Abstract
Oseltamivir, a pro-drug, is the best option for treatment and chemoprophylaxis for influenza outbreaks. However, many patients treated with oseltamivir developed adverse reactions, including hypersensitivity, gastritis, and neurological symptoms. The aim of this study was to determine the adverse drug reactions (ADRs) in Mexican patients treated with oseltamivir and whether these ADRs are associated with SNPs of the genes involved in the metabolism, transport, and interactions of oseltamivir. This study recruited 310 Mexican patients with acute respiratory diseases and treated them with oseltamivir (75 mg/day for 5 days) because they were suspected to have influenza A/H1N1 virus infection. Clinical data were obtained from medical records and interviews. Genotyping was performed using real-time polymerase chain reaction and TaqMan probes. The association was assessed under genetic models with contingency tables and logistic regression analysis. Out of 310 patients, only 38 (12.25%) presented ADRs to oseltamivir: hypersensitivity (1.9%), gastritis (10%), and depression and anxiety (0.9%). The polymorphism ABCB1-rs1045642 was associated with adverse drug reactions under the recessive model (P = 0.017); allele C was associated with no adverse drug reactions, while allele T was associated with adverse drug reactions. The polymorphisms SLC15A1-rs2297322, ABCB1-rs2032582, and CES1-rs2307243 were not consistent with Hardy–Weinberg equilibrium, and no other associations were found for the remaining polymorphisms. In conclusion, the polymorphism rs1045642 in the transporter encoded by the ABCB1 gene is a potential predictive biomarker of ADRs in oseltamivir treatment.
Subject terms: Predictive markers, Genetic markers
Introduction
Acute respiratory diseases are a serious global public health problem due to their high rates of morbidity and mortality. These diseases are the most frequent reason why patients use health services. Acute respiratory diseases are caused by microorganisms that affect the respiratory system for less than 15 days [1]. In Mexico, acute respiratory diseases are a priority health problem because they are among the ten main causes of illness and death in different age groups (Sistema Nacional de Vigilancia Epidemiológica, Secretaría de Salud, at http://www.sinave.gob.mx/). During 6 years, 108,830 deaths due to acute respiratory diseases were reported in Mexico [2].
The influenza A virus is one of the microorganisms that cause acute respiratory infections [3]. This virus is responsible for annual seasonal epidemics worldwide. In the last century, there have been four significant worldwide outbreaks of influenza A with high mortality rates for humans [4]. To treat influenza, the U.S. Food and Drug Administration (FDA) approved the use of oseltamivir, which is a controversial [5, 6] antiviral agent that selectively inhibits the neuraminidases of both the influenza A and B viruses [7]. Viral neuraminidase promotes the release of progeny viruses from infected cells by cleaving the terminal sialic acid residues from carbohydrate moieties on the surfaces of host cells and influenza virus envelopes [8]. Oseltamivir was the best option for treatment and chemoprophylaxis during the influenza A/H1N1 2009 pandemic (influenza A/H1N1-pdm2009) outbreak because it could be administered to patients as young as 1-year old [9]. However, patients treated with oseltamivir have developed adverse reactions such as vertigo, dizziness, sickness, insomnia, headache, behavioural alterations, and numbness [10]. The U.S. FDA reported that oseltamivir’s adverse effects on the central nervous system include delirium, hallucinations, confusion, abnormal behaviour, convulsions, and encephalitis [11] (http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm107840.htm). Moreover, oseltamivir treatment has been associated with abnormal behaviour and death in patients from Japan and the United Kingdom [12–14]. However, the pharmaceutical industry has reported no adverse events [15]. In Mexico, oseltamivir phosphate was the only pro-drug that was distributed and prescribed for influenza treatment during the influenza A/H1N1-pdm2009 pandemic [16], and no information is available for adverse reactions in the Mexican population.
This individual susceptibility to oseltamivir adverse reactions might be explained by the genetic polymorphisms of proteins involved in its uptake, activation, and elimination. To be effective, oseltamivir phosphate must be taken up by peptide transporter 1 (PepT1), and activated by carboxylesterase 1 (CES1) to form oseltamivir carboxylate; furthermore oseltamivir phosphate, before activation, can be eliminated from cells through P-glycoprotein (Fig. 1) [17–20]. Besides, oseltamivir carboxylate not only inhibits viral neuraminidase but can also inhibit human sialidases [21]. The genes that encode for Pept1, CES1, P-glycoprotein, and human neuraminidase (NEU2) harbour SNPs that affect their functions [22–29].
Fig. 1. Transport, metabolism, and interactions of oseltamivir.
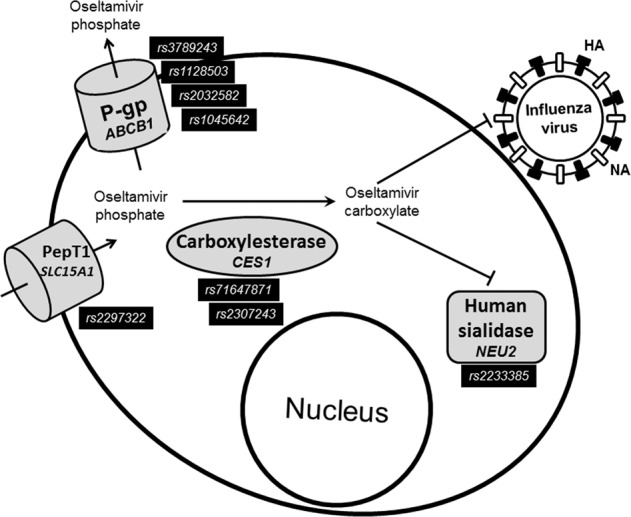
The pro-drug oseltamivir phosphate is taken up by the peptide transporter 1 (PepT1) and is effluxed by P-glycoprotein (P-gp) before its activation by carboxylesterase 1 to oseltamivir carboxylate. Oseltamivir carboxylate inhibits the release of progeny viruses from infected cells by blocking the viral neuraminidase but also inhibits the activity of human sialidase 2. The SNPs evaluated in this study are depicted in each protein. Pept1 peptide transporter 1 (encoded by SLC15A1 gene), P-gp P-glycoprotein 1 (encoded by ABCB1 gene), HA hemagglutinin, NA neuroaminidase.
The aim of this study was to determine the adverse drug reactions (ADRs) in Mexican patients treated with oseltamivir and whether these ADRs are associated with SNPs of the genes involved in the metabolism, transport, and interactions of oseltamivir.
Methods
Design
A retrospective study with convenience sampling was carried out on patients with acute respiratory disease who were suspected to have pandemic influenza virus A/H1N1 infections and who were treated with oseltamivir for at least 5 days. This study was conducted according to Good Clinical Practice standards. It meets all criteria contained in the Declarations of Helsinki by the World Medical Association in 1964; those criteria were modified in 2013. The protocol was approved by the ethical and scientific committees of the Mexican Social Security Institute (protocol number R-2012-785-095).
Patient selection
This study included male and female patients who were treated at the Instituto Mexicano del Seguro Social clinics, which are located in the state of Nuevo Leon, Mexico. The inclusion criteria were as follows: patients with acute respiratory diseases who were suspected to be infected with pandemic influenza virus A/H1N1 and who were treated with oseltamivir at 75 mg/kg/day for at least 5 days. Patients were excluded when they had any of the following: clinically documented renal or hepatic failure, withdrawn written informed consent, low DNA quality, incomplete clinical records, incomplete genetic data, or family relations with each other.
Clinical data and biological samples were collected between December 2009 and December 2012. The biological samples consisted of pharyngeal and oropharyngeal swabs, which were obtained during the recruitment period and were stored at −80 °C until analysis. A total of 310 samples were selected for evaluation.
The diagnosis of influenza A/H1N1 was determined by real-time reverse transcription polymerase chain reaction assays, which were conducted in accordance with the Centers for Disease Control and Prevention protocol [30]. Clinical and sociodemographic information was obtained through personal interviews and medical records at primary, secondary, or tertiary care facilities. We studied drug hypersensitivity and adverse effects related to the central nervous and gastric systems.
DNA extraction and SNP analysis
Genomic DNA extraction was performed with a MagNA Pure LC Total Nucleic Acid Isolation Kit (Roche Diagnostics, Indianapolis, Indiana, USA) using Magna Pure LC 2.0® equipment from Roche. DNA samples were stored at −20 °C until analysis.
Genetic analysis was performed to assess four clinically relevant variants of the ABCB1 gene (rs3789243, rs1128503, rs2032582, and rs1045642), two variants of the CES1 gene (rs71647871 and rs2307243), one variant of the SLC15A1 gene (rs2297322), and one variant of the NEU2 gene (rs2233385). Allelic discrimination by real-time PCR was performed with specific primers and TaqMan® probes. Approximately 50 ng of genomic DNA was added to a master mix (Applied Biosystems, Foster City, CA, USA) that contained 200 nmol of primers and 40 nmol of probes for each polymorphism. Samples were placed in a 96-well plate and amplified as follows: pre-PCR read at 60 °C for 30 s, then 10 min at 95 °C, followed by 50 cycles of 95 °C for 15 s, and 60 °C for 1 min. Post-read analyses were performed at 60 °C for 30 s. All assays were carried out in a 7500 Fast PCR System (Applied Biosystems), and analyses were performed with 7500 Fast System SDS software.
Statistical analysis
For sample size calculation, the incidence of 36.1% of ADRs reported by Dalvi et al. was considered [31]. It was assumed that the incidence of ADRs in our study population was 13%. Considering a confidence level of 95%, a significance level of 5%, and a minimum power of 80%, a sample size of 245 subjects would be sufficient. The Hardy–Weinberg equilibrium (HWE) was estimated for all genetic polymorphisms by comparing their genotype frequencies. The associations were assessed using χ2 and exact Fisher tests in genetic models (codominant, dominant, overdominant, and recessive) and were validated by logistic regression analysis. The odds ratio was calculated with a 95% confidence interval (95% CI). The P values were two-tailed and adjusted by Bonferroni correction (Pc). Next, to exclude spurious association the Benjamini–Hochberg test was used for multiple test correction [32]. Pairwise linkage disequilibrium (LD, D′) analysis between polymorphisms and haplotype reconstruction were performed. The associations between haplotypes and ADRs were determined by the Exact Fisher test. The HWE and haplotype reconstructions were performed using SNPstats software (https://www.snpstats.net/start.htm). The association analysis was conducted with SPSS software V.25 (IBM Corp., NY, USA). P values < 0.05 were considered statistically significant.
Results
Table 1 depicts the basic characteristics of the group of 310 patients who were suspected to have a pandemic influenza virus A/H1N1 infection. Of the samples, 56% were from females, and 44% were from males. No significant differences were found between the ages of the female and male patients. The proportions of hospitalised female and male patients were similar (42% and 44%, respectively), and only five patients died (two females and three males). The patients’ common clinical features (>70% of patients) were fever, cough, and the sudden onset of symptoms and headache. The majority of patients presented rhinorrhoea, prostration, shaking chills, myalgia, arthralgia, and odynophagia (Table 2). Of the total group, 130 (42%) tested positive for influenza A/H1N1, 11 (3.5%) were positive for seasonal influenza, and 169 (54.5%) were negative for influenza. The aetiological agent for patients with negative samples remained unknown. All patients were treated with oseltamivir at 75 mg/kg/day for at least 5 days, and the signs and symptoms that were provoked by oseltamivir were recorded. Only 38 patients presented ADRs to oseltamivir (Table 3), although some cases counted more than one adverse event in a given subject. Serious effects such as depression and anxiety were observed in three (0.9%) patients, but no seizures or hallucination effects were reported (Table 3). Thirty-one patients (10%) manifested gastritis, and six patients (1.9%) manifested hypersensitivity.
Table 1.
Characteristics of the Mexican patients with acute respiratory diseases included in this study.
| Gender | N (%) | Age ± SD | Hospitalised (%) |
|---|---|---|---|
| Female | 173 (55.8) | 29.3 ± 19.6 | 74 (42.8) |
| Male | 137 (44.2) | 30.5 ± 22.9 | 61 (44.5) |
| Total | 310 (100) | 29.8 ± 21.1 | 135 (43.5) |
SD standard deviation.
Table 2.
Clinical features of the Mexican patients with acute respiratory diseases (N = 310).
| Signs/symptoms | N | % |
|---|---|---|
| Fever | 264 | 85.2 |
| Cough | 254 | 81.9 |
| Sudden onset | 223 | 71.9 |
| Headache | 219 | 70.6 |
| Rhinorrhoea | 212 | 68.4 |
| Prostration | 200 | 64.5 |
| Shaking chills | 190 | 61.3 |
| Myalgia | 190 | 61.3 |
| Arthralgia | 185 | 59.7 |
| Odynophagia | 168 | 54.2 |
| Dyspnoea | 153 | 49.4 |
| Chest pain | 121 | 39.0 |
| Abdominal pain | 83 | 26.8 |
| Coryza | 65 | 21.0 |
| Conjunctivitis | 50 | 16.1 |
| Irritability | 33 | 10.6 |
| Diarrhoea | 32 | 10.3 |
| Polypnea | 26 | 8.4 |
| Cyanosis | 26 | 8.4 |
Table 3.
Adverse drug reactions associated with oseltamivir administration in Mexican patients (N = 310).
| Adverse drug reactionsa | N | % |
|---|---|---|
| Gastritis | 31 | 10 |
| Hypersensitivity | 6 | 1.9 |
| Anxiety | 2 | 0.6 |
| Depression | 1 | 0.3 |
| Seizures | 0 | 0 |
| Hallucination | 0 | 0 |
aCase counts may reflect multiple adverse events in a given subject.
For the genetic tests, the polymorphisms rs2297322 of the SLC15A1 gene, rs2032582 (G/A) of the ABCB1 gene, and rs2307243 of the CES1 gene were found to be in Hardy–Weinberg disequilibrium (P < 0.05). Therefore, only ABCB1-rs3789243, ABCB1-rs1128503, ABCB1-rs2032582 (G/T), ABCB1-rs2032582 (A/T), ABCB1-rs1045642, CES1-rs71647871, and NEU2-rs2233385 were included in subsequent analysis. Table 4 depicts the genotypes. A χ2 analysis was performed to establish whether the identified variants explain the ADRs observed in the Mexican population. Only rs1045642 of ABCB1 gene (C3435T) was found to be significantly associated with ADRs (P = 0.031) under the codominant model (Table 4). Then, the polymorphism ABCB1-rs1045642 was subjected to logistic regression analysis for each ADR observed, and a trend of association was found between this SNP and gastritis (P = 0.057) under the codominant model, but it was significantly associated under the recessive model. Allele C is a protective factor (OR: 0.395, P = 0.017), and allele T had an increased risk of gastritis (OR: 2.532, Table 5).
Table 4.
Genotype frequencies and association between genotypes and ADRs.
| Genotypes gene-SNP | N | HWE | Association to ADRs | |||
|---|---|---|---|---|---|---|
| χ2 | P value | Yes (N) | No (N) | P value | ||
| SLC15A1-rs2297322 | ||||||
| C/C | 144 | 6.025 | 0.138 | 14 | 130 | ¥ |
| C/T | 120 | 21 | 99 | |||
| T/T | 46 | 3 | 43 | |||
| ABCB1-rs3789243 | ||||||
| C/C | 71 | 3.18 | 0.0746 | 11 | 60 | 0.12 |
| C/T | 138 | 20 | 118 | |||
| T/T | 101 | 7 | 94 | |||
| ABCB1-rs1128503 | ||||||
| C/C | 73 | 0.412 | 0.5209 | 8 | 65 | 0.949 |
| C/T | 149 | 19 | 130 | |||
| T/T | 88 | 11 | 77 | |||
| ABCB1-rs2032582 | ||||||
| G/G | 99 | 4.4522 | 0.0348 | 13 | 86 | ¥ |
| G/A | 112 | 15 | 97 | |||
| A/A | 54 | 7 | 47 | |||
| G/G | 99 | 0.6428 | 0.4226 | 13 | 86 | |
| G/T | 16 | 1 | 15 | 0.909 | ||
| T/T | 0 | |||||
| A/A | 54 | 3.7191 | 0.0534 | 7 | 47 | |
| A/T | 29 | 2 | 27 | 0.789 | ||
| T/T | 0 | |||||
| ABCB1-rs1045642 | ||||||
| C/C | 71 | 1.58 | 0.2088 | 7 | 64 | 0.031*¤ |
| C/T | 143 | 12 | 131 | |||
| T/T | 96 | 19 | 77 | |||
| CES1-rs71647871 | ||||||
| C/C | 306 | 0.013 | 0.9089 | 38 | 268 | 1 |
| C/T | 4 | 0 | 4 | |||
| T/T | 0 | |||||
| CES1-rs2307243 | ||||||
| G/G | 67 | 114.73 | 9.01−27 | 5 | 62 | ¥ |
| G/A | 239 | 33 | 206 | |||
| A/A | 4 | 0 | 239 | |||
| NEU2-rs2233385 | ||||||
| G/G | 267 | 1.144 | 0.2848 | 36 | 231 | 0.343 |
| G/A | 40 | 2 | 38 | |||
| A/A | 3 | 0 | 3 | |||
SNP single nucleotide polymorphism, HWE Hardy–Weinberg equilibrium, ADRs Adverse drug reactions.
¥Not included in association analysis because these markers are in HWE–disequilibrium; ¤lower than Benjamini–Hocheberg P value (0.041); *statistically significant.
Table 5.
Association between genotypes and ADRs under genetic models.
| Gene | SNP | Model | OR (95% CI) | P value | Pc value |
|---|---|---|---|---|---|
| ABCB1 | rs1045642 | Recessive (C/C + C/T vs. T/T) | C/C + C/T: no ADRs | 0.017* | 0.021* |
| 0.395 (0.180–0.867) | |||||
| T/T: ADRs | |||||
| 2.532 (1.154–5.560) |
SNP single nucleotide polymorphism, OR odds ratio.
*Statistically significant and supported by logistic regression analysis.
Haplotype analysis revealed that 19.4% of patients were distributed mainly among four different haplotypes (Table 6). Table 7 depicts the results of a LD analysis of the SNPs. The associations of nonfunctional variants that depend on LD were heterogenic. The analysis demonstrated that ABCB1-rs3789243 and CES1-rs71647871 were in LD (P = 0.029). Similarly, ABCB1-rs1045642 and NEU2-rs2233385 were in LD (P = 0.0435). Thus, our results suggest the identification of two haplotypes. No association was found between haplotypes and ADRs.
Table 6.
Haplotypes for all polymorphisms in Mexican populations with acute respiratory diseases (N = 310).
| Haplotypes | N | % | P value |
|---|---|---|---|
| CC-CT-CT-GA-CT-CC-AG-GA | 23 | 7.4 | 0.85 |
| CT-CT-CT-GA-CT-CC-AG-GA | 16 | 5.2 | |
| CC-TT-CC-AA-CC-CC-AG-GG | 12 | 3.9 | |
| CT-TT-CC-AA-CC-CC-AG-GG | 9 | 2.9 | |
| Others ≤ 2% | 250 | 80.6 |
Order of genotypes: SLC15A1-rs2297322, ABCB1-rs3789243, ABCB1-rs1128503, ABCB1-rs2032582, ABCB1-rs1045642, CES1-rs71647871, CES1-rs2307243, and NEU2-rs2233385.
Table 7.
Linkage disequilibrium analysis of the ABCB1, CES1, and NEU2 gene polymorphisms.
| Gene | CES1 | NEU2 | |
|---|---|---|---|
| SNP | rs71647871 | rs2233385 | |
| ABCB1 | rs3789243 | 0.029* | 0.0871 |
| rs1128503 | 0.0595 | 0.3741 | |
| rs1045642 | 0.0683 | 0.0435* | |
| CES1 | rs71647871 | 0.4015 |
*Statistically significant.
Discussion
Oseltamivir was the most suitable treatment for the influenza A/H1N1 2009 pandemic. However, several side effects were reported. The prevention of those effects requires an understanding of how genetic polymorphisms affect oseltamivir’s interaction with neuraminidases, metabolism, and transport. There was no record of its side effects in Mexico, despite its extensive use for influenza treatment. This study identified that only three patients (0.96%) presented side effects to oseltamivir treatment that were associated with neuropsychiatric adverse events (NPAEs) such as anxiety and depression. In contrast, during the 2009 influenza pandemic period in the USA and Japan, only ~0.002% of patients presented NPAE [10]. That percentage is less than two orders of magnitude of the results of our study.
P-glycoprotein and PepT1 are transporters that facilitate oseltamivir bioavailability. Both are expressed in the luminal membrane of the intestine and in brain capillaries [10, 17, 18, 33]. Oseltamivir phosphate but not oseltamivir carboxylate is a substrate for P-glycoprotein [17]. Genetic variants of the ABCB1 gene, which encodes human P-glycoprotein, have been identified, and some of those variants modify the transport rate of anticancer and anti-cholesterol drugs [22, 34]. The rs1045642 and rs2032582ABCB1 gene variants, which are the most extensively studied, have been associated with differences in expression and function [31, 32, 35, 36]. The frequency of the rs1045642 allele in the present population was not significantly different from its frequency in several European populations [35]. However, African populations have exhibited an elevated presence of the mutated allele (i.e. 73–83%) [36].
The P-glycoprotein polymorphism rs2032582 is strongly associated with rs1045642. Previous studies have demonstrated the influence of this mutation in neuropsychiatric diseases [26] and on the pharmacological effect of paroxetine [37]. Moreover, the rs1128503 polymorphism, which is related to abnormal folding of the protein, can segregate with rs1045642 and rs2032582 [36, 38]. This polymorphism of rs1128503has higher frequencies among South and East Asian populations (59% and 63% respectively) than our population (52.4%) and has been related to different clinical manifestations such as epilepsy [39] and adverse reactions to anticancer treatment [40]. In addition, an increased frequency of the T allele has been found in patients who presented with myalgia, but no association was reported [41].
The SNP rs3789243 is a polymorphism that is located in intron 3 of the ABCB1 gene. Although the function of rs3789243 is not well defined, studies have associated it with epilepsy [42] and the risk of suffering some types of cancer [43]. Our study determined that this allele occurs in 54.8% of the Mexican population (Table 6), which is similar to the frequency in American, African, and European populations (see 1000 Genomes Project Phase 3 allele frequencies at http://grch37.ensembl.org/Homo_sapiens/Info/Index). In contrast to a previous study that demonstrated that oseltamivir NPAEs might be related to the presence of rs2032582 and rs1045642 ABCB1 gene polymorphisms [26], our data indicated that only rs1045642 was associated with oseltamivir adverse effects.
PepT1 plays an important role in the absorption of dipeptides; tripeptides; and compounds that mimic peptides, including antibiotics administered orally such as β-lactams [44]. PepT1 also plays an important role in the transport of oseltamivir [18]. SLC15A1, the gene that encodes PepT1, presents several allelic forms. In particular, the rs2297322 polymorphism significantly reduces the transport capacity of PepT1 [45]. The objective of this study was to evaluate whether this polymorphism is associated with the adverse effects caused by treatment with oseltamivir. However, the data demonstrate that the allelic and genotypic frequencies of the SLC15A1 gene were not in Hardy–Weinberg equilibrium, and analysis of this mutation was therefore omitted from this paper.
In addition to bioavailability alterations, oseltamivir adverse effects have also been associated with deficiencies in its metabolism. CES1, the main enzyme responsible for oseltamivir metabolism [46], presents genetic polymorphisms that change the conformation of the protein, which results in enzymes with slow or no activity. In particular, in vitro and in vivo studies have demonstrated that the allelic variant rs71647871 presents a decreased oseltamivir metabolism [19]. As Table 4 demonstrates, the genotypic and allelic frequencies of the CES1 rs71647871 polymorphism in our Mexican population were <1%, similar to the frequencies reported in Americans [47]. Other genetic studies have revealed mutation frequencies in CES1 of 3.7% in white populations, 4.3% in black populations, 2.0% in Hispanic populations, and 0% in Asian populations [47]. Shi et al. suggested that this mutation could explain ~4% of the variability in the activation of oseltamivir [19]. While the rs2233385 NEU2 polymorphism has not been reported in European and African Americans, our results establish that the allelic frequency of this variant in the Mexican population was 7.4%, which is very similar to the frequency observed in Asian populations (9.2%) [29]. However, no association with oseltamivir side effects was found. Additional factors, such as oseltamivir’s interactions with other drugs and alterations of inflammatory cytokines, could influence the activity of receptors and enzymes. These factors might contribute to an increase in the accumulation of oseltamivir, thus increasing the occurrence of adverse effects in humans. The limitations of this study were that it analysed a small number of samples and observed a low number of adverse effects. For these reasons, it is necessary to validate our results in a larger number of samples to suggest to the polymorphism in the transporter as a potential predictive biomarker of ADRs in oseltamivir treatment.
In conclusion, the polymorphism rs1045642 in the transporter encoded by the ABCB1 gene is a potential predictive biomarker of ADRs in oseltamivir treatment.
Acknowledgements
Authors thank Mrs. Ana María Garza and Lic. Israel R. Benavides Páramo for their administrative support. RNGS was supported by CONACyT (Scholarship no. 182607).
Funding
This study was supported by Consejo Nacional de Ciencia y Tecnología, México (Grant number 162243).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fernandes-Matano L, Monroy-Munoz IE, Angeles-Martinez J, Sarquiz-Martinez B, Palomec-Nava ID, Pardave-Alejandre HD, et al. Prevalence of non-influenza respiratory viruses in acute respiratory infection cases in Mexico. PLoS ONE. 2017;12:e0176298. doi: 10.1371/journal.pone.0176298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Secretaria de Salud. Prevención y Control de las Enfermedades Respiratorias e Influenza 2013-2018, Programa Sectorial de Salud (page 43). https://www.gob.mx/cms/uploads/attachment/file/37944/PAE_PrevencionControlEnfermedadesRespiratoriasInfluenza2013_2018.pdf.
- 3.Panda S, Mohakud NK, Pena L, Kumar S. Human metapneumovirus: review of an important respiratory pathogen. Int J Infect Dis. 2014;25:45–52. doi: 10.1016/j.ijid.2014.03.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang J, Nawata K. A comparative study on predicting influenza outbreaks. Biosci Trends. 2017;11:533–41. [DOI] [PubMed]
- 5.Jefferson T, Jones M, Doshi P, Spencer EA, Onakpoya I, Heneghan CJ. Oseltamivir for influenza in adults and children: systematic review of clinical study reports and summary of regulatory comments. BMJ. 2014;348:g2545. doi: 10.1136/bmj.g2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta YK, Meenu M, Mohan P. The Tamiflu fiasco and lessons learnt. Indian J Pharm. 2015;47:11–16. doi: 10.4103/0253-7613.150308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dobson J, Whitley RJ, Pocock S, Monto AS. Oseltamivir treatment for influenza in adults: a meta-analysis of randomised controlled trials. Lancet. 2015;385:1729–37. doi: 10.1016/S0140-6736(14)62449-1. [DOI] [PubMed] [Google Scholar]
- 8.von Itzstein M, Wu WY, Kok GB, Pegg MS, Dyason JC, Jin B, et al. Rational design of potent sialidase-based inhibitors of influenza virus replication. Nature. 1993;363:418–23. doi: 10.1038/363418a0. [DOI] [PubMed] [Google Scholar]
- 9.Fiore AE, Fry A, Shay D, Gubareva L, Bresee JS, Uyeki TM. Antiviral agents for the treatment and chemoprophylaxis of influenza–recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2011;60:1–24. [PubMed] [Google Scholar]
- 10.Donner B, Bader-Weder S, Schwarz R, Peng MM, Smith JR, Niranjan V. Safety profile of oseltamivir during the 2009 influenza pandemic. Pharmacoepidemiol Drug Saf. 2011;20:532–43. doi: 10.1002/pds.2136. [DOI] [PubMed] [Google Scholar]
- 11.Gupta YK, Padhy BM. Issues in pharmacotherapy of 2009 H1N1 influenza infection. J Postgrad Med. 2010;56:321–7. doi: 10.4103/0022-3859.70945. [DOI] [PubMed] [Google Scholar]
- 12.Kitching A, Roche A, Balasegaram S, Heathcock R, Maguire H. Oseltamivir adherence and side effects among children in three London schools affected by influenza A(H1N1)v, May 2009—an internet-based cross-sectional survey. Eur Surveill. 2009;14:19287. doi: 10.2807/ese.14.30.19287-en. [DOI] [PubMed] [Google Scholar]
- 13.Toovey S, Rayner C, Prinssen E, Chu T, Donner B, Thakrar B, et al. Assessment of neuropsychiatric adverse events in influenza patients treated with oseltamivir: a comprehensive review. Drug Saf. 2008;31:1097–114. doi: 10.2165/0002018-200831120-00006. [DOI] [PubMed] [Google Scholar]
- 14.Ueda N, Umetsu R, Abe J, Kato Y, Nakayama Y, Kato Z, et al. Analysis of neuropsychiatric adverse events in patients treated with oseltamivir in spontaneous adverse event reports. Biol Pharm Bull. 2015;38:1638–44. doi: 10.1248/bpb.b15-00253. [DOI] [PubMed] [Google Scholar]
- 15.Blumentals WA, Song X. The safety of oseltamivir in patients with influenza: analysis of healthcare claims data from six influenza seasons. Med Gen Med. 2007;9:23. [PMC free article] [PubMed] [Google Scholar]
- 16.Gutierrez-Mendoza LM, Schwartz B, Mendez de Lira Jde J, Wirtz VJ. Oseltamivir storage, distribution and dispensing following the 2009 H1N1 influenza outbreak in Mexico. Bull World Health Organ. 2012;90:782–7. doi: 10.2471/BLT.11.101733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morimoto K, Nakakariya M, Shirasaka Y, Kakinuma C, Fujita T, Tamai I, et al. Oseltamivir (Tamiflu) efflux transport at the blood-brain barrier via P-glycoprotein. Drug Metab Dispos. 2008;36:6–9. doi: 10.1124/dmd.107.017699. [DOI] [PubMed] [Google Scholar]
- 18.Ogihara T, Kano T, Wagatsuma T, Wada S, Yabuuchi H, Enomoto S, et al. Oseltamivir (tamiflu) is a substrate of peptide transporter 1. Drug Metab Dispos. 2009;37:1676–81. doi: 10.1124/dmd.109.026922. [DOI] [PubMed] [Google Scholar]
- 19.Shi J, Wang X, Eyler RF, Liang Y, Liu L, Mueller BA, et al. Association of oseltamivir activation with gender and carboxylesterase 1 genetic polymorphisms. Basic Clin Pharmacol Toxicol. 2016;119:555–61. doi: 10.1111/bcpt.12625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poirier A, Belli S, Funk C, Otteneder MB, Portmann R, Heinig K, et al. Role of the intestinal peptide transporter PEPT1 in oseltamivir absorption: in vitro and in vivo studies. Drug Metab Dispos. 2012;40:1556–65. doi: 10.1124/dmd.112.044990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chavas LM, Tringali C, Fusi P, Venerando B, Tettamanti G, Kato R, et al. Crystal structure of the human cytosolic sialidase NEU2. Evidence for the dynamic nature of substrate recognition. J Biol Chem. 2005;280:469–75. doi: 10.1074/jbc.M411506200. [DOI] [PubMed] [Google Scholar]
- 22.George J, Dharanipragada K, Krishnamachari S, Chandrasekaran A, Sam SS, Sunder E. A single-nucleotide polymorphism in the MDR1 gene as a predictor of response to neoadjuvant chemotherapy in breast cancer. Clin Breast Cancer. 2009;9:161–5. doi: 10.3816/CBC.2009.n.026. [DOI] [PubMed] [Google Scholar]
- 23.Lewis JP, Horenstein RB, Ryan K, O’Connell JR, Gibson Q, Mitchell BD, et al. The functional G143E variant of carboxylesterase 1 is associated with increased clopidogrel active metabolite levels and greater clopidogrel response. Pharmacogenetics Genom. 2013;23:1–8. doi: 10.1097/FPC.0b013e32835aa8a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tarkiainen EK, Backman JT, Neuvonen M, Neuvonen PJ, Schwab M, Niemi M. Carboxylesterase 1 polymorphism impairs oseltamivir bioactivation in humans. Clin Pharmacol Ther. 2012;92:68–71. doi: 10.1038/clpt.2012.13. [DOI] [PubMed] [Google Scholar]
- 25.Chen F, Zhang B, Parker RB, Laizure SC. Clinical implications of genetic variation in carboxylesterase drug metabolism. Expert Opin Drug Metab Toxicol. 2018;14:131–42. doi: 10.1080/17425255.2018.1420164. [DOI] [PubMed] [Google Scholar]
- 26.L’Huillier AG, Ing Lorenzini K, Crisinel PA, Rebsamen MC, Fluss J, Korff CM, et al. ABCB1 polymorphisms and neuropsychiatric adverse events in oseltamivir-treated children during influenza H1N1/09 pandemia. Pharmacogenomics. 2011;12:1493–501. doi: 10.2217/pgs.11.91. [DOI] [PubMed] [Google Scholar]
- 27.Petryszyn PW, Wiela-Hojenska A. The importance of the polymorphisms of the ABCB1 gene in disease susceptibility, behavior and response to treatment in inflammatory bowel disease: a literature review. Adv Clin Exp Med. 2018;27:1459–63. doi: 10.17219/acem/92936. [DOI] [PubMed] [Google Scholar]
- 28.Liu S, Wang C, Chen Y, Peng S, Chen X, Tan Z. Association of SLC15A1 polymorphisms with susceptibility to dyslipidaemia in a Chinese Han population. J Clin Pharm Ther. 2019;44:868-74. [DOI] [PubMed]
- 29.Li CY, Yu Q, Ye ZQ, Sun Y, He Q, Li XM, et al. A nonsynonymous SNP in human cytosolic sialidase in a small Asian population results in reduced enzyme activity: potential link with severe adverse reactions to oseltamivir. Cell Res. 2007;17:357–62. doi: 10.1038/cr.2007.27. [DOI] [PubMed] [Google Scholar]
- 30.WHO. CDC protocol of realtime RTPCR for influenza A(H1N1). 2009. http://www.who.int/csr/resources/publications/swineflu/CDCRealtimeRTPCR_SwineH1Assay-2009_20090430.pdf.
- 31.Dalvi PS, Singh A, Trivedi HR, Mistry SD, Vyas BR. Adverse drug reaction profile of oseltamivir in children. J Pharmacol Pharmacother. 2011;2:100–3. doi: 10.4103/0976-500X.81901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B (Methodol) 1995;57:289–300. [Google Scholar]
- 33.Saito H, Okuda M, Terada T, Sasaki S, Inui K. Cloning and characterization of a rat H+/peptide cotransporter mediating absorption of beta-lactam antibiotics in the intestine and kidney. J Pharmacol Exp Ther. 1995;275:1631–7. [PubMed] [Google Scholar]
- 34.Leon-Cachon RBR, Ascacio-Martinez JA, Gamino-Pena ME, Cerda-Flores RM, Meester I, Gallardo-Blanco HL, et al. A pharmacogenetic pilot study reveals MTHFR, DRD3, and MDR1 polymorphisms as biomarker candidates for slow atorvastatin metabolizers. BMC Cancer. 2016;16:74. doi: 10.1186/s12885-016-2062-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silva-Zolezzi I, Hidalgo-Miranda A, Estrada-Gil J, Fernandez-Lopez JC, Uribe-Figueroa L, Contreras A, et al. Analysis of genomic diversity in Mexican Mestizo populations to develop genomic medicine in Mexico. Proc Natl Acad Sci USA. 2009;106:8611–6. doi: 10.1073/pnas.0903045106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ameyaw MM, Regateiro F, Li T, Liu X, Tariq M, Mobarek A, et al. MDR1 pharmacogenetics: frequency of the C3435T mutation in exon 26 is significantly influenced by ethnicity. Pharmacogenetics. 2001;11:217–21. doi: 10.1097/00008571-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 37.Kato M, Fukuda T, Serretti A, Wakeno M, Okugawa G, Ikenaga Y, et al. ABCB1 (MDR1) gene polymorphisms are associated with the clinical response to paroxetine in patients with major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:398–404. doi: 10.1016/j.pnpbp.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 38.Tanabe M, Ieiri I, Nagata N, Inoue K, Ito S, Kanamori Y, et al. Expression of P-glycoprotein in human placenta: relation to genetic polymorphism of the multidrug resistance (MDR)-1 gene. J Pharmacol Exp Ther. 2001;297:1137–43. [PubMed] [Google Scholar]
- 39.Oros MM. Genetic performance criteria for valproate in patients with epilepsy. Likars’ka Sprava. 2011;3–4:113–9. [PubMed] [Google Scholar]
- 40.Gonzalez-Haba E, Garcia MI, Cortejoso L, Lopez-Lillo C, Barrueco N, Garcia-Alfonso P, et al. ABCB1 gene polymorphisms are associated with adverse reactions in fluoropyrimidine-treated colorectal cancer patients. Pharmacogenomics. 2010;11:1715–23. doi: 10.2217/pgs.10.159. [DOI] [PubMed] [Google Scholar]
- 41.Hoenig MR, Walker PJ, Gurnsey C, Beadle K, Johnson L. The C3435T polymorphism in ABCB1 influences atorvastatin efficacy and muscle symptoms in a high-risk vascular cohort. J Clin Lipidol. 2011;5:91–6. doi: 10.1016/j.jacl.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 42.Haerian BS, Lim KS, Mohamed EH, Tan HJ, Tan CT, Raymond AA, et al. Lack of association of ABCB1 haplotypes on five loci with response to treatment in epilepsy. Seizure. 2011;20:546–53. doi: 10.1016/j.seizure.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 43.Andersen V, Ostergaard M, Christensen J, Overvad K, Tjonneland A, Vogel U. Polymorphisms in the xenobiotic transporter multidrug resistance 1 (MDR1) and interaction with meat intake in relation to risk of colorectal cancer in a Danish prospective case-cohort study. BMC Cancer. 2009;9:407. doi: 10.1186/1471-2407-9-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ganapathy ME, Brandsch M, Prasad PD, Ganapathy V, Leibach FH. Differential recognition of beta-lactam antibiotics by intestinal and renal peptide transporters, PEPT 1 and PEPT 2. J Biol Chem. 1995;270:25672–7. doi: 10.1074/jbc.270.43.25672. [DOI] [PubMed] [Google Scholar]
- 45.Zucchelli M, Torkvist L, Bresso F, Halfvarson J, Hellquist A, Anedda F, et al. PepT1 oligopeptide transporter (SLC15A1) gene polymorphism in inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:1562–9. doi: 10.1002/ibd.20963. [DOI] [PubMed] [Google Scholar]
- 46.Merali Z, Ross S, Pare G. The pharmacogenetics of carboxylesterases: CES1 and CES2 genetic variants and their clinical effect. Drug Metab Drug Interact. 2014;29:143–51. doi: 10.1515/dmdi-2014-0009. [DOI] [PubMed] [Google Scholar]
- 47.Zhu HJ, Patrick KS, Yuan HJ, Wang JS, Donovan JL, DeVane CL, et al. Two CES1 gene mutations lead to dysfunctional carboxylesterase 1 activity in man: clinical significance and molecular basis. Am J Hum Genet. 2008;82:1241–8. doi: 10.1016/j.ajhg.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]


