Abstract
The pig is an omnivorous, monogastric species with many advantages to serve as an animal model for human diseases. There are very high similarities to humans in anatomy and functions of the immune system, e g., the presence of tonsils, which are absent in rodents. The porcine immune system resembles man for more than 80% of analyzed parameters in contrast to the mouse with only about 10%. The pig can easily be bred, and there are less emotional problems to use them as experimental animals than dogs or monkeys. Indwelling cannulas in a vein or lymphatic vessel enable repetitive stress-free sampling. Meanwhile, there are many markers available to characterize immune cells. Lymphoid organs, their function, and their role in lymphocyte kinetics (proliferation and migration) are reviewed. For long-term experiments, minipigs (e.g., Göttingen minipig) are available. Pigs can be kept under gnotobiotic (germfree) conditions for some time after birth to study the effects of microbiota. The effects of probiotics can be tested on the gut immune system. The lung has been used for extracorporeal preservation and immune engineering. After genetic modifications are established, the pig is the best animal model for future xenotransplantation to reduce the problem of organ shortage for organ transplantation. Autotransplantation of particles of lymphnodes regenerates in the subcutaneous tissue. This is a model to treat secondary lymphedema patients. There are pigs with cystic fibrosis and severe combined immune deficiency available.
Keywords: Pig, Animal model, Lymphoid organs, Lymph nodes regeneration, Xenotransplantation
Introduction
The domestic pig (Sus scrofa domesticus) is a world-wide domestic animal. For biomedical research, pigs have been bred to get pigs of smaller size (minipig). There are many different minipigs, e.g., Minnesota, Yukatan, Hanford, Mini-Lewe, and the widely used Göttingen minipigs (Glodeck et al. 1977; Bollen and Ellegaard 1996). The genetic management of the Göttingen minipig has been documented by Simianer and Köhn (2010). A minor disadvantage in comparison with normal piglets is the smaller ears in the Göttingen minipigs, which make it more difficult to take blood samples or intravenous (iv) injection via the ear vein. A great advantage of the pig as an experimental animal is that it can be kept under gnotobiotic /germfree conditions for some weeks after birth (Waldmann 1988). Such gnotobiotic pigs are an excellent model to study the dramatic kidney problems (hemolytic uremic syndrome) after oral infection with enterohemorrhagic Escherichia coli (Gunzer et al. 2002).
Pigs in general are more difficult to handle than the docile sheep, and they are much more interested in each other. Therefore, in experiments with indwelling venous or intralymphatic cannula, the pigs have to be kept individually. When a piglet is handled gently, rubbing the neck and belly enables repeated blood sampling without giving a sedative drug. While on the other hand if a pig is lifted up, placed on its back, and fixed, it well fight with enormous stress effects, e.g., stress leukocytosis which has sometimes been misinterpreted.
In this review, examples of different lymphoid organs will be discussed (Fig. 1) in respect to use them as models. Furthermore, the genetic manipulations are summarized which are of great relevance, e.g., xenotransplantation of pig organs to reduce the lack of human organs in transplantations compared with large animals in vaccine development and documented the advantages of the pig. The porcine immune system resembles humans in > 80% in contrast to mice with only 10% (Dawson 2011).
Fig. 1.
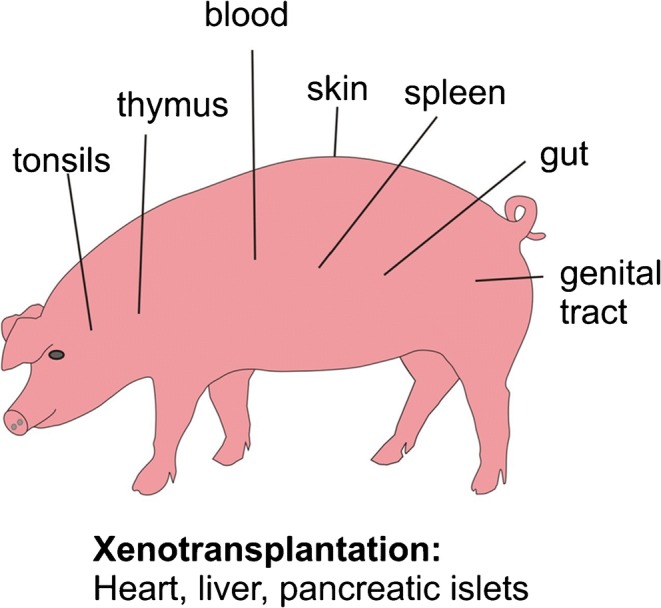
Schematic drawing of important organs of the pig as models for immunology
Many years ago, every few year, congresses were organized on the topic “Swine in biomedical research”. There were sections included on the immune system of the pigs. These proceedings were published, e.g., Tumbleson 1986 (Tumbleson and Schook 1996). Examples from these books are reviews on the migration and homing of lymphocytes in the pig (Binns and Pabst 1988) and the productions of lymphocyte in different lymphoid organs in pigs (Pabst and Binns 1986) and the behavior of lymphocytes in vivo (Binns et al. 1986). In 2009, there was a special issue on porcine immunology in the journal of developmental and comparative immunology. In the introductory article, Summerfield documented the continuous increase of annual publications on porcine immunology reaching more than 450 in 2008 (Summerfield and McCullough 2009). In the most detailed book on immunology, the encyclopedia immunology, Saalmüller and Gerner (2016) wrote a chapter on the immune system of swine with enormous numbers of references. The reader is recommended to check that review for many details. The pig is an excellent model for developmental immunology (Rothkötter et al. 2002). The immunology of the pig is of particular interest from the point of view of a physician (Rothkötter 2009).
Characterization of immune cells in the pig
The complexity of cellular immune reaction can be characterized by the cluster of differentiation (CD) of different immune cell. Initially the mouse and man leukocytes were studied. CD workshops were organized to find a common nomenclature. For the pig, the first workshop was organized in 1991. In man, 419 proteins have been defined, and for the pig, 259 corresponding CD are characterized. The reader is referred to the review (Dawson and Lunney 2018). See also the review of Piroiou-Guzylack and Salmon 2008. Porcine T cells can be subdivided into several different subsets very (NC) similar to other species, e.g., alpha, beta T cells (Gerner et al. 2015). There are also natural killer cells that can respond to various cytokines and can lyse virus-infected cells (summarized by Gerner et al. 2009). NC cells are present in increased numbers in the lung of influenza-infected pigs (Mair et al. 2016). An interesting marker for pig lymphocytes is CD 27. CD 27+ lymphocytes are mature T cells in the thymus, and blood B lymphocytes lacked CD 27 expression and NK cells expressed it at intermediate level (Reutner et al. 2012). A more recent study (Reutner et al. 2013) documented the differences of certain subsets in organ distribution and functions. The subset CDP alpha+ CD 27− cells resemble terminally differentiated effects or memory cells in man. In CD 8 alpha+ CD27+ helper cells homing receptors were increased. These data can be the basis for functional, such as migration kinetic studies which are not possible in man. However, there are also species’ differences. As an example, interleukin 4 is not a stimulatory factor for B cells but is a blocked antibody and L-6 secretion and suppressed antigen-stimulated proliferation of B cells (Murtaugh et al. 2009). Therefore the pigs should not be used as a model for IL4 research.
Organ distribution of lymphocytes and their subsets in the pig
The total number of lymphoid cells in a body obviously depends on the size and the age of the animal (e.g., thymus). There are technical problems to quantify lymphocytes in the different organs. The content of DNA per organ divided by the average content of DNA of a lymphocyte or counting lymphoid cells on histology sections revealed that a total number of lymphocyte in a conventional pig of 26 kg body weight was about 320 × 1010 lymphocytes. In the blood, the most easily accessible compartment was only 3 % (Pabst and Trepel 1975a, b). Much later markers for B, T, and Null lymphocytes in different lymphoid and non-lymphoid organs were quantified in Göttingen minipigs: IgA-positive cells were found in only small numbers. Surface IgM-positive cells were up to 24% in the iliac lymph node (Boeker et al. 1999). After defining and characterization of any new marker for a lymphocyte subset, it would be optimal when the organ distribution and age dependence would be determined. These aspects are also true for rodents. However, for the young pig, there are more data available on the organ distribution of lymphocytes than in most other species including man.
Immunoglobulins, antibody repertoire, and B Cell development
The B cell system in pigs is unique. The fetus is born without antibodies, because of the tight placental barriers. However, Intrauterine injection of fetal and germfree pigs with polyclonal B cell activators resulted in immunoglobulin level, in sera. Also, specific antibody responses could be detected 7 days after immunization in 55-day-old fetuses (Tlaskalova-Hogenova et al. 1994). This group (Sterzl et al.) in Prague was very active in germfree techniques in the pig. Therefore, the newborn piglet depends on suckling colostrum. Until the gut epithelium stops to be permeable for proteins (gut closure), enormous amounts of immunoglobulins are taken up in the piglet’s gut. Microbial colonization influences creating the antibody repertoire (Butler et al. 2008 and Butler et al. 2009) the addition of oral antigen and antibody exposure was studied in neonatal pigs (Haverson et al. 2009) and also before in germfree pigs (Haverson et al 2007).
Therefore, the pig is not a good model for man with the permeable placenta. Butler et al. have reviewed the situation in piglets, and the reader is referred to these detailed papers (Butler et al. 2016; Butler et al. 2017a, b).
Porcine dendritic cells
As in other species, dendritic cells (DC) are heterogeneous in pigs: the “conventional DC” has primarily an antigen-sensing function, and the “plasmacytoid DC” is the professional interferon alpha producer. The comparisons of the phenotype and the tissue localization as well as the antigen presenting cells have been summarized by Summerfield and McCullough (2009). DC has also antiviral response and is stimulated by Toll-like receptors. In pigs, the expression of DC11b and SIRP alpha (CD 172a) was documented (Bimczok et al. 2005). In the wall of the small intestine of the pigs, there are multiple subsets of MHC class II+ antigen presenting cells. The alpha integrin C11R1 was most frequently expressed. The often used adjuvant cholera toxin resulted in a twofold increase in the expression of maturation markers CD 80/CD 86 (Bimczok et al. 2010). The phenotype and distribution in the small intestine mucosa of pigs and the spatial relationship to epithelial cells were studied by Bimczok et al. (2006).
Dendritic cells can also be used to induce immune reactions in vaccination strategies. There are many data published in the human system or in mice to use particle-based vaccines of both viral and synthetic origin. These should be tested also in the pig as reviewed in detail by McCullough and Summerfield (2009). There are tables of successful test using a vaccinia virus, pox virus vector, pseudorabies vector, or adenovirus vector as well as nanoparticle vaccine delivery systems and different adjuvant delivery systems in pigs (McCullough and Summerfield 2009).
Host defense peptides and Toll-like receptors in pigs
A part of the innate immune effectors is called antimicrobial peptides, which are produced by epithelial cells. Best known are the alpha, beta defensins. A comparison of the different host defense peptides in the pig with those in man has been summarized by Sang and Blecha (2009). These peptides have party also in antiviral activities as documented for the porcine reproductive and respirator syndrome virus. There was an upregulation of genes of different host defense peptides in 2-week-old congenitally infected pigs (Sang and Blecha 2009; Sang et al. 2009). A mycotoxin is often found in cereals and pig feed. The expression of porcine beta defensin was depressed. The intestinal permeability increased and the weight gain decreased (Wang et al. 2018). Thus, antimicrobial peptides are critical in the pig epithelial barrier. Pathogen mentoring is critical in inflammation and infection. The most studied pattern recognition receptors (TLR) are Toll-like receptors, which are essential in activation of the immune system. In humans, 10 TLR genes have fully been identified and cloned in the pig. These are important in vaccine development (Uenishi and Shinhai 2009). Rogers et al. (2008c) have published a table of 16 peptides, which are unique to pigs or have a human orthologue. Porcine Toll-like receptors are of great relevance in disease resistance in pigs (Uenishi and Shinhai 2009).
Many details of the porcine innate immune system such as pattern recognition receptors, humoral innate responses and the contribution of the different cells have been summarized recently (Mair et al. 2014). A detailed review on the innate immune system in pigs and the porcine reproductive and respiratory virus has been published by Sang and Blecha 2009. Porcine B cell expresses TLR receptors depending on the subsets. For example, TLR 2, TLR 7/8, and TLR 9 promote antibody responses (Braun et al. 2017).
The severe combined immunodeficiency pig
When T and B lymphocytes are absent, severe combined immunodeficiency results (SCID). In the mouse, the genes RAG1 and RAG2 have been successfully knocked out, and these mice are taken as an excellent animal model for SCID in man. As pointed about before, man and mice are different in many aspects of physiology and anatomy (Mestas and Hughes 2004). Therefore, it was a great break through when a RAG1/2 knockout pigs were published (Huang et al. 2014). These miniature pigs lack mature T and B lymphocytes, have hypoplastic of immune organs, and failed to perform the VCDJ rearrangement. These pigs will be extremely useful of vaccination studies and other biomedical and translational research (Huang et al. 2014).
Thymus
As in other mammals, the epithelial cells of the thymus of the pig are derived from the pharyngeal endoderm and ectoderm from the third brachial cleft. Stem cells from the bone marrow enter this epithelial structure to form the two compartments: cortex and medulla. The role of the cortex is responsible for T lymphocyte proliferation and selection. During the development, the thymus anlage moves downward to the thorax and is finally placed in the retrosternal area. In the pig, there are also two cervical parts of the thymus on both sides of the trachea, which are easily accessible without an opening of the thorax. The thymus is extremely stress dependent, thus resulting in reduction of lymphocyte number and partly in a different subset composition. Therefore, we did not use local injections of DNA precursors to study lymphocyte production but applied the metaphase arrest technique using vincristine. Vincristine was injected intravenously ( 0.25 mg/kg body weight) after a control sample was excised and 4 (1, 2, 2.5, 3.5 h) biopsies are taken. On histological sections, the number of mitotic figures was counted in the medulla and the cortex of the thymus. The increase of the mitotic index with time was higher in the cortex (rm = 0.49%) than in the medulla (rm = 0.32%). An obvious drawback of this technique is that no subsets of lymphocytes can be determined (Pabst and Fritz 1986). In other experiments in pigs, secondary lymphoid organs (spleen, mesenteric lymph nodes, Peyer’s patches) or bone marrow were labelled selectively with the DNA precursor tritiated thymidine and evaluation with autoradiography of thymic sections revealed an influx of newly formed lymphocyte from all these organs. The immigrants were always near to venules in the cortico-medullary area. These venules had an unusual high endothelium similar to high endothelial venules in lymph nodes or tonsils. Such venules are documented in the recently published review (Pabst 2019). In the pig, the emigration of lymphocyte via the thymic vein was characterized and quantified (Binns et al. 1988). There were differences in lymphocyte subsets when peripheral blood, thymus, or thymic emigrants were characterized. The group of Bianchi et al. (1992) studied the development of B and T cell compartments in porcine lymphoid organs from birth to adult life. Mesenteric lymph nodes of pigs were selectively labbeled with the DNA precursor tritiated thymidene. Thus, the local proliferation and the export of nearly-formed lymphoid cells to lymphoid and non-lymphoid organs were quantified by autroradiography (Pabst and Trepel 1979). One interesting finding was the presence of few immunoglobulin-positive B lymphocytes already before birth with an increase until group adult life. Recently Sinkorova et al. 2019, documented that in the pig, B cell lymphogenesis in the thymus is similar to that in the bone marrow in respect to the rearrangement of immunoglobulin heavy chain genes (Sinkora et al. 2019). The presence of a relatively high incidence of IgA-positive cells in the pig thymus had already been described before (Allan and Porter 1973). In the young pig, there is a virus with severely effects on the respiratory and reproductive system: porcine reproductive and respiratory syndrome (PRRS). This virus infects thymic antigen presenting cells in the thymus and interacts with the double position T cells resulting in a loss of T helper cells (Butler et al. 2019). To my knowledge only in pigs vascularized thymic lobe transplantation has been successfully established to support thymopoiesis (La Mattina et al. 2002). When xenotransplantation of pig organs will be established (see paragraph on xenotransplantation), this might be a clinically interesting topic.
In conclusion, the pig is a model for studying the lymphocyte cell traffic in and out of the thymus.
Bone marrow
The bone marrow is described in textbooks of anatomy and immunology as a typical primary lymphoid organ producing lymphoid cells independent of antigens. However, enormous numbers of lymphocytes migrate from secondary lymphoid organs such as the spleen, lymph nodes, and tonsils to the bone marrow. This was documented in the pig after selective labelling of the lymphocytes in these organs either by isolated perfusion or minor injection of label. Many of the cells later leave the bone marrow again. Plasma cell precursors migrate from the spleen to the bone marrow and have longer survival time there than in the spleen. These notions mainly obtained in the pig have recently been summarized. For reference details, see Pabst et al. 1988.
The nose as a route for drug delivery to the central nervous system
When therapuetic antibodies have to be used in the treatment of neurological diseases, it is essential to bypass the blood-brain barrier. In man, the nose has three bilateral ducts. In the superior duct, there are nerve endings of the olfactory nerve, the first cranial nerve. There are functional FC receptors for IgG. In the pig, this is very similar to man. Allogeneic porcine IgG were found time-dependent in the lamina propria and along axonal bundles, when tested in ex vivo porcine olfactory mucosa. Thus, the pig seems to be an adequate model for the transport of immunoglobulins to the CNS (Ladel et al. 2018).
Tonsils
At a very important strategic site where the respiratory and alimentary tract both start, we find the so-called Waldeyer´s ring of lymphoid issue, the tonsils. There is a single pharyngeal tonsil at the roof of the pharynx, two palatine tonsils on each site, the lingual tonsil at the root of the tongue and two accumulations of lymphoid tissue on the lateral wall of the pharynx next to the openings of the auditory tube the tubal tonsils. For the details on the development of the tonsils in the pig, see Liebler-Tenorio and Pabst 2006. All these tonsils are also found in the pig in contrast to the mouse and rat, which lack tonsils. Thus, the pig is an excellent model to study their structure and function. A further advantage is the accessibility of the palatine tonsils. Lymphocyte proliferation in the different compartments, this tonsil has been studied by the metaphase and arrest technique using vincristine. After a biopsy was taken in anesthetized pigs, vincristine was injected with iv resulting in an arrest of cells in the cell cycle in the metaphase and four biopsies were excised for up to 3.5 h, the mitotic rate (%/h) was highest in the follicle (3.4) but lower in the interfollicular region (0.2) and the corona (0.2) and in the reticular epithelium of the crypts (0.3) (Pabst and Fritz 1986). After multiple local injections of fluorescein isothiocyanate into the palatine tonsils in young pigs, the emigration of lymphocyte to other organs like different lymph nodes spleen and gut wall was quantified (Pabst and Nowara 1984). The palatine tonsil in the pig is ideal to study antigen uptake and vaccination protocols due to its acceptability. The nasopharyngeal tonsil of the pig has been studied by typical histological techniques and also scanning EM and M cells documented (Kumar et al. 2015).
Larynx and trachea transplantation
In patients with advanced carcinoma of the larynx, the removal of the larynx or the trachea is the only option of treatment. The laryngectomy has to be combined with a trachea stoma which often has several problems for the patient. The transplantation of the larynx is an alternative (Khazraee et al. 2018). The pig is an important model for this treatment. The group of Birchall in England focused on this topic. The first step was to describe the vascular, myological situation, and functional outcomes (Birchall et al. 2011; Birchall et al. 2017). The immunological outcomes with the lymphocyte subset composition in the supraglottic, glottic, and infraglottic region have been characterized in minipigs (Birchall et al. 2011). Tracheal replacement had been reviewed (Delaere and van Raemdonck 2016). In pigs, the necessity of both epithelial cells and mesenchymal stem cell–derived chondrocytes contribute to the tissue-engineered airway transplant in pigs (Go et al. 2010). Such a tissue-engineered cadaveric trachea transplant has been used in a 12-year-old child and a follow up of 2 years documented (Elliott et al. 2012). The problems to develop a new trachea (pig revascularization) by tissue engineering have been summarized (Law et al. 2016). In the future, these will be two alternatives, A. genetic-modified “humaniest” pigs as larynx donors or B. tissue-engineered larynx (Hamilton and Birchall 2017; Ogunhade et al. 2019).
In conclusion, the pig is a clinically relevant model for larynx and trachea transplantation.
Lymph nodes
All over the body tissue, fluid is transported from the tissues via afferent lymphatic vessels to an aggregation of lymphoid issue, the lymph nodes. There are enormous numbers named by the region (e.g., axillary lymph node), the localization next to a structure (parailiac, paraaortic), or by the organs they drain (e.g., splenic, renal). The fluid leaves the lymph node via the efferent lymph vessel, which mostly leads to another lymph node then to a lymphatic duct and finally via the thoracic duct entering the blood system in the angel of the subclavian jugular vein on the left side of the body. All of this is also true for the pig. The histological structure is, however, is different: it is the so-called inverted lymph node which is also present in elephants, dolphins, hippopotamus, and warthogs (see Binns 1982).
In the afferent lymph, there are lymphocytes, e. g., in skin draining lymph (a 35–200/mm3), very few neutrophils and some “veiled cells” as they were called initially, now known as dendritic cells. There are T and B lymphocytes (for details and early references, see Binns 1982). The efferent lymph coming from a lymph node in the pig contains very few lymphocytes in comparison with other species (see paragraph on lymphocyte migration via HEV). In the pig, the medullary tissue is in the periphery of the lymph node and the cortical part in the central area. Thus, T and B cells are mainly found in an atypical localization. Each compartment can be stimulated, e.g., by injecting an antigen in the draining area, and “lymphocyte depletion, e. g., by giving anti-lymphocyte serum or whole body irradiation results in a reduction of lymphocytes” in the lymph nodes (for details see Binns 1982). The cortex, paracortex, and medulla are distinct microanatomical structure. The cortex contains primary follicles and germinal centers surrounded by a corona. These are called secondary follicles which are surrounded by a sinus similar to the subcapsular sinus in other species. Neonatal germfree piglets do not have germinal centers. The paracortex or sometimes called deep diffuse cortex contains tightly packed small lymphocytes. This compartment can easily be identified by venules with a high endothelium (High endothelial venules=HEV). See also paragraph on lymphocyte migration. The medulla is formed by a reticulum which a sparse distribution of lymphocytes and plasma cells (Binns 1982). A comparison of the structure of lymph nodes in different species has been summarized by Rothkötter 2009 and Haley 2017. A further positive aspect of pig lymph nodes is that one lymph node drains to the next one, e.g., superficial inguinal to deep inguinal, and this is similar to the structure in man but not typical in the mouse. Hoshi et al. (1986) documented large central cisternae and intratrabecular lymph channels with lymphatic valves in pig lymph nodes. These are very unique findings not known from the lymph nodes in other species. The development of the B and T lymphocyte compartments from birth to adult was studied by Bianchi et al. (1992).
HEV—High endothelial venules in pig lymph nodes are not only entry but also exit routes of lymphocytes
In secondary lymphoid organs like lymph nodes, Peyer’s patches or tonsils lymphocytes leave the blood in large numbers due to a cascade of steps of adhesion molecules, which have largely be characterized in the mouse and also in man. In sheep, the endothelial cells of the postcapillary vein are flat. However, there is also a high rate of lymphocyte emigration. Thus, the height of the endothelial cells cannot be the essential criteria. After leaving the blood via HEV, lymphocytes migrate into efferent lymphatics, lymphatic trunks, and finally the thoracic duct, which ends at the left jugular-subclavian angle. This route is called lymphocyte recirculation. In pigs, a paucity of lymphocytes in the thoracic duct lymph had been described about 60 years ago (for details, see Binns et al. 1985). Bennell and Husband (1981a, b) documented that in pigs, lymphocytes from the afferent gut lymphatics do not migrate from the lymph node by efferent lymphatics ramified over the endothelial junctions, and lymphocytes were identified in different phases of migration between endothelial cells and intercellular junctions. In such a study, the migration of the lymphocytes into or out of the vessel human cannot be decided (Sasaki et al. 1994). The following in vivo experiments documented the exit of lymphocytes from lymph nodes. Mesenteric lymph nodes (after the gut was tied off) were perfused via the artery with a normothermic cell-free perfusate; the venous effluent was collected for up to 3 h. The mesenteric lymph was collected in parallel. In pigs, lymphocytes left lymph nodes continuously although no lymphocyte could have entered the lymph node. In sheep on the other hand, lymphocytes left via the efferent lymphatics and not the blood. After 3 h of cell-free perfusion, there were still lymphocytes in the wall of HEV in pigs (Binns et al. 1985). Already in 1990, Binns and Licence have published the route of pig peripheral blood lymphocytes in fetal sheep lymph nodes and sheep blood lymphocytes in unsuckled newborn piglet lymph node. The xenogenic lymphocytes emigrated as the homologous lymphocytes. Thus, there must be an exit signal in pig lymph nodes. An open question remains: do the HEV work as a revolving donor that is an entry and exit site or are there HEV for entry and stress for exit? (Fig. 2).
Fig. 2.
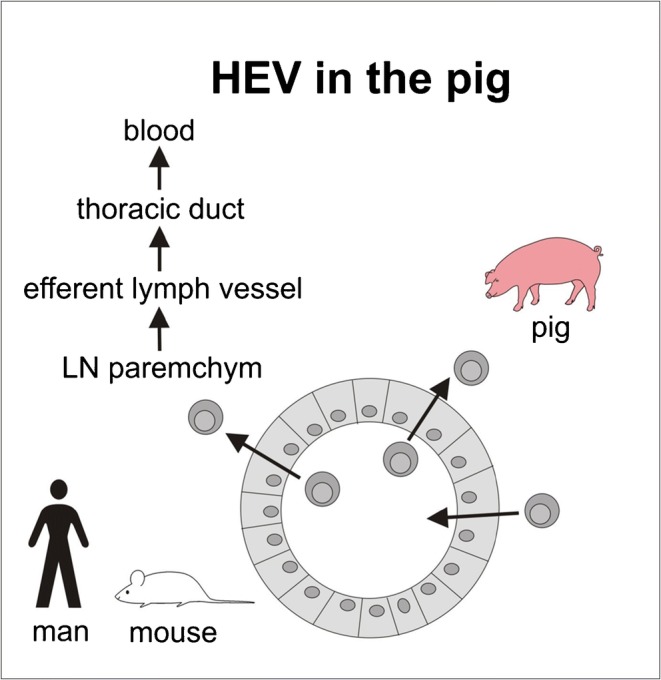
High endothelial venules (HEV) and their role in lymphocytes migration in rodents and in the pig indicating the entry and exit rout of lymphocytes in pig HEV
Regenerated autotransplanted lymph node fragments as a treatment option in secondary lymph edema
Secondary lymph edema is a frequent complication after surgical or radiological damage or removal of lymph nodes. This happens, for example, in many patients with mammary carcinoma despite the increasing frequency of sentinel lymph node resection. Until now, secondary lymphedema can only be treated symptomatically by manual lymph drainage or wearing a garment. All sophisticated surgical tricks such as lymph-lymph or lymph-venous anastomosis had no long-lasting effects. There are high numbers of women suffering from postmastectomy lymphedema of the arm when the axillar lymph nodes were exited. The risk factors for lymphedema of the arm are documented (Rupp et al. 2019). Splenic tissue regenerates in the greater omentum in the pig. Autotransplanted lymph node fragments, however, did not regenerate. Particles under the kidney capsule or muscular fascia did not regenerate (Rothkötter and Pabst 1990). In pouches of subcutaneous fat in the inguinal region, regenerated lymph node structures with afferent lymphatic and germinal centers developed (Pabst and Rothkötter 1988). The subcutaneous regeneration happened in young piglets and also in adult animals. The transplanted fragments undergo necrosis first and then the regeneration started. An autotransplanted tissue cannot result in a graft versus host reaction.
Lymph nodes were cut into small pieces, and the sizes of the transplanted fragments and with or without the capsule were compared. At 5 and 8 months after surgery, Berlin blue was injected in the draining area and the lymph flow was documented and quantified by SPECT/CT. All lymph node fragments showed and regained structure histologically. Thus, the lymph flow was restored (Blum et al. 2007; 2010). In rats, the regeneration was quantified by 3D-active contour segmentation by magnetic resonance imaging (Sommer et al. 2012a, b). The regeneration was enhanced by injecting the growth factor VEGF-C in the draining area (Schindewolffs et al. 2014).
Fragments of inguinal lymph nodes of minipigs were cryopreserved and autotransplanted after 1 month. The typical lymph node structure regenerated and the draining capacity was documented by SPECT-CT hybrid imaging (Hadamitzky et al. 2018). Recently nanofibirillar collagen scaffolds have been used successfully in guiding lymphangiogenesis in the treatment of acquired lymphedema in pigs (Hadamitzky et al. 2016). In conclusion, after all the experiments in the pig, this technique might be applied in the clinic in the future, as a compassionate use.
The pig spleen in lymphocyte kinetics
There are major differences in structure and function of the spleen. On the one hand, there is the “storage spleen” with many muscles in trabeculae and the capsule, e.g., in the dog. That can result in a rapid contraction and mobilization of up to 20% of erythrocytes into the blood. On the other hand, there is the spleen which consists of large amounts of immune cells (“protection spleen”). The pig spleen is similar to the human spleen. To test the role of an organ in any function is to remove it and look for the effects. Autologous blood lymphocytes were labelled and reinjected via an iv cannula. Then a splenectomy performed and the retransfusion experiments repeated. The residence time of the lymphocytes in the blood was prolonged by the dramatically of after splenectomy (Pabst and Trepel 1976). The data obtained in normal pigs were the base for the statement that more lymphocytes recirculate through the spleen than through all other lymphoid organs with high endothelial venules (Pabst and Westermann 1998). The pigs’ spleen had been selectively labelled with the DNA precursor 3H-thymidin. In serial samples of splenic tissue, the cell cycle parameters G2-S-G1-M phase were determined by autoradiography. Thus, the proliferation in the normal microenvironment was characterized in contrast to many in vitro techniques (Pabst and Trepel 1975a, b). These data obtained in pigs was the base for the perfusion of isolated human spleens, and the cell cycle length, G2 and S phase, could be determined (Pabst and Reinecke 1981) and documenting the production of the coagulation factor VIII by the spleen (Pabst et al. 1977a). All nucleated cells in the spleen were labelled by selective perfusion of the pig spleen with an RNA precursor (H3-cytidine) and the emigrated lymphocytes identified in other organs and quantified (Pabst et al. 1978). The lymphocyte production and the emigration of the newly formed lymphocytes were studied by using the DNA precursor tritiated thymidine (Pabst et al. 1977a, b). Thus, in no other species, the role of the spleen in lymphocyte production and migration is in more detail studied than in the pig.
Splenic autotransplantation and regeneration
The spleen is critical in filtering certain bacteria. There is a dramatic situation in the overwhelming post-splenectomy infection (OPSI). This sepsis is lethal in about 30%. In most cases, pneumococci enter the blood. These bacteria are normally removed by the filter function of the spleen. In a spleen person or after a splenectomy due to splenic rupture or hematologic reasons, pneumococcal vaccinations are recommended. When there is trauma to the spleen, a partial splenectomy can be an alternative. When splenic fragments are transplanted, they will regenerate and function as a spleen. The pig is an excellent model because the tissue regenerates after arterial ligation or partial splenectomy (Pabst et al. 1984). The splenic blood flow could be measured, and therefore the pig was much more comparable to man than experiments in rodents (Pabst et al. 1984).
There was no difference in splenic regeneration whether small splenic particulates or thin slices were placed in a pouch of the great omentum (Pabst and Kamran 1986). The regeneration of autortransplanted splenic tissue in the greater omentum was not proportional to total weight and size of the transplanted fragments (Pabst and Westermann 1987). The regeneration had been documented by scintigraphy of autologous; heat altered 99 mTc-labeled red blood cells (Reilmann et al. 1983). The immunoarchitecture of regenerated splenic tissue was similar to a normal spleen Pabst et al. (1991). Thus, the pig (normal piglets or Göttingen minipigs) is an excellent model for splenic regeneration.
Selective labelling of lymphoid organs by extracorporeal perfusion in the pig
Lymphocytes are mostly produced in one organ, but later most of them leave that organ and migrate in the blood to other lymphoid organs. The blood pool is the most easy accessible pool. At any given time, only about 2% of all lymphocytes of the body are in the blood. To study the role of an individual organ in lymphocyte migration, a general cell marker like the RNA precursor tritiated cytidine or fluorescein isotiocyanate for all cells has been used. For proliferating lymphocyte, radioactively labelled tritiated thymidine has been usefull. The evaluation was performed by scintillation counting or autoradiography. The size of the vessels in the young pig is large enough to be cannulated and connected to an extracorporeal perfusion system. Thus, labelling lymphocytes in their normal environment at body temperature is possible in the pig. By this technique, the spleen, the bone marrow, and the small intestine with or without mesenteric lymph nodes were studied (Pabst et al. 1980). The interaction between innate immunity and porcine reproductive and respiratory syndrome was studied (Sanger et al. 2011)
The selectivity of the label was documented. The number and localization of lymphocytes labelled in one organ could be quantified of different times and the life span measured (e.g., lymphoid cells labelled in the pig spleen emigrated in large numbers to the bone marrow and developed into mature plasma cells (Pabst and Nowara 1982)). This technique is advantageous to many local injections of the label as the perfusion is more homogenous. This technique is not applicate in rodents, and therefore normally lymphoid organs were teased to cell suspensive, labelled in vivo, and reinjected into recipients.
Mucosal Immunology
All organs with a mucosa, e.g., mouth, respiratory tract, gastrointestinal tract, and genital tract, are often called common mucosal lymphoid tissue (c MALT). This overstresses the similarities, e.g., vaccinating the nose does not imply a protective immune reaction in all other mucosal organs. Therefore, we recommend the term i MALT: Integrated mucosal lymphoid tissue (Pabst and Brandtzaeg 2020), because there is preferential migration of lymphoid cells, e.g., from the gut wall to the bronchial tract. An example is to protect pigs from the bacterial lung infection by Actinobacillus pleuropneumoniae by giving an oral dose of encapsulated bacteria (Hensel et al. 1994; Hensel et al. 1996). A surprising finding was a dramatic increase of plasma cells in the bronchiolar lavage (Delventhal et al. 1992). In this paragraph, I will focus on the gut wall in the pig.
There were some differences in MALT structure and functions in farm animals (Liebler-Tenorio and Pabst 2006). The pig as a monogastric omnivorous species has several similarities to man (Bailey et al. 2005a, b). In the gut wall, there are organized accumulations of lymphocytes: isolated follicles and aggregated follicles called Peyer’s patches (PP), which differ in cell composition and overall structure between “conventional” PP, e.g., in the jejunum (jej PP) and upper ileum (jej PP) and a continuous patch in the distal ileum (il PP). In the mouse, there are also the so-called crypto-patches and in the rat and man lymphocyte filled villi (Pabst and Brandtzaeg 2020). The development of the number, size, structure, and proliferative capacity of PP in the jejunum and ileum was studied at different postnatal ages in conventional and germfree pigs (Pabst et al. 1988). The length of the jej PP and ileal PP increased with age. This was due to an increase in follicle size and the number of follicles. In older pigs (4, 5 years), the ileal PP regressed to small follicles. In germfree pigs of 39 and 59 days of age, longer PP were found than in newborns but significantly shorter than in conventional pigs. The lymphocyte production was studied by the metaphase arrest technique using vincristine. Lymphocyte proliferation increase dramatically in germinal centers with age, while in the interfollicular area and dome, an age-independent lymphocyte production was documented with no differences between jej PP and il PP (Pabst et al. 1988). In another study, the PP in pigs were studied in 1-, 1.5-, and 2-month-old pigs. The interfollicular area was wider (0.1 ± 0.04 mm) in jej PP in comparison with ileal PP (0.04 ± 0.03 mm). In the jej PP, there were more T cells than in il PP. In germfree pigs, there was a preference of IgM+ cells over IgA+ (Barman et al. 1997). A potential effect of resecting the il PP or transposing it to the oral part of the small intestine in the pig. There was no compensatory growth after resection. However, the number of CD8 lymphocytes was reduced in the blood, spleen, tonsils, and lymph nodes (Rothkötter et al. 1994). The dome epithelium is covered not only by enterocytes but also by membranous cells (M cell) which are the preferential cells for the uptake of particulate antigens. In the pig, the marker for M cells is cytokeratin 18 (Gebert et al. 1994). Fluorescent yeast particles were placed in the gut lumen and the uptake by M cells in jeg PP studied. Electron microscopy documented the kinetic of uptake with very few particles in macrophages in the epithelium, but after 4 h, the yeast was below the basal lamina and 89% in macrophages (Beier and Gebert 1998).
When fluorescinated particles were instilled in isolated loops of the jejunum with several jeg PP and these excised at 30, 60, and 120 min, a surprising finding was that in each PP, one done area was full of fluorescent particles and the domes next to it, no uptake was seen. Based on this observation, we hypothesize that it would be a great advantage to develop a stimulus for the induction of M cell so that all PP are active for antigen uptake. In a second step, an oral vaccine should be given, and the antibody response would be much more pronounced (Pabst and Rothkötter, unpublished). Braun et al. (2017) summarized all arguments, why the il PP in the pig differs from those in sheep, which might have the potential of a primary lymphoid organ for B lymphocytes.
The effector site of the gut immune system is the epithelium with the intraepithelial lymphocytes (IEL) and the lymphoid cells in the lamina propria. The gut of pigs was treated with EDTA first to recover the IEL, and then an incubation with collagenase followed to get the lymphoid cells of the lamina propria. In conventional pigs, the number of IEL increased with age, and the numbers in the jejunum per gram were higher than in the ileum. In germfree piglets of 45 days of age, they were comparable with 5 days old conventional piglets. The thymidine analogue bromodeoxyuridin was used to study lymphocyte proliferation. The IEL had a labelling index of 3 to 7%, but in the lamina propria, only 1% were labelled (Rothkötter et al. 1994). In the lamina propria, lymphocytes proliferated more in the crypt region than in the villous part. The CD2 T lymphocytes increased tenfold from day 1 to day 40.
Ig-positive cells appeared later than T cells. In 40 days old piglets, there were more Ig A4 than IgM+ cells. In germfree pigs, the numbers were comparable to 5 days old conventional piglets (Rothkötter et al. 1991). Already in 1981, data were published on the localization of IgA, IgM, and IgG in the gut of suckled pigs. The site of absorption of immunoglobulins of colostrum in suckled pigs was obvious in the villous epithelium but absent from the crypts (Butler et al. 1981). A single dose of antigen (ovalbumin) in neonatal pigs reduced the subsequent immune response (Haverson et al. 2009). Environmental factors increase the productions of IgA (Levast et al. 2014). The pig has also been used as a model for the development and compartmentalization of the TCR delta repertoir and the effect of age and microbial factors (Holtmeier et al. 2002). The migration pattern of T and B lymphocytes from the gut lymph was quantified and characterized in pigs. Only IgA-positive cells accumulate in the lamina propria (Rothkötter et al. 1999).
Cannulation of intestinal lymphatics in the pig
Bennell and Husband (1981a, b) described the experiments in rats and pigs resecting mesenteric lymph nodes and later cannulating the intestinal lymphatic trunk in pigs or the thoracic duct in rats. The technique enabled the sampling of lymph coming from the gut without the filter station of mesenteric lymph nodes. Thus, this was afferent lymph. In rats, the animals have to be strictly restraint in a Bollman cage. The animals cannot move, and it is extremely stressful. Therefore, this technique is not allowed since several years in Germany. In sheep, a restraining cage holds the animals in a standing position. Bennell and Husband (1981a, b) had not described whether the pigs were restrained.
We optimized this technique. At 3 months of age, the Göttingen minipigs were laparotomized and the mesenteric lymph nodes carefully removed from both sides of the mesentery and the blood supply of the lymph nodes stopped by electrocautery. After such an operation, the afferent and efferent lymphatics anastomose by physiological regeneration (called “pseudoafferent lymphatic”). At about 8 months of age, a venous cannula was placed in an external jugular vein. The cannula was pulled through a silastic tube and exteriorized in the neck. The intestinal lymph duct could easily be identified because the pigs were fed with olive oil the evening before the operation. The absorbed fat changed the color of the lymph to white. The intestinal lymph duct was below the pancreas near to the confluence of the left renal vein and the caudal vena cava (Rothkötter et al. 1993). The canula in the intestinal lymphatic was pulled through a silastic tube which avoided kinking and could be fixed to the skin, where a 250 ml bottle was placed in a bag. The lymph volume and the lymphocyte numbers and different cell subsets were measured. The pigs were placed in nonrestraining conditions with full access to food and water each in a separate box as the pigs otherwise started chewing on the cannula of the other pigs. In some pigs, the DNA precursor BrdU (bromodeoxyuridine) was given iv to measure the proliferation of the cells in the gut. By double labelling, the subsets of BrdU positive cells could be determined: IgA+ cells reached a maximum of > 50% of 12 k (Thielke et al. 1999). In other experiments with cannulating intestinal lymphatics, the surprising data were obtained that in total numbers, more newly formed T cells and B cells left the gut wall via lymphatics (Rothkötter et al. 1995). B and T lymphocyte migrate from the gut lymph to other parts of the gut wall and all other lymphoid organs. However, only IgA+ cells accumulate in the intestinal mucosa (Rothkötter et al. 1999). Proliferating intestinal gamma/delta cells recirculated recently and formed mainly the pool of these cells in the blood (Thielke et al. 2003). In conclusion, the pig is an excellent model to study intestinal lymph and the lymphoid cells coming from the gut wall under conditions not possible in other species.
Probiotics and Prebiotics tested in pig
Prebiotics and probiotics are recommended to treat patients in different inflammations and infections of the intestinal tract. The monogastric omnivorous pig was therefore tested. When Enterococcus faecium was added to the food, the subsets of CD4+ and CD25+ cells in the ileal Peyer´s patches were tested. There were some changes in the T helper cell responses but not convincing effects after Salmonella typhimurium infection (Kreuzer et al. 2014). A probiotic Bacillus cerens stain activated the Natural killer receptor (NUG2D) resulting in differences in the subset composition of intraepithelial cells (Altmeyer et al. 2014). In another study, the prebiotic feeding of Enterococcus faecium resulted in a longer and wider villi and higher numbers of intraepithelial lymphocytes. This effect is only present at day 28 postinfection (Rieger et al. 2015). In pre-weaning piglets, the probiotic affected intestinal immune-associated gene expression (Siepert et al. 2014). In 2011, there was an outbreak of infections with an E. coli bacterium producing Shiga toxin resulting in diarrhea and a hemolytic syndrome (HUS) in humans. Neonatal gnotobiotic pigs were used as an animal model for this strain. Unexpectedly, this strain caused only mild symptoms and minor changes in histology and blood parameter. Thus, in this case, the pig did not show symptoms as in man (Wöchtl et al. 2017). The effects of dietary factors on gut homeostasis and early gut maturation have been reviewed by Everaert et al. 2017 and Roselli et al. 2017.
Lymphocyte compartments in the lung
There are five compartments where lymphocytes are found in the lung: (1) intraepithelial, (2) lamina propria, (3) bronchus-associated lymphoid tissue (BALT), (4) intravascular pool, and (5) bronchoalveolar space (Pabst 2000). The dynamics of lymphocyte in the lung had been summarized already 10 years earlier (Pabst 1990). In the pig, the composition of lymphocytes in the bronchial epithelium and lamina propria is not different to other species. BALT is hardly present in healthy pigs but very obvious in mycoplasma infected animals. The intravascular pool is of much more interest. When lymphocytes are labelled and injected intravenously, the majority will be found in the lung initially. This is often interpreted as a sticking of the lymphocytes to the intrapulmonary capillary bed. This is not the case as documented in pigs. Blood lymphocytes were labelled in vitro with a fluorescent dye and injected either intravenously or into the left heart. Comparable numbers were of lymphocytes found in the lung after 30 min indicating a specific homing of lymphocytes to the lung vascular bed. When the left lung of the pig was perfused with a cell-free medium for up to 4 h, a continuous venous release of lymphocytes was measured. This resulted in 1.5 × 109 lymphocytes. Also labelling peripheral blood lymphocytes with 51Cr documented a high intravascular pool of lymphocytes (Pabst et al. 1987). The bronchoalveolar space is often lavage in the clinics, and the numbers and subsets of lymphocytes are taken as an indicator for different lung diseases. The majority of cells in the bronchoalveolar lavage are macrophages. The question was often, what is the origin and fate of the lymphocytes in the airspace. Therefore, autologous blood lymphocytes of normal young pigs were labelled with 51Cr or fluorescein isothiocyanate (FITC) and instilled into a single segmental bronchus. By immunohistology, FITC-positive lymphocytes were found in the sinuses of the draining nodes. Combined with cell surface staining (B, T, TH, and Ts lymphocytes), the lymphocyte subsets were quantified and showed a preferential migration out of the bronchoalveolar space to the draining bronchial lymph node (Pabst and Binns 1995). The lymphatic drainage of the pig lung is very comparable to man (Riquet et al. 2000).In the pig lung, there are also intravascular macrophages which had for longtime believed is only the case in goats (Winkler 1988). These macrophages pick up and clear bacteria and particulates from the blood.
The pig lung a model for lung preservation before transplantation
The anatomy and bronchoscopy of the pig lung and the similarity to man have been summarized by Indge et al. (2014). Lungs of organ donors have to be prepared often for transport to the recipient. Roman et al. (2015) compared a cell-free or cellular perfused in a pig model. Lung histology and ultrastructure were largely preserved (Becker et al. 2015). Cellular and acellular perfusate and its effect on the ultrastructure of the lung were studied in 20 pigs (Steinmeyer et al. 2018). In vivo treatment of the isolated perfused lung was tested in minipigs by autoretransplantation (Krüger et al. 2016). Another application of the isolated perfused lung is to treat infected lungs by perfusion with high doses of antibiotics followed by autotransplantation (Zinne et al. 2018). The pig lung has also been used for silencing MHC expression by applying short-hairpin RNAs targeting ß2-microglobulin and class II transactivator transcripts. The feasibility of this technique was demonstrated achieving a targeted silencing effect of 67% and 52%, respectively, without effecting cell viability or lung tissue integrity (Figueiredo et al. 2019). A functional repair of the donor lungs was tested by IL-10 gene therapy (Cypel et al. 2009). Negative pressure ventilation in normothermic ex vivo lung perfusion was tested with pig lungs. Irrespective of the perfusate, the solution there was less inflammation and less lung injury (Aboelnazar et al. 2018). The safety and efficacy of ex vivo donor lung adenoviral IL-10 gene therapy for a transplant survival model has also been tested successfully in pigs (Machuca et al. 2017). Meanwhile normothermic perfusion of the lung is possible also in man (Warnecke et al. 2012; Fildes et al. 2015).
Pig model of Cystic Fibrosis
Cystic fibrosis is the most frequent lethal genetic disease in Caucasian men. There is no causal treatment available for most of them. Symptomatic treatments from birth have extended the life expectancy over the last decades. Cystic fibrosis is an autosomal recessive disease in man caused by mutations of the gene encoding the cystic fibrosis transmembrane conductance regulation (CFTR) ion channel. CFTR is expressed in epithelia of many organs especially the lung, pancreas, liver, intestine and vas deferens. A sweat test with pilocarpine is a strong indicator for this devastating disease. The mouse models do not show lung disease. Therefore, it was a great breakthrough when the group of Rogers published the pig model in 2008 (Rogers et al. 2008a; b) and summarized by Rogers et al. 2008c. All these data document the advantage of the pig model of this disease. The very relevant function of bacterial eradication from the lung was described by Stoltz et al. (2010). There are more than 2000 mutations known of the CFTR gene. One of the most frequent mutations is the ΔF508 mutation. Therefore, a further advantage was to develop pigs with a mutation of ΔF508 (Ostedgaard et al. 2011). Thus, the pig is now the animal model for future experiments to treat this disease.
Xenotransplantation of pig organs
In all countries with organ transplantation programs, there is a deficiency of organ donor, and thousands of patients die on the waiting list. Already in 1985, Hammer et al. produced transgenic pigs by microinjection. The pig is the favorite species in xenotransplantation: The major histocompatibility complex in pigs is well characterized (Lunney et al. 2009). Genome editing has revolutionized genetically modified pigs (Klymiuk et al. 2010; Yao et al. 2016; Fischer et al. 2016; Fischer et al. 2019). The different techniques and also in combination have resulted in more than 40 different genetic alterations (Cooper et al. 2016). Some examples are interleukin-2 receptor gamma gene knockout pigs by zinc finger nuclease—encoding in RNA (Watanabe et al. 2013) or the development of inducible transgene expressions in pigs (Klymiuk et al. 2012). There are examples for genetic modifications with relevance to xenogeneic organ transplantation. The production of transgenic pigs for xenotransplantation has been summarized (Niemann and Petersen 2016). For the detail on gene stacking and gene editing in pigs, see the paper of Fischer et al. 2016. In the preclinical situation, pig to primate transplantation is the first step, which has been explored for pancreatic islet transplantation (Matsunari et al. 2014; Hering and Walawalkar 2009), kidney transplantation (Iwase and Kobayashi 2015) and heart transplantation (Mohiuddin et al. 2015; Längin et al. 2018). In particular, the study of Längin et al. is impressive because the pig hearts had life supporting functions for up to 945 days than in the monkeys. The pigs were genetically multi-modified: they lacked the galactose alpha-1,3 galactose epitopes and expressed the human membrane cofactor protein (CD46) and human thrombomodulin (Längin et al. 2018). Initially there was the trend of transforming viruses from pigs to man when xenotransplantation of pig organs and immunosuppression will be needed after transplanting an organ normally called prevention for porcine endogenous retrovirus (PERV). So far, no such phenomenon has been documented in pig to baboon transplantation.
Porcine endogenous retrovirus in xenotransplantation
A potential obstacle in using pig organs for xenotransplantation is the risk of the transmission of infections agents. This can more or less be excluded by using specific-pathogen-free (SPF) animals. Viruses can be more dangerous in immunosuppressed patient, which are vertically transmitted like the ones found in all pig breeds (Dieckhoff et al. 2009). However, there are techniques to solve this problem. One is to knockdown PERV (Dieckhoff et al. 2008) or remove it by the modern techniques, e.g., with the CRISPR/Cas technique, which also works in pigs (Gerlach et al. 2018).
Skin
The pig skin is similar to the human skin. Therefore, pigs can get sunburn. The importance of the pig skin for cutaneous pharmacology and toxicology has been summarized by Monteiro-Riviere and Riviere (1996). Different techniques have been used such as the isolated perfused porcine skin flaps with the advantage of viable. Full thickness skin with an intact blood supply have also been used. After, some differences to human skin are discussed (Monteiro-Riviere and Riviere 1996). The histological structure of the pig skin has been studied in detail by Meyer et al. (2007). They stress in particular the comparable microvascular structure. The thickness of the different layers varies between body areas as in man. The outer side of the ear is recommended for drug testing. They state to store the pig ear at 4 °C for 4–6 h after obtaining the material from the slaughterhouse and thus avoiding the use of experimental animals. The isolated blood perfused ear of the pig has been recommended for skin penetration studies (de Lange et al. 1992). Skin abrasion as a technique to treat sulfur mustard injuries had been tested in Yucatan minipigs (Rice et al. 2000). The immunology of the pig skin as a model for human skin has been summarized by (Summerfield et al. 2015). Delayed-type hypersensitivity reactions have been studied in the young pigs by the local injection of phytohemagglutinin. There was an accumulation of CD8+ lymphocytes. All lymphocytes subsets showed a high rate of proliferation locally as documented by the incorporation of the DNA-precursor bromodeoxyuridine (BrdU) (Fritz et al. 1990).
Sexually transmitted infections
There are estimations that per day, there are more than a million sexually transmitted infections worldwide (Gottlieb and Johnson 206). Therefore, there is an urgent need for adequate animal models, e.g., to develop vaccines. Lorenzen et al. (2017) have summarized the differences and similarities of the hormonal cycle between pigs and humans. A recent review summarized details on experiments on different causes like Trichomonas vaginalis, herpesviruses, Hepatitis B and C virus, as well as Treponema pallidum and Neisseria gonorrhoeae. The infection with Chlamydia suis and Chlamydia trachomatis induce multifunctional CD4 cells in pigs (Käser et al. 2018). A multi-subunit Chlamydia vaccine was protective (Bøje et al. 2016). The reader is referred to the recent review by Käser et al. (2017). The pig has also been used as a model for the infection with the Zika virus with its effects on the fetus (Darbellay et al. 2017), because neonatal pigs are susceptible to experimental Zika virus infection.
The pig in toxicology research
In regulatory toxicity testing in addition to rodents, another species has to be used. The pig is much more acceptable to the public than the dog or monkeys (Bode et al. 2010; Forster et al. 2010). The minipig has many advantages to the domestic pig. The phylogeny and classification of vaccine including different strain of minipigs used in Europe, Japan, and North America are documented by Ganderup et al. (2012). The use of minipigs includes the different routes (oral, intramuscular, intravenous, transdermal); safety pharmacology and juvenile toxicology are all mentioned. There is a problem in reproductive toxicology, because in the pig, large molecules do not pass the placenta.
Vamathevan et al. (2013) compared the genomes of minipigs and beagles and the relevance for toxicology studies. In the dog, there were more pseudogenized targets (41 genes) than in the minipig (19 genes). More recently, the advantages of the pig in a safety assessment trial of single stranded oligonucleotides have been documented. (Braendli-Baiocco et al. 2017). Feuen et al. (2012) stressed the practical aspects when minipigs are used in toxicological research in a company. These papers are extremely helpful for starting experiments with handling minipigs.
Limitations of the pig as a model for human diseases
In animal facilities, the pig needs much more space than rodents. The maintenance is more expensive. When indwelling cannulas, e.g., venous, biliary, intestinal, or lymphatic are needed, each animal has to be placed individually. The handling of the pig, e.g., for injections needs careful training to avoid stress effects. The placenta is tight and therefore large molecules as antibodies cannot pass the barrier to the fetus. The unique structure of the lymph node is a limitation for the studies on the functional microanatomy of the lymph node.
Perspectives and conclusions
As a monogastric, omnivorous species, the pig is ideal for studying intestinal immunology, e.g., testing prebiotic and probiotic food additives and effects of microbial colonization. The pig can be kept in a germfree, gnotobiotic environment for several weeks. The skin is very similar to the human skin. There are tonsils in the pig in contrast to rodents. The intestine, pancreatic and bile ducts, vessels (artery, vein, lymphatics), and ureter can be cannulated for some time to collect samples without stress. Pigs breed at all times of the year and have a large litter size. For longtime experiments, well-characterized minipigs (e.g., Göttingen minipig) are available all over the world. There are less ethical problems to use pig organs for transplantation than of pet animals (like the dog or large primates like chimpanzee, orangutan, or gorilla). The organ size is another advantage of the pig. The enormous recent progress in genetic engineering (“Hominidae”) of the pig is the reason for the prognosis that the pig will be the organ donor of the future for xenotransplantation in man.
Acknowledgments
Several colleagues sent me recent publications such as Armin Saalmüller, Veterinary University Vienna, Austria; Doris Höltig, Veterinary University Hannover, Germany, and others. Thanks to Marita Peter who prepared the figures and in particular Silke Wallbaum who transformed my handwriting to the readable computer version. My apology to all the authors whose interesting publications are not quoted due to restrictions in the number of references.
Funding information
My own studies were supported by several grants of the German Research Foundation (DFG).
Compliance with ethical standards
Conflict of interest
I declare that there is no conflict of interest. This paper does not contain any unpublished studies with human participants nor animals. Therefore no ethical approval is necessary.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aboelnazar NS, Himmat S, Hatami S, White CW, Burhani MS, Dromparis P, Matsumura N, Tian G, Dyck JRB, Mengel M, Freed DH, Nagendran J. Negative pressure ventilation decreases inflammation and lung edema during normothermic ex-vivo lung perfusion. J Heart Lung Transplant. 2018;37:520–530. doi: 10.1016/j.healun.2017.09.007. [DOI] [PubMed] [Google Scholar]
- Alan WD, Porter P. The relative distribution of IgM and IgA cells in intestinal mucosa and lymphoid tissues of the young unweaned pig and their significance in ontogenesis of secretory immunity. Immunology. 1973;24:4963–4501. [PMC free article] [PubMed] [Google Scholar]
- Altmeyer S, Kröger S, Vahjen W, Zentek J, Scharek-Tedin L. Impact of a probiotic Bacillus cereus strain on the jejunal epithelial barrier and on the NKG2D expressing immune cells during the weaning phase of piglets. Vet Immunol Immunopathol. 2014;161:57–65. doi: 10.1016/j.vetimm.2014.07.001. [DOI] [PubMed] [Google Scholar]
- Bailey M, Haverson K, Inman C, Harris C, Jones P, Corfield G, Miller B, Stokes C. The development of the mucosal immune system pre- and post-weaning: balancing regulatory and effector function. Proc Nutr Soc. 2005;64:451–457. doi: 10.1079/pns2005452. [DOI] [PubMed] [Google Scholar]
- Bailey M, Haverson K, Inman C, Harris C, Jones P, Corfield G, Miller B, Stokes C (2005b) The influence of environment on development of the mucosal immune system. Vet Immunol Immunopathol. 108:189–198 [DOI] [PubMed]
- Barman NN, Bianchi AT, Zwart RJ, Pabst R, Rothkötter HJ. Jejunal and ileal Peyer's patches in pigs differ in their postnatal development. Anat Embryol (Berl) 1997;195:41–50. doi: 10.1007/s004290050023. [DOI] [PubMed] [Google Scholar]
- Becker S, Steinmeyer J, Avsar M, Höffler K, Salman J, Haverich A, Warnecker G, Ochs M, Schnapper A. Evaluation acellular versus cellular perfusate composition during prolonged ex vivo lung perfusion after initial cold ischaemia for 24 hours. Transpl Int. 2015;29:88–97. doi: 10.1111/tri.12649. [DOI] [PubMed] [Google Scholar]
- Beier R, Gebert A. Kinetics of particle uptake in the domes of Peyer's patches. Am J Phys. 1998;275:G130–G137. doi: 10.1152/ajpgi.1998.275.1.G130. [DOI] [PubMed] [Google Scholar]
- Bennell MA, Husband AJ. Route of lymphocyte migration in pigs I Lymphocyte circulation in gut-associated lymphoid tissue. Immunology. 1981;42:469–474. [PMC free article] [PubMed] [Google Scholar]
- Bennell MA, Husband AJ. Route of lymphocyte migration in pigs. II Migration to the intestinal lamina propria of antigen-specific cells generated in response to intestinal immunization in the pig. Immunology. 1981;42:475–479. [PMC free article] [PubMed] [Google Scholar]
- Bianchi AT, Zwart RJ, Jeurissen SH, Moonen-Leusen HW. Development of the B- and T-cell compartments in porcine lymphoid organs from birth to adult life: an immunohistological approach. Vet Immunol Immunopathol. 1992;33:201–221. doi: 10.1016/0165-2427(92)90182-p. [DOI] [PubMed] [Google Scholar]
- Bimczok D, Sowa EN, Faber-Zuschratter H, Pabst R, Rothkötter HJ. Site-specific expression of CD11b and SIRPalpha (CD172a) on dendritic cells: implications for their migration patterns in the gut immune system. Eur J Immunol. 2005;35:1418–1427. doi: 10.1002/eji.200425726. [DOI] [PubMed] [Google Scholar]
- Bimczok D, Post A, Tschernig T, Rothkötter HJ. Phenotype and distribution of dendritic cells in the porcine small intestinal and tracheal mucosa and their spatial relationship to epithelial cells. Cell Tissue Res. 2006;325:461–468. doi: 10.1007/s00441-006-0195-3. [DOI] [PubMed] [Google Scholar]
- Bimczok D, Verdonck F, Hartig R, Cox E, Rothkötter HJ. Primary porcine CD11R1+ antigen-presenting cells isolated from small intestinal mucosa mature but lose their T cell stimulatory function in response to cholera toxin treatment. Vet Immunol Immunopathol. 2010;134:239–248. doi: 10.1016/j.vetimm.2009.10.022. [DOI] [PubMed] [Google Scholar]
- Binns RM. Organisation of the lymphoreticular system and lymphocyte markers in the pig. Vet Immunol Immunopathol. 1982;3:95–146. doi: 10.1016/0165-2427(82)90033-2. [DOI] [PubMed] [Google Scholar]
- Binns RM, Licence ST. Exit of recirculating lymphocytes from lymph nodes is directed by specific exit signals. Eur J Immunol. 1990;20:449–452. doi: 10.1002/eji.1830200234. [DOI] [PubMed] [Google Scholar]
- Binns RM, Pabst R. Lymphoid cell migration and homing in the young pig: Alternative immune mechanisms in action. In: Husband AJ, editor. Migration and homing of lymphoid cells. Boca Raton: CRC Press; 1988. pp. 137–174. [Google Scholar]
- Binns RM, Pabst R, Licence ST. Lymphocyte emigration from lymph nodes by blood in the pig and efferent lymph in the sheep. Immunology. 1985;54:105–111. [PMC free article] [PubMed] [Google Scholar]
- Binns RM, Pabst R, Licence ST. The behavior of pig lymphocyte populations in vivo. In: Tumbleson ME, editor. Swine in biomedical research. New York: Plenum Press; 1986. pp. 1837–1853. [Google Scholar]
- Binns RM, Pabst R, Licence ST. Subpopulations of T lymphocytes emigrating in venous blood draining pig thymus labelled in vivo with fluorochrome. Immunology. 1988;63:261–267. [PMC free article] [PubMed] [Google Scholar]
- Birchall MA, Kingham PJ, Murison PJ, Ayling SM, Burt R, Mitchard L, Jones A, Lear P, Stokes CR, Terenghi G, Bailey M, Macchiarini P. Laryngeal transplantation in minipigs: vascular, myologic and functional outcomes. Eur Arch Otorhinolaryngol. 2011;268:405–414. doi: 10.1007/s00405-010-1355-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchall MA, Kingham PJ, Murison PJ, Ayling SM, Burt R, Mitchard L, Jones A, Lear P, Stokes CR, Terenghi G, Bailey M, Macchiarini P. Erratum to: Laryngeal transplantation in minipigs: vascular, myologic and functional outcomes. Eur Arch Otorhinolaryngol. 2017;274:595–596. doi: 10.1007/s00405-016-4357-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum KS, Radtke C, Knapp WH, Pabst R, Gratz KF. SPECT-CT: a valuable method to document the regeneration of lymphatics and autotransplanted lymph node fragments. Eur J Nucl Med Mol Imaging. 2007;34:1861–1867. doi: 10.1007/s00259-007-0458-6. [DOI] [PubMed] [Google Scholar]
- Blum KS, Hadamitzky C, Gratz KF, Pabst R. Effects of autotransplanted lymph node fragments on the lymphatic system in the pig model. Breast Cancer Res Treat. 2010;120:59–66. doi: 10.1007/s10549-009-0367-4. [DOI] [PubMed] [Google Scholar]
- Bode G, Clausing P, Gervais F, Loegsted J, Luft J, Nogues V, Sims J, Steering Group of the RETHINK Project The utility of the minipig as an animal model in regulatory toxicology. J Pharmacol Toxicol Methods. 2010;62:196–220. doi: 10.1016/j.vascn.2010.05.009. [DOI] [PubMed] [Google Scholar]
- Boeker M, Pabst R, Rothkötter HJ. Quantification of B, T and null lymphocyte subpopulations in the blood and lymphoid organs of the pig. Immunobiology. 1999;201:74–87. doi: 10.1016/S0171-2985(99)80048-5. [DOI] [PubMed] [Google Scholar]
- Bøje S, Olsen AW, Erneholm K, Agerholm JS, Jungersen G, Andersen P, Follmann F. A multi-subunit Chlamydia vaccine inducing neutralizing antibodies and strong IFN-γ+ CMI responses protects against a genital infection in minipigs. Immunol Cell Biol. 2016;94:185–195. doi: 10.1038/icb.2015.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollen PJA, Ellegaard L. Development in Breeding Göttingen Minipigs. In: Tumbleson ME, Schook LB, editors. Advances in Swine in Biomedical Research. New York: Plenum Press; 1996. [Google Scholar]
- Braendli-Baiocco A, Festag M, Dumong Erichsen K, Persson R, Mihatsch MJ, Fisker N, Funk J, Mohr S, Constien R, Ploix C, Brady K, Berrera M, Altmann B, Lenz B, Albassam M, Schmitt G, Weiser T, Schuler F, Singer T, Tessier Y. From the cover: the minipig is a suitable non-rodent model in the safety assessment of single stranded oligonucleotides. Toxicol Sci. 2017;157:112–128. doi: 10.1093/toxsci/kfx025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun RO, Python S, Summerfield A. Porcine B cell subset responses to Toll-like receptor ligands. Front Immunol. 2017;8:1044. doi: 10.3389/fimmu.2017.01044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JE, Klobasa F, Werhahn E. The differential localization of IgA, IgM and IgG in the gut of suckled neonatal piglets. Vet Immunol Immunopathol. 1981;2:53–65. doi: 10.1016/0165-2427(81)90038-6. [DOI] [PubMed] [Google Scholar]
- Butler JE, Zhao Y, Sinkora M, Wertz N, Kacskovics I. Immunoglobulins, antibody repertoire and B cell development. Dev Comp Immunol. 2008;33:321–333. doi: 10.1016/j.dci.2008.06.015. [DOI] [PubMed] [Google Scholar]
- Butler JE, Zhao Y, Sinkora M, Wertz N, Kacskovics I. Immunoglobulins, antibody repertoire and B cell development. Dev Comp Immunol. 2009;33:321–333. doi: 10.1016/j.dci.2008.06.015. [DOI] [PubMed] [Google Scholar]
- Butler JE, Santiage-Mateo K, Wertz N, Sun X, Sinkora M, Francis DL. Antibody repertoire development in fetal and neonatal piglets. XXIV. Hypothesis: the ileal Peyer patches (IPP) are the major source of primary, undiversified IgA antibodies in newborn piglets. Elsevier. Dev Comp Immunol. 2016;65:340–351. doi: 10.1016/j.dci.2016.07.020. [DOI] [PubMed] [Google Scholar]
- Butler JE, Wertz N, Sinkora M. Antibody repertoire development in swine. Annu Rev Anim Biosci. 2017;5:255–279. doi: 10.1146/annurev-animal-022516-022818. [DOI] [PubMed] [Google Scholar]
- Butler JE, Wertz N, Sinkora M. Antibody repertoire development in swine. Annu Rev AnimBiosci. 2017;5:3.1–3.25. doi: 10.1146/annurev-animal-022516-022818. [DOI] [PubMed] [Google Scholar]
- Butler JE, Sinkora M, Wang G, Stepanova K, Li Y, Cai X. Perturbation of thymocyte development underlies the PRRS pandemic: a testable hypothesis. Front Immunol. 2019;10:1077. doi: 10.3389/fimmu.2019.01077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper DK, Ekser B, Ramsoondar J, Phelps C, Ayares D. The role of genetically engineered pigs in xenotransplantation research. J Pathol. 2016;238:288–299. doi: 10.1002/path.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypel M, Liu M, Rubacha M, Yeung JC, Hirayama S, Anraku M, Sato M, Medin J, Davidson BL, de Perrot M, Waddell TK, Slutsky AS, Keshavjee S. Functional repair of human donor lungs by IL-10 gene therapy. Sci Transl Med. 2009;1:4ra9. doi: 10.1126/scitranslmed.3000266. [DOI] [PubMed] [Google Scholar]
- Darbellay J, Cox B, Lai K, Delgado-Ortega M, Wheler C, Wilson D, Walker S, Starrak G, Hockley D, Huang Y, Mutwiri G, Potter A, Gilmour M, Safronetz D, Gerdts V, Karniychuk U. Zika virus causes persistent infection in porcine concept uses and may impair health in offspring. EBioMedicine. 2017;25:73–86. doi: 10.1016/j.ebiom.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson H. A comparative assessment of the pig, mouse and human genomes: structural and functional analysis of genes involved in immunity an inflammation. In: Mc Anultiy PA, Dawjan Canderup ADWC, Hassintgs KL, editors. The Minipig In Biomedical Research. Boca Raton: CRC Press; 2011. p. 664. [Google Scholar]
- Dawson HD, Lunney JK. Porcine cluster of differentiation (CD) markers 2018 update. Res Vet Sci. 2018;118:199–246. doi: 10.1016/j.rvsc.2018.02.007. [DOI] [PubMed] [Google Scholar]
- De Lange J, van Eck P, Elliott GR, de Kort WLAM, Wolthuis OL (1992) The isolated blood-perfused pig ear: an inexpensive and animal-saving model for skin penetration studies. JPM:71–77 [DOI] [PubMed]
- Delaere P, Van Raemdonck D. Tracheal replacement. J Thorac Dis. 2016;8(Suppl2):186–196. doi: 10.3978/j.issn.2072-1439.2016.01.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delventhal S, Hensel A, Petzoldt K, Pabst R. Cellular changes in the bronchoalveolar lavage (BAL) of pigs, following immunization by the enteral or respiratory route. Clin Exp Immunol. 1992;90:223–227. doi: 10.1111/j.1365-2249.1992.tb07933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieckhoff B, Petersen B, Kues WA, Kurth R, Niemann H, Denner J. Knockdown of porcine endogenous retrovirus (PERV) expression by PERV-specific shRNA in transgenic pigs. Xenotransplantation. 2008;15:36–45. doi: 10.1111/j.1399-3089.2008.00442.x. [DOI] [PubMed] [Google Scholar]
- Dieckhoff B, Kessler B, Jobst D, Kues W, Petersen B, Pfeifer A, Kurth R, Niemann H, Wolf E, Denner J. Distribution and expression of porcine endogenous retroviruses in multi-transgenic pigs generated for xenotransplantation. Xenotransplantation. 2009;16:64–73. doi: 10.1111/j.1399-3089.2009.00515.x. [DOI] [PubMed] [Google Scholar]
- Elliott MJ, De Coppi P, Speggiorin S, Roebuck D, Butler CR, Samuel E, Crowley C, McLaren C, Fierens A, Vondrys D, Cochrane L, Jephson C, Janes S, Beaumont NJ, Cogan T, Bader A, Seifalian AM, Hsuan JJ, Lowdell MW, Birchall MA. Stem-cell-based, tissue engineered tracheal replacement in a child: a 2-year follow-up study. Lancet. 2012;380:994–1000. doi: 10.1016/S0140-6736(12)60737-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everaert N, Van Cruchten S, Westräm B, Bailey M, Van Gginneken C, Thymann T, Pieper R. A review on early gut maturation and colonization in pigs, including biological and dietary factors affecting gut homeostasis. Anim Feed Sci Technol. 2017;233:89–133. [Google Scholar]
- Feuen B, Roels K, Witters J, Verbraeken F, Peeters T, Kinpe A, Vynckier A, Lammens L, van Bekkum Y. Minipigs in toxicological research at Janssen: practical aspects. Janssen Newsletter. 2012;28:18–21. [Google Scholar]
- Figueiredo C, Carvalho Oliveira M, Chen-Wacker C, Jansson K, Höffler K, Yuzefovych Y, Pogozhykh O, Jin Z, Kühnel M, Jonigk D, Wiegmann B, Sommer W, Haverich A, Warnecke G, Blasczyk R. Immunoengineering of the vascular endothelium to silence MHC expression during normothermic ex vivo lung perfusion. Hum Gene Ther. 2019;30:485–496. doi: 10.1089/hum.2018.117. [DOI] [PubMed] [Google Scholar]
- Fildes JE, Archer LD, Blaikley J, Ball AL, Stone JP, Sjöberg T, Steen S, Yonan N. Clinical outcome of patients transplanted with marginal donor lungs via ex vivo lung perfusion compared to standard lung transplantation. Transplantation. 2015;99:1078–1083. doi: 10.1097/TP.0000000000000462. [DOI] [PubMed] [Google Scholar]
- Fischer K, Kraner-Scheiber S, Petersen B, Rieblinger B, Buermann A, Flisikowska T, Flisikowski K, Christan S, Edlinger M, Baars W, Kurome M, Zakhartchenko V, Kessler B, Plotzki E, Szczerbal I, Switonski M, Denner J, Wolf E, Schwinzer R, Niemann H, Kind A, Schnieke A. Efficient production of multi-modified pigs for xenotransplantation by 'combineering', gene stacking and gene editing. Sci Rep. 2016;6:29081. doi: 10.1038/srep29081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer K, Rieblinger B, Hein R, Sfriso R, Zuber J, Fischer A, Klinger B, Liang W, Flisikowski K, Kurome M, Zakhartchenko V, Kessler B, Wolf E, Rieben R, Schwinzer R, Kind A, Schnieke A. Viable pigs after simultaneous inactivation of porcine MHC class I and three xenoreactive antigen genes GGTA1, CMAH and B4GALNT2. Xenotransplantation. 2019;8:e12560. doi: 10.1111/xen.12560. [DOI] [PubMed] [Google Scholar]
- Forster R, Bode G, Ellegaard L, van der Laan JW, Steering Group of the RETHINK Project The RETHINK project--minipigs as models for the toxicity testing of new medicines and chemicals: an impact assessment. J Pharmacol Toxicol Methods. 2010;62:158–159. doi: 10.1016/j.vascn.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Fritz FJ, Pabst R, Binns RM. Lymphocyte subsets and their proliferation in a model for a delayed-type hypersensitivity reaction in the skin. Immunology. 1990;71:508–516. [PMC free article] [PubMed] [Google Scholar]
- Ganderup NC, Harvey W, Mortensen JT, Harrouk W. The minipig as nonrodent species in toxicology--where are we now? Int J Toxicol. 2012;31:507–528. doi: 10.1177/1091581812462039. [DOI] [PubMed] [Google Scholar]
- Gebert A, Rothkötter HJ, Pabst R. Cytokeratin 18 is an M-cell marker in porcine Peyer's patches. Cell Tissue Res. 1994;276:213–221. doi: 10.1007/BF00306106. [DOI] [PubMed] [Google Scholar]
- Gerlach M, Kraft T, Brenner B, Petersen B, Niemann H, Montag J. Efficient knock-in of a point mutation in porcine fibroblasts using the CRISPR/Cas9-GMNN fusion gene. Genes. 2018;9:296. doi: 10.3390/genes9060296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerner W, Käser T, Saalmüller A. Porcine T lymphocytes and NK cells--an update. Dev Comp Immunol. 2009;33:310–320. doi: 10.1016/j.dci.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Gerner W, Talker SC, Koinig HC, Sedlak C, Mair KH, Saalmüller A. Phenotypic and functional differentiation of porcine αβ T cells: Current knowledge and available tools. Mol Immunol. 2015;66:3–13. doi: 10.1016/j.molimm.2014.10.025. [DOI] [PubMed] [Google Scholar]
- Glodeck P, Bruns E, Oldys B, Holtz W. Das Göttingen Minischwein, ein Laboratoriumstier mit weltweiter Bedeutung. Züchtungskunde. 1977;49:21–32. [Google Scholar]
- Go T, Jungebluth P, Baiguero S, Asnaghi A, Martorell J, Ostertag H, Mantero S, Birchall M, Bader A, Macchiarini P. Both epithelial cells and mesenchymal stem cell-derived chondrocytes contribute to the survival of tissue-engineered airway transplants in pigs. J Thorac Cardiovasc Surg. 2010;139:437–443. doi: 10.1016/j.jtcvs.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Gunzer F, Hennig-Pauka I, Waldmann KH, Sandhoff R, Gröne HJ, Kreipe HH, Matussek A, Mengel M. Gnotobiotic piglets develop thrombotic microangiopathy after oral infection with enterohemorrhagic Escherichia coli. Am J Clin Pathol. 2002;118:364–375. doi: 10.1309/UMW9-D06Q-M94Q-JGH2. [DOI] [PubMed] [Google Scholar]
- Hadamitzky C, Zaitseva TS, Bazalova-Carter M, Paukshto MV, Hou L, Strassberg Z, Ferguson J, Matsuura Y, Dash R, Yang PC, Kretchetov S, Vogt PM, Rockson SG, Cooke JP, Huang NF. Aligned nanofibrillar collagen scaffolds - guiding lymphangiogenesis for treatment of acquired lymphedema. Biomaterials. 2016;102:259–267. doi: 10.1016/j.biomaterials.2016.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadamitzky C, Perić H, Theobald SJ, Gratz KF, Spohr H, Pabst R, Vogt PM. Effect of cryopreservation on lymph node fragment regeneration after autologous transplantation in the minipig model. Innov Surg Sci. 2018;3:139–146. doi: 10.1515/iss-2018-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley PJ. The lymphoid system: a review of species differences. J Toxicol Pathol. 2017;30:111–123. doi: 10.1293/tox.2016-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton NJI, Birchall MA. Tissue-Engineered Larynx: Future applications in laryngeal cancer. Curr Otorhinolaryngol Rep. 2017;5:42–48. doi: 10.1007/s40136-017-0144-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer RE, Pursel VG, Rexroad CE, Jr, Wall RJ, Bolt DJ, Ebert KM, Palmiter RD, Brinster RL. Production of transgenic rabbits, sheep and pigs by microinjection. Nature. 1985;315:680–683. doi: 10.1038/315680a0. [DOI] [PubMed] [Google Scholar]
- Haverson K, Rehakova Z, Sinkora J, Sver L, Bailey M. Immune development in jejunal mucosa after colonization with selected commensal gut bacteria: a study in germ-free pigs. Vet Immunol Immunopathol. 2007;119:243–253. doi: 10.1016/j.vetimm.2007.05.022. [DOI] [PubMed] [Google Scholar]
- Haverson K, Corfield G, Jones PH, Kenny M, Fowler J, Bailey M, Stokes CR, Miller BG. Effect of oral antigen and antibody exposure at birth on subsequent immune status. A study in neonatal pigs. Int Arch Allergy Immunol. 2009;150:192–204. doi: 10.1159/000218123. [DOI] [PubMed] [Google Scholar]
- Hensel A, Pabst R, Bunka S, Petzoldt K. Oral and aerosol immunization with viable or inactivated Actinobacillus pleuropneumoniae bacteria: antibody response to capsular polysaccharides in bronchoalveolar lavage fluids (BALF) and sera of pigs. Clin Exp Immunol. 1994;96:91–97. doi: 10.1111/j.1365-2249.1994.tb06236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensel A, van Leengoed LA, Szostak M, Windt H, Weissenböck H, Stockhofe-Zurwieden N, Katinger A, Stadler M, Ganter M, Bunka S, Pabst R, Lubitz W. Induction of protective immunity by aerosol or oral application of candidate vaccines in a dose-controlled pig aerosol infection model. J Biotechnol. 1996;44:171–181. doi: 10.1016/0168-1656(95)00150-6. [DOI] [PubMed] [Google Scholar]
- Hering BJ, Walawalkar N. Pig-to-nonhuman primate islet xenotransplantation. Transpl Immunol. 2009;21:81–86. doi: 10.1016/j.trim.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Holtmeier W, Käller J, Geisel W, Pabst R, Caspary WF, Rothkötter HJ. Development and compartmentalization of the porcine TCR delta repertoire at mucosal and extraintestinal sites: the pig as a model for analyzing the effects of age and microbial factors. J Immunol. 2002;169:1993–2002. doi: 10.4049/jimmunol.169.4.1993. [DOI] [PubMed] [Google Scholar]
- Hoshi N, Hashimoto Y, Kitagawa H, Kon Y, Kudo N. Histological and immunohistochemical studies on the architecture of lymph nodes in pig. Nihon Juigaku Zasshi. 1986;48:1097–1107. doi: 10.1292/jvms1939.48.1097. [DOI] [PubMed] [Google Scholar]
- Huang J, Guo X, Fan N, Song J, Zhao B, Ouyang Z, Liu Z, Zhao Y, Yan Q, Yi X, Schambach A, Frampton J, Esteban MA, Yang D, Yang H, Lai L. RAG1/2 knockout pigs with severe combined immunodeficiency. J Immunol. 2014;193:1496–1503. doi: 10.4049/jimmunol.1400915. [DOI] [PubMed] [Google Scholar]
- Iwase H, Kobayashi T. Current status of pig kidney xenotransplantation. Int J Surg. 2015;23:229–233. doi: 10.1016/j.ijsu.2015.07.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge EP, Hughes JM, Egan JJ, Maguire M, Molloy EL, O'Dea S. Anatomy and bronchoscopy of the porcine lung. A model for translational respiratory medicine. Am J Respir Cell Mol Biol. 2014;51:334–343. doi: 10.1165/rcmb.2013-0453TR. [DOI] [PubMed] [Google Scholar]
- Käser T, Pasternak JA, Delgado-Ortega M, Hamonic G, Lai K, Erickson J, Walker S, Dillon JR, Gerdts V, Meurens F. Chlamydia suis and Chlamydia trachomatis induce multifunctional CD4 T cells in pigs. Vaccine. 2017;35:91–100. doi: 10.1016/j.vaccine.2016.11.050. [DOI] [PubMed] [Google Scholar]
- Käser T, Renois F, Wilson HL, Cnudde T, Gerdts V, Dillon JR, Jungersen G, Agerholm JS, Meurens F. Contribution of the swine model in the study of human sexually transmitted infections. Infect Genet Evol. 2018;66:346–360. doi: 10.1016/j.meegid.2017.11.022. [DOI] [PubMed] [Google Scholar]
- Khazraee SP, Marashi SM, Kaviani M, Azarpira N. Stem cell-based therapies and tissue engineering of trachea as promising therapeutic methods in mustard gas exposed patients. Int J Organ Transplant Med. 2018;9:145–154. [PMC free article] [PubMed] [Google Scholar]
- Klymiuk N, Aigner B, Brem G, Wolf E. Genetic modification of pigs as organ donors for xenotransplantation. Mol Reprod Dev. 2010;77:209–221. doi: 10.1002/mrd.21127. [DOI] [PubMed] [Google Scholar]
- Klymiuk N, Böcker W, Schönitzer V, Bähr A, Radic T, Fröhlich T, Wünsch A, Keßler B, Kurome M, Schilling E, Herbach N, Wanke R, Nagashima H, Mutschler W, Arnold GJ, Schwinzer R, Schieker M, Wolf E. First inducible transgene expression in porcine large animal models. FASEB J. 2012;26:1086–1099. doi: 10.1096/fj.11-185041. [DOI] [PubMed] [Google Scholar]
- Kreuzer S, Rieger J, Strucken EM, Thaben N, Hünigen H, Nöckler K, Janczyk P, Plendl J, Brockmann GA. Characterization of CD4+ subpopulations and CD25+ cells in ileal lymphatic tissue of weaned piglets infected with Salmonella Typhimurium with or without Enterococus faecium feeding. Vet Immunol Immunopathol. 2014;158:143–155. doi: 10.1016/j.vetimm.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Krüger M, Zinne N, Biancosino C, Höffler K, Rajab TK, Waldmann KH, Jonigk D, Avsar M, Haverich A, Hoeltig D. Porcine pulmonary auto-transplantation for ex vivo therapy as a model for new treatment strategies. Internat Cardiovasc Thorac Surg. 2016;23:358–366. doi: 10.1093/icvts/ivw160. [DOI] [PubMed] [Google Scholar]
- Kumar RP, Kumar P, Singh G. Histology, histochemistry and scanning electron microscopy of nasopharyngeal tonsil in the pig. Indian J Vet Anat. 2015;27:29–32. [Google Scholar]
- Ladel S, Flamm J, Zadeh AS, Filzwieser D, Walter JC, Schlossbauer P, Kinscherf R, Lischka K, Luksch H, Schindowski K. Allogenic Fc Domain-Facilitated Uptake of IgG in Nasal Lamina Propria: Friend or Foe for Intranasal CNS Delivery? Pharmaceutics. 2018;10:107. doi: 10.3390/pharmaceutics10030107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Mattina JC, Kumagai N, Barth RN, Yamamoto S, Kitamura H, Moran, SC, Mezrich JD, Sachs D, Yamada K (2002) Transplantation 73:826-831. [DOI] [PubMed]
- Längin M, Mayr T, Reichart B, Michel S, Buchholz S, Guethoff S, Dashkevich A, Baehr A, Egerer S, Bauer A, Mihalj M, Panelli A, Issl L, Ying J, Fresch AK, Buttgereit I, Mokelke M, Radan J, Werner F, Lutzmann I, Steen S, Sjöberg T, Paskevicius A, Qiuming L, Sfriso R, Rieben R, Dahlhoff M, Kessler B, Kemter E, Kurome M, Zakhartchenko V, Klett K, Hinkel R, Kupatt C, Falkenau A, Reu S, Ellgass R, Herzog R, Binder U, Wich G, Skerra A, Ayares D, Kind A, Schönmann U, Kaup FJ, Hagl C, Wolf E, Klymiuk N, Brenner P, Abicht JM. Consistent success in life-supporting porcine cardiac xenotransplantation. Nature. 2018;564:430–433. doi: 10.1038/s41586-018-0765-z. [DOI] [PubMed] [Google Scholar]
- Law JX, Liau LL, Aminuddin BS, Ruszymah BH. Tissue-engineered trachea: a review. Int J Pediatr Otorhinolaryngol. 2016;91:55–63. doi: 10.1016/j.ijporl.2016.10.012. [DOI] [PubMed] [Google Scholar]
- Levast B, Berri M, Wilson HL, Meurens F, Salmon H. Development of gut immunoglobulin A production in piglet in response to innate and environmental factors. Dev Comp Immunol. 2014;44:235–244. doi: 10.1016/j.dci.2013.12.012. [DOI] [PubMed] [Google Scholar]
- Liebler-Tenorio EM, Pabst R. MALT structure and function in farm animals. Vet Res. 2006;37:257–280. doi: 10.1051/vetres:2006001. [DOI] [PubMed] [Google Scholar]
- Lorenzen E, Follmann F, Secher JO, Goericke-Pesch S, Hansen MS, Zakariassen H, Olsen AW, Andersen P, Jungersen G, Agerholm JS. Intrauterine inoculation of minipigs with Chlamydia trachomatis during diestrus establishes a longer lasting infection compared to vaginal inoculation during estrus. Microbes Infect. 2017;19:334–342. doi: 10.1016/j.micinf.2017.01.008. [DOI] [PubMed] [Google Scholar]
- Lunney JK, Ho CS, Wysocki M, Smith DM. Molecular genetics of the swine major histocompatibility complex, the SLA complex. Dev Comp Immunol. 2009;33:362–374. doi: 10.1016/j.dci.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Machuca TN, Cypel M, Bonato R, Yeung JC, Chun YM, Juvet S, Guan Z, Hwang DM, Chen M, Saito T, Harmantas C, Davidson BL, Waddell TK, Liu M, Keshavjee S. Safety and efficacy of ex vivo donor lung adenoviral IL-10 gene therapy in a large animal lung transplant survival model. Hum Gene Ther. 2017;28:757–765. doi: 10.1089/hum.2016.070. [DOI] [PubMed] [Google Scholar]
- Mair KH, Sedlak C, Käser T, Pasternak A, Levast B, Gerner W, Saalmüller A, Summerfield A, Gerdts V, Wilson HL, Meurens F. The porcine innate immune system: an update. Dev Comp Immunol. 2014;45:321–343. doi: 10.1016/j.dci.2014.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair KH, Stadler M, Talker SC, Forberg H, Storset AK, Müllebner A, Duvigneau JC, Hammer SE, Saalmüller A, Gerner W. Porcine CD3(+)NKp46(+) lymphocytes have NK-cell characteristics and are present in increased frequencies in the lungs of influenza-infected animals. Front Immunol. 2016;7:263. doi: 10.3389/fimmu.2016.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunari H, Kobayashi T, Watanabe M, Umeyama K, Nakano K, Kanai T, Matsuda T, Nagaya M, Hara M, Nakauchi H, Nagashima H. Transgenic pigs with pancreas-specific expression of green fluorescent protein. J Reprod Dev. 2014;60:230–237. doi: 10.1262/jrd.2014-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough KC, Summerfield A. Targeting the porcine immune system--particulate vaccines in the 21st century. Dev Comp Immunol. 2009;33:394–409. doi: 10.1016/j.dci.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestas J, Hughes CCW. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- Meyer W, Kacza J, Zschemisch NH, Godynicki S, Seeger J. Observations on the actual structural conditions in the stratum superficiale dermidis of porcine ear skin, with special reference to its use as model for human skin. Ann Anat. 2007;189:143–156. doi: 10.1016/j.aanat.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Mohiuddin MM, Reichart B, Byrne GW, McGregor CGA. Current status of pig heart xenotransplantation. Int J Surg. 2015;23:234–239. doi: 10.1016/j.ijsu.2015.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro-Riviere NA, Riviere J. The pig as a model for cutaneous pharmacology and toxicology research. In: Tumbleson ME, Schook LB, editors. Advances in Swine in biomedical Research. New York: Plenum Press; 1996. pp. 425–458. [Google Scholar]
- Murtaugh MP, Johnson CR, Xiao Z, Scamurra RW, Zhou Y. Species specialization in cytokine biology: Is interleukin-4 central to the TH1-TH2 paradigm in swine? Dev Comp Immunol. 2009;33:344–352. doi: 10.1016/j.dci.2008.06.014. [DOI] [PubMed] [Google Scholar]
- Niemann H, Petersen B. The production of multi-transgenic pigs: update and perspectives for xenotransplantation. Transgenic Res. 2016;25:361–374. doi: 10.1007/s11248-016-9934-8. [DOI] [PubMed] [Google Scholar]
- Ogunlade O, Ho JOY, Kalber TL, Hynds RE, Zhang E, Janes SM, Birchall MA, Butler CR, Beard P. Monitoring neovascularization and integration of decellularized human scaffolds using photoacoustic imaging. Photoacoustics. 2019;13:76–84. doi: 10.1016/j.pacs.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostedgaard LS, Meyerholz DK, Chen JH, Pezzulo AA, Karp PH, Rokhlina T, Ernst SE, Hanfland RA, Reznikov LR, Ludwig PS, Rogan MP, Davis GJ, Dohrn CL, Wohlford-Lenane C, Taft PJ, Rector MV, Hornick E, Nassar BS, Samuel M, Zhang Y, Richter SS, Uc A, Shilyansky J, Prather RS, McCray PB, Jr, Zabner J, Welsh MJ, Stoltz DA. The ΔF508 mutation causes CFTR misprocessing and cystic fibrosis-like disease in pigs. Sci Transl Med. 2011;3:74ra24. doi: 10.1126/scitranslmed.3001868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabst R. The spleen in lymphocyte migration. Immunol Today. 1988;9:43–45. doi: 10.1016/0167-5699(88)91258-3. [DOI] [PubMed] [Google Scholar]
- Pabst R. Compartmentalization and kinetics of lymphoid cells in the lung. Reg Immunol. 1990;3:62–71. [PubMed] [Google Scholar]
- Pabst R. Mucosa-associated lymphoid tissue in the lung: localization, numbers and dynamics in five different compartments. In: Busse WW, Holgate ST, editors. Asthma and Rhinitis. 2. Oxford: Blackwell Science; 2000. pp. 543–556. [Google Scholar]
- Pabst R. The bone marrow is not only a primary lymphoid organ: The critical role for T lymphocyte migration and housing of long-term memory plasma cells. Eur J Immunol. 2018;48:1096–1100. doi: 10.1002/eji.201747392. [DOI] [PubMed] [Google Scholar]
- Pabst R. The thymus is relevant in the migration of mature lymphocytes. Cell Tissue Res. 2019;376:19–24. doi: 10.1007/s00441-019-02994-z. [DOI] [PubMed] [Google Scholar]
- Pabst R, Binns RM (1986) Comparison of lymphocyte production and migration in pig lymph nodes, tonsils, spleen, bone marrow and thymus In: Swine in Biomedical Research (ed) Tumblesom ME. Plenum Press, New York, vol 3 pp. 1865–1871
- Pabst R, Binns RM. Lymphocytes migrate from the bronchoalveolar space to regional bronchial lymph nodes. Am J Respir Crit Care Med. 1995;151:495–499. doi: 10.1164/ajrccm.151.2.7842212. [DOI] [PubMed] [Google Scholar]
- Pabst R, Brandtzaeg P. Overview of the mucosal immune system structure. In: MC Donald TT, Blumberg ST, Smith PD, editors. Principles in mucosal immunology. 2. New York: Garland Sciences; 2020. pp. 3–19. [Google Scholar]
- Pabst R, Fritz FJ. Comparison of lymphocyte production in lymphoid organs and their compartments using the metaphase-arrest technique. Cell Tissue Res. 1986;245:423–30.a. doi: 10.1007/BF00213950. [DOI] [PubMed] [Google Scholar]
- Pabst R, Kamran D. Autotransplantation of splenic tissue. J Pediatr Surg. 1986;21:120–124. doi: 10.1016/s0022-3468(86)80062-8. [DOI] [PubMed] [Google Scholar]
- Pabst R, Nowara E. Organ Distribution and fate of newly formed splenic lymphocytes in the pig. Anat Rec. 1982;202:85–94. doi: 10.1002/ar.1092020110. [DOI] [PubMed] [Google Scholar]
- Pabst R, Nowara E. The emigration of lymphocytes from palatine tonsils after local labelling. Arch Otorhinolaryngol. 1984;240:7–13. doi: 10.1007/BF00464338. [DOI] [PubMed] [Google Scholar]
- Pabst R, Reinecke G. Proliferation of human lymphoid cells. Lymphocytopoiesis and cell cycle parameters in isolated perfused human spleens. Scand J Haematol. 1981;26:41–49. doi: 10.1111/j.1600-0609.1981.tb01622.x. [DOI] [PubMed] [Google Scholar]
- Pabst R, Rothkötter HJ. Regeneration of autotransplanted lymph node fragments. Cell Tissue Res. 1988;251:597–601. doi: 10.1007/BF00214008. [DOI] [PubMed] [Google Scholar]
- Pabst R, Trepel F. The predominant role of the spleen in lymphocyte recirculation. I Homing of lymphocytes to and release from the isolated perfused pig spleen. Cell Tissue Kinet. 1975;8:529–541. [PubMed] [Google Scholar]
- Pabst R, Trepel F. Quantitative evaluation of the total number and distribution of lymphocytes in young pigs. Blut. 1975;31:77–86. doi: 10.1007/BF01633723. [DOI] [PubMed] [Google Scholar]
- Pabst R, Trepel F. The predominant role of the spleen in lymphocyte recirculation. II. Pre- and postsplenectomy retransfusion studies in young pigs. Cell Tissue Kinet. 1976;9:179–189. [PubMed] [Google Scholar]
- Pabst R, Trepel F. Selective labeling of mesenteric lymph nodes: cell production and emigration of newly formed lymphocytes to other organs. Anat Rec. 1979;195:341–355. doi: 10.1002/ar.1091950208. [DOI] [PubMed] [Google Scholar]
- Pabst R, Westermann J. Regeneration of autotransplanted splenic tissue is not proportional to total weight and size of transplanted fragments. Pediatr Surg Int. 1987;2:161–163. [Google Scholar]
- Pabst R, Westermann J (1998) Lmyphocyte trafficking. In: Encyclopedia Immunology (Eds Delves Pf. Roidt) Academic Press, London. 2nd Ed. pp 1616–1620
- Pabst R, Heyes H, Rasche H, Schick P, Trepel F. Factor VIII coagulant activity and factor VIII-related antigen released from isolated perfused human spleens. Blut. 1977;34:27–30. doi: 10.1007/BF00997035. [DOI] [PubMed] [Google Scholar]
- Pabst R, Munz D, Trepel F. Splenic lymphocytopoiesis and migration pattern of splenic lymphocytes. Cell Immunol. 1977;33:33–44. doi: 10.1016/0008-8749(77)90132-0. [DOI] [PubMed] [Google Scholar]
- Pabst R, Munz D, Trepel F. Migration pattern of splenic lymphocytes after local labelling of the pig spleen with 3H-cytidine. Scand J Haematol. 1978;20:181–192. doi: 10.1111/j.1600-0609.1978.tb02445.x. [DOI] [PubMed] [Google Scholar]
- Pabst R, Reilmann H, Neuhaus P. Selective labelling of lymphoid tissues by extracorporeal perfusion. Immunol Methods. 1980;33:23–32. doi: 10.1016/0022-1759(80)90079-4. [DOI] [PubMed] [Google Scholar]
- Pabst R, Kamran D, Creutzig H. Splenic regeneration and blood flow after ligation of the splenic artery or partial splenectomy. Am J Surg. 1984;147:382–386. doi: 10.1016/0002-9610(84)90172-7. [DOI] [PubMed] [Google Scholar]
- Pabst R, Binns RM, Licence ST, Peter M. Evidence of a selective major vascular marginal pool of lymphocytes in the lung. Am Rev Respir Dis. 1987;136:1213–1218. doi: 10.1164/ajrccm/136.5.1213. [DOI] [PubMed] [Google Scholar]
- Pabst R, Geist M, Rothkötter HJ, Fritz FJ. Postnatal development and lymphocyte production of jejunal and ileal Peyer's patches in normal and gnotobiotic pigs. Immunology. 1988;64:539–544. [PMC free article] [PubMed] [Google Scholar]
- Pabst R, Westermann J, Rothkötter HJ. Immunoarchitecture of regenerated splenic and lymph node transplants. Int Rev Cytol. 1991;128:215–260. doi: 10.1016/s0074-7696(08)60500-8. [DOI] [PubMed] [Google Scholar]
- Piroiou-Guzylack L, Salmon H. Membrane markers of the immune cells in swine: an update. Vet Res. 2008;39:54. doi: 10.1051/vetres:2008030. [DOI] [PubMed] [Google Scholar]
- Reilmann H, Pabst R, Creutzig H. Regeneration and function of autologous splenic grafts in pigs. Eur Surg Res. 1983;15:168–175. doi: 10.1159/000128349. [DOI] [PubMed] [Google Scholar]
- Reutner K, Leitner J, Essler SE, Witter K, Patzl M, Steinberger P, Saalmüller A, Gerner W. Porcine CD27: identification, expression and functional aspects in lymphocyte subsets in swine. Dev Comp Immunol. 2012;38:321–331. doi: 10.1016/j.dci.2012.06.011. [DOI] [PubMed] [Google Scholar]
- Reutner K, Leitner J, Müllebner A, Ladinig A, Essler SE, Duvigneau JC, Ritzmann M, Steinberger P, Saalmüller A, Gerner W. CD27 expression discriminates porcine T helper cells with functionally distinct properties. Vet Res. 2013;44:18. doi: 10.1186/1297-9716-44-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice P, Brown RF, Lam DG, Chilcott RP, Bennett NJ. Dermabrasion--a novel concept in the surgical management of sulphur mustard injuries. Burns. 2000;26:34–40. doi: 10.1016/s0305-4179(99)00096-0. [DOI] [PubMed] [Google Scholar]
- Rieger J, Janczyk P, Hünigen H, Neumann K, Plendl J. Intraepithelial lymphocyte numbers and histomorphological parameters in the porcine gut after Enterococcus faecium NCIMB 10415 feeding in a Salmonella Typhimurium challenge. Vet Immunol Immunopathol. 2015;164:40–50. doi: 10.1016/j.vetimm.2014.12.013. [DOI] [PubMed] [Google Scholar]
- Riquet M, Souilamas R, Hubsch JP, Brière J, Colomer S, Hidden G. Lymphatic drainage of heart and lungs: comparison between pig and man. Surg Radiol Anat. 2000;22:47–50. doi: 10.1007/s00276-000-0047-x. [DOI] [PubMed] [Google Scholar]
- Rogers CS, Hao Y, Rokhlina T, Samuel M, Stoltz DA, Li Y, Petroff E, Vermeer DW, Kabel AC, Yan Z, Spate L, Wax D, Murphy CN, Rieke A, Whitworth K, Linville ML, Korte SW, Engelhardt JF, Welsh MJ, Prather RS. Production of CFTR-null and CFTR-DeltaF508 heterozygous pigs by adeno-associated virus-mediated gene targeting and somatic cell nuclear transfer. J Clin Invest. 2008;118:1571–1577. doi: 10.1172/JCI34773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers CS, Stoltz DA, Meyerholz DK, Ostedgaard LS, Rokhlina T, Taft PJ, Rogan MP, Pezzulo AA, Karp PH, Itani OA, Kabel AC, Wohlford-Lenane CL, Davis GJ, Hanfland RA, Smith TL, Samuel M, Wax D, Murphy CN, Rieke A, Whitworth K, Uc A, Starner TD, Brogden KA, Shilyansky J, McCray PB, Jr, Zabner J, Prather RS, Welsh MJ. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science. 2008;321:1837–1841. doi: 10.1126/science.1163600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers CS, Abraham WM, Brogden KA, Engelhardt JF, Fisher JT, McCray PB, Jr, McLennan G, Meyerholz DK, Namati E, Ostedgaard LS, Prather RS, Sabater JR, Stoltz DA, Zabner J, Welsh MJ. The porcine lung as a potential model for cystic fibrosis. Am J Phys Lung Cell Mol Phys. 2008;295:L240–L263. doi: 10.1152/ajplung.90203.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman M, Gjorgjimajkoska O, Neil D, Nair S, Colah S, Parmar J, Tsui S. Comparison between cellular and acellular perfusates for ex vivo lung perfusion in a porcine model. J Heart Lung Transplant. 2015;34:978–987. doi: 10.1016/j.healun.2015.03.023. [DOI] [PubMed] [Google Scholar]
- Roselli M, Pieper R, Rogel-Gaillard C, de Vires H, Bailey M, Smidt H, Lauridsen C. Immunomodulating effects of probiotics for microbiota modulation, gut health and disease resistance in pigs. Anim Feed Sci Technol. 2017;233:104–119. [Google Scholar]
- Rothkötter HJ. Anatomical particularities of the porcine immune system--a physician's view. Dev Comp Immunol. 2009;33:267–272. doi: 10.1016/j.dci.2008.06.016. [DOI] [PubMed] [Google Scholar]
- Rothkötter HJ, Pabst R. Autotransplantation of lymph node fragments Structure and function of regenerated tissue. Scand J Plast Reconstr Surg Hand Surg. 1990;24:101–105. doi: 10.3109/02844319009004528. [DOI] [PubMed] [Google Scholar]
- Rothkötter HJ, Zimmermann HJ, Pabst R (1990) Size of jejunal Peyer's patches and migration of lymphocyte subsets in pigs after resection or transposition of the continuous ileal Peyer's patch. [DOI] [PubMed]
- Rothkötter HJ, Ulbrich H, Pabst R. The postnatal development of gut lamina propria lymphocytes: number, proliferation, and T and B cell subsets in conventional and germ-free pigs. Pediatr Res. 1991;29:237–242. doi: 10.1203/00006450-199103000-00004. [DOI] [PubMed] [Google Scholar]
- Rothkötter HJ, Huber T, Barman NN, Pabst R. Lymphoid cells in afferent and efferent intestinal lymph: lymphocyte subpopulations and cell migration. Clin Exp Immunol. 1993;92:317–322. doi: 10.1111/j.1365-2249.1993.tb03398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothkötter HJ, Kirchhoff T, Pabst R. Lymphoid and non-lymphoid cells in the epithelium and lamina propria of intestinal mucosa of pigs. Gut. 1994;35:1582–1589. doi: 10.1136/gut.35.11.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothkötter HJ, Hriesik C, Pabst R. More newly formed T than B lymphocytes leave the intestinal mucosa via lymphatics. Eur J Immunol. 1995;25:866–869. doi: 10.1002/eji.1830250336. [DOI] [PubMed] [Google Scholar]
- Rothkötter HJ, Hriesik C, Barman NN, Pabst R. B and also T lymphocytes migrate via gut lymph to all lymphoid organs and the gut wall, but only IgA+ cells accumulate in the lamina propria of the intestinal mucosa. Eur J Immunol. 1999;29:327–333. doi: 10.1002/(SICI)1521-4141(199901)29:01<327::AID-IMMU327>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Rothkötter HJ, Sowa E, Pabst R. The pig as a model of developmental immunology. Hum Exp Toxicol. 2002;21:533–536. doi: 10.1191/0960327102ht293oa. [DOI] [PubMed] [Google Scholar]
- Rupp J, Hadamitzky C, Henkenberens C, Christiansen H, Steinmann D, Bruns F. Frequency and risk factors for arm lymphedema after multimodal breast-conserving treatment of nodal positive breast Cancer - a long-term observation. Radiat Oncol. 2019;14:39. doi: 10.1186/s13014-019-1243-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saalmüller A, Gerner W (2016) The immune system of swine. Inst. of Immunol. Department of Patholbiol. Uni of Veterinary medicine Vienna, Austria
- Sang Y, Blecha F. Porcine host defense peptides: expanding repertoire and functions. Dev Comp Immunol. 2009;33:334–343. doi: 10.1016/j.dci.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Sang Y, Ruchala P, Lehrer RI, Ross CR, Rowland RR, Blecha F. Antimicrobial host defense peptides in an arteriviral infection: differential peptide expression and virus inactivation. Viral Immunol. 2009;22:235–242. doi: 10.1089/vim.2009.0005. [DOI] [PubMed] [Google Scholar]
- Sang Y, Rowland RR, Blecha F. Interaction between innate immunity and porcine reproductive and respiratory syndrome virus. Anim Health Res Rev. 2011;12:149–167. doi: 10.1017/S1466252311000144. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Pabst R, Rothkötter HJ. The unique ultrastructure of high-endothelial venules in inguinal lymph nodes of the pig. Cell Tissue Res. 1994;276:85–90. doi: 10.1007/BF00354787. [DOI] [PubMed] [Google Scholar]
- Schindewolffs L, Breves G, Buettner M, Hadamitzky C, Pabst R. VEGF-C improves regeneration and lymphatic reconnection of transplanted autologous lymph node fragments: An animal model for secondary lymphedema treatment. Immun Inflamm Dis. 2014;2:152–161. doi: 10.1002/iid3.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siepert B, Reinhardt N, Kreuzer S, Bondzio A, Twardziok S, Brockmann G, Nöckler K, Szabó I, Janczyk P, Pieper R, Tedin K. Enterococcus faecium NCIMB 10415 supplementation affects intestinal immune-associated gene expression in post-weaning piglets. Vet Immunol Immunopathol. 2014;157:65–77. doi: 10.1016/j.vetimm.2013.10.013. [DOI] [PubMed] [Google Scholar]
- Simianer H, Köhn F. Genetic management of the Göttingen Minipig population. J Pharmacol Toxicol Methods. 2010;62:221–226. doi: 10.1016/j.vascn.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Sinkorova J, Stepanova K, Butler JE, Sinkora M. T cells in swine completely rearrange immunoglobulin heavy chain genes. Dev Comp Immunol. 2019;99:103396. doi: 10.1016/j.dci.2019.103396. [DOI] [PubMed] [Google Scholar]
- Sommer T, Buettner M, Bruns F, Breves G, Hadamitzky C, Pabst R. Improved regeneration of autologous transplanted lymph node fragments by VEGF-C treatment. Anat Rec (Hoboken) 2012;295:786–791. doi: 10.1002/ar.22438. [DOI] [PubMed] [Google Scholar]
- Sommer T, Meier M, Bruns F, Pabst R, Breves G, Hadamitzky C. Quantification of lymphedema in a rat model by 3D-active contour segmentation by magnetic resonance imaging. Lymphat Res Biol. 2012;10:25–29. doi: 10.1089/lrb.2011.0010. [DOI] [PubMed] [Google Scholar]
- Steinmeyer J, Becker S, Avsar M, Salman J, Höffler K, Haverich A, Warnecke G, Mühlfeld C, Ochs M, Schnapper-Isl A. Cellular and acellular ex vivo lung perfusion preserve functional lung ultrastructure in a large animal model: a stereological study. Respir Res. 2018;19:238. doi: 10.1186/s12931-018-0942-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltz DA, Meyerholz DK, Pezzulo AA, Ramachandran S, Rogan MP, Davis GJ, Hanfland RA, Wohlford-Lenane C, Dohrn CL, Bartlett JA, Nelson GA, 4th, Chang EH, Taft PJ, Ludwig PS, Estin M, Hornick EE, Launspach JL, Samuel M, Rokhlina T, Karp PH, Ostedgaard LS, Uc A, Starner TD, Horswill AR, Brogden KA, Prather RS, Richter SS, Shilyansky J, McCray PB, Jr, Zabner J, Welsh MJ. Cystic fibrosis pigs develop lung disease and exhibit defective bacterial eradication at birth. Sci Transl Med. 2010;2:29ra31. doi: 10.1126/scitranslmed.3000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerfield A, McCullough KC. The porcine dendritic cell family. Dev Comp Immunol. 2009;33:299–309. doi: 10.1016/j.dci.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerfield A, Meurens F, Ricklin ME. The immunology of the porcine skin and its value as a model for human skin. Mol Immunol. 2015;66:14–21. doi: 10.1016/j.molimm.2014.10.023. [DOI] [PubMed] [Google Scholar]
- Thielke KH, Pabst R, Rothkötter HJ. Quantification of proliferating lymphocyte subsets appearing in the intestinal lymph and the blood. Clin Exp Immunol. 1999;117:277–284. doi: 10.1046/j.1365-2249.1999.00974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thielke KH, Hoffmann-Moujahid A, Weisser C, Waldkirch E, Pabst R, Holtmeier W, Rothkötter HJ. Proliferating intestinal gamma/delta T cells recirculate rapidly and are a major source of the gamma/delta T cell pool in the peripheral blood. Eur J Immunol. 2003;33:1649–1656. doi: 10.1002/eji.200323442. [DOI] [PubMed] [Google Scholar]
- Tlaskalova-Hogenova H, Mandel L, Trebichavsky I, Kovaru F, Barot R, Sterzl J. Development of immune responses in early pig ontogeny. Vet Immunol Immunopathol. 1994;43:135–142. doi: 10.1016/0165-2427(94)90129-5. [DOI] [PubMed] [Google Scholar]
- Tumbleson ME, editor. Swine in biomedical research. New York: Plenum press; 1986. p. 3. [Google Scholar]
- Tumbleson ME, Schook LB. Advances in swine in biomedical research. New York: Planum Press; 1996. [Google Scholar]
- Uenishi H, Shinkai H. Porcine Toll-like receptors: the front line of pathogen monitoring and possible implications for disease resistance. Dev Comp Immunol. 2009;33:353–361. doi: 10.1016/j.dci.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Vamathevan JJ, Hall MD, Hasan S, Woollard PM, Xu M, Yang Y, Li X, Wang X, Kenny S, Brown JR, Huxley-Jones J, Lyon J, Haselden J, Min J, Sanseau P. Minipig and beagle animal model genomes aid species selection in pharmaceutical discovery and development. Toxicol Appl Pharmacol. 2013;270:149–157. doi: 10.1016/j.taap.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Waldmann KH. Gnotobiotic delivery and raising of piglets of the Göttinger miniature swine breed. Tierarztl Prax Suppl. 1988;3:84–92. [PubMed] [Google Scholar]
- Warnecke G, Moradiellos J, Tudorache I, Kühn C, Avsar M, Wiegmann B, Sommer W, Ius F, Kunze C, Gottlieb J, Varela A, Haverich A. Normothermic perfusion of donor lungs for preservation and assessment with the Organ Care System Lung before bilateral transplantation: a pilot study of 12 patients. Lancet. 2012;380:1851–1858. doi: 10.1016/S0140-6736(12)61344-0. [DOI] [PubMed] [Google Scholar]
- Wang S, Yang J, Zhang B, Wu K, Yang LC, Zhang J, Zhang C, Rajput SA, Zhang N, Sun L, Qi D. Toxins Deoxynivalenol impairs porcine intestinal host defense peptide expression in weaned pnd IPEC-J2 cells. Toxins. 2018;541:10. doi: 10.3390/toxins10120541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Nakano K, Matsunari H, Matsuda T, Maehara M, Kanai T, Kobayashi M, Matsumura Y, Sakai R, Kuramoto M, Hayashida G, Asano Y, Takayanagi S, Arai Y, Umeyama K, Nagaya M, Hanazono Y, Nagashima H. Generation of interleukin-2 receptor gamma gene knockout pigs from somatic cells genetically modified by zinc finger nuclease-encoding mRNA. PLoS One. 2013;8(10):e76478. doi: 10.1371/journal.pone.0076478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler GC. Pulmonary intravascular macrophages in domestic animal species: review of structural and functional properties. Am J Anat. 1988;181:217–234. doi: 10.1002/aja.1001810302. [DOI] [PubMed] [Google Scholar]
- Wöchtl B, Gunzer F, Gerner W, Gasse H, Koch M, Bagó Z, Ganter M, Weissenböck H, Dinhopl N, Coldewey SM, von Altrock A, Waldmann KH, Saalmüller A, Zimmermann K, Steinmann J, Kehrmann J, Klein-Hitpass L, Blom J, Ehricht R, Engelmann I, Hennig-Pauka I. Comparison of clinical and immunological findings in gnotobiotic piglets infected with Escherichia coli O104:H4 outbreak strain and EHEC O157:H7. Gut Pathog. 2017;9:30. doi: 10.1186/s13099-017-0179-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Huang J, Zhao J. Genome editing revolutionize the creation of genetically modified pigs for modeling human diseases. Hum Genet. 2016;135:1093–1105. doi: 10.1007/s00439-016-1710-6. [DOI] [PubMed] [Google Scholar]
- Zinne N, Krueger M, Hoeltig D, Tuemmler B, Boyle EC, Biancosino C, Hoeffler K, Braubach P, Rajab TK, Ciubotaru A, Rohde J, Waldmann KH, Haverich A. Treatment of infected lungs by ex vivo perfusion with high dose antibiotics and autotransplantation: A pilot study in pigs. PLoS One. 2018;13:e0193168. doi: 10.1371/journal.pone.0193168. [DOI] [PMC free article] [PubMed] [Google Scholar]


