises and reaches or exceeds the critical closing pressure at the arteriolar level, other contributors to the calculated CrCP become less important and the discrepancy between the estimated and actual CPP decreases [41]. Following decompressive craniotomy, ICP in general is assumed to not reach critical levels. Sample sizes did not allow for matched comparisons at various ICP values between children with and without DC, but a difference in ICP/higher ICP may have contributed to the slightly improved agreement in patients who had not undergone DC. However, wide limits of agreement in this group of children would still prohibit its use clinically. Additionally, bias between the methods was slightly better when the PaCO2 values were low (30–35 mmHg) and progressively worsened as PaCO2 values rose (> 45 mmHg). Autoregulation is known to be most effective and CVR highest at lower PaCO2 values [42, 43]. The overall effect of these factors on measured CBFVs may account for the modest improvement in agreement at relative hypocapnea.
Another important clinical need is the ability to monitor non-invasively, not just at a single point but over time, the actual cerebral perfusion of these critically ill children. The components that contribute to the calculation of the CrCP, and thus, the CrCP/CPPe include not just the tissue pressure (ICP) but also the cerebrovascular resistance/vasomotor tone (CVR), and the downstream venous pressure [30, 31]. Thus, the calculation of the CrCP/CPPe reflects the net effect of local and systemic physiologic or pathologic alterations on the cerebral circulation. If trended over time, it may be useful to assist in determining an individual’s overall response to progressive or improving pathologic states and the effects of therapeutic strategies on the effective cerebral perfusion pressure. In two small experimental animal studies, this has been done successfully. Varsos et al. calculated CrCP during 38 episodes of ICP plateau waves induced by lumbar infusion. ICP increased during infusion on average by 24 mmHg and a concomitant increase in CrCP of 27% from baseline was identified (mean CrCP 51.9 ± 8.76 at baseline ICP; mean CrCP 63.31 ± 10.83 at the top of the plateau waves) [34]. The same author, in a different study, determined that alterations to the calculated baseline CrCP and CrCP/CPPe accurately reflected the overall net effect of changes to ICP, mean arterial pressure, and ventilation in rabbits [44]. Three patients in our cohort had serial TCD examinations around the time of significant pathophysiologic changes and treatment interventions that allowed for the repeated calculation of CrCP and thus CrCP/CPPe. In all 3 children, CrCP and CrCP/CPPe trended in the expected direction based on the measurement of simultaneously captured invasive parameters. This is a very limited sample, however, so the importance of these results must not be overstated. Future studies in larger numbers of children should determine the utility of CrCP calculated non-invasively in this way to monitor for changing physiology and as a means to measure the response to therapeutic interventions in children with acute, severe, neurologic illness.
Limitations
This study involved a relatively small cohort of patients who experienced a limited range of CPP variations. Due to the limited number of participants, we were unable to determine if there were certain patient characteristics that determined good versus poor agreement between the presented methods and the invasively measured CPP. Future studies could involve larger groups of children and attempt to capture agreement data with different subsets of patients and determine if CPPe or CrCP/CPPe can be used to non-invasively predict CPP in some children. Furthermore, this study did not evaluate the techniques to non-invasively estimate CPP in children with disease processes outside TBI. Other disease processes that have different pathophysiologic mechanisms and more diffuse versus heterogeneous neurologic injury may have different levels of agreement.
If any of these studies were to find closer agreement between estimated CPP and invasively measured CPP, other considerations would need to be taken into account before widespread clinical use of the technique began. One limitation of TCD use in general is the considerable variation that can be seen between different operators. It would therefore be imperative that all operators at a single institution would have to undergo regular evaluation to ensure minimal variation in technique and results of their TCD examinations. This would help to ensure accurate and interpretable results when repeated examinations are done on a single patient over time by multiple operators. Furthermore, as CPP is highly dynamic and can fluctuate widely within minutes, improved technology that allows for continuous reliable TCD measurement and subsequent real-time non-invasive CPP calculation in children would be required in order to use the technique as anything other than a one-time screening tool.
Conclusions
CPPe and CrCP/CPPe do not have clinical values in the non-invasive estimation of the absolute CPP measured invasively or to detect cerebral hypoperfusion below desired thresholds of 50–60 mmHg in children with severe TBI.
Compliance with ethical standards
This study was approved by the Institution Review Board.
Footnotes
All work was performed at Nationwide Children’s Hospital, Columbus, OH, USA.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Nicole F O’Brien, Email: nicole.obrien@nationwidechildrens.org.
Marlina E. Lovett, Email: marlina.lovett@nationwidechildrens.org
Melissa Chung, Email: melissa.chung@nationwidechildrens.org.
Tensing Maa, Email: tensing.maa@nationwidechildrens.org.
References
- 1.Udomphorn Y, Armstead WM, Vavilala MS. Cerebral blood flow and autoregulation after pediatric traumatic brain injury. Pediatr Neurol. 2008;38:225–234. doi: 10.1016/j.pediatrneurol.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strauss G, Hansen BA, Kirkgaard P, et al. Liver function, cerebral blood flow autoregulation, and hepatic encephalopathy in fulminant hepatic failure. Hepatology. 1997;25:837–839. doi: 10.1002/hep.510250409. [DOI] [PubMed] [Google Scholar]
- 3.Moller K, Larsen FS, Qvist J, et al. Dependency of cerebral blood flow on mean arterial pressure in patients with acute bacterial meningitis. Crit Care Med. 2000;28:1027–1032. doi: 10.1097/00003246-200004000-00019. [DOI] [PubMed] [Google Scholar]
- 4.Moller K, Qvist T, Tofteng F, et al. Cerebral blood flow and metabolism during infusion of norepinephrine and propofol in patients with bacterial meningitis. Stroke. 2004;35:1333–1339. doi: 10.1161/01.STR.0000128418.17312.0e. [DOI] [PubMed] [Google Scholar]
- 5.Newell DW, Aaslid R, Lam A, Mayberg TS, Winn HR. Comparison of flow and velocity during dynamic autoregulation testing in humans. Stroke. 1994;25:793–797. doi: 10.1161/01.str.25.4.793. [DOI] [PubMed] [Google Scholar]
- 6.Adelson PD, Srinivas R, Chang Y, Bell M, Kochanek PM. Cerebrovascular response in children following severe traumatic brain injury. Childs Nerv Syst. 2011;27:1465–1476. doi: 10.1007/s00381-011-1476-z. [DOI] [PubMed] [Google Scholar]
- 7.Adelson PD, Clyde B, Kochanek PM, Wisniewski SR, Marion DW, Yonas H. Cerebrovascular response in infants and young children following severe traumatic brain injury: a preliminary report. Pediatr Neurosurg. 1997;26:200–207. doi: 10.1159/000121192. [DOI] [PubMed] [Google Scholar]
- 8.Allen BB, Chiu YL, Gerber LM, et al. Age-specific cerebral perfusion pressure thresholds and survival in children and adolescents with severe traumatic brain injury. Ped Crit Care Med. 2014;15:62–70. doi: 10.1097/PCC.0b013e3182a556ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prabhakaran P, Reddy AT, Oakes WJ, King WD, Winkler MK, Givens TG. A pilot trial comparing cerebral perfusion pressure-targeted therapy to intracranial pressure-targeted therapy in children with severe traumatic brain injury. J Neurosurg. 2004;100:454–459. doi: 10.3171/ped.2004.100.5.0454. [DOI] [PubMed] [Google Scholar]
- 10.Kumar R, Singhi S, Singhi P, Jayashree M, Bansal A, Bhatti A. Randomized controlled trial comparing cerebral perfusion pressure-targeted therapy versus intracranial pressure-targeted therapy for raised intracranial pressure due to acute CNS infections in children. Crit Care Med. 2014;42:1775–1787. doi: 10.1097/CCM.0000000000000298. [DOI] [PubMed] [Google Scholar]
- 11.Shetty R, Singhi S, Singhi P, Jayashree M. Cerebral perfusion pressure–targeted approach in children with central nervous system infections and raised intracranial pressure: is it feasible? J Child Neurol. 2008;23:192–198. doi: 10.1177/0883073807308716. [DOI] [PubMed] [Google Scholar]
- 12.Kochanek PM, Carney N, Adelson PD, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents- second edition. Ped Crit Care Med. 2012;13:S11–S23. doi: 10.1097/PCC.0b013e31823f435c. [DOI] [PubMed] [Google Scholar]
- 13.Howlett JA, Northington FJ, Gilmore MM, et al. Cerebrovascular autoregulation and neurologic injury in neonatal hypoxic ischemic encephalopathy. Pediatr Res. 2013;74:525–535. doi: 10.1038/pr.2013.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chesnut RM, Temkin N, Carney N, Dikmen S, Rondina C, Videtta W, Petroni G, Lujan S, Pridgeon J, Barber J, Machamer J, Chaddock K, Celix JM, Cherner M, Hendrix T, Global Neurotrauma Research Group Global Neurotrauma Research Group: a trial of intracranial-pressure monitoring in traumatic brain injury. N Engl J Med. 2012;367:2471–2481. doi: 10.1056/NEJMoa1207363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bennett TD, DeWitt PE, Greene TH, Srivastava R, Riva-Cambrin J, Nance ML, Bratton SL, Runyan DK, Dean JM, Keenan HT. Functional outcome after intracranial pressure monitoring for children with severe traumatic brain injury. JAMA Pediatr. 2017;171:965–971. doi: 10.1001/jamapediatrics.2017.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horvat CM, Kochanek PM. Big data not yet big enough to determine the influence of intracranial pressure monitoring on outcome in children with severe traumatic brain injury. JAMA Pediatr. 2017;171:942–943. doi: 10.1001/jamapediatrics.2017.2390. [DOI] [PubMed] [Google Scholar]
- 17.Anderson RCE, Kan P, Klimo P, Brockmeyer DL, Walker ML, Kestle JR. Complications of intracranial pressure monitoring in children with head trauma. J Neurosurg. 2004;101:53–58. doi: 10.3171/ped.2004.101.2.0053. [DOI] [PubMed] [Google Scholar]
- 18.Bennett TD, Riva-Cambrin J, Keenan HT, et al. Variation in intracranial pressure monitoring and outcomes in pediatric traumatic brain injury. Arch Pediatr Adolesc Med. 2012;116:641–647. doi: 10.1001/archpediatrics.2012.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dixon RR, Nocera MN, Zolotor AJ, et al. Intracranial pressure monitoring in infants and young children with traumatic brain injury. Ped Crit Care Med. 2016;17:1064–1072. doi: 10.1097/PCC.0000000000000937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Odetola FO, Tilford JM, Davis MM. Variation in the use of intracranial-pressure monitoring and mortality in critically ill children with meningitis in the United States. Pediatrics. 2006;117:1893–1900. doi: 10.1542/peds.2005-2179. [DOI] [PubMed] [Google Scholar]
- 21.Goodson CM, Rosenblatt K, Rivera-Lara L, et al. Cerebral blood flow autoregulation in sepsis for the intensivist: why its monitoring may be the future of individualized care. J Int Care Med. 2018;33:63–73. doi: 10.1177/0885066616673973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Helbok R, Olson DM, LeRoux PD, et al. Intracranial pressure and cerebral perfusion pressure monitoring in non-TBI patients: special considerations. Neurocrit Care. 2014;21:S85–S94. doi: 10.1007/s12028-014-0040-6. [DOI] [PubMed] [Google Scholar]
- 23.O’Brien NF, Hall M. Extracorporeal membrane oxygenation and cerebral blood flow velocity in children. Ped Crit Care Med. 2013;14:126–134. doi: 10.1097/PCC.0b013e3182712d62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rilinger JF, Smith CM, de Regnier RA, et al. Transcranial Doppler identification of neurologic injury during pediatric extracorporeal membrane oxygenation therapy. J Stroke and Cerebrovasc Dis. 2017;26:2336–2345. doi: 10.1016/j.jstrokecerebrovasdis.2017.05.022. [DOI] [PubMed] [Google Scholar]
- 25.Aaslid R, Markwalder TM, Nornes H. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg. 1982;57:769–774. doi: 10.3171/jns.1982.57.6.0769. [DOI] [PubMed] [Google Scholar]
- 26.Aaslid R, Lindegaard KF, Sorteberg W, Nornes H. Cerebral autoregulation dynamics in humans. Stroke. 1989;20:45–52. doi: 10.1161/01.str.20.1.45. [DOI] [PubMed] [Google Scholar]
- 27.Czosnyka M, Matta BF, Smielewski P, Kirkpatrick PJ, Pickard JD. Cerebral perfusion pressure in head injured patients: a noninvasive assessment using transcranial Doppler ultrasonography. J Neurosurg. 1998;88:802–808. doi: 10.3171/jns.1998.88.5.0802. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt EA, Czosnyka M, Gooskens I, et al. Preliminary experience of the estimation of cerebral perfusion pressure using transcranial Doppler ultrasonography. J Neurol Neurosurg Psychiatry. 2001;70:198–204. doi: 10.1136/jnnp.70.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smielewski P, Czosnyka M, Kirkpatrick P, McEroy H, Rutkowska H, Pickard JD. Assessment of cerebral autoregulation using carotid artery compression. Stroke. 1996;27:2197–2203. doi: 10.1161/01.str.27.12.2197. [DOI] [PubMed] [Google Scholar]
- 30.Panerai RB, Kelsall AW, Rennie JM, Evans DH. Estimation of critical closing pressure in the cerebral circulation of newborns. Neuropediatrics. 1995;26:168–173. doi: 10.1055/s-2007-979748. [DOI] [PubMed] [Google Scholar]
- 31.Lopez-Magana JA, Richards HK, Radolovich D, et al. Critical closing pressure: comparison of three methods. J of Cereb Blood Flow and Metab. 2009;29:987–993. doi: 10.1038/jcbfm.2009.24. [DOI] [PubMed] [Google Scholar]
- 32.Thees C, Scholz M, Schaller C, et al. Relationship between intracranial pressure and critical closing pressure in patients with neurotrauma. Anesthesiology. 2002;96:595–599. doi: 10.1097/00000542-200203000-00014. [DOI] [PubMed] [Google Scholar]
- 33.Aaslid R, Lash SR, Bardy GH, Gild WH, Newell DW. Dynamic pressure-flow velocity relationships in the human cerebral circulation. Stroke. 2003;34:1645–1649. doi: 10.1161/01.STR.0000077927.63758.B6. [DOI] [PubMed] [Google Scholar]
- 34.Varsos GV, deRiva N, Smiewlewski P, et al. Critical closing pressure during intracranial pressure plateau waves. Neurocrit Care. 2013;18:341–348. doi: 10.1007/s12028-013-9830-5. [DOI] [PubMed] [Google Scholar]
- 35.Ar E, Vanhille E, Le Moigno S, et al. Non-invasive assessment of cerebral perfusion pressure in brain injured patients with moderate intracranial hypertension. Br J Anaesth. 2005;94:216–221. doi: 10.1093/bja/aei034. [DOI] [PubMed] [Google Scholar]
- 36.Abecasis F, Cardim D, Czosnyka M, Robba C, Agrawal S (2020) Transcranial Doppler as a non-invasive method to estimate cerebral perfusion pressure in children with severe traumatic brain injury. Childs Nerv Syst 36:125–131 [DOI] [PubMed]
- 37.Early C, Dewey R, Pieper HP, Hunt WE. Dynamic pressure-flow relationships of brain blood flow in the monkey. J Neurosurg. 1974;41:590–596. doi: 10.3171/jns.1974.41.5.0590. [DOI] [PubMed] [Google Scholar]
- 38.Dewey R, Pieper HP, Hunt WE. Experimental cerebral hemodynamics. J Neurosurg. 1974;41:597–606. doi: 10.3171/jns.1974.41.5.0597. [DOI] [PubMed] [Google Scholar]
- 39.Panerai RB. The critical closing pressure of the cerebral circulation. Med Eng Physics. 2003;25:621–632. doi: 10.1016/s1350-4533(03)00027-4. [DOI] [PubMed] [Google Scholar]
- 40.Figaji AA, Zwane E, Fieggen AG, Siesjo P, Peter JC. Transcranial Doppler pulsatility index is not a reliable indicator of intracranial pressure in children with severe traumatic brain injury. Surg Neurol. 2009;72:389–394. doi: 10.1016/j.surneu.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 41.Vanhille EA, LeMoigno S, Benhamou D, Mazoit J. Non-invasive assessment of cerebral perfusion pressure in brain injured patients with moderate intracranial hypertension. Br J of Anesth. 2005;94:216–221. doi: 10.1093/bja/aei034. [DOI] [PubMed] [Google Scholar]
- 42.Taccone FS, Castaneres-Zapatero D, Peres-Bota D, Vincent JL, Berre J, Melot C. Cerebral autoregulation is influenced by carbon dioxide levels in patients with septic shock. Neurocrit Care. 2010;12:35–42. doi: 10.1007/s12028-009-9289-6. [DOI] [PubMed] [Google Scholar]
- 43.Lee JH, Kelly DF, Oertel M, McArthur DL, Glenn TC, Vespa P, Boscardin WJ, Martin NA. Carbon dioxide reactivity, pressure autoregulation, and metabolic suppression reactivity after head injury: a transcranial Doppler study. J Neurosurg. 2001;95:222–232. doi: 10.3171/jns.2001.95.2.0222. [DOI] [PubMed] [Google Scholar]
- 44.Varsos GV, Richards H, Kasprowicz M, Budohoski KP, Brady KM, et al. Critical closing pressure determined with a model of cerebrovascular impedence. J Cereb Blood Flow Metab. 2013;33:235–243. doi: 10.1038/jcbfm.2012.161. [DOI] [PMC free article] [PubMed] [Google Scholar]


