Abstract
Introduction
Little has been published on respiratory syncytial virus (RSV) among Medicare patients at high risk (HR) of RSV complications due to age or comorbidity.
Methods
Adult patients (at least 18 years of age) with at least 1 diagnostic code for RSV were identified using the 5% US Medicare database from 2011 through 2015. Patients were required to have continuous health plan enrollment for 180 days pre- and 180 days post-RSV diagnosis (baseline and follow-up periods, respectively). HR was defined as diagnosis of chronic lung disease, congestive heart failure, or weakened immune system for 180 days during the baseline period. Patients were categorized as initially hospitalized if hospitalized within 1 day of RSV diagnosis. Logistic regression models were developed to determine predictors of initial hospitalization. Healthcare utilization and costs for 180 days pre- and post-RSV diagnosis were compared.
Results
The study included 756 HR patients who were initially hospitalized with RSV diagnoses. Among these, 61.7% were diagnosed in the emergency department vs 15.3% in a physician’s office, with hypertension (76.3%), chronic obstructive pulmonary disease (COPD) (53.7%), and high cholesterol (52.0%) observed as the most prevalent comorbidities. Of these, COPD, congestive heart failure, chronic kidney disease, and previous evidence of pneumonia were significant predictors of hospitalization. Other significant predictors of hospitalization included older age, hematological malignancies, stroke, and baseline healthcare resource use. Among both HR and non-HR hospitalized patients, there was a significant increase in healthcare resource utilization following hospitalization, including the number of inpatient admissions and longer hospital stays post-RSV diagnosis. The total mean all-cause healthcare costs among HR hospitalized patients increased by $9210 per patient (p < 0.0001) post-RSV diagnosis.
Conclusion
Hospitalized Medicare beneficiaries with RSV infections pose a significant healthcare burden as compared with non-hospitalized patients, mainly driven by higher comorbidity, higher likelihood of multiple inpatient admissions, and costly medical interventions.
Keywords: Infectious disease, Medicare, Predictors of hospitalization, Respiratory syncytial virus
Key Summary Points
| Why carry out this study? |
| There is a gap in the literature regarding the burden of respiratory syncytial virus (RSV) among elderly Medicare patients who have a high risk of RSV complications due to age or comorbidity. |
| The purpose of this study was to evaluate predictors of hospitalization and quantify the clinical and economic burden of RSV among Medicare patients in the USA. |
| What was learned from the study? |
| Hospitalized elderly patients with RSV had higher associated healthcare resource utilization, mainly driven by a higher proportion of comorbidities, higher likelihood of multiple inpatient admissions, and costly medical interventions as compared with patients who were never hospitalized. |
| The study may help providers to identify patients at highest risk for hospitalization, morbidity, and mortality and take appropriate measures regarding RSV management. |
Introduction
Human respiratory syncytial virus (RSV) is common among elderly patients and can cause lower respiratory tract infection (LRTI) including pneumonia, exacerbate congestive heart failure, asthma, or chronic obstructive pulmonary disease (COPD), leading to hospitalization [1]. According to the Centers for Disease Control and Prevention (CDC), 177,000 hospitalizations yearly have been attributed to RSV infections alone among adults aged over 65 years [2]. In general, patients aged 65 years or older had 3–5 times higher hospitalization rates with longer length of stay (LOS), and twice as many emergency department (room) (ER) and ambulatory visits compared to those aged 18–49 years. RSV is also the cause for LRTI in many immunocompromised patients, such as hematopoietic stem cell transplant (HSCT) and solid organ transplant (SOT) recipients, including lung transplant recipients, and those with leukemia, lymphoma, or other cancer patients receiving chemotherapy, and those with advanced HIV [3].
At the time of initial presentation to a healthcare provider, it is difficult to differentiate RSV from other respiratory virus infections on the basis of clinical parameters alone; therefore, it is important for healthcare providers to identify the disease so that management of RSV infection can be more effective [4]. Worldwide, no specific treatment for RSV has received marketing authorization for use in adults; therefore, the standard of care is supportive treatment, such as intravenous fluid hydration and oxygen supplementation. The only treatment options with antiviral activity are aerosolized, intravenous, or orally administered ribavirin [5–7]. Inhaled ribavirin has limited evidence of effectiveness and carries potential risks such as teratogenicity, bronchospasm, or anemia [8] for both the patient and their care providers. While approved in some countries for severely ill young children, the treatment is not generally recommended in pediatric patients [6, 9]. Ribavirin has been used in adult patients, primarily among those who are immunocompromised, but supporting data are limited [10, 11].
The CDC has classified adult patients aged 65 years and older and those with chronic cardiac or pulmonary disease or weakened immune systems as high-risk patients who are more likely to experience serious complications due to RSV-related infection and have a higher risk of mortality [12]. Elderly patients are also more prone to RSV infections due to their decreased immunologic response to the virus [13]. The morbidity, mortality, and economic burden of RSV may therefore also be driven through the exacerbation of underlying illness as well as the infection itself [14, 15]. Current research on healthcare resource utilization of RSV-related infection focuses mainly on either pediatric or adult patients with private healthcare insurance [16–18]. Less has been published on healthcare utilization and burden of disease among elderly Medicare patients diagnosed with RSV-related infections in the USA. To address these data gaps, the purpose of this study was to identify characteristics of adult RSV-infected patients in the inpatient and outpatient setting as well as predictors of hospitalization, and to describe the current clinical and economic burden of RSV among elderly Medicare patients in the USA.
Methods
Data Source
This real-world observational study was conducted using the Medicare 5% national sample administrative database from January 2011 through December 2015. All patient identifiers in the database have been fully encrypted; therefore, neither institutional review board approval nor consent was necessary for this study, as it was conducted in the USA with depersonalized claims data and does not meet criteria for studies with human participants; it is therefore exempt from approval per the provision for unidentifiable personal data in the Federal Policy for the Protection of Human Subjects (1991).
The Medicare claims data files used for this study included inpatient and outpatient (Parts A and B), Medicare carrier, prescription (Part D) drug events, skilled nursing facility (SNF), home health agency (HHA), hospice, durable medical equipment (DME), and the Medicare denominator file, which contains demographic and enrollment information of Medicare beneficiaries.
Patient Selection
Patients with a medical claim for RSV diagnosis (International Classification of Disease, Ninth Revision, Clinical Modification [ICD-9-CM] codes 079.6, 466.11, 480.01 and ICD-10-CM codes B97.4, J20.5, J12.1, J21.0) were identified between July 1, 2011 and June 30, 2015. The date of the first observed RSV diagnosis during this period was designated as the index date.
Patients were required to be aged 18 years or older at the time of diagnosis, with continuous Medicare medical and pharmacy benefits for at least 180 days prior to the index date (baseline period) and at least 180 days after the index date (follow-up period). Patients who died during the follow-up period were also included in the study. Patients were excluded if they had an RSV diagnosis during the baseline period or an influenza or human metapneumovirus (hMPV) diagnosis during the study period. Patients were categorized as hospitalized if they were diagnosed with RSV during hospitalization or hospitalized within 1 day of RSV diagnosis. The hospital admission date was captured as the start of the index hospitalization.
Among patients who were hospitalized, participants were further categorized as high-risk and non-high-risk patients. High-risk patients were identified if they met any of the following criteria: diagnosis of chronic lung disease (including asthma and COPD), prior pneumonia, congestive heart failure (CHF), or immune compromise on or within 180 days before the date of RSV diagnosis. A patient was considered to be immunocompromised if they had evidence of SOT, HSCT, or hematological malignancies (leukemia, lymphoma, and plasma cell neoplasms). All conditions were identified using ICD-9-CM and ICD-10-CM codes. The non-high-risk patients were the remaining hospitalized patients in the study population.
Patients were categorized as outpatients if they were diagnosed with RSV in the outpatient setting and not hospitalized within 1 day of diagnosis. Patients diagnosed in the outpatient setting were also categorized into high-risk and non-high-risk cohorts.
Study Variables
Baseline patient demographics including age, sex, US geographic region, comorbidities, and setting of diagnosis during the 180 days prior to the index date (baseline period) were assessed for all patients. The diagnosis setting included ER (combined ER and/or inpatient setting), physician’s office, or other Medicare settings (DME, HHA, hospice, SNF). Among outpatients, patients were further classified as later hospitalized (diagnosed in the outpatient setting and hospitalized at least 2 days from index diagnosis) or never hospitalized. Comorbidities including HSCT, SOT, hematological malignancies, stroke, osteoporosis, anxiety, asthma, cancer, depression, osteoarthritis, chronic kidney disease (CKD), coronary artery disease (CAD), CHF, diabetes, high cholesterol, COPD, hypertension, and previous evidence of pneumonia were identified using ICD-9-CM or ICD-10-CM codes during the baseline period (6 months prior to the index date).
Complications, all-cause mortality, death during inpatient stay, and 30-day readmission rates during the follow-up period were evaluated. Complications of interest—respiratory failure, chest pain, hypoxia, cardiac arrhythmia, cough, myocardial infarction, dyspnea, pneumonia, lower and upper respiratory tract infection (excluding influenza, RSV, and hMPV)—were identified using ICD-9-CM and ICD-10-CM codes. The proportion of patients who were discharged to an SNF as well as LOS in an SNF were also evaluated.
All-cause healthcare utilization and costs during the pre- (baseline) and post-index (follow-up) periods were analyzed, including hospital LOS, number of office visits, pharmacy use, ER, inpatient (and hospital LOS across all hospitalization), and outpatient visits. Costs were adjusted to 2015 US dollars using the medical care component of the Consumer Price Index (CPI).
Statistical Methods
All baseline and outcome variables were analyzed descriptively. Percentages and counts were provided for categorical variables. Means and standard deviations (SDs) were computed for continuous variables. Healthcare costs and utilizations were analyzed descriptively and compared between 180 days pre- and post-RSV diagnosis. Bivariate comparison between pre- and post-index date periods were made using a paired t test to evaluate the statistical significance for continuous variables, including average number of resource utilization and costs.
Multivariate logistic regression was conducted to examine potential predictors of hospitalization among hospitalized patients vs those who were never hospitalized. Purposeful model selection was used to identify variables to be included in the logistic regression. First, all independent variables with p < 0.25 in bivariate testing were initially included in the model. Secondly, variables with p > 0.10 were dropped from the model sequentially unless they were identified as confounders (i.e., variables that, when dropped from the model, resulted in at least a 20% change in parameter estimates for 1 or more of the other variables, when compared to the original model). The final model included confounders, variables that had p ≤ 0.10, and high-risk conditions. Covariates in the final model included demographics, comorbidities, previous evidence of pneumonia, number of conditions, and the number of inpatient and ER visits during the baseline period.
Statistical analyses were conducted using the Statistical Analysis System (SAS) v.9.3. (Cary, North Carolina, USA). The threshold for p value significance was set at α-level 0.05 for pre–post analysis and logistic regression.
Results
Patient Population
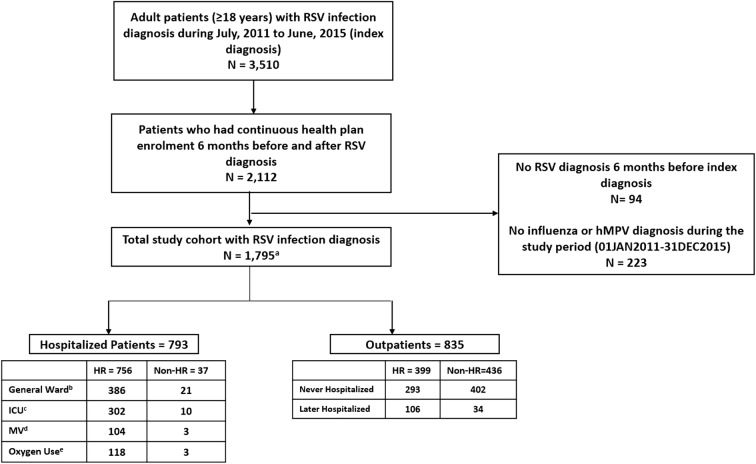
A total of 3510 patients diagnosed with RSV were identified from the Medicare 5% national sample administrative database. Figure 1 shows the selection criteria to identify patients with RSV. After the inclusion and exclusion criteria were applied, the final analytical sample comprised 1795 patients, including 793 (44.2%) patients who were hospitalized (within 1 day of RSV diagnosis), 835 (46.5%) diagnosed in the outpatient setting (not hospitalized within 1 day of RSV diagnosis), and 167 (9.3%) diagnosed in other Medicare settings (DME, HHA, hospice, SNF). The study population included 756 (42.1%) high-risk patients who were initially hospitalized and 37 (2.1%) non-high-risk patients who were hospitalized. Among patients diagnosed in the outpatient setting, 399 (47.8%) were classified as high-risk patients, whereas 436 (52.2%) were classified as non-high-risk patients. Among patients diagnosed in the outpatient setting, 140 (16.8%) were later hospitalized (i.e., hospitalized after 1 day of RSV diagnosis) and 695 (83.2%) of patients were never hospitalized during the follow-up period (Fig. 1).
Fig. 1.
Flow chart for patient inclusion criteria. HR high risk, hMPV human metapneumovirus, ICU intensive care unit, MV mechanical ventilation, RSV respiratory syncytial virus. aThe total study cohort includes patients diagnosed in inpatient, outpatient, or other Medicare settings. bPatients who did not use services in the ICU or MV during the index hospitalization. cPatients who used services in the ICU during index hospitalization; ICU and MV are not mutually exclusive. dPatients who used MV during index hospitalization; ICU and MV are not mutually exclusive. ePatients who used supplemental oxygen during index hospitalization
Baseline Characteristics
Patient characteristics and diagnosis setting are summarized in Table 1. Among hospitalized high-risk patients, 61.7% were diagnosed in the ER setting, whereas 15.3% and 23.0% were diagnosed in a physician’s office or other Medicare settings, respectively. On average, high-risk hospitalized patients were older (mean age 77 years; SD 13.4 years) than those who were not hospitalized (mean age 74 years; SD 14.5 years). Most of the total sample population were female patients residing in the southern US region. Of high-risk patients initially hospitalized, hypertension (76.3%), COPD (53.7%), high cholesterol (52.0%), diabetes (41.1%), CHF (41.1%), CAD (39.8%), CKD (31.0%), and previous evidence of pneumonia (42.3%) were among the most prevalent comorbid conditions during the baseline period. These conditions were also the most common comorbidities occurring among high-risk patients in the outpatient cohort (Table 1).
Table 1.
Descriptive baseline characteristics for patients diagnosed with RSV
| Hospitalized high-riska | Hospitalized non-high-risk | Outpatient high-risk | Outpatient non-high-risk | |
|---|---|---|---|---|
| N = 756 | N = 37 | N = 399 | N = 436 | |
| Age, mean (SD) | 77.1 (13.4) | 71.9 (17.1) | 73.6 (14.5) | 71.1 (12.3) |
| Age group (years), n (%) | ||||
| < 65 | 112 (14.8%) | 11 (29.7%) | 83 (20.8%) | 93 (21.3%) |
| 65–74 | 167 (22.1%) | 8 (21.6%) | 111 (27.8%) | 177 (40.6%) |
| 75–84 | 222 (29.4%) | 10 (27.0%) | 107 (26.8%) | 111 (25.5%) |
| ≥ 85 | 255 (33.7%) | 8 (21.6%) | 98 (24.6%) | 55 (12.6%) |
| Sex, n (%) | ||||
| Male | 267 (35.3%) | 9 (24.3%) | 123 (30.8%) | 164 (37.6%) |
| Female | 489 (64.7%) | 28 (75.7%) | 276 (69.2%) | 272 (62.4%) |
| US geographic region, n (%) | ||||
| Northeast | 221 (29.2%) | 11 (29.7%) | 94 (23.6%) | 126 (28.9%) |
| North central | 189 (25.0%) | 9 (24.3%) | 82 (20.6%) | 73 (16.7%) |
| South | 264 (34.9%) | 10 (27.0%) | 143 (35.8%) | 145 (33.3%) |
| West | 80 (10.6%) | 7 (18.9%) | 72 (18.0%) | 89 (20.4%) |
| Unknown | 2 (0.3%) | 0 (0.0%) | 8 (2.0%) | 3 (0.7%) |
| Diagnosis setting, n (%) | ||||
| ER settingb | 466 (61.7%) | 37 (100.0%) | 35 (8.8%) | 9 (2.1%) |
| Outpatient setting | 116 (15.3%) | 0 (0.0%) | 364 (91.2%) | 427 (97.9%) |
| Other settingsc | 174 (23.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Comorbidities, n (%) | ||||
| Anxiety | 131 (17.3%) | 7 (18.9%) | 44 (11.0%) | 17 (3.9%) |
| Asthma | 139 (18.4%) | 0 (0.0%) | 97 (24.3%) | 0 (0.0%) |
| Cancer | 147 (19.4%) | 10 (27.0%) | 54 (13.5%) | 34 (7.8%) |
| Chronic obstructive pulmonary disease | 406 (53.7%) | 0 (0.0%) | 253 (63.4%) | 0 (0.0%) |
| Congestive heart failure | 311 (41.1%) | 0 (0.0%) | 109 (27.3%) | 0 (0.0%) |
| Coronary artery disease | 301 (39.8%) | 6 (16.2%) | 102 (25.6%) | 62 (14.2%) |
| Depression | 200 (26.5%) | 7 (18.9%) | 79 (19.8%) | 36 (8.3%) |
| Diabetes | 311 (41.1%) | 12 (32.4%) | 142 (35.6%) | 123 (28.2%) |
| High cholesterol | 393 (52.0%) | 17 (45.9%) | 204 (51.1%) | 173 (39.7%) |
| Hypertension | 577 (76.3%) | 19 (51.4%) | 273 (68.4%) | 217 (49.8%) |
| Chronic kidney disease | 234 (31.0%) | 10 (27.0%) | 46 (11.5%) | 12 (2.8%) |
| Osteoarthritis | 219 (29.0%) | 8 (21.6%) | 110 (27.6%) | 71 (16.3%) |
| Osteoporosis | 100 (13.2%) | 1 (2.7%) | 39 (9.8%) | 46 (10.6%) |
| Stroke | 82 (10.8%) | 1 (2.7%) | 18 (4.5%) | 9 (2.1%) |
| Hematological malignancies | 54 (7.1%) | 0 (0%) | 10 (2.2%) | 0 (0%) |
| Solid organ transplant | 40 (5.3%) | 0 (0%) | 5 (1.3%) | 0 (0%) |
| Stem cell transplant | 10 (1.3%) | 0 (0%) | 1 (0.3%) | 0 (0%) |
| Previous evidence of pneumonia, n (%) | 320 (42.3%) | 0 (0.0%) | 95 (23.8%) | 0 (0.0%) |
COPD chronic obstructive pulmonary disease, ER emergency room
aSee “Methods” and “Patient Selection” for high-risk criteria
bER includes patients diagnosed in inpatient and ER settings
cOther settings includes durable medical equipment (DME), home health agency (HHA), hospice, and skilled nursing facility (SNF)
Predictors of Initial Hospitalization
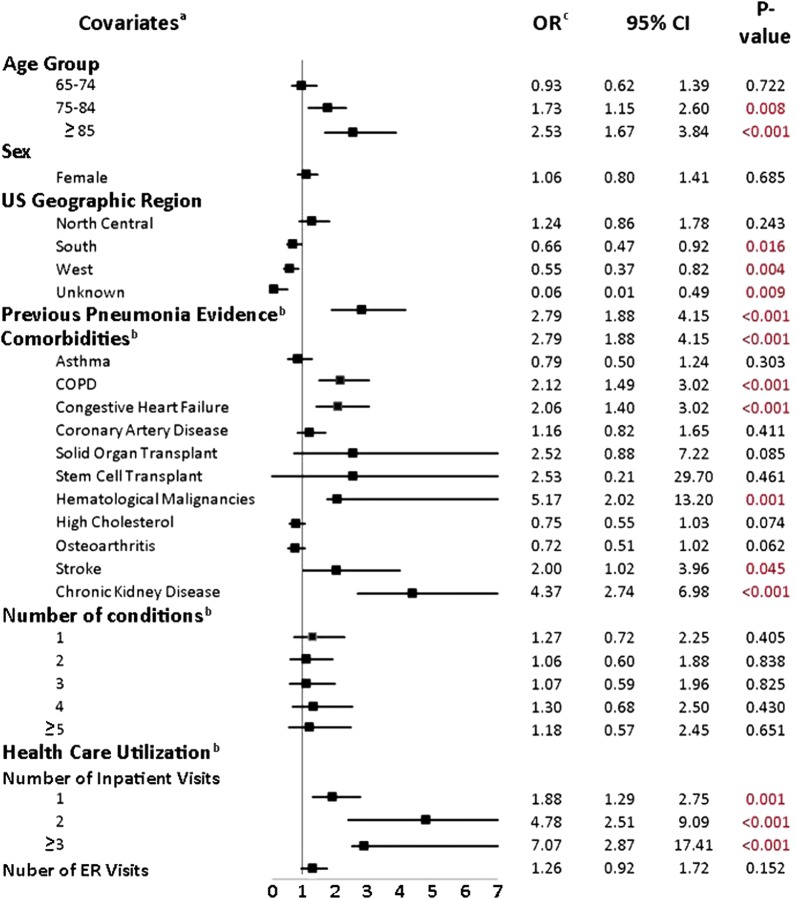
Figure 2 shows predictors of hospitalization using a logistic regression model. Comorbidities such as hematological malignancies (odds ratio [OR] 5.17; p < 0.001), CKD (OR 4.37; p < 0.001), COPD (OR 2.12; p < 0.001), CHF (OR 2.06; p = 0.002), and stroke (OR 2.00; p = 0.045) as well as previous evidence of pneumonia (OR 2.79; p < 0.001) were significant predictors of hospitalization, after adjusting for other covariates. Other significant predictors of hospitalization included older age and healthcare resource use during the baseline period (Fig. 2).
Fig. 2.
Predictors of initial hospitalization. CI confidence interval, COPD chronic obstructive pulmonary disease, ER emergency room, OR odds ratio. aThe following were used as references: age (< 65 years), sex (male), US geographic region (northeast), number of comorbidities (n = 0), number of inpatient visits (n = 0). bVariables were evaluated during the baseline period. cOR > 1 indicates that the variable is a positive predictor of hospitalization among patients who were initially hospitalized (N = 793) as compared with patients who were never hospitalized (N = 695)
Outcomes Assessment
Figure 3 illustrates outcomes of patients with RSV during the follow-up period. During the 180 days post-RSV diagnosis, upper and lower respiratory tract infections (88.1% excluding influenza, RSV, and hMPV), pneumonia (77.2%), dyspnea (74.7%), and arrhythmias (51.1%) were predominant complications among high-risk hospitalized patients. These conditions were also the most common complications among hospitalized non-high-risk patients and high-risk outpatients. The complication rates were much lower in non-high-risk outpatients (Fig. 3).
Fig. 3.
Complications during the follow-up period among patients with RSV. COPD chronic obstructive pulmonary disease, HR high risk, RTI respiratory tract infection. aExcluding influenza, RSV, and hMPV. bSee “Methods” and “Patient Selection” for high-risk criteria
Outcomes are described in Table 2. Mortality and readmission rates were highest in the high-risk hospitalized patients (Table 2). Deaths during inpatient stay (hospitalization) occurred among 4.2% of the high-risk hospitalized patients and 0% among hospitalized non-high-risk patients. The all-cause mortality within 30 days of RSV diagnosis was 13.4% among high-risk hospitalized patients and 8.1% among hospitalized non-high-risk patients. Among high-risk patients discharged from a hospital, 24.7% were discharged to an SNF with a mean LOS of 7.8 days; of those, 71.1% did not reside in an SNF prior to hospitalization, whereas 13.5% of hospitalized non-high-risk patients were discharged to an SNF with a mean LOS of 5.1 days and 80% of those did not utilize an SNF prior to hospitalization. The 30-day hospital readmission rate was 20.4% and 16.2% for hospitalized high-risk and non-high-risk patients, respectively (Table 2).
Table 2.
Descriptive outcomes assessment for patients diagnosed with RSV
| Hospitalized high-riska | Hospitalized non-high-risk | |
|---|---|---|
| N = 756 | N = 37 | |
| Length of stay during initial hospitalization, mean (SD) | 7.0 (10.7) | 5.5 (4.6) |
| 30-day readmission, N (%) | 154 (20.4%) | 6 (16.2%) |
| Patients who were discharged to SNF, N (%) | 187 (24.7%) | 5 (13.5%) |
| % of patients not in SNF prior to hospitalization | 133 (71.1%) | 4 (80.0%) |
| % of patients in SNF prior to hospitalization | 54 (28.9%) | 1 (20.0%) |
| Length of stay in skilled nursing facility, mean (SD) | 7.8 (32.2) | 5.1 (17.9) |
| All-cause mortality, N (%) | ||
| Within 30 days of RSV diagnosis | 101 (13.4%) | 3 (8.1%) |
| Within 90 days of RSV diagnosis | 178 (23.5%) | 3 (8.1%) |
| Within 180 days of RSV diagnosis | 221 (29.2%) | 4 (10.8%) |
| Death during inpatient stay, N (%) | ||
| Within 30 days of RSV diagnosis | 32 (4.2%) | 0 (0.0%) |
| Within 90 days of RSV diagnosis | 57 (7.5%) | 0 (0.0%) |
| Within 180 days of RSV diagnosis | 69 (9.1%) | 1 (2.7%) |
RSV respiratory syncytial virus, SD standard deviation, SNF skilled nursing facility
aSee “Methods” and “Patient Selection” for high-risk criteria
Pre–Post Analysis
Table 3 depicts pre- and post-RSV diagnosis analysis of healthcare resource utilization and costs among high-risk and non-high-risk hospitalized patients as well as outpatients. Among HR hospitalized patients, significant increases in the number of inpatient visits (from 1.2 to 1.5 visits; p < 0.001) and longer LOS (from 9.0 to 12.2 days; p < 0.001) were observed across all hospitalizations (including the index hospitalization), whereas the number of outpatient visits decreased post-RSV diagnosis (from 17.8 to 15.2 visits; p < 0.001), along with pharmacy visits (from 21.1 to 16.0 visit; p < 0.001). Similarly, hospitalized non-high-risk outpatients incurred an increased number of inpatient visits (from 0.6 to 1.9 visits; p < 0.001) and prolonged LOS (from 3.1 days to 11.4 days; p < 0.001) following the RSV diagnosis.
Table 3.
Pre- and post-RSV diagnosis healthcare costs and utilization among Medicare patients with RSV
| Hospitalized high-riska, N = 756 | Hospitalized non-high-risk, N = 37 | |||||
|---|---|---|---|---|---|---|
| Pre-index | Post-index | p valueb | Pre-index | Post-index | p valueb | |
| All-cause healthcare utilization mean (SD) | ||||||
| # of inpatient visits | 1.2 (1.4) | 1.5 (1.4) | < 0.0001* | 0.6 (1.2) | 1.9 (1.7) | < 0.0001* |
| # of outpatient visit | 17.8 (12.8) | 15.2 (13.5) | < 0.0001* | 14.2 (10.4) | 13.6 (9.0) | 0.737 |
| # of pharmacy uses | 21.1 (14.7) | 16.0 (14.1) | < 0.0001* | 15.5 (11.6) | 12.8 (8.8) | 0.149 |
| Length of stay | 9.0 (15.0) | 12.2 (19.2) | < 0.0001* | 3.1 (7.9) | 11.4 (16.5) | 0.000* |
| All-cause healthcare costs mean (SD) | ||||||
| Outpatient costs | $6540 ($10,783) | $5544 ($10,113) | 0.003* | $5421 ($0) | $3924 ($4619) | 0.252 |
| Inpatient costs | $16,604 ($27,664) | $23,172 ($33,438) | < 0.0001* | $6365 ($14,828) | $21,810 ($26,005) | 0.000* |
| Pharmacy costs | $3776 ($6834) | $3002 ($6445) | < 0.0001* | $1678 ($2244) | $1605 ($2140) | 0.760 |
| Other costsc | $4793 ($10,434) | $9205 ($13,619) | < 0.0001* | $3598 ($11,849) | $8866 ($16,311) | 0.062 |
| Total costsd | $31,713 ($36,718) | $40,923 ($41,152) | < 0.0001* | $17,063 ($27,446) | $36,204 ($32,597) | 0.000* |
| Outpatient high-risk, N = 399 | Outpatient non-high-risk, N = 436 | |||||
|---|---|---|---|---|---|---|
| Pre-index | Post-index | p valueb | Pre-index | Post-index | p valueb | |
| All-cause healthcare utilization mean (SD) | ||||||
| # of inpatient visits | 0.5 (0.9) | 0.5 (1.0) | 0.967 | 0.1 (0.4) | 0.2 (0.5) | 0.028* |
| # of outpatient visit | 15.9 (11.8) | 16.6 (11.1) | 0.170 | 10.8 (10.0) | 12.3 (9.7) | < 0.0001* |
| # of pharmacy uses | 17.3 (11.3) | 18.1 (12.2) | 0.110 | 11.9 (10.0) | 12.6 (9.2) | 0.029* |
| Length of stay | 3.0 (8.5) | 3.1 (10.0) | 0.868 | 0.5 (2.9) | 0.9 (3.6) | 0.052 |
| All-cause healthcare costs mean (SD) | ||||||
| Outpatient costs | $3886 ($5534) | $3891 ($4888) | 0.987 | $2452 ($4436) | $2836 ($4603) | 0.056 |
| Inpatient costs | $5648 ($15,138) | $5874 ($16,425) | 0.831 | $922 ($4221) | $1940 ($7027) | 0.009* |
| Pharmacy costs | $2454 ($3590) | $2777 ($6408) | 0.225 | $2036 ($4138) | $2314 ($5708) | 0.260 |
| Other costsc | $2510 ($6686) | $3567 ($8856) | 0.020* | $437 ($2250) | $829 ($3628) | 0.030* |
| Total costsd | $14,498 ($21,611) | $16,108 ($23,688) | 0.252 | $5847 ($9090) | $7919 ($12,976) | 0.000* |
RSV respiratory syncytial virus, SD standard deviation
*p ≤ 0.05
aSee “Methods” and “Patient Selection” for high-risk criteria
bp values were calculated using paired t tests
cOther costs includes durable medical equipment (DME), home health agency (HHA), hospice, and skilled nursing facility (SNF)
dTotal costs consists of the sum of inpatient costs, outpatient costs, pharmacy costs, and other costs
A significant difference in healthcare visits was not observed between the pre- and post-RSV diagnosis period among high-risk outpatients. However, as compared with the pre-RSV diagnosis period, incremental increases in the number of inpatient (from 0.1 to 0.2; p = 0.028), outpatient (from 10.8 to 12.3; p < 0.001), and pharmacy visits (from 11.9 to 12.6; p = 0.029) were observed post-RSV diagnosis among non-high-risk outpatients.
Total average per patient all-cause healthcare costs increased by $9210 (p < 0.001) for high-risk hospitalized patients, $19,141 (p < 0.001) for hospitalized non-high-risk patients, and $2072 (p < 0.001) for non-high-risk outpatients. Total average per patient all-cause healthcare costs were similar ($1610) for high-risk outpatients (p = 0.252). The primary cost drivers were related to all-cause hospitalization costs, which increased for hospitalized high-risk patients (from $16,604 to $23,172; p < 0.001), hospitalized non-high-risk patients (from $6365 to $21,810; p < 0.001), and outpatient non-high-risk patients (from $922 to $1940; p = 0.009) during the follow-up period (Table 3).
Discussion
To our knowledge, this is the first study to identify characteristics of adult patients with RSV in the inpatient and outpatient setting and predictors of hospitalization as well as to describe the current clinical and economic burden of RSV in the US Medicare population. Our study demonstrates that hematological malignancies, number of inpatient visits prior to hospitalization for RSV and chronic kidney disease (CKD), previous evidence of pneumonia (during the baseline period), congestive heart failure (CHF), stroke, and older age were among the predictors of hospitalization. All-cause mortality among high-risk hospitalized patients was 4.2% during inpatient stays and 13.4% within 30 days of diagnosis. Among high-risk and non-high-risk hospitalized patients, there was a significant increase in healthcare resource utilization including the number of subsequent inpatient admissions and longer hospital stays post-RSV diagnosis, as compared with non-hospitalized patients. The total mean all-cause healthcare costs among high-risk hospitalized and non-high-risk hospitalized patients increased by $9210 (p < 0.0001) per patient and $19,141 (p < 0.001) per patient post-RSV diagnosis, respectively.
RSV leads to in significant morbidity and mortality, with 14,000 reported deaths, and more than 177,000 hospitalizations annually in the USA among adults 65 years of age and older [2]. In addition, elderly patients with underlying high-risk conditions are prone to complications related to LRTIs, resulting in prolonged hospitalization, and therefore potentially death [15, 19, 20]. Although RSV typically presents with mild cold-like symptoms, RSV can sometimes lead to more severe complications including LRTI, pneumonia, and exacerbation of underlying conditions such as CHF, asthma, and COPD [21–25]. Similarly, most hospitalized high-risk patients in our study were older (average age 77 years) and diagnosed with LRTI (84.3%, excluding influenza, RSV, or hMPV), and had previous evidence of pneumonia (77.2%), dyspnea (74.7%), or COPD (65.5%) in the 180 days pre-RSV infection, which may have been caused by secondary RSV-related complications.
Most available research on the economic burden of RSV in the USA predominantly focuses on infants or adult patients in private insurance populations that largely comprise patients less than 65 years of age. These studies highlight the increased burden of RSV due to the higher utilization of healthcare resources including intensive care and ventilator support, and the costs incurred as a result of underlying conditions [26]. In a study by Han et al., the annual total costs related to RSV pneumonia hospitalization in all elderly patients aged 65 years and older were estimated in the range of $150–680 million [27]. Pastula et al. also indicated that the average hospitalization costs associated with RSV infection among all adults in the USA were $38,828 [28]. Amand et al. found that the incremental differences in adjusted annual healthcare costs between patients with and without RSV was higher among patients aged 65 years and older ($12,030–23,194) than among those aged less than 65 years old ($2251–5391) [18]. The current analysis observed significant increases in medical resource utilization after RSV diagnosis, including an increase in the number of subsequent inpatient admissions and prolonged hospital LOS among high-risk hospitalized patients. The higher resource utilization observed in this study also translated to increased healthcare costs. Total mean all-cause healthcare costs per patient increased by $9210 per patient (p < 0.0001) for high-risk hospitalized patients, $19,141 (p < 0.001) for hospitalized non-high-risk patients, and $2072 (p < 0.001) for non-high-risk outpatients from the baseline period to the follow-up period, while hospitalization costs were the main cost driver (57%). This is consistent Amand et al.’s findings that patients 65 years of age and older had 3–5 times more hospitalizations as compared with those aged 18–49 years.
ER visits, hospitalizations, ICU admissions, and death due to respiratory complications play an important role in the clinical burden of RSV infection. Emergency departments are especially likely to be the first point of care for patients with RSV. Overall, 4.2% of the admitted high-risk patients died during an inpatient stay and 13.4% within 30 days of diagnosis. At discharge, 24.7% of the admitted high-risk patients in the current study were discharged to an SNF where they remained on average for 7.8 days. Among high-risk patients discharged to an SNF, 71.1% did not reside in an SNF prior to hospitalization. The burden of an RSV outbreak is high in long-term care facilities such as SNFs [29].
Despite the substantial disease burden, the importance of RSV may not be recognized by admitting physicians, and RSV management may not be initiated promptly. This may impact health outcomes among elderly patients, increasing the burden of their disease. Rapid identification of RSV may influence decision-making for treatment, which in turn can optimize effective patient management, thereby reducing hospital admission rates and LOS [30–32]. Rapid molecular tests for viral pathogen detection have been shown to decrease hospital LOS [32]. It is important for attending physicians to identify patients with RSV who require more than the current standard of care at the time of diagnosis. The results of this study demonstrate that hematological malignancies, the number of inpatient visits, CKD, pneumonia, CHF, CAD, stroke, and older age were predictors of hospitalizations, which may help healthcare providers to treat high-risk patients more effectively.
Limitations
Certain limitations should be acknowledged regarding the results of this study. As with all retrospective claims analyses, the study utilized claims data that were primarily coded for administrative purposes rather than clinical accuracy, and was therefore susceptible to coding errors and diagnosis discrepancies. In claims database analysis, only associations rather than casual inferences can be inferred.
In this study specifically, the RSV diagnosis was captured on the basis of ICD-9/10-CM codes and not diagnostic testing. This methodology may have led to the underestimation or overestimation of the actual number of RSV cases, as there could be an inherent difficulty of distinguishing between RSV and RSV-like illness, although we did exclude patients with influenza and hMPV to get a more exclusive sample of patients with RSV. Complications were identified as conditions that occurred 6 months following an RSV diagnosis date (ICD-9/ICD-10 codes); therefore, we could not ascertain whether the conditions were an actual complication of RSV or a progression of an underlying disease that may have been exacerbated as a result of RSV. In addition, RSV complications related to the disease progression or exacerbation of underlying chronic conditions such as COPD or CHF were not captured because of the lack of relevant diagnostic codes in the data. Therefore, we might have underestimated the rate of complications in this population. We also could not distinguish between community-acquired and hospital-acquired RSV infection because of lack of ICD codes to differentiate between 2 settings. Moreover, this study used a fee-for-service (FFS) Medicare database that includes information on Medicare FFS beneficiaries only (approx. 69% of Medicare beneficiaries in 2015) [33], which may limit the generalizability of the study results to patients with commercial insurance plans.
Conclusion
Hospitalized Medicare beneficiaries with RSV infections pose a substantial burden on the healthcare system, primarily due to comorbidities that require more intensive management related to RSV-mediated exacerbations, a higher likelihood of multiple inpatient admissions, and costly medical interventions, such as ICU admission and/or mechanical ventilation. Emergency departments and general wards are likely to be the first point of care for these patients. This study demonstrates a significant burden of RSV among elderly patients that is consistent with existing literature. The incremental all-cause total healthcare costs were estimated at $9210 post-RSV diagnosis, compared to pre-RSV diagnosis, for high-risk hospitalized patients.
RSV-infected elderly patients with high-risk underlying conditions experience greater morbidity and generate a substantial economic burden to the healthcare system. The findings from this research may help policymakers, healthcare providers, and other stakeholders in identifying patients at highest risk for hospitalization, morbidity, and mortality, so as to make more informed decisions regarding RSV management.
Acknowledgements
Funding
This study was funded, without restriction, by Janssen Scientific Affairs, LLC, including all expenses and fees associated with publication. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Medical Writing, Editorial, and Other Assistance
Research assistance in the preparation of this article was provided by Janvi Sah of SIMR, LLC. Editorial assistance was provided by Michael Kane of SIMR, LLC. This assistance was funded by Janssen Scientific Affairs, LLC.
Disclosures
Furaha Kariburyo is an employee of SIMR, LLC, a paid consultant to Janssen Scientific Affairs, LLC. Veronique Wyffels and Sandra Gavart are employees of Janssen Pharmaceutica NV and might be stockholders of Johnson & Johnson. Roman Fleischhackl is an employee of Janssen-Cilag Pharma and might be a stockholder of Johnson & Johnson. Huseyin Yuce has no potential conflicts to declare.
Compliance with Ethics Guidelines
Since the core study did not involve the collection, use, or transmittal of individual identifiable data, institutional review board approval was not required, as data that is de-identified a priori is exempt from the Federal Policy for the Protection of Human Subjects ([1991]; 45CFR46.101(b)(4): Existing Data & Specimens—No Identifiers), and further, does not meet the identification criteria necessary to be privileged under the Health Insurance Portability and Accountability Act of 1996 (HIPAA). Nonetheless, both the data set and the security of the premises where we kept the data set meet the requirements of HIPAA, and by default, this study is in accord with the same.
Data Availability
The datasets generated and analyzed during the current study are not publicly available due to a data licensing agreement with the Centers for Medicare & Medicaid Services (CMS).
Footnotes
Enhanced Digital Features
To view enhanced digital features for this article go to 10.6084/m9.figshare.11567205.
References
- 1.RSV in Older Adults and Adults with Chronic Medical Conditions|CDC [Internet]. Cdc.gov. 2019. https://www.cdc.gov/rsv/high-risk/older-adults.html. Accessed 6 Aug 2019.
- 2.Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. New Engl J Med. 2005;352(17):1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 3.Simões EA, DeVincenzo JP, Boeckh M, et al. Challenges and opportunities in developing respiratory syncytial virus therapeutics. J Infect Dis. 2015;211(Suppl 1):S1–S20. doi: 10.1093/infdis/jiu828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zambon MC, Stockton JD, Clewley JP, Fleming DM. Contribution of influenza and respiratory syncytial virus to community cases of influenza-like illness: an observational study. Lancet. 2001;358(9291):1410–1416. doi: 10.1016/S0140-6736(01)06528-X. [DOI] [PubMed] [Google Scholar]
- 5.Simões EA, Carbonell-Estrany X, Rieger CH, et al. The effect of respiratory syncytial virus on subsequent recurrent wheezing in atopic and nonatopic children. J Allergy Clin Immunol. 2010;126(2):256–262. doi: 10.1016/j.jaci.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedman JN, Rieder MJ, Walton JM, Canadian Paediatric Society. Acute Care Committee. Drug Therapy and Hazardous Substances Committee Bronchiolitis: recommendations for diagnosis, monitoring and management of children one to 24 months of age. Paediatr Child Health. 2014;19(9):485–498. doi: 10.1093/pch/19.9.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134:e1474–1502. doi: 10.1542/peds.2014-2742. [DOI] [PubMed] [Google Scholar]
- 8.Krilov LR. Safety issues related to the administration of ribavirin. Pediatr Infect Dis J. 2002;21(5):479–481. doi: 10.1097/00006454-200205000-00037. [DOI] [PubMed] [Google Scholar]
- 9.Accessdata.fda.gov. 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/021511s029lbl.pdf. Accessed 7 Aug 2019.
- 10.Shah JN, Chemaly RF. Management of RSV infections in adult recipients of hematopoietic stem cell transplantation. Blood. 2011;117(10):2755–2763. doi: 10.1182/blood-2010-08-263400. [DOI] [PubMed] [Google Scholar]
- 11.von Lilienfeld-Toal M, Berger A, Christopeit M, et al. Community acquired respiratory virus infections in cancer patients—guideline on diagnosis and management by the Infectious Diseases Working Party of the German Society for haematology and Medical Oncology. Eur J Cancer. 2016;67:200–212. doi: 10.1016/j.ejca.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Respiratory Syncytial Virus Infection (RSV): People at high risk for severe RSV infection. Centers for Disease Control and Prevention website. https://www.cdc.gov/rsv/high-risk/index.html. Accessed 3 Dec 2018. (Updated 26 June 2018).
- 13.Russell CD, Unger SA, Walton M, Schwarze J. The human immune response to respiratory syncytial virus infection. Clin Microbiol Rev. 2017;30(2):481–502. doi: 10.1128/CMR.00090-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Crit Care Med. 2000;161(5):1501–1507. doi: 10.1164/ajrccm.161.5.9906076. [DOI] [PubMed] [Google Scholar]
- 15.Díez-Domingo J, Pérez-Yarza EG, Melero JA, et al. Social, economic, and health impact of the respiratory syncytial virus: a systematic search. BMC Infect Dis. 2014;14:544. doi: 10.1186/s12879-014-0544-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McLaurin KK, Farr AM, Wade SW, Diakun DR, Stewart DL. Respiratory syncytial virus hospitalization outcomes and costs of full-term and preterm infants. J Perinatol. 2016;36(11):990. doi: 10.1038/jp.2016.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stewart DL, Romero JR, Buysman EK, Fernandes AW, Mahadevia PJ. Total healthcare costs in the US for preterm infants with respiratory syncytial virus lower respiratory infection in the first year of life requiring medical attention. Curr Med Res Opin. 2009;25(11):2795–2804. doi: 10.1185/03007990903290894. [DOI] [PubMed] [Google Scholar]
- 18.Amand C, Tong S, Kieffer A, Kyaw MH. Healthcare resource use and economic burden attributable to respiratory syncytial virus in the United States: a claims database analysis. BMC Health Serv Res. 2018;18(1):294. doi: 10.1186/s12913-018-3066-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee N, Lui GC, Wong KT, et al. High morbidity and mortality in adults hospitalized for respiratory syncytial virus infections. Clin Infect Dis. 2013;57(8):1069–1077. doi: 10.1093/cid/cit471. [DOI] [PubMed] [Google Scholar]
- 20.Billings JL, Hertz MI, Wendt CH. Community respiratory virus infections following lung transplantation. Transpl Infect Dis. 2001;3(3):138–148. doi: 10.1034/j.1399-3062.2001.003003138.x. [DOI] [PubMed] [Google Scholar]
- 21.Nicholson KG, Kent J, Hammersley V, Cancio E. Acute viral infections of upper respiratory tract in elderly people living in the community: comparative, prospective, population based study of disease burden. BMJ. 1997;315(7115):1060–1064. doi: 10.1136/bmj.315.7115.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Respiratory Syncytial Virus (RSV): RSV infection in older adults. Centers for Disease Control and Prevention website. https://www.cdc.gov/rsv/factsheet-older-adults.html. Accessed 3 Dec 2018. (Updated 26 June 2018).
- 23.Falsey AR, Walsh EE. Respiratory syncytial virus infection in adults. Clin Microbiol Rev. 2000;13(3):371–384. doi: 10.1128/CMR.13.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Falsey AR, Cunningham CK, Barker WH, et al. Respiratory syncytial virus and influenza A infections in the hospitalized elderly. J Infect Dis. 1995;172(2):389–394. doi: 10.1093/infdis/172.2.389. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention: RSV in oloder adults and adults with chronic medical conditions. https://www.cdc.gov/rsv/high-risk/older-adults.html. Accessed 15 Mar 2019.
- 26.Binder W, Thorsen J, Borczuk P. RSV in adult ED patients do emergency providers consider RSV as an admission diagnosis? Am J Emergency Med. 2017;35(8):1162–1165. doi: 10.1016/j.ajem.2017.06.022. [DOI] [PubMed] [Google Scholar]
- 27.Han LL, Alexander JP, Anderson LJ. Respiratory syncytial virus pneumonia among the elderly: an assessment of disease burden. J Infect Dis. 1999;179(1):25–30. doi: 10.1086/314567. [DOI] [PubMed] [Google Scholar]
- 28.Pastula ST, Hackett J, Coalson J, et al. Hospitalizations for respiratory syncytial virus among adults in the United States, 1997–2012. Open Forum Infect Dis. 2017;4(1):ofw270. doi: 10.1093/ofid/ofw270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caram LB, Chen J, Taggart EW, et al. Respiratory syncytial virus outbreak in a long-term care facility detected using reverse transcriptase polymerase chain reaction: an argument for real-time detection methods. J Am Geriatr Soc. 2009;57(3):482–485. doi: 10.1111/j.1532-5415.2008.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brendish NJ, Schiff HF, Clark TW. Point-of-care testing for respiratory viruses in adults: the current landscape and future potential. J Infect. 2015;71:501–510. doi: 10.1016/j.jinf.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oosterheert JJ, van Loon AM, Schuurman R, et al. Impact of rapid detection of viral and atypical bacterial pathogens by real-time polymerase chain reaction for patients with lower respiratory tract infection. Clin Infect Dis. 2005;41:1438–1444. doi: 10.1086/497134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vos LM, Bruning AH, Reitsma JB, et al. Rapid molecular tests for influenza, respiratory syncytial virus, and other respiratory viruses: a systematic review of diagnostic accuracy and clinical impact studies. Clin Infect Dis. 2019;69:1243–1253. doi: 10.1093/cid/ciz056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.The Kaiser Family Foundation Medicare Advantage. http://kff.org/medicare/fact-sheet/medicare-advantage/. Accessed 22 Mar 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the current study are not publicly available due to a data licensing agreement with the Centers for Medicare & Medicaid Services (CMS).