Abstract
Cancer vaccine development has proven challenging with the exception of some virally induced cancers for which prophylactic vaccines exist. Currently, there is only one FDA approved vaccine for the treatment of prostate cancer and as such prostate cancer continues to present a significant unmet medical need. In this study, we examine the effectiveness of a therapeutic cancer vaccine that combines the ISCOMATRIX™ adjuvant (ISCOMATRIX) with the Toll-like receptor 3 agonist, polyinosinic–polycytidylic acid (Poly I:C), and Flt3L, FMS-like tyrosine kinase 3 ligand. We employed the TRAMP-C1 (transgenic adenocarcinoma of the mouse prostate) model of prostate cancer and the self-protein mPAP (prostatic acid phosphatase) as the tumor antigen. ISCOMATRIX™–mPAP–Poly I:C–Flt3L was delivered in a therapeutic prime-boost regime that was consistently able to achieve complete tumor regression in 60% of animals treated and these tumor-free animals were protected upon rechallenge. Investigations into the underlying immunological mechanisms contributing to the effectiveness of this vaccine identified that both innate and adaptive responses are elicited and required. NK cells, CD4+ T cells and interferon-γ were all found to be critical for tumor control while tumor infiltrating CD8+ T cells became disabled by an immunosuppressive microenvironment. There is potential for broader application of this cancer vaccine, as we have been able to demonstrate effectiveness in two additional cancer models; melanoma (B16-OVA) and a model of B cell lymphoma (Eµ-myc-GFP-OVA).
Electronic supplementary material
The online version of this article (10.1007/s00262-020-02597-6) contains supplementary material, which is available to authorized users.
Keywords: Prostate cancer, Adjuvant, Therapeutic vaccine, NK cells, CD4+ T cells, TRAMPC1
Introduction
Evidence suggests that it is possible to vaccinate against cancer, at least in the case of virally induced cancers. In these instances, the vaccine targets the viral causative agent and not the cancer itself. Indirect evidence to support the possibility of using host immunity in the fight against non-virally induced cancers comes in the form of the success story that are the checkpoint inhibitors. These immunotherapies show us that an anti-tumor T cell response is mounted but becomes impaired along the way by the immunosuppressive microenvironment. ‘Taking the brakes off the system’ with these checkpoint inhibitors reveals just how effective an anti-tumor immune response can be [1].
Prostate cancer continues to present a significant unmet medical need with approximately 30,000 men dying from the disease each year in the USA alone, second only to lung cancer [2]. Active surveillance, radiation and radical prostatectomy are the most common treatment options in the management of early stages of prostate cancer. Although these treatments have very good 5-year survival rates (approaching 100%), albeit with a range of potential side effects, it is estimated that 20–30% of patients will show signs of disease recurrence after the 5-year follow-up period (www.pcf.org). Priming the immune system so that it may be able to assist in ‘mopping up’ cancerous cells that remain following radiation or surgery, reveals a window of opportunity for adjunctive vaccination in the treatment/management of prostate cancer. A challenge to this opportunity is the immunologically ‘cold’ nature of prostate cancer and this challenge is clear when you consider that only one prostate cancer vaccine has been approved since 2010. Patients vaccinated with the FDA approved PROVENGE®-PAP-GM-CSF loaded antigen presenting cells, an autologous cellular cancer immunotherapy for the treatment of asymptomatic or minimally symptomatic metastatic castrate-resistant prostate cancer (mCRPC), experienced median survival benefits of 4.1 months [3]. The phase III PROSPECT trial for the prostate vaccine development by Bavarian Nordic, PROSTVAC, failed to show an overall survival benefit as a monotherapy [4] but clinical trials exploring its potential in combination strategies are ongoing.
Currently, it is well accepted that cancer treatment will require multiple therapy combinations, including for instance, multi-adjuvanted vaccines and check point inhibitors [1, 5–7]. Combination strategies aim to elicit anti-tumor immune responses of greater magnitude and quality capable of overcoming tumor immunosuppression or resistance mechanisms. Most immunomodulatory strategies have focussed on reactivating T cells but there is scope to expand on these strategies to boost innate immune responses. NK cells have long been recognized for their potential to kill cancer cells and have been exploited in a range of therapeutic approaches such as autologous cell transfer, monoclonal antibodies engineered for increased ADCC (antibody-dependent cellular cytotoxicity) activity, chimeric antigen receptors [8–10] and are potential targets for checkpoint inhibitors [11–13]. Designing immunotherapies that stimulate NK cells in addition to effector T cells could result in more effective therapies by harnessing the full complement of NK-driven anti-tumor effector mechanisms [14, 15].
In the last years, we have investigated the use of the saponin-based adjuvant, ISCOMATRIX™ adjuvant, in cancer vaccines due to its ability to induce both tumor Ag-specific cellular and Ab responses, as well as, NK cell activation. Initial studies in cancer models demonstrated that ISCOMATRIX™ adjuvanted vaccines, comprising tumor antigen and adjuvant, were efficacious in the prophylactic setting [16, 17] but for effectiveness in the therapeutic setting, it is likely that additional vaccine components would be required. Recently, we and others have sought to combine multiple adjuvants in an effort to improve the efficacy of vaccines containing poorly immunogenic antigens and cancer vaccines [18–22]. The addition of PolyI:C and CpG Oligodeoxynucleotides (CpG) and a tumor antigen revealed the potential of an ISCOMATRIX™ adjuvant and TLR agonist combination cancer vaccine [22]. In this present study, we examine the effectiveness of a prostate cancer vaccine that combines the ISCOMATRIX™ adjuvant and the tumor antigen mPAP with the TLR3 agonist, Poly I:C, and the immunostimulatory cytokine Flt3L. The goal of this study was to identify a vaccine that could provide therapeutic protection against a poorly immunogenic tumor. Moreover, the mechanism of action (MoA) of this combination therapeutic prostate cancer vaccine was probed. These studies revealed that NK cells and CD4+ T cells, optimally stimulated by this vaccine combination, can drive an effective anti-tumor immune response that can overcome the need for CD8+ T cells that were found to acquire an exhausted phenotype over time. The vaccine revealed striking effects on NK cell activation and function and induced a robust protective memory response. Importantly, the vaccine showed impact on prostate weight in the pre-metastatic phase in a model of spontaneous prostate cancer that more closely reflects the human disease. In addition, the vaccine has potential for broader application as we demonstrated significant efficacy in the melanoma (B16-OVA) and lymphoma (Eµ-myc-OVA) mouse models.
Materials and methods
Animals and cell lines
TRAMP-C1 cells [23, 24] were grown in TRAMP tissue culture medium (Dulbecco’s Modified Eagle Medium (DMEM, Sigma Aldrich, St Louis, MO, USA) supplemented with 10% (w/v) FBS (Sigma Aldrich), 5 µg/ml insulin, cell-culture grade 0.01 nM dihydrotestosterone (Sigma) and 100 U/ml penicillin/100 µg/ml streptomycin) in a 37 °C, 5% CO2 incubator and were harvested by trypsinization when cells were ~ 80–90% confluent. B16-OVA cells were grown in RPMI-1640 complete media [10% (w/v)] FBS (Sigma Aldrich St Louis, MO, USA), 100 U/ml penicillin/100 µg/ml streptomycin supplemented with 0.4 mg/ml of G418/Geneticin selection in a 37 °C, 10% CO2 incubator and were harvested with Accutase (Sigma Aldrich, St Louis, MO, USA) when cells were ~ 80–90% confluent. Thawed Eµ-myc-OVA [25], B cell lymphoma, cells were injected directly into mice. C57BL/6J (CD45.2), B6.SJL/J-PTPRCa (CD45.1), C57BL/6-Tg(TRAMP)8247Ng/J (TRAMP Transgenic-Tg) and B6.129S7-Ifngtm1Ts/J (IFNγ KO) mice were used in experiments. In all cases, sex matched mice at 7–12 weeks of age were used except in memory experiments where control mice were aged to match memory mice.
Antigens and adjuvants
ISCOMATRIX™ adjuvant (CSL Biotherapies, King of Prussia, PA; ISCOMATRIX is a registered trademark of ISCOTEC Ab a CSL company; ISCO is a registered trademark of CSL) was prepared as described previously and used at 3.8 ISCO units/dose [26]. Poly I:C (InvivoGen, San Diego, CA, USA) was used at 5 µg/dose and low-endotoxin chicken OVA (Hyglos, Germany) was used at 30 µg/dose. mPAP was produced as previously described [22] and was used at 300 µg/dose.
Vaccination and tumor challenge protocols
For therapeutic tumor experiments anesthetized mice were injected s.c. with 5 × 105 B16-OVA and 3 × 106 TRAMP-C1 cells on the right flank. Vaccinations were performed on days 2 and 9. 10 µg of Flt3L (BioXCell, West Lebanon, NH, USA) was injected s.c. at the scruff of the neck, daily for 9 days from the day of tumor inoculation. Tumor size (subcutaneous tumor area) was estimated by measuring two bisecting diameters of the tumor. Mice were euthanized when the tumor mass reached 100 mm2. The percentage of survival and tumor-free mice were also determined. For memory experiments, mice were rechallenged ~ 100 days after complete regression of primary tumors with 3 × 106 TRAMP-C1 cells on the right flank. For Eµ-myc B cell lymphoma experiments, PtpRCa mice (CD45.1+) were injected i.v. with 1 × 103 Eµ-myc-GFP-OVA lymphoma cells (CD45.2+). Mice were prime-boosted as described above. Tumor mice were monitored daily for signs of illness and euthanized when advanced ill-health was observed. Spleens were harvested for analysis of tumor burdens that were determined by the number of CD45.2+CD19+ cells per spleen. For analysis of TRAMP Tg mice and littermate controls, animals were primed at 6 weeks of age and boosted a week later. Mice received Flt3L for 9 consecutive days from the day of prime. At week 15 or weeks 21–24 mice were weighed and prostates, ampullary glands and seminal vesicles were collected, weighed and fixed for his histological analysis. Histological analysis was performed by VepaLabs (Rowville, Australia).
In vivo NK cell, CD4+ and CD8+ T cell depletions
On day 0, C57BL/6 mice were inoculated with B16-OVA tumor cells as described previously. For NK cell depletion, mice were injected with 100 µg i.p. anti-NK1.1 mAb (PK136; BioXCell, West Lebanon, NH, USA) or isotype control on days 1, 2, and 6 and then every 5 days thereafter for the duration of the experiment. For depletion of CD4+ T cells, mice were injected with 100 µg i.p. anti-GK1.5 mAb (GK1.5; Walter and Eliza Hall Institute, Melbourne, Victoria Australia) or isotype control 1 day prior to tumor implantation and then daily for 3 days. For maintenance of CD4+ T cell depletion mice were injected weekly for the duration of the experiment. For depletion of CD8+ T cells, mice were injected with 100 µg per mouse rat anti-CD8β mAb (53-5.8; Walter and Eliza Hall Institute, Melbourne, Victoria, Australia) or isotype control, i.p., 1 day prior to tumor implantation and then for the following 2 days. Blood was sampled 4 days later to check for CD8+ T cell depletion using anti-CD8α Ab (53-6.7, BD Biosciences, Franklin Lakes, NJ, USA). Depletion efficiency was > 95%. For maintenance of CD8+ T cell depletion mice were injected weekly for the duration of the experiment.
Isolation and staining of TILs
Tumors were excised from mice and minced finely using a scalpel blade. Minced tumors were placed in 5 ml of RPMI 1640:DMEM media (1:1), containing collagenase type 4 (1 mg/ml, Sigma St Louis, MO, USA) and DNase I (20 μg/ml, Roche, Basel, Switzerland). Tumors were digested at 37 °C for 45 min, rotating and rolling gently using a MACSmix Tube rotator (Miltenyi Biotec, Germany). Tumor suspensions were then passed through a 40-μm cell strainer and washed twice in RPMI 1640 complete media. Isolated TILs were then stained with anti-CD45 (30-F11), anti-CD11b (M1/70), anti-CD8 (53-6.7), anti-CD4 (RM4-5), anti-NK1.1 (PK136), anti-PD1 (EH12.1) and anti-TIM3 (5D12) (all BD Pharmingen, San Diego, CA, USA). TILs were analyzed by flow cytometry using a FACS Canto instrument or Fortessa (BD Biosciences, Franklin Lakes, NJ, USA).
Analysis of NK cell phenotype and function
Mice were vaccinated as described above. Twenty-four hours after priming draining axial/brachial LN was harvested and the number of CD3+NK1.1+ NK cells was enumerated. NK cells were stained with anti-CD3 (17A2) and anti-NK1.1 (PK136) (both from BD Biosciences, Franklin Lakes, NJ, USA). NK cell activation was determined by surface staining using anti-CD69 (H1.2F3, Biolegend, San Diego, CA, USA). Eight hours after priming, NK cells from single-cell-suspensions of the draining axial/brachial LN were stained for intracellular IFNγ. Cells were cultured ex vivo for 4 h in the presence of brefeldin A1 (1000x, eBioscience, San Diego, CA, USA). Cells were stained with anti–CD3 (17A2) and anti–NK1.1 (PK136) (both from BD Biosciences, Franklin Lakes, NJ, USA) washed, fixed, and permeabilized (Perm/Wash; BD Biosciences, Franklin Lakes, NJ, USA). Cells were then stained with anti–IFN-g (XMG1.2; BD Biosciences, Franklin Lakes, NJ, USA). Similarly, 24 h after vaccination NK cells from single-cell-suspensions of the spleen were stained for intracellular Granzyme B (GzmB). GzmB was detected using anti-human GzmB (GB12; Invitrogen, Waltham, MA, USA) using a Cytofix/Cytoperm kit (eBioscience, San Diego, CA, USA). Direct killing of TRAMP-C1 cells by NK cells was assessed using an in vitro killing assay. MACS purified NK cells (NK cell isolation kit II, Miltenyi Biotec, Germany) isolated 24 h post-prime or stimulated in vitro for 4 days with IL-15 and IL-18 were cultured with 5000 TRAMP-C1 cells for ~ 21 h. The remaining viable cells were enumerated by flow cytometry using 7-AAD exclusion. All flow cytometric analysis was carried out using a FACSCanto or Fortessa instrument (BD Biosciences, Franklin Lakes, NJ, USA) and data were analyzed using FlowJo flow cytometry analysis software.
Results
ISCOMATRIX™ adjuvant formulated with mPAP, Poly I:C and Flt3L provides therapeutic protection against TRAMP-C1 tumors
As prostate cancer is considered an immunologically ‘cold’ tumor, we speculated that adaptive immune responses to TRAMP-C1 tumors could be poor. In considering how we could boost innate immune responses and/or stimulate more robust adaptive immune responses, we devised a combination of adjuvant (ISCOMATRIX™), TLR ligand (Poly I:C) and a hematopoietin (Flt3L). Belonging to different classes of immunomodulators, we speculated these molecules could synergise to stimulate a broad anti-tumor immune response in TRAMP-C1 tumor-bearing mice.
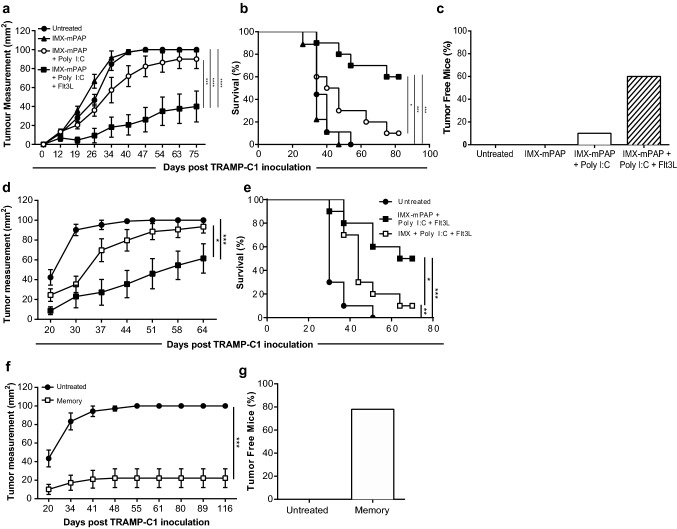
Prime-Boost vaccination with ISCOMATRIX™–mPAP–Poly I:C–Flt3L induced significant protection in mice bearing TRAMP-C1 tumors compared to animals receiving only some of the vaccine components (Fig. 1a). The vaccine improved survival (Fig. 1b) and impaired tumor growth and remarkably was also capable of inducing complete responses against TRAMP-C1 tumors in 60% of vaccinated mice (Fig. 1c). The vaccine response was very durable with survival advantages maintained out to over 75 days. Therapeutic efficacy was also observed when mice were subjected to a delayed vaccination schedule (prime-boost vaccination on days six and thirteen, respectively) also resulted in excellent control of tumor growth, overall survival and percentage tumor-free mice over an extended time frame (Supplementary Fig. 1). Comparable results led to the selection of day two and day nine prime-boost vaccinations for all subsequent experiments.
Fig. 1.
ISCOMATRIX™ adjuvant formulated with mPAP, Poly I:C and Flt3L provides therapeutic protection against TRAMP-C1 tumors. Animals that were untreated were compared to animals that were vaccinated with either, IMX–mPAP, IMX–mPAP + pIC or IMX–mPAP + pIC + Flt3. Day 2 prime and day 9 boost (a–c). a Tumor growth. b Percent survival. c Tumor free mice at conclusion of experiment. d, e Mice vaccinated with IMX + pIC + Flt3 plus or minus antigen-mPAP were compared for d growth of TRAMP-C1 tumor and e percent survival. f, g Mice previously challenged with TRAMP-C1 tumors that had received the combination vaccine IMX–mPAP + pIC + Flt3L, that had become tumor free and had remained so for at least 90 days, were rechallenged with TRAMP-C1 cells. f Tumor growth in protected animals was compared to age-matched naïve mice inoculated with TRAMP-C1 g Tumor free mice. Data are presented as mean ± SEM where n = 10 mice/group from one representative experiment of three equivalent experiments (a–e) or where n = 10 (Untreated) and 18 (memory) (f, g) mice/group from one representative experiment of two equivalent experiments. Statistical significance in tumor growth (a, d, f) was determined using a two-way ANOVA with Tukey’s multiple comparisons test. Percent survival (b, e) was plotted as a Kaplan–Meier curve and the log-rank (Mantel–Cox) test was used to calculate statistical significance. p < 0.05*, p < 0.01**, p < 0.001*** and p < 0.0001****. IMX = ISCOMATRIX® adjuvant
Antigen specificity and memory immune responses upon rechallenge are hallmarks of vaccination. To show that the effectiveness of this vaccine was at least in part due to antigen-specific mechanisms, TRAMP-C1 tumor growth in animals vaccinated with the full ISCOMATRIX™–mPAP–Poly I:C–Flt3L combination were compared to animals vaccinated with the combination of ISCOMATRIX™–Poly I:C–Flt3L, lacking the mPAP tumor antigen. When the tumor antigen mPAP was absent from the vaccine combination, tumor growth proceeded unchecked, whereas when mPAP was included, tumor growth was better controlled (Fig. 1d) and animals experienced improved survival (Fig. 1e). To assess the potential of the ISCOMATRIX™–mPAP–Poly I:C–Flt3L vaccine to induce memory, vaccinated mice previously challenged with TRAMP-C1 tumors that had become tumor-free and had remained so for ~ 100 days, were rechallenged with TRAMP-C1 tumors. Vaccinated animals were protected from rechallenge with TRAMP-C1 tumors (Fig. 1f), with 80% of animals remaining free of tumor (Fig. 1g). As expected all age-matched unvaccinated animals receiving a primary TRAMP-C1 challenge succumbed to their tumor burdens. The resistance to rechallenge with TRAMP-C1 tumors indicates that the ISCOMATRIX™–mPAP–Poly I:C–Flt3L vaccine is indeed able to induce a robust and persistent protective memory response.
Vaccine stimulated NK cells and CD4+ T cells drive anti-tumor immune responses as tumor-infiltrating CD8+ T cells are disabled by exhaustion
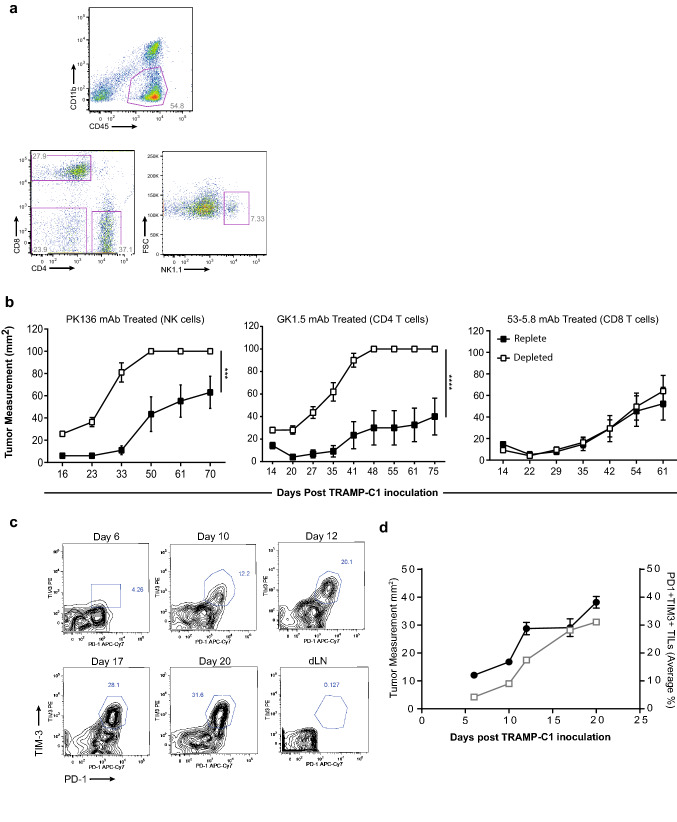
To understand the underlying immunological mechanisms contributing to the effectiveness of the ISCOMATRIX™–mPAP–Poly I:C–Flt3L vaccine we focused on the major immune effector cells that mediate tumor-killing responses; CD4+ and CD8+ T cells and NK cells. Analysis of TRAMPC1 tumor infiltrating lymphocytes (TILs) by staining of dissociated tumors identified that CD4+ T cells, CD8+ T cells and NK cells all infiltrate TRAMP-C1 tumors (Fig. 2a) TRAMP-C1 tumor growth was monitored in vaccinated animals that had been depleted of NK cells, CD4+ T cells or CD8+ T cells. In the absence of both NK cells (Fig. 2, left panel) and CD4+ T cells (Fig. 2, middle panel), the ISCOMATRIX™–mPAP–Poly I:C–Flt3L vaccine was no longer able to control TRAMP-C1 tumor growth. All animals lacking NK cells or CD4+ T cells were euthanized with maximal tumor burdens, and the tumor grew with kinetics similar to that of untreated animals. This observation indicates that both NK and CD4 T+ cell-mediated mechanisms contributed to the anti-tumor response induced by this vaccine. Surprisingly vaccinated animals depleted of CD8+ T cells were not impaired in their capacity to control tumor growth (Fig. 2, right panel). CD8+ T cells were shown to infiltrate TRAMPC1 tumors (Fig. 2a) but a time-course analysis of CD8+ TILs from unvaccinated animals revealed that these cells acquired an exhausted PD1+TIM3+ phenotype [27] overtime (Fig. 2d) limiting their ability to participate in the anti-tumor immune response. While CD8+ T cells may not contribute significantly to reducing the volume of tumor material as measured in these experiments, CD8+ T cells that have been instructed by the ISCOMATRIX™–mPAP–Poly I:C–Flt3L vaccine are likely responsible for the robust and persistent memory responses observed.
Fig. 2.
Vaccine stimulated NK cells and CD4+ T cells control tumor burden while an immunosuppressive tumor microenvironment drives CD8+ TIL exhaustion. a CD4+ T cells, CD8+ T cells and NK cells infiltrate TRAMP-C1 tumors, b mice were depleted of either NK cells, CD4+ T cells or CD8+ T cells. Tumor growth in NK depleted mice (left panel), CD4+ T cell depleted mice (middle panel) and CD8+ T cell depleted mice (right panel), c time-course of representative CD8+PD1+TIM3+ TIL profiles d Proportion of PD1+TIM3+ TILs (open squares) as a function of time and tumor size (filled circles). Data are presented as mean ± SEM where n = 9–10 mice/group from one representative experiment of two equivalent experiments. Statistical significance in tumor growth was determined using a two-way ANOVA. p < 0.01** and p < 0.001***. IMX = ISCOMATRIX® adjuvant
ISCOMATRIX™–mPAP–Poly I:C–Flt3L vaccine induces strong phenotypic and functional activation of NK cells
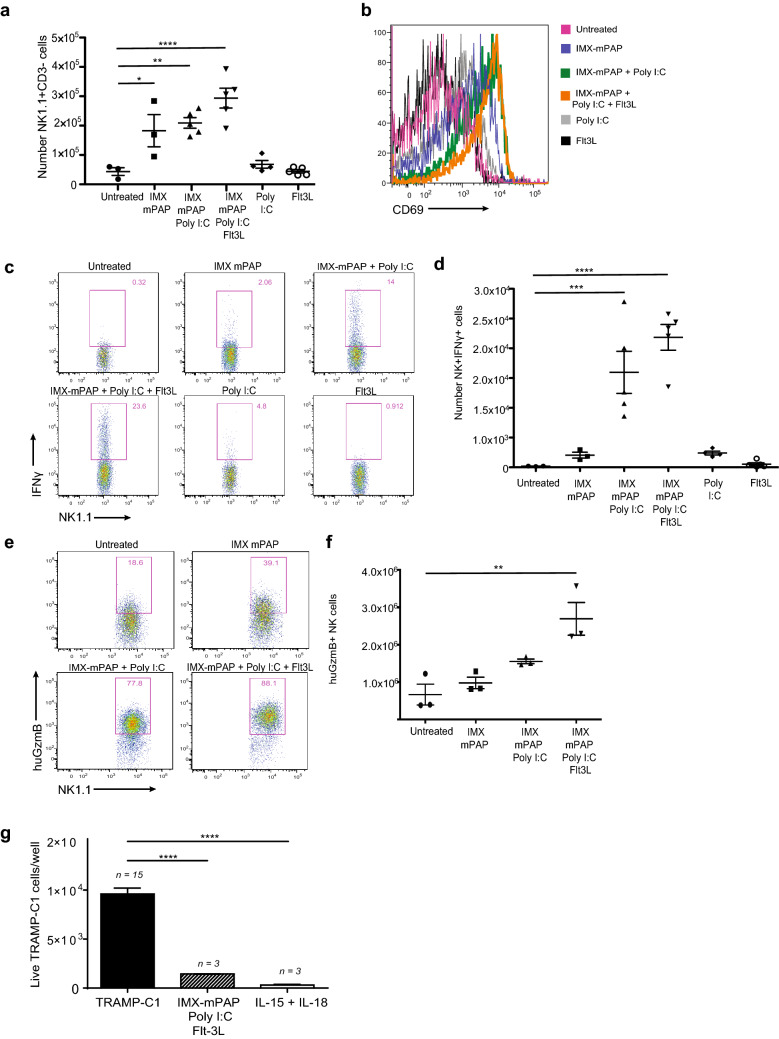
NK cells were first identified for their ability to kill tumor cells [28–30] and have since been implicated in the elimination of many murine and human cancers. With such a well described role in the anti-tumor immune response and a clear indication that NK cells contribute significantly to TRAMP-C1 tumor control following vaccination with ISCOMATRIX™–mPAP–Poly I:C–Flt3L (Fig. 2b, left panel), we further investigated how these critical innate cells responded to our combination vaccine. The ISCOMATRIX™–mPAP–Poly I:C–Flt3L vaccine induced the greatest numbers of NK cells (Fig. 3a) compared to animals vaccinated with simplified vaccine combinations or Poly I:C/Flt3L alone. NK cell expansion is not unexpected given repeat administration of soluble human Flt3L has been shown to expand NK cell numbers [31]. Administration of Flt3L alone does indeed increase NK cell numbers as seen post-boost in the spleen (after which mice have received 10ug of Flt3L over 9 days) (Supplementary Fig. 2) but the increase of the NK cell numbers with the ISCOMATRIX™–mPAP–Poly I:C–Flt3L vaccine is even greater. NK cells displayed higher levels of the early activation marker CD69 when exposed to the full vaccine combination (Fig. 3b). Poly I:C itself is a strong stimulus for CD69 expression on NK cells [32] and we did find that CD69 expression went up on cells treated with Poly I:C alone or the vaccine combination ISCOMATRIX™–mPAP–Poly I:C (Fig. 3b). These findings suggest that the components of our vaccine synergise to stimulate NK cells of greater magnitude and superior activation than can be attributed to Poly I:C alone.
Fig. 3.
Vaccination induces strong phenotypic and functional activation of NK cells that are prepared for tumor elimination. a Enumeration of NK cells. b NK cell activation was assessed using the early activation marker CD69. Representative histograms showing the geometric mean fluorescence intensity of CD69 staining. c Representative profiles of NK cells producing IFNγ (ex vivo) 8 h after priming. d Enumeration of IFNγ-producing NK cells. e Representative profiles of NK cells producing Granzyme B (ex vivo), 24 h after boosting, detected by ICS. f Granzyme B-producing NK cells were enumerated. g in vitro TRAMP-C1 killing assay. n, indicates the number of replicate wells. Data are presented as mean ± SEM where n = 3–5 mice/group from one representative experiment of two equivalent experiments. a, d, f, g Statistical significance was determined using a one-way ANOVA with Tukey’s multiple comparisons test. p < 0.05*, p < 0.01**, p < 0.001*** and p < 0.0001****. IMX = ISCOMATRIX® adjuvant
We explored further the effect of the ISCOMATRIX™–mPAP–Poly I:C–Flt3L vaccine on the function of NK cells. NK cells secrete cytokines, such as IFN-γ, that participate in the shaping of the adaptive immune response [33]. Following vaccination, NK cells express more IFN-γ (Fig. 3c, d) than animals having received ISCOMATRIX™–mPAP or ISCOMATRIX™–mPAP–Poly I:C. Poly I:C alone can directly stimulate NK cells to produce IFN-γ [32]. We detected IFN-γ production by NK cells following Poly I:C stimulation alone but IFN-γ levels were lower in this group as compared to animals vaccinated with the full combination vaccine (Fig. 3c). As another measure of NK function we assessed the expression of the cytotoxic mediator GzmB. As was the case for NK cell expansion, CD69 and IFN-γ expression, GzmB levels were greatest in animals vaccinated with ISCOMATRIX™–mPAP–Poly I:C–Flt3L (Fig. 3e, f). Taking together these findings indicate that vaccination with ISCOMATRIX™–mPAP–Poly I:C–Flt3L generates NK cells that are phenotypically and functionally primed for elimination of TRAMP-C1 tumors. Dissecting NK cell responsiveness after exposure to individual vaccine components in ISCOMATRIX™–mPAP, PolyI:C and Flt3L, compared to the full vaccine combination, highlights that the immunogenicity of the combination is greater than just the sum of its parts.
Given the numerical, phenotypic and functional activation of NK cells by the ISCOMATRIX™–mPAP–Poly I:C–Flt3L vaccine, we asked if NK cells were capable of directly killing TRAMP-C1 tumor cells? To test this, we purified NK cells from mice that had been vaccinated with ISCOMATRIX™–mPAP–Poly I:C–Flt3L and cultured these with TRAMP-C1 cells in vitro and measured TRAMP-C1 cell death. Ex vivo, NK cells from ISCOMATRIX™–mPAP–Poly I:C–Flt3L vaccinated animals were able to directly kill TRAMP-C1 cells (Fig. 3g). Overall, the data strongly support that NK cell mediated killing of TRAMP-C1 tumor cells contributes to the MoA of the ISCOMATRIX™–mPAP–Poly I:C–Flt3L vaccine.
Interferon-gamma is critical for tumor control following vaccination
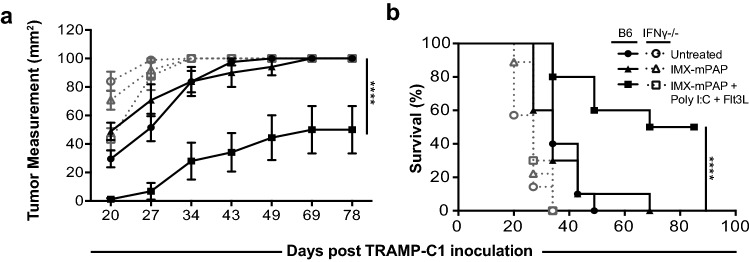
The critical role NK cells and CD4+ T cells play in the anti-tumor response led us to investigate the role of IFN-γ, as a key cytokine produced by both of these cells. We took mice deficient in IFN-γ (IFN-γ KO) and monitored TRAMP-C1 tumor growth following vaccination with ISCOMATRIX™–mPAP–Poly I:C–Flt3L. Vaccinated IFN-γ KO mice were severely impaired in their ability to control tumor growth compared to their vaccined WT controls (Fig. 4a). This was also reflected in the poor overall survival (Fig. 4b). These data indicate that IFN-γ produced following vaccination with ISCOMATRIX™–mPAP–Poly I:C–Flt3L is important for TRAMP-C1 tumor control and contributes significantly to the MoA of this therapeutic combination vaccine.
Fig. 4.
Interferon-gamma is critical for tumor control following vaccination. a Tumor growth in IFNγ-/- mice and b percent survival were monitored for the duration of the experiment. Data are presented as mean ± SEM where n = 10 mice/group from one representative experiment of two equivalent experiments. Statistical significance in tumor growth a was determined using a two-way ANOVA with Tukey’s multiple comparisons test. Percent survival b was plotted as a Kaplan–Meier curve and the log-rank (Mantel–Cox) test was used to calculate statistical significance. p < 0.05*, p < 0.01**, p < 0.001*** and p < 0.0001****. IMX = ISCOMATRIX® adjuvant
Vaccination with ISCOMATRIX™–mPAP + Poly I:C + Flt3L leads to reduced prostate weight in a model of spontaneous prostate cancer
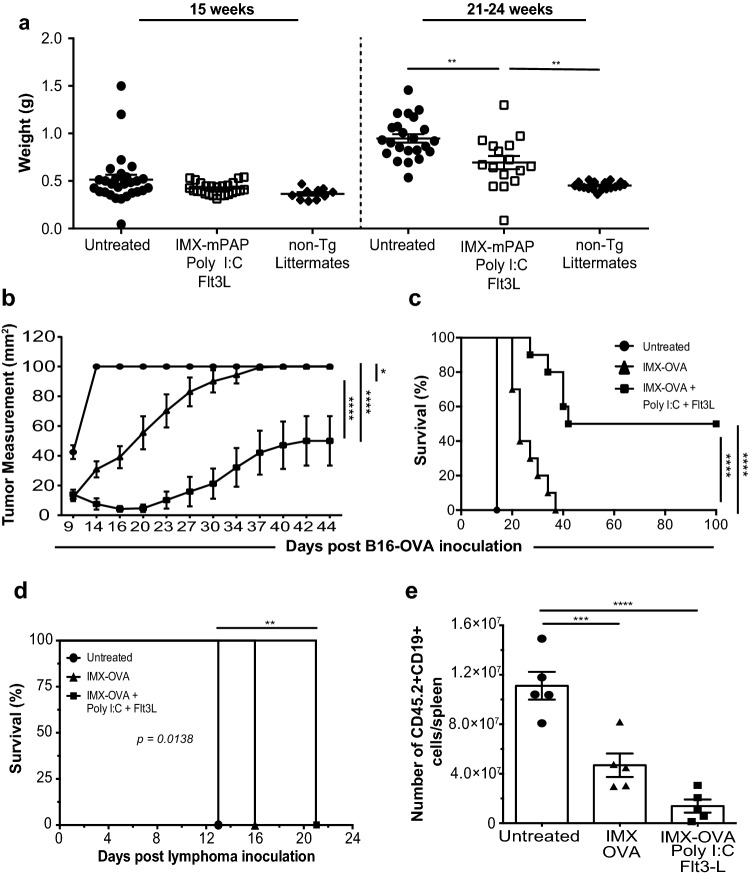
Having demonstrated good efficacy for the ISCOMATRIX™–mPAP + Poly I:C + Flt3L vaccine against the transplantable TRAMP-C1 cell line, we wanted to test the vaccines potential to suppress denovo prostate tumor growth in a spontaneous model that more closely reflects the human disease. To do this we moved to the TRAMP Tg mice, from which the TRAMP-C1 cell line was derived. These mice express the SV40 large T antigen oncogene under transcriptional control of the prostate-specific rat probasin promoter. Orthotopic prostate tumors spontaneously develop from the age of 10 weeks, which histologically resemble human prostate cancer [24]. TRAMP Tg mice were vaccinated with a prime at 6 weeks and 1 week later were boosted with ISCOMATRIX™–mPAP + Poly I:C + Flt3L. Mice received nine consecutive doses of Flt3L from the day of prime. At week 15 (well-moderately differentiated carcinomas) or weeks 21–24 (poorly differentiated adenocarcinoma) [34] mice were weighed and prostates, ampullary glands and seminal vesicles were collected, weighed and fixed for histological analysis. These time points were selected for analysis as they represented primary disease and not metastatic disease. Metastatic disease is a setting in which the disease burden would likely be too great to be impacted by adjunctive vaccination as is the concept presented in this study. At week 15 there was no difference in prostate weights, an indicator of disease burden, compared to littermate controls, however by 21–24 weeks, mice vaccinated with ISCOMATRIX™–mPAP + Poly I:C + Flt3L showed a reduction in prostate + seminal vesicle weight compared to littermate controls (Fig. 5a). Although a reduction in prostate/seminal vesicle wet weight indicates an impact of macroscopic disease burden, histological analysis indicated differentiation to adenocarcinoma was not interrupted (data not shown) by vaccination with ISCOMATRIX™–mPAP + Poly I:C + Flt3L.
Fig. 5.
IMX–mPAP + Poly I:C–Flt3 vaccine protects against both solid and non-solid cancers. a Prostates and seminal vesicles weights of vaccinated TRAMP Tg mice and littermate controls at week 15 or weeks 21–24. Data are presented as mean ± SEM where n = 22–30 mice/group pooled from eleven experiments. Statistical significance was determined using a one-way ANOVA using Tukey’s multiple comparisons test. p < 0.01**. b B16-OVA tumor growth and c Percent survival d Eµ-myc-GFP-OA lymphoma growth and e percent survival. Statistical significance was determined using a one-way ANOVA using Tukey’s multiple comparisons test. Data are presented as mean ± SEM where n = 10 (b, c) or n = 5 (d, e) mice/group from one representative experiment of three equivalent experiments. Statistical significance in tumor growth b was determined using a two-way ANOVA with Tukey’s multiple comparisons test. Percent survival c, d) was plotted as a Kaplan–Meier curve and the log-rank (Mantel–Cox) test was used to calculate statistical significance. p < 0.05*, p < 0.01**, p < 0.001*** and p < 0.0001****. IMX = ISCOMATRIX® adjuvant
ISCOMATRIX™–OVA–Poly I:C–Flt3L vaccination can also protect against B16-OVA melanoma and Eµ-myc-GFP-OVA B cell lymphoma
To further extend the significance of these studies we examined the therapeutic efficacy of the ISCOMATRIX™–tumor Ag–Poly I:C–Flt3L vaccine in two additional mouse models of cancer; B16-OVA melanoma and the B cell lymphoma model Eµ-myc-GFP-OVA that models human Burkitt’s lymphoma. In these tumor models, the surrogate tumor antigen OVA was used as the vaccine antigen. B16-OVA challenged animals that had received the ISCOMATRIX™–OVA–Poly I:C–Flt3L vaccine were better able to control tumor growth (Fig. 5b) and demonstrated excellent overall survival (Fig. 5c). Lymphoma-bearing mice that were left untreated developed advanced illness on day 13 post-lymphoma inoculation (Fig. 5d). In contrast, mice vaccinated with ISCOMATRIX™–OVA–Poly I:C–Flt3L did not develop signs of advanced illness until day 21 (Fig. 5d), a 60% extension of lifespan over unvaccinated littermates. To confirm tumor elimination in vaccinated mice, spleens were analyzed for tumor burden (CD45.2+CD19+ cells) 13 days post-lymphoma inoculation. ISCOMATRIX™–OVA–Poly I:C–Flt3L vaccinated animals showed a significant reduction in lymphoma burden compared to untreated mice or mice vaccinated with ISCOMATRIX™–OVA (Fig. 5e). These results highlight the potential broad application for this cancer vaccine.
Discussion
We have previously shown that a multi-adjuvanted vaccine strategy combining the self-Ag PAP and ISCOMATRIX™ adjuvant, with Poly I:C and CpG can provide some therapeutic efficacy against TRAMP-C1 tumors [22]. This Poly I:C and CpG containing vaccine combined multiple TLR ligands that are well known for their adjuvant-like properties, however, the more restricted expression of TLR9 to pDCs and B cells in humans [35] may limit the effectiveness of CpG for inducing T cell immunity when used in patients [36]. We speculated that by combining immunomodulators from different classes, not just TLR ligands, a more protective vaccine response could be induced with better translational potential. With this aim we identified the vaccine combination ISCOMATRIX™–mPAP–Poly I:C–Flt3L. This therapeutic combination vaccine shows efficacy in the TRAMP-C1 model of prostate cancer when delivered in a prime-boost regime. This vaccine: (1) is effective against a high tumor burden, as a function of either a large tumour inoculum or a delayed vaccine schedule (2) generates a memory response that can protect against rechallenge (3) can limit prostate tumor burden in a model in which progression to adenocarcinoma is genetically predetermined and (4) demonstrates effectiveness in solid tumors and a model of B cell lymphoma.
We demonstrate the effectiveness of a combination approach that contains, in addition to an adjuvant and tumor antigen, the TLR3 agonist, Poly I:C and the hemopoietic cytokine, Flt3L. Direct stimulation of TRAMP-C1 cells with Poly I:C has been shown to have no direct effect on tumor cell growth but TRAMP tumor growth in TLR3-/- animals implicates signalling through TLR3 on immune and or stromal cells in anti-tumor immunity [37]. Others have shown administration of Poly I:C to suppress the growth of TRAMP tumors and lead to the recruitment of T cells and NK cells to the tumor site. Orthotopic prostate tumors from Poly I:C treated TRAMP mice were also found to be impaired histologically in their state of adenocarcinoma progression [37]. It is important to note that these results were achieved using serial doses of fifty times more Poly I:C than used in this present study. Although we were unable to prevent the programmed progression of adenocarcinoma in TRAMP-Tg mice we have potentially impacted the number of tumor cells undergoing these changes. Our vaccinated animals showed reduced prostate weight, a surrogate indicator of disease burden. Ciavarra et al. [38] demonstrated that TRAMP-C1 tumor growth could be slowed by 21 or 35 days of systemic administration of Flt3L protein given at an equivalent dose as used in this current study. This effect was lost upon withdrawal of treatment indicating that Flt3L administration alone does not elicit persistent protective immunity against the TRAMP-C1 tumors. Flt3L is a potent hemopoietic cytokine which in addition to enhancing antitumor priming by promoting host dendritic cell expansion [39, 40], promotes expansion of NK cell progenitors [31]. In the setting of vaccination specifically, elevated serum Flt3L levels are known to be detected soon after s.c. immunization with adjuvant (TLR ligands) and protein antigen and the ensuing Flt3 signalling is required for optimal immunity. In this study, Flt3L dosed for nine consecutive days did expand NK cell numbers and combined with the other vaccine components was able to synergistically enhance NK cell activation.
In exploring the immune mechanisms contributing to this vaccine efficacy, we found that animals lacking NK cells and CD4+ T cells lost their capacity to control tumor growth but this was not the case in animals depleted of CD8+ T cells. NK cells are thought to play a role in the TRAMP prostate cancer mouse model with NKG2D-deficient TRAMP mice exhibiting a higher incidence of early-arising prostate adenocarcinomas, suggesting a role for early NKG2D-driven NK cell immune-surveillance in these mice [41]. Similarly, humanized transgenic TRAMP mice expressing the soluble NKG2G ligand—MICB—exhibited increased incidence of progressed carcinomas and metastasis [42]. A study investigating NK cells that infiltrate human prostate cancer showed NK cells to have an immature phenotype with low cytotoxic potential. This study also found that the balance between activatory and inhibitory receptors on prostate tumor infiltrating NK cells was altered and this tip towards increased expression of inhibitory receptors was more pronounced with metastatic progression [43]. These findings highlight that an immunotherapy able to improve NK cell anti-tumor activity, such as our vaccine, could contribute to overcoming this prostate-driven NK cell immunosuppression observed in humans.
The non-essential role for CD8+ T cells in control of tumor growth was unexpected given previous in vivo studies formulating ISCOMATRIX with a surrogate tumor antigen, OVA—B16-OVA melanoma, have shown enhanced CD8+ T-cell cross-priming enabling prophylactic and therapeutic tumoricidal activity [44]. In addition, using an ISCOMATRIX™–OVA–Poly I:C–CpG vaccine in B16-OVA tumor-bearing animals, found CD8+ T cells to be essential to the effectiveness of this vaccine [22]. We have three lines of evidence to support a limited role for CD8+ T cells in the primary immune response, paving the way for greater contributions by CD4+ T cells and NK cells as we have shown. Firstly, primary human prostate cancers have been shown to have low HLA class I expression, with 85% of primary tumors displaying HLA class I downregulation. This level of MHC class I downregulation is greater than that observed in other tumor types [45]. Secondly, TRAMPC1 tumors have low MHC class I expression and that this expression can be improved with exposure to IFN-γ [38] limiting the ability of CD8+ T cells to directly engage with tumors. Thirdly, CD8+ T cells infiltrating TRAMPC1 tumors were found to acquire an exhausted phenotype suggesting they are unable to participate in the anti-tumor immune response to their fullest potential. Our vaccine combination was unable to overcome this exhaustion and engage CD8+ T cells drawing them into the response. It would be interesting to see if the addition of anti-PD1 to the combination would release CD8+ T cell from their supressed state and allow them to contribute to the response.
Upon rechallenge with tumors animals demonstrated the presence of a robust and persistent memory response. The ability to mount a protective memory response suggests that antigen-specific memory CD8+ T cells are generated during the primary tumor challenge and these cells are successfully recalled upon rechallenge. This does not exclude that memory CD4+ T cells are also recalled or the possibility that NK cells are again activated following rechallenge. A study by Cheadle et al. [46] investigating the potential of TLR7 agonism in improving anti-tumor efficacy of Obinutuzumab (glygoengineered α-CD20) in murine lymphoma models demonstrated that primary anti-tumor immunity was dependent on both NK cells an CD4+ T cells but not CD8+ T cells. The improved response observed is thought to reflect the ability of R848 to potentiate Obinutuzumab’s effector mechanism, in particular Ab-dependent cell-mediated cytotoxicity by NK cells. Similarly, the anti-tumor immune response generated by our combination vaccine produces NK cells with enhanced effector function. Despite the observed non-participation of CD8+ T cells in the primary anti-lymphoma response, the conclusion was reached that both CD4+ and CD8+ T cells were necessary for the generation of long-term immunological memory.
This current study shows effectiveness of the ISCOMATRIX™–OVA–Poly I:C–Flt3L vaccine in two additional models of cancer—B16-OVA melanoma and Eµ-myc-GFP-OVA lymphoma. It is likely that vaccine-induced tumor control in these models, where the vaccine antigen stimulates an immunodominant CD8+ T cell response, may be CD8+ T cell-dependent. In support of this are immunogenicity studies conducted in the absence of tumor that show strong induction of OVA-specific CD8+ T cells (using OVA-tetramer) and numerous CD8+IFNγ+ cells (data not shown) following prime-boost vaccination with ISCOMATRIX™–OVA–Poly I:C–Flt3L. Differential requirement for CD8+ T cells in control of tumor growth across different tumor models may reflect the difference between highly immunogenic tumor antigens like OVA and less immunogenic tumor antigens like PAP. We also demonstrate the impact of the tumor environment on the spectrum of anti-tumor immune response. We show here that CD8+ T cells are present in TRAMP tumors but they accumulate an exhausted phenotype overtime. It is likely that CD8+ TILs isolated from B16-OVA tumors would not show such a phenotype.
We show that with the appropriate NK cell stimulation, provided by our combination vaccine, NK cells can be very efficacious against a poorly immunogenic tumor. These results highlight the potential for harnessing NK cells in the solid tumor setting where CD8+ T cells are either exhausted or fail to ‘see’ tumor antigen. The efficacy of this vaccine is also reliant on CD4+ T cells. It is possible that CD4+ T cells provide critical help that allow NK cells to perform optimally and potentially maintain long-term anti-tumor activity. From these studies where both the immunogenicity of the tumor is known and the immune mechanisms behind a particular vaccine combination is largely understood, we can begin to tailor future vaccine combinations to trigger the immune responses that are likely to be most effective against a given tumor. In the setting of a limited tumor burden, i.e., minimal residual disease following medical interventions such as radiation or surgery or in the setting where disease is slow-growing and non-progressive, there is a significant opportunity for the application of an immune-stimulating vaccine.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the following CSL colleagues for experimental support and for critical inputs: Cathy Owczarek, Matt Hardy, Pierre Scotney and teams for protein production and purification. Catherine Tarlinton for assistance with flow cytometry. We thank Max Walker and team (University of Melbourne, Australia) for expert animal care. Helen Pearson (Peter McCallum Cancer Centre, Melbourne, Australia) for demonstrating mouse prostate dissections.
Abbreviations
- Flt3L
FMS-like tyrosine kinase 3 ligand
- GzmB
Granzyme B
- IFNγ
Interferon gamma
- ISCOMATRIX™/IMX
ISCOMATRIX™ adjuvant
- MoA
Mechanism of action
- mPAP
Murine prostatic acid phosphatase
- OVA
Ovalbumin
- Poly I:C
Polyinosinic–polycytidylic acid
- TILs
Tumor infiltrating lymphocytes
- Tg
Transgenic
- TLR
Toll-like receptor
- TRAMP
Transgenic adenocarcinoma of the mouse prostate
Author contributions
Experimental design: AB and ABM. Execution of experiments: AB, AS and SP. Data analyses: AB, AS and SP. AB wrote the manuscript. ABM, EM and GTB provided direction. Manuscript review: all authors.
Funding
At the time of completing this work, Adele Barr was the recipient of National Health and Medical Research Council Fellowship CDA APP1006663. Gabrielle T. Belz is supported by a fellowship from the National Health and Medical Research Council (NHMRC) of Australia (APP1135898).
Compliance with ethical standards
Conflict of interest
Adele Barr, Sandro Prato, Adriana Baz Morelli and Eugene Maraskovsky are currently employees of CSL Ltd. At the time of completing this work Anabel Silva was an employee of CSL Ltd. The authors declare that there are no other conflicts of interest.
Ethical approval
This work was carried out according to the ‘Australian Code for the Responsible Conduct of Research 2018’. All animal studies were approved by the CSL Limited/Pfizer Australia Animal Ethics Committee (Melbourne, VIC, Australia). Experiments performed were outlined in approved animal ethics applications AEC (Animal Ethics Committee) register numbers 863, 831, 880, 966 and 981.
Animal source
C57BL/6J and B6.SJL/J-PTPRCa mice used in these experiments were bred at the Bio21 Institute (Melbourne, VIC, Australia). C57BL/6 Tg(TRAMP)8247Ng/J and B6.129S7-Ifngtm1Ts/J mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA).
Cell line authentication
The B16-OVA melanoma cell line (clone MO5, [47]) was kindly provided by A. Surhbier (QIMR Berghofer, Brisbane, QLD, Australia). The TRAMP-C1 cell line was purchased from American Type Culture Collection (ATCC®) (CRL-2730). The Eµ-myc-GFP-OVA lymphoma cell line was kindly provided by the creator of this lymphoma cell line, J. Villadangos [25] (The University of Melbourne, Melbourne, Australia). Tumor cell lines have been cultured no longer than 3 weeks after thawing and used for the experiments without any further authentication. All cell lines were routinely tested for mycoplasma contamination with PCR technique.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Adele M. Barr, Email: adele.barr@csl.com.au
Adriana Baz Morelli, Email: adriana.bazmorellli@csl.com.au.
References
- 1.Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161(2):205–214. doi: 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD. Jemal A (2019) Cancer statistics. CA Cancer J Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 3.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF, Investigators IS. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 4.Gulley JL, Borre M, Vogelzang NJ, Ng S, Agarwal N, Parker CC, Pook DW, Rathenborg P, Flaig TW, Carles J, Saad F, Shore ND, Chen L, Heery CR, Gerritsen WR, Priou F, Langkilde NC, Novikov A, Kantoff PW. Phase III trial of PROSTVAC in asymptomatic or minimally symptomatic metastatic castration-resistant prostate cancer. J Clin Oncol. 2019;37(13):1051–1061. doi: 10.1200/JCO.18.02031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348(6230):56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 6.Collins JM, Redman JM, Gulley JL. Combining vaccines and immune checkpoint inhibitors to prime, expand, and facilitate effective tumor immunotherapy. Expert Rev Vaccines. 2018;17(8):697–705. doi: 10.1080/14760584.2018.1506332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ilyinskii PO, Kovalev GI, O'Neil CP, Roy CJ, Michaud AM, Drefs NM, Pechenkin MA, Fu FN, Johnston LPM, Ovchinnikov DA, Kishimoto TK. Synthetic vaccine particles for durable cytolytic T lymphocyte responses and anti-tumor immunotherapy. PLoS ONE. 2018;13(6):e0197694. doi: 10.1371/journal.pone.0197694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dahlberg CI, Sarhan D, Chrobok M, Duru AD, Alici E. Natural killer cell-based therapies targeting cancer: possible strategies to gain and sustain anti-tumor activity. Front Immunol. 2015;6:605. doi: 10.3389/fimmu.2015.00605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rezvani K, Rouce RH. The application of natural killer cell immunotherapy for the treatment of cancer. Front Immunol. 2015;6:578. doi: 10.3389/fimmu.2015.00578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehta RS, Rezvani K. Chimeric antigen receptor expressing natural killer cells for the immunotherapy of cancer. Front Immunol. 2018;9:283. doi: 10.3389/fimmu.2018.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chester C, Fritsch K, Kohrt HE. Natural killer cell immunomodulation: targeting activating, inhibitory, and co-stimulatory receptor signaling for cancer immunotherapy. Front Immunol. 2015;6:601. doi: 10.3389/fimmu.2015.00601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pahl J, Cerwenka A. Tricking the balance: NK cells in anti-cancer immunity. Immunobiology. 2017;222(1):11–20. doi: 10.1016/j.imbio.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 13.Andre P, Denis C, Soulas C, Bourbon-Caillet C, Lopez J, Arnoux T, Blery M, Bonnafous C, Gauthier L, Morel A, Rossi B, Remark R, Breso V, Bonnet E, Habif G, Guia S, Lalanne AI, Hoffmann C, Lantz O, Fayette J, Boyer-Chammard A, Zerbib R, Dodion P, Ghadially H, Jure-Kunkel M, Morel Y, Herbst R, Narni-Mancinelli E, Cohen RB, Vivier E. Anti-NKG2A mAb is a checkpoint inhibitor that promotes anti-tumor immunity by unleashing both T and NK Cells. Cell. 2018;175(7):1731–1743. doi: 10.1016/j.cell.2018.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cerwenka A, Lanier LL. Natural killers join the fight against cancer. Science. 2018;359(6383):1460–1461. doi: 10.1126/science.aat2184. [DOI] [PubMed] [Google Scholar]
- 15.Rautela J, Souza-Fonseca-Guimaraes F, Hediyeh-Zadeh S, Delconte RB, Davis MJ, Huntington ND. Molecular insight into targeting the NK cell immune response to cancer. Immunol Cell Biol. 2018;96(5):477–484. doi: 10.1111/imcb.12045. [DOI] [PubMed] [Google Scholar]
- 16.Lenarczyk A, Le TT, Drane D, Malliaros J, Pearse M, Hamilton R, Cox J, Luft T, Gardner J, Suhrbier A. ISCOM based vaccines for cancer immunotherapy. Vaccine. 2004;22(8):963–974. doi: 10.1016/j.vaccine.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 17.Maraskovsky E, Sjolander S, Drane DP, Schnurr M, Le TT, Mateo L, Luft T, Masterman KA, Tai TY, Chen Q, Green S, Sjolander A, Pearse MJ, Lemonnier FA, Chen W, Cebon J, Suhrbier A. NY-ESO-1 protein formulated in ISCOMATRIX adjuvant is a potent anticancer vaccine inducing both humoral and CD8+ T-cell-mediated immunity and protection against NY-ESO-1+ tumors. Clin Cancer Res. 2004;10(8):2879–2890. doi: 10.1158/1078-0432.CCR-03-0245. [DOI] [PubMed] [Google Scholar]
- 18.Thompson EA, Ols S, Miura K, Rausch K, Narum DL, Spangberg M, Juraska M, Wille-Reece U, Weiner A, Howard RF, Long CA, Duffy PE, Johnston L, O'Neil CP, Lore K. TLR-adjuvanted nanoparticle vaccines differentially influence the quality and longevity of responses to malaria antigen Pfs25. JCI Insight. 2018 doi: 10.1172/jci.insight.120692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Julier Z, de Titta A, Grimm AJ, Simeoni E, Swartz MA, Hubbell JA. Fibronectin EDA and CpG synergize to enhance antigen-specific Th1 and cytotoxic responses. Vaccine. 2016;34(21):2453–2459. doi: 10.1016/j.vaccine.2016.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mount A, Koernig S, Silva A, Drane D, Maraskovsky E, Morelli AB. Combination of adjuvants: the future of vaccine design. Expert Rev Vaccines. 2013;12(7):733–746. doi: 10.1586/14760584.2013.811185. [DOI] [PubMed] [Google Scholar]
- 21.Shirota H, Tross D, Klinman DM. CpG Oligonucleotides as cancer vaccine adjuvants. Vaccines (Basel) 2015;3(2):390–407. doi: 10.3390/vaccines3020390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silva A, Mount A, Krstevska K, Pejoski D, Hardy MP, Owczarek C, Scotney P, Maraskovsky E, Baz Morelli A. The combination of ISCOMATRIX adjuvant and TLR agonists induces regression of established solid tumors in vivo. J Immunol. 2015;194(5):2199–2207. doi: 10.4049/jimmunol.1402228. [DOI] [PubMed] [Google Scholar]
- 23.Foster BA, Gingrich JR, Kwon ED, Madias C, Greenberg NM. Characterization of prostatic epithelial cell lines derived from transgenic adenocarcinoma of the mouse prostate (TRAMP) model. Cancer Res. 1997;57(16):3325–3330. [PubMed] [Google Scholar]
- 24.Greenberg NM, DeMayo F, Finegold MJ, Medina D, Tilley WD, Aspinall JO, Cunha GR, Donjacour AA, Matusik RJ, Rosen JM. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci USA. 1995;92(8):3439–3443. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prato S, Mintern JD, Lahoud MH, Huang DC, Villadangos JA. Induction of antigen-specific effector-phase tolerance following vaccination against a previously ignored B-cell lymphoma. Immunol Cell Biol. 2011;89(5):595–603. doi: 10.1038/icb.2010.131. [DOI] [PubMed] [Google Scholar]
- 26.Drane D, Gittleson C, Boyle J, Maraskovsky E. ISCOMATRIX adjuvant for prophylactic and therapeutic vaccines. Expert Rev Vaccines. 2007;6(5):761–772. doi: 10.1586/14760584.6.5.761. [DOI] [PubMed] [Google Scholar]
- 27.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207(10):2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herberman RB, Nunn ME, Lavrin DH. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic acid allogeneic tumors. I. Distribution of reactivity and specificity. Int J Cancer. 1975;16(2):216–229. doi: 10.1002/ijc.2910160204. [DOI] [PubMed] [Google Scholar]
- 29.Kiessling R, Klein E, Wigzell H. "Natural" killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975;5(2):112–117. doi: 10.1002/eji.1830050208. [DOI] [PubMed] [Google Scholar]
- 30.Oldham RK, Herberman RB. Evaluation of cell-mediated cytotoxic reactivity against tumor associated antigens with 125I-iododeoxyuridine labeled target cells. J Immunol. 1973;111(6):862–871. [PubMed] [Google Scholar]
- 31.Shaw SG, Maung AA, Steptoe RJ, Thomson AW, Vujanovic NL. Expansion of functional NK cells in multiple tissue compartments of mice treated with Flt3-ligand: implications for anti-cancer and anti-viral therapy. J Immunol. 1998;161(6):2817–2824. [PubMed] [Google Scholar]
- 32.Schmidt KN, Leung B, Kwong M, Zarember KA, Satyal S, Navas TA, Wang F, Godowski PJ. APC-independent activation of NK cells by the Toll-like receptor 3 agonist double-stranded RNA. J Immunol. 2004;172(1):138–143. doi: 10.4049/jimmunol.172.1.138. [DOI] [PubMed] [Google Scholar]
- 33.Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331(6013):44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gingrich JR, Barrios RJ, Kattan MW, Nahm HS, Finegold MJ, Greenberg NM. Androgen-independent prostate cancer progression in the TRAMP model. Cancer Res. 1997;57(21):4687–4691. [PubMed] [Google Scholar]
- 35.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5(10):987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 36.Hochrein H, Wagner H. Of men, mice and pigs: looking at their plasmacytoid dendritic cells [corrected] Immunology. 2004;112(1):26–27. doi: 10.1111/j.1365-2567.2004.01878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chin AI, Miyahira AK, Covarrubias A, Teague J, Guo B, Dempsey PW, Cheng G. Toll-like receptor 3-mediated suppression of TRAMP prostate cancer shows the critical role of type I interferons in tumor immune surveillance. Cancer Res. 2010;70(7):2595–2603. doi: 10.1158/0008-5472.CAN-09-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ciavarra RP, Somers KD, Brown RR, Glass WF, Consolvo PJ, Wright GL, Schellhammer PF. Flt3-ligand induces transient tumor regression in an ectopic treatment model of major histocompatibility complex-negative prostate cancer. Cancer Res. 2000;60(8):2081–2084. [PubMed] [Google Scholar]
- 39.Maraskovsky E, Brasel K, Teepe M, Roux ER, Lyman SD, Shortman K, McKenna HJ. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. J Exp Med. 1996;184(5):1953–1962. doi: 10.1084/jem.184.5.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maraskovsky E, Daro E, Roux E, Teepe M, Maliszewski CR, Hoek J, Caron D, Lebsack ME, McKenna HJ. In vivo generation of human dendritic cell subsets by Flt3 ligand. Blood. 2000;96(3):878–884. doi: 10.1182/blood.V96.3.878. [DOI] [PubMed] [Google Scholar]
- 41.Guerra N, Tan YX, Joncker NT, Choy A, Gallardo F, Xiong N, Knoblaugh S, Cado D, Greenberg NM, Raulet DH. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity. 2008;28(4):571–580. doi: 10.1016/j.immuni.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu G, Lu S, Wang X, Page ST, Higano CS, Plymate SR, Greenberg NM, Sun S, Li Z, Wu JD. Perturbation of NK cell peripheral homeostasis accelerates prostate carcinoma metastasis. J Clin Investig. 2013;123(10):4410–4422. doi: 10.1172/JCI69369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pasero C, Gravis G, Guerin M, Granjeaud S, Thomassin-Piana J, Rocchi P, Paciencia-Gros M, Poizat F, Bentobji M, Azario-Cheillan F, Walz J, Salem N, Brunelle S, Moretta A, Olive D. Inherent and tumor-driven immune tolerance in the prostate microenvironment impairs natural killer cell antitumor activity. Cancer Res. 2016;76(8):2153–2165. doi: 10.1158/0008-5472.CAN-15-1965. [DOI] [PubMed] [Google Scholar]
- 44.Wilson NS, Yang B, Morelli AB, Koernig S, Yang A, Loeser S, Airey D, Provan L, Hass P, Braley H, Couto S, Drane D, Boyle J, Belz GT, Ashkenazi A, Maraskovsky E. ISCOMATRIX vaccines mediate CD8+ T-cell cross-priming by a MyD88-dependent signaling pathway. Immunol Cell Biol. 2012;90(5):540–552. doi: 10.1038/icb.2011.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blades RA, Keating PJ, McWilliam LJ, George NJ, Stern PL. Loss of HLA class I expression in prostate cancer: implications for immunotherapy. Urology. 1995;46(5):681–686. doi: 10.1016/S0090-4295(99)80301-X. [DOI] [PubMed] [Google Scholar]
- 46.Cheadle EJ, Lipowska-Bhalla G, Dovedi SJ, Fagnano E, Klein C, Honeychurch J, Illidge TM. A TLR7 agonist enhances the antitumor efficacy of obinutuzumab in murine lymphoma models via NK cells and CD4 T cells. Leukemia. 2017 doi: 10.1038/leu.2016.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Falo LD, Jr, Kovacsovics-Bankowski M, Thompson K, Rock KL. Targeting antigen into the phagocytic pathway in vivo induces protective tumour immunity. Nat Med. 1995;1(7):649–653. doi: 10.1038/nm0795-649. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.