Abstract
Background:
Abnormal thyroid hormone levels (high or low) and autoimmunity from autoimmune thyroid disease (AITD) may increase dementia risk.
Methods:
We examined the associations of thyroid dysfunction or possible AITD in 1990 - 1992 with dementia through 2017 in the Atherosclerosis Risk in Communities (ARIC) Neurocognitive Study. Thyroid dysfunction (subclinical and overt hypo- or hyperthyroidism and euthyroidism) was categorized from serum thyroid-stimulating hormone (TSH) and free thyroxine (FT4) cut-points and AITD from anti-thyroid peroxidase (anti-TPO) antibody positivity. Dementia was identified primarily based on cognitive test performance, neuropsychological examinations and clinician review of suspected cases. Additional cases of dementia were ascertained through telephone interviews or relevant hospital and death certificate codes. Cox regression with multivariable adjustment was used for analysis.
Results:
After exclusions for missing data, 12,481 participants were included in the analysis (mean index exam age 57 ± 5.7 (44% male, 25% black)), and 2,235 incident dementia cases were identified. AITD was not significantly associated with dementia. Subclinical hypothyroidism was associated with a lower risk of dementia (hazard ratio (HR) (95% confidence interval (CI)): 0.74 (0.60 - 0.92)), while overt hyperthyroidism was associated with a higher risk of dementia (HR (95% CI): 1.40 (1.02 - 1.92)) compared to euthyroid participants. Participants with serum FT4 concentrations above the 95th percentile were at an increased risk of dementia compared to those in the middle 90% of FT4 (HR (95% CI): 1.23 (1.02 - 1.48)).
Conclusions:
Subclinical hypothyroidism was associated with reduced risk of dementia, whereas overt hyperthyroidism, particularly very elevated FT4, was associated with increased risk of dementia. The association between subclinical hypothyroidism and reduced risk of dementia cannot be explained, but may have been an artifact due to change. By extrapolation, effective treatment of overt hyperthyroidism may modestly reduce dementia risk in older adults.
Keywords: Thyroid, Dementia, Epidemiology, Cohort
Introduction
The thyroid gland produces triiodothyronine (T3) and thyroxine (T4) from dietary iodine [1, 2]. Thyroid-stimulating hormone (TSH) promotes the release of free T3 (FT3) and free T4 (FT4), the biologically active forms of T3 and T4, to stimulate cellular energy use and increase metabolism [2–5]. In iodine-replete countries like the USA, autoimmune thyroid disease (AITD) is the most coimnon cause of thyroid dysfunction and most often leads to hypothyroidism [6]. Among Americans 12 years and older, prevalence of thyroid dysfunction is approximately 5.8%, with 4.6% of cases presenting as hypothyroidism and 1.2% of cases presenting as hyperthyroidism [7, 8]. A combination of genetic susceptibility and enviromnental factors, including radiation, smoking, infection and stress, can trigger the autoimmune response causing AITD [6, 9]. All forms of AITD are associated with the presence of serum anti-thyroid peroxidase (anti-TPO), although the presence of antibodies does not necessitate clinically detectible disease [10,11].
Thyroid dysfunction can cause a range of mood and cognitive disturbances, especially in severe cases, including anxiety, depression, irritability and deficits in executive function [12]. Increased screening and better treatment has reduced the rate of short-term thyroid-related cognitive symptoms by reducing the incidence of severe thyroid disorders and reversing cognitive symptoms with effective treatment of thyroid abnormalities [12]. However, there is interest in the link between thyroid disorders and dementia due to the thyroid’s well-established influence on brain development and function [13, 14]. Dementia is most common among those aged 65 years and older and is characterized by severe cognitive impairment interfering with daily functioning and independence. Dementia and thyroid dysfunction are both associated with advancing age and are more prevalent in women [15, 16].
There are two mechanisms by which thyroid disorders may be associated with dementia: action of abnormal thyroid hormone concentrations (high TSH causing low FT4 or high FT4 causing low TSH) on the brain causing impairment [17] or autoimmunity causing AITD and encephalopathy leading to permanent brain damage [18]. While some studies have found a relation between elevated TSH levels and increased rates of dementia or cognitive decline, the literature regarding other thyroid hormones (necessary for diagnosing dysfunction) and AITD (measured via anti-TPO positivity) is mixed, limited by modest sample sizes (n < 3,000) and focused on only older participants (aged 65 years and older at baseline) [13, 19–26]. Using data from the Atherosclerosis Risk in Communities (ARIC): Neurocognitive Study (NCS), we tested the hypothesis that AITD (anti-TPO antibodies) and abnormal thyroid hormone (TSH and FT4) levels are associated with increased incidence rates of dementia over 20 years of follow-up.
Materials and Methods
ARIC is a prospective cohort study that enrolled 15,792 primarily white and African American participants from Forsyth County, NC, Jackson, Mississippi, the northwest suburbs of Minneapolis, Minnesota and Washington County, Maryland from 1987 to 1989. After IRB approval and informed consent, ARIC has followed participants continuously for hospitalization and mortality. For this analysis, baseline started at ARIC visit 2 (1990 - 1992) at the time of thyroid assessments and cognitive status was ascertained through visit 6 (2016 - 2017). Participants were excluded from follow-up if they were nonwhite or African American or African Americans from MD or MN (n = 103), did not attend visit 2 (n = 1,432), had missing serum TSH, FT4, or anti-TPO antibody measures (n = 1,769), or had prevalent dementia at visit 2 (n = 4) for a final analytic sample of 12,481 participants. This study is in ethical compliance with human study guidelines.
Thyroid function was assessed using serum samples stored at −70 °C since collection at visit 2 that were thawed and tested at Advanced Research Diagnostics Laboratory (University of Minnesota) between 2011 and 2013. Assays from Roche Diagnostics were used on an Elecsys 2010 analyzer using a sandwich iimnunoassay method for TSH and competition immunoassay methods for FT4 and anti-TPO antibodies [27]. Interassay coefficients of variation were ≤ 10% [27]. Anti-TPO antibody positivity was defined as > 34 kIU/L, based on assay manufacturer guidelines [28]. Five clinical categories (subclinical hypothyroidism, subclinical hyperthyroidism, overt hypothyroidism, overt hyperthyroidism and euthyroidism) were used to define thyroid dysfunction based on ARIC-derived cut-points associated with thyroid-related genes and genetic risk score (Table 1) [7, 28]. These categories differ from traditional clinical cut-points and were derived biochemically. We also examined categorical variables based on the lowest 5%, middle 90% and highest 5% of TSH and FT4 levels (to compare levels in the normal and non-normal ranges of the distribution), as well as continuously.
Table 1.
Baseline Characteristics Stratified by Clinical Categories of Thyroid Dysfunction, ARIC 1990 - 1992
| Risk factors | Hypothyroidism |
Euthyroidism (n = 10,956) | Hyperthyroidism |
||
|---|---|---|---|---|---|
| Overt (n = 281) | Subclinical (n = 581) | Subclinical (n = 429) | Overt (n = 234) | ||
| TSH > 5.1 mIU/L; FT4 < 0.85 ng/dL | TSH > 5.1 mIU/L; FT4 = 0.85 - 1.4 ng/dL | TSH = 0.56 - 5.1 mIU/L; FT4 = 0.85 - 1.4 ng/dL | TSH < 0.56 mIU/L; FT4 = 0.85 - 1.4 ng/dL | TSH < 0.56 mIU/L; FT4 > 1.4 ng/dL | |
| Age, years | 58.4 ± 5.4 | 58.4 ± 5.7 | 56.8 ± 5.7 | 56.5 ± 5.8 | 57.1 ± 5.8 |
| Men, % | 22.8 | 33.4 | 45.9 | 33.6 | 19.7 |
| African American, % | 12.8 | 11.0 | 25.1 | 41.5 | 23.1 |
| APOE4 carriers, % | 29.2 | 29.6 | 29.9 | 27.3 | 28.2 |
| Basic educationa, % | 23.1 | 16.8 | 21.5 | 29.2 | 16.2 |
| Family income < $16,000b, % | 24.2 | 21.3 | 25.2 | 34.3 | 22.2 |
| Current alcohol drinker, % | 48.8 | 58.7 | 57.1 | 49.2 | 56.0 |
| Current tobacco smoker, % | 13.9 | 12.9 | 22.3 | 29.6 | 25.2 |
| BMI, kg/m2 | 28.9 ± 5.7 | 27.7 ± 5.5 | 28.0 ± 5.4 | 27.6 ± 5.4 | 27.4 ± 5.4 |
| Hypertensionc, % | 34.1 | 30.9 | 35.8 | 40.0 | 32.6 |
| Diabetesd, % | 7.5 | 10.3 | 11.3 | 15.9 | 12.8 |
| HDL cholesterol, mg/dL | 49.4 ± 16.6 | 49.7 ± 16.3 | 49.4 ± 16.8 | 52.8 ± 17.9 | 52.1 ± 15.7 |
| Total cholesterol, mg/dL | 229.2 ± 48.9 | 214.3 ± 41.2 | 209.7 ± 39.2 | 208.1 ± 38.0 | 199.5 ± 35.2 |
| Prevalent CVDe, % | 8.5 | 8.1 | 8.8 | 11.0 | 9.8 |
| Thyroid medication usef, % | 40.9 | 28.6 | 2.8 | 27.5 | 71.4 |
| TSH, mIU/L | 26.3 ± 32.7 | 8.0 ± 4.4 | 2.0 ± 1.0 | 0.3 ± 0.2 | 0.1 ± 0.2 |
| FT4, ng/dL | 0.7 ± 0.2 | 1.0 ± 0.1 | 1.1 ± 0.1 | 1.2 ± 0.1 | 1.8 ± 0.6 |
| Anti-TPO positive (> 34 IU/L), % | 79.4 | 51.1 | 9.3 | 13.8 | 27.4 |
Mean ± standard deviation.
Based on self-report of some high school education or less at visit 1 (1987 - 1989).
Based on self-report of family income at visit 1 (1987 - 1989).
Defined as diastolic blood pressure ≥ 90 mm Hg, systolic blood pressure ≥ 140 mm Hg, or use of hypertensive medication.
Defined as non-fasting blood glucose ≥ 200 mg/dL, fasting blood glucose ≥ 126 mg/dL, self-report of diabetes, or reporting taking medication for diabetes or high blood sugar.
Defined as prevalent stroke, coronary heart disease, myocardial infarction, or atrial fibrillation at visit 2.
Participants who reported thyroid medication use at baseline. ARIC: Atherosclerosis Risk in Communities; BMI: body mass index; HDL: high-density lipoprotein; CVD: cardiovascular disease; TSH: thyroid-stimulating hormone; FT4: free thyroxine; TPO: thyroid peroxidase.
Covariates included sex, race-center (MS-blacks, NC-wliites, NC-blacks, MN-whites and MD-whites), APOE ε4, income and education from visit 1 (1987 - 1989). At visit 2 baseline (1990 - 1992), age, body mass index (BMI), tobacco smoking status, hypertension, diabetes, alcohol drinking status, high-density lipoprotein (HDL) cholesterol, total cholesterol, prevalent cardiovascular disease (CVD) and thyroid medication use were measured. BMI was calculated from measured weight and height. Hypertension was defined as having a systolic blood pressure ≥ 140 mm Hg, diastolic blood pressure ≥ 90 mm Hg, or self-report of antihypertensive medication use. Diabetes was defined as non-fasting serum glucose ≥ 200 mg/dL, fasting glucose ≥ 126 mg/dL, self-report of diabetes diagnosis from a physician, or report of taking medication for diabetes or high blood sugar. Prevalent CVD was defined as having prevalent stroke, coronary heart disease, myocardial infarction (MI), or atrial fibrillation (AF) at visit 2 ascertained via hospital surveillance, self-report, or detected at an ARIC clinic visit (MI and AF), with clinical events other than AF adjudicated by a panel of experts [29–32]. Participants were asked to bring medication containers for any medication taken in the past 4 weeks prior to each clinic visit, from which thyroid medication use was determined at baseline and throughout follow-up.
For participants that attended visit 5 (2011 - 2013) and 6 (2016 - 2017), dementia cases were primarily identified based on data collected from the ARIC-NCS clinic examinations. Additional dementia cases were ascertained through surveillance of hospitalization and death certificate codes, informant interviews and dementia screening during annual telephone follow-up calls [33]. A brief cognitive assessment consisting of the delayed word recall test, digit symbol substitution test and word fluency test was administered to all participants at visits 2 (1990 - 1992) and 4 (1996 - 1998). At visit 5, the cognitive battery was expanded to include eight additional tests. Cognitive tests were administered using standardized protocols, and scores were converted to z-scores. We identified cognitively impaired participants as those with significant cognitive decline from visits 2 through 6 or with a mini-mental state examination (MMSE) score < 21 for whites and < 19 for African Americans (methods described in detail elsewhere) [33]. These participants, as well as a random sample of cognitively normal participants, were given additional physical and neurological exams, including brain magnetic resonance imaging (MRI) at visit 5, and their informants were interviewed using the clinical dementia rating (CDR) scale and the functional activities questionnaire (FAQ) [33]. Information about suspected cases was reviewed by a committee of clinicians and participants were classified as cognitively normal, having mild cognitive impairment (MCI), or having dementia [33].
Dementia surveillance methods were in place for those participants that did not attend the cohort examination. Dementia cases were identified at annual and semi-annual telephone follow-up calls with the administration of the modified telephone interview for cognitive status (TICSm) to participants who were alive and did not attend visits 5 or 6, and informant interviews for deceased participants suspected to have had dementia. Additional dementia cases were identified via surveillance of hospital discharge ICD-9 codes and death certificate codes related to dementia throughout the entire follow-up period.
Statistical analysis
We described means and prevalences of baseline covariates, thyroid hormone levels and AITD status stratified by clinical categories of thyroid dysfunction. To characterize the association between abnormal thyroid function and dementia, we used Cox regression to assess the hazard of incident dementia between visits 2 and 6. We modeled thyroid function in several ways: 1) anti-TPO antibody status (positive/negative); 2) clinical categories of thyroid dysfunction with euthyroidism as the reference; 3) categorical TSH and FT4 hormone levels (i.e. three categories, comparing participants whose hormone levels fell within the middle 90% (reference), lowest 5%, and highest 5%); and 4) per standard deviation difference in TSH or FT4 level.
For each analysis, three models were tested. Model 1 adjusted for age, sex, center-race, APOE ε4, income and education. Model 2 adjusted for model 1 covariates as well as BMI, smoking status, hypertension, diabetes, prevalent coronary heart disease (CHD), drinking status, HDL cholesterol and total cholesterol. Model 3 adjusted for model 2 covariates in addition to prevalent CVD and baseline thyroid medication use. Sensitivity analyses were conducted to assess whether taking thyroid medication throughout follow-up (versus at baseline) was associated with risk of dementia; however, medication use was not associated with dementia and results did not change. We verified that the proportional hazards assumption was met by testing the interaction between each measure of abnormal thyroid function by log follow-up time. We also used a restricted cubic spline model to investigate the continuous non-linear relationship between thyroid hormone level and dementia with knots specified at the fifth, 50th and 95th percentiles. All statistical analyses were conducted using SAS 9.4 (SAS Inc., Cary, NC).
Results
Of the 15,792 ARIC participants, 12,481 were included in the analysis after exclusions. The mean age of participants was 57 ± 5.7 at visit 2. Among these, 13.3% were anti-TPO positive, 2.3% had overt hypothyroidism, 4.7% subclinical hypothyroidism, 3.4% overt hyperthyroidism, 1.9% subclinical hyperthyroidism, 87.8% were euthyroid and 7% were taking thyroid medication. Overall, participants with hypo- or hyperthyroidism had higher prevalences of risk factors for dementia compared to those with euthyroidism (Table 1). Participants with hypo- or hyperthyroidism were more likely to be women. Participants with hypothyroidism were less likely to be African American, while participants with hyperthyroidism were more likely to be African American compared to those with euthyroidism. Those with hypothyroidism were less likely to be current tobacco smokers, less likely to have diabetes, and had higher mean total cholesterol concentrations compared to participants in euthyroid or hyperthyroid categories. Participants with hyperthyroidism had higher HDL cholesterol and more prevalent CVD than those who were euthyroid or hypothyroid. Thyroid medication use was most coimnon among participants with overt dysfunction.
A total of 2,235 dementia events occurred over a median follow-up of 21.9 (maximum 27.7) years. Participants identified as anti-TPO antibody positive, a marker of AITD, did not have a statistically significant increased hazard of dementia compared to participants who were anti-TPO antibody negative even after multivariable adjustment (hazard ratio (HR) (95% confidence interval (CI)): 0.90 (0.80 - 1.03)) (Table 2). No significant association was found between overt hypothyroidism and dementia, compared to euthyroidism (Table 3). Subclinical hypothyroidism was associated with a 26% reduced hazard of dementia after full adjustment for covariates compared to participants with euthyroidism (HR (95% CI):0. 74 (0.60 - 0.92)). Overt hyperthyroidism was associated with a 40% increased hazard of dementia compared to participants with euthyroidism (HR (95% CI): 1.40 (1.02 - 1.92)), while subclinical hyperthyroidism was not statistically significantly associated.
Table 2.
HRs (95% CI) of Dementia by Anti-TPO Positivity Status, ARIC 1990 - 2017
| Anti-TPO negative ≤ 34 IU/L (number of total = 10,821; number of events = 1,932) | Anti-TPO positive > 34 IU/L (number of total = 1,660; number of events = 303) | |
|---|---|---|
| Model 1 HR | 1 (Ref) | 0.92 (0.82 - 1.04) |
| Model 2 HR | 1 (Ref) | 0.93 (0.82 - 1.05) |
| Model 3 HR | 1 (Ref) | 0.90 (0.80 - 1.03) |
Model 1: age, sex, race-center, APOE ε4, income and education. Model 2: model 1 + BMI, smoking status, hypertension, diabetes, drinking status, HDL cholesterol and total cholesterol. Model 3: model 2 + prevalent CVD and baseline thyroid medication use. HR: hazard ratio; CI: confidence interval; TPO: thyroid peroxidase; ARIC: Atherosclerosis Risk in Communities; BMI: body mass index; HDL: high-density lipoprotein; CVD: cardiovascular disease.
Table 3.
HRs (95% CI) of Dementia by Clinical Categories of Thyroid Dysfunction, ARIC 1990 - 2017
| Hypothyroidism |
Euthyroidism | Hyperthyroidism |
|||
|---|---|---|---|---|---|
| Overt | Subclinical | Subclinical | Overt | ||
| TSH > 5.1 mIU/L; FT4 < 0.85 ng/dL (number of total = 281; number of events = 60) | TSH > 5.1 mIU/L; FT4 = 0.85 - 1.4 ng/dL (number of total = 581; number of events = 98) | TSH= 0.56 - 5.1 mIU/L; FT4 = 0.85 - 1.4 ng/dL (number of total = 10,956; number of events = 1,952) | TSH < 0.56 mIU/L; FT4 = 0.85 - 1.4 ng/dL (number of total = 429; number of events = 74) | TSH < 0.56 mIU/L; FT4 > 1.4 ng/dL (number of total = 234; number of events = 51) | |
| Model 1 HR | 1.03 (0.80 - 1.33) | 0.78* (0.64 - 0.96) | 1 (Ref) | 1.07 (0.85 - 1.35) | 1.51* (1.14 - 1.99) |
| Model 2 HR | 1.01(0.78 - 1.31) | 0.76* (0.61 - 0.93) | 1 (Ref) | 1.03 (0.81 - 1.31) | 1.49* (1.12 - 1.98) |
| Model 3 HR | 0.96 (0.73 - 1.26) | 0.74* (0.60 - 0.92) | 1 (Ref) | 1.01 (0.79 - 1.29) | 1.40* (1.02 - 1.92) |
P-value < 0.05. Model 1: age, sex, race-center, APOE ε4, income and education. Model 2: model 1 + BMI, smoking status, hypertension, diabetes, drinking status, HDL cholesterol and total cholesterol. Model 3: model 2 + prevalent CVD and baseline thyroid medication use. HR: hazard ratio; CI: confidence interval; ARIC: Atherosclerosis Risk in Communities; BMI: body mass index; HDL: high-density lipoprotein; CVD: cardiovascular disease.
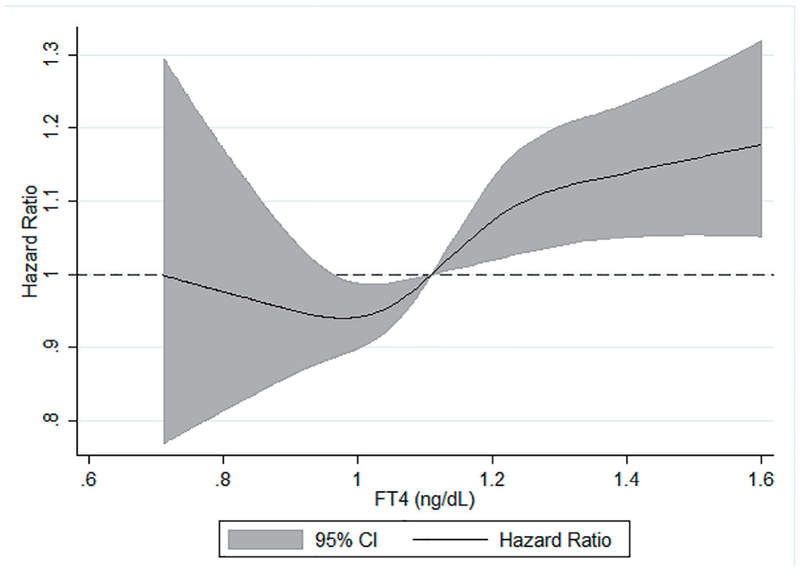
We also examined the association between categorical serum TSH and FT4 levels and dementia (Table 4). There was no significant association between continuous TSH level and dementia after multivariable adjustment including adjustment for serum FT4 levels. There was also no significant association between having TSH levels in the lowest 5% or highest 5% of categorical distribution and hazard of dementia compared to those in the middle 90% of TSH levels. A one standard deviation greater FT4 concentration was associated with a 5% greater hazard of dementia after multivariable adjustment including adjustment for TSH (HR (95% CI): 1.05 (1.01 - 1.09)). Correspondingly, compared to participants in the middle 90% of FT4 level, having serum FT4 in the highest 5% of the categorical distribution was associated with a 23% increased hazard of dementia after full adjustment (HR (95% CI): 1.23 (1.02-1.48)). In contrast, participants in the lowest 5% of FT4 level were not at increased hazard of dementia. Using a restricted cubic spline model, we found levels of FT4 ≥ 1.1 ng/dL were positively, linearly associated with risk of dementia (Fig. 1). In an ad hoc analysis, we tested a TSH*FT4 interaction term. The interaction term was not statistically significant in any of the TSH or FT4 analyses.
Table 4.
HRs (95% CI) of Dementia by Categorical Distribution of Thyroid Hormone Levels, ARIC 1990 - 2017
| TSH | Lowest 5%, ≤ 0.54 mIU/L (number of total = 626; number of events = 116) | Middle 90%, 0.55 - 5.97 mIU/L (number of total = 11,226; number of events = 1,998) | Highest 5%, ≥ 5.98 mIU/L (number of total = 629; number of events = 121) | TSH per 6.35 mIU/L (number of total = 12,481; number of events = 2,235) |
|---|---|---|---|---|
| Model 1 HR | 1.24* (1.03 - 1.50) | 1 (Ref) | 0.93 (0.77 - 1.12) | 1.03 (0.98 - 1.07) |
| Model 2 HR | 1.13 (0.92 - 1.39) | 1 (Ref) | 0.94 (0.77 - 1.14) | 1.03 (0.98 - 1.07) |
| Model 3 HR | 1.09 (0.87 - 1.36) | 1 (Ref) | 0.90 (0.73 - 1.11) | 1.02 (0.97 - 1.07) |
| FT4 | Lowest 5%, ≤ 0.89 ng/dL (number of total = 628; number of events = 123) | Middle 90%, 0.90 - 1.35 ng/dL (number of total = 11,170; number of events = 1,981) | Highest 5%, ≥ 1.36 ng/dL (number of total = 683; number of events = 131) | FT4 per SD (0.19 ng/dL) (number of total = 12,481; number of events = 2,235) |
| Model 1 HR | 0.96 (0.79 - 1.18) | 1 (Ref) | 1.30* (1.08 - 1.55) | 1.06* (1.02 - 1.09) |
| Model 2 HR | 0.96 (0.78 - 1.17) | 1 (Ref) | 1.26* (1.05 - 1.51) | 1.05* (1.01 - 1.09) |
| Model 3 HR | 0.95 (0.77 - 1.16) | 1 (Ref) | 1.23* (1.02 - 1.48) | 1.05* (1.01 - 1.09) |
P-value < 0.05. Model 1: age, sex, race-center, APOE ε4, income, education and TSH or FT4. Model 2: model 1 + BMI, smoking status, hypertension, diabetes, drinking status, HDL cholesterol and total cholesterol. Model 3: model 2 + prevalent CVD and baseline thyroid medication use. HR: hazard ratio; CI: confidence interval; ARIC: Atherosclerosis Risk in Communities; TSH: thyroid-stimulating hormone; FT4: free thyroxine; BMI: body mass index; HDL: high-density lipoprotein; CVD: cardiovascular disease.
Figure 1.
Age, sex, race-center, APOE, income, education and TSH adjusted HR (95% CI) of incident dementia in relation to serum FT4*, ARIC, 1990 - 2017. *Analyzed using restricted cubic splines with knots at the fifth (0.90 ng/dL), 50th (1.11 ng/dL) and 95th (1.35 ng/dL) percentiles of the FT4 distribution. FT4 hormone levels were truncated at the first (0.71 ng/dL) and 99th (1.61 ng/dL) centiles to minimize the influence of extreme values. TSH: thyroid-stimulating hormone; HR: hazard ratio; CI: confidence interval; FT4: free thyroxine; ARIC: Atherosclerosis Risk in Communities.
Discussion
In this prospective cohort study of community-dwelling adults who were followed for 22 years from middle age to older adulthood, subclinical hypothyroidism was associated with a reduced risk of dementia and overt hyperthyroidism with an increased risk of dementia, compared to euthyroid participants. We also found that neither continuous TSH nor categorical TSH was associated with increased risk of dementia. However, a standard deviation increase in FT4 was associated with an increased risk of dementia and those in the highest 5% of categorical FT4 were at increased risk.
Our findings suggest that overt hyperthyroidism and elevated FT4 (the hormone used to diagnose hyperthyroidism) are associated with increased of dementia. These results are consistent with previous studies that found an association between elevated serum FT4 levels and dementia risk [19, 20, 23, 24]. We did not find an association between overt hypothyroidism and dementia nor TSH and dementia. This was inconsistent with the literature [13, 20, 25, 26], which found a significant association between serum TSH (the hormone used to define hypothyroidism) and dementia. In addition, we found that subclinical hypothyroidism was associated with a reduced risk of dementia, an association that we cannot explain. However, these results for clinical hypothyroidism may be related to lack of power. Despite ARIC’s large sample size, the vast majority of participants fell within the euthyroid category reducing the precision of our effect estimates. These findings are important given the American Academy of Neurology recommendation to screen patients undergoing initial assessment for dementia for thyroid dysfunction, particularly hypothyroidism [34]. While their recommendation focuses on age-related hypothyroidism, our findings emphasize the importance of diagnosing and treating hyperthyroidism [34].
Strengths of this analysis include the long follow-up period, large sample size, as well as comprehensive ascertainment of dementia cases; however, there are some limitations to our analysis that warrant consideration. In our assessment of AITD, we only had one measure of autoimmunity, anti-TPO antibody levels. Yet, anti-TPO autoantibodies are found in over 90% of patients with AITD and likely allowed us to capture the most AITD cases [35]. Another limitation is the change in thyroid hormone levels with aging that may have led to misclassification of subclinical hypothyroidism. As shown in our data and reported by others [22], in healthy adults aged 60 and older, average TSH levels rise with advancing age, while FT4 concentrations remain fairly stable [36]. Subclinical hypothyroidism is characterized by elevated TSH with FT4 in the normal range. Older ARIC participants may have had age-related changes in TSH levels that caused them to be mis-classified as having euthyroidism when they had subclinical hypothyroidism. We were also only able to use one measure of thyroid hormone levels and could not adjust for age-related changes. However, these changes were likely modest and misclassification would have pushed effect estimates towards the null. Thus, misclassification does not likely explain the paradoxical inverse association between subclinical hypothyroidism and dementia, in the face of no significant association between overt hypothyroidism and dementia. In addition, our measure of AITD status should not be affected by age-related misclassification, and our analysis of thyroid hormone levels still allowed us to determine whether thyroid hormone levels affect risk of incident dementia regardless of the cause of thyroid hormone dysfunction.
Participants may have developed thyroid dysfunction over the follow-up period, and we tried to address this by adjusting for thyroid medication use at baseline as well as over follow-up. In these analyses, medication use was not associated with dementia and results did not change. Finally, while the ascertainment of dementia was extensive and included several different methods including adjudicated cases at clinic visits, surveillance of hospital and death certificate codes, and telephone interviews for cognitive status, there is still potential for either selection bias due to attrition or misclassification of cases. Yet, dementia cases were ascertained throughout the entire ARIC-NCS follow-up period, and our results do corroborate previous findings in the literature. We were also unable to examine the association of abnormal thyroid function with specific dementia etiologies. The suspected association between thyroid dysfunction and CVD suggests that there may be an association with cerebrovascular disease etiology specifically [27].
Our analysis suggests that subclinical hypothyroidism may be associated with reduced risk of dementia, although the biological pathway is unclear and this potential association warrants further investigation. Additionally, our results indicate that overt hyperthyroidism may be a risk factor for dementia. By extrapolation from these observational data, it may be that effective treatment and management of thyroid hormone levels in overt hyperthyroidism could modestly reduce the risk of incident dementia.
Acknowledgments
We would like to thank the staff and participants of the ARIC study for their important contributions. We would also like to thank Dr. Lisa S. Chow at the University of Minnesota Department of Medicine, Division of Diabetes, Endocrinology, and Metabolism for technical support.
Financial Disclosure
Dr. George was supported by National Heart, Lung, and Blood Institute (NHLBI) Training Grant T32HL007779. Dr. Selvin was supported by NIH/NIDDK grants K24DK106414 and R01DK089174. Dr. Palta was supported by NIH/NIA grant K99AG052830. The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700004I, HHSN268201700005I). Neurocognitive data were collected by U01 2U01HL096812, 2U01HL096814, 2U01HL096899, 2U01HL096902, 2U01HL096917 from the NIH (NHLBI, NINDS, NIA and NIDCD), and with previous brain MRI examinations funded by R01-HL70825 from the NHLBI. Reagents for the thyroid function tests were donated by Roche Diagnostics.
Footnotes
Conflict of Interest
The authors have no conflict of interest.
Informed Consent
Informed consent was obtained from the participants.
References
- 1.How does the thyroid gland work? 2010; https://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0072572/. Accessed April 12, 2018.
- 2.Santisteban P Werner & Ingbar’s the thyroid a fundamental and clinical text. Tenth ed. Philadelphia: Lippincott Williams & Wilkins; 2013. [Google Scholar]
- 3.Mullur R, Liu YY, Brent GA. Thyroid hormone regulation of metabolism. Physiol Rev. 2014;94(2):355–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rousset B, Dupuy C, Miot F, Dumount J. Endotext In: De Groot LJ, Grossman A, Korbonits M, et al. , eds. Chapter 2 thyroid hormone synthesis and secretion. South Dartmouth, MA: Endotext [Internet]; 2000. [Google Scholar]
- 5.Hiller-Sturmhofel S, Bartke A. The endocrine system: an overview. Alcohol Health Res World. 1998;22(3):153–164. [PMC free article] [PubMed] [Google Scholar]
- 6.Antonelli A, Ferrari SM, Corrado A, Di Domenicantonio A, Fallahi P. Autoimmune thyroid disorders. Autoimmun Rev. 2015;14(2):174–180. [DOI] [PubMed] [Google Scholar]
- 7.Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, Braverman LE. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489–499. [DOI] [PubMed] [Google Scholar]
- 8.Visser WE, Visser TJ, Peeters RP. Thyroid disorders in older adults. Endocrinol Metab Clin North Am. 2013;42(2):287–303. [DOI] [PubMed] [Google Scholar]
- 9.Pearce EN, Farwell AP, Braverman LE. Thyroiditis. N Engl J Med. 2003;348(26):2646–2655. [DOI] [PubMed] [Google Scholar]
- 10.McLeod DS, Cooper DS. The incidence and prevalence of thyroid autoimmunity. Endocrine. 2012;42(2):252–265. [DOI] [PubMed] [Google Scholar]
- 11.Frohlich E, Wahl R. Thyroid autoimmunity: role of anti-thyroid antibodies in thyroid and extra-thyroidal diseases. Front Immunol. 2017;8:521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samuels MH. Thyroid disease and cognition. Endocrinol Metab Clin North Am. 2014;43(2):529–543. [DOI] [PubMed] [Google Scholar]
- 13.Beydoun MA, Beydoun HA, Rostant OS, Dore GA, Fanelli-Kuczmarski MT, Evans MK, Zonderman AB. Thyroid hormones are associated with longitudinal cognitive change in an urban adult population. Neurobiol Aging. 2015;36(11):3056–3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith JW, Evans AT, Costall B, Smythe JW. Thyroid hormones, brain function and cognition: a brief review. Neurosci Biobehav Rev. 2002;26(1):45–60. [DOI] [PubMed] [Google Scholar]
- 15.Gharib H, Tuttle RM, Baskin HJ, Fish LH, Singer PA, McDermott MT, American Association of Clinical E, et al. Consensus Statement #1: Subclinical thyroid dysfunction: a joint statement on management from the American Association of Clinical Endocrinologists, the American Thyroid Association, and The Endocrine Society. Thyroid. 2005;15(1):24–28; response 32-23. [DOI] [PubMed] [Google Scholar]
- 16.Hugo J, Ganguli M. Dementia and cognitive impairment: epidemiology, diagnosis, and treatment. Clin Geriatr Med. 2014;30(3):421–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brent GA. Mechanisms of thyroid hormone action. J Clin Invest. 2012;122(9):3035–3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flanagan EP, Caselli RJ. Autoimmune encephalopathy. Semin Neurol. 2011;31(2):144–157. [DOI] [PubMed] [Google Scholar]
- 19.de Jong FJ, Masaki K, Chen H, Remaley AT, Breteler MM, Petrovitch H, White LR, et al. Thyroid function, the risk of dementia and neuropathologic changes: the Honolulu-Asia aging study. Neurobiol Aging. 2009;30(4):600–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chaker L, Wolters FJ, Bos D, Korevaar TI, Hofman A, van der Lugt A, Koudstaal PJ, et al. Thyroid function and the risk of dementia: The Rotterdam Study. Neurology. 2016;87(16):1688–1695. [DOI] [PubMed] [Google Scholar]
- 21.Napthali K, Boyle M, Tran H, Schofield PW, Peel R, McEvoy M, Oldmeadow C, et al. Thyroid antibodies, autoimmunity and cognitive decline: is there a population-based link? Dement Geriatr Cogn Dis Extra. 2014;4(2):140–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pasqualetti G, Pagano G, Rengo G, Ferrara N, Monzani F. Subclinical hypothyroidism and cognitive impairment: systematic review and meta-analysis. J Clin Endocrinol Metab. 2015;100(11):4240–4248. [DOI] [PubMed] [Google Scholar]
- 23.Yeap BB, Alfonso H, Chubb SA, Puri G, Hankey GJ, Flicker L, Almeida OP. Higher free thyroxine levels predict increased incidence of dementia in older men: the Health in Men Study. J Clin Endocrinol Metab. 2012;97(12):E2230–2237. [DOI] [PubMed] [Google Scholar]
- 24.Volpato S, Guralnik JM, Fried LP, Remaley AT, Cappola AR, Launer LJ. Serum thyroxine level and cognitive decline in euthyroid older women. Neurology. 2002;58(7):1055–1061. [DOI] [PubMed] [Google Scholar]
- 25.Moon JH, Park YJ, Kim TH, Han JW, Choi SH, Lim S, Park DJ, et al. Lower-but-normal serum TSH level is associated with the development or progression of cognitive impairment in elderly: Korean Longitudinal Study on Health and Aging (KLoSHA). J Clin Endocrinol Metab. 2014;99(2):424–432. [DOI] [PubMed] [Google Scholar]
- 26.Tan ZS, Beiser A, Vasan RS, Au R, Auerbach S, Kiel DP, Wolf PA, et al. Thyroid function and the risk of Alzheimer disease: the Framingham Study. Arch Intern Med. 2008;168(14):1514–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin SS, Daya N, Lutsey PL, Matsushita K, Fretz A, McEvoy JW, Blumenthal RS, et al. Thyroid function, cardiovascular risk factors, and incident atherosclerotic cardiovascular disease: the Atherosclerosis Risk in Communities (ARIC) Study. J Clin Endocrinol Metab. 2017;102(9):3306–3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schultheiss UT, Teumer A, Medici M, Li Y, Daya N, Chaker L, Homuth G, et al. A genetic risk score for thyroid peroxidase antibodies associates with clinical thyroid disease in community-based populations. J Clin Endocrinol Metab. 2015;100(5):E799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goff DC Jr., Howard G, Wang CH, Folsom AR, Rosamond WD, Cooper LS, Chambless LE. Trends in severity of hospitalized myocardial infarction: the atherosclerosis risk in communities (ARIC) study, 1987-1994. Am Heart J. 2000;139(5):874–880. [DOI] [PubMed] [Google Scholar]
- 30.White AD, Folsom AR, Chambless LE, Sharret AR, Yang K, Conwill D, Higgins M, et al. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years’ experience. J Clin Epidemiol. 1996;49(2):223–233. [DOI] [PubMed] [Google Scholar]
- 31.Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ, Folsom AR. Incidence of atrial fibrillation in whites and African-Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2009;158(1):111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosamond WD, Folsom AR, Chambless LE, Wang CH, McGovern PG, Howard G, Copper LS, et al. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke. 1999;30(4):736–743. [DOI] [PubMed] [Google Scholar]
- 33.Knopman DS, Gottesman RF, Sharrett AR, Wruck LM, Windham BG, Coker L, Schneider AL, et al. Mild cognitive impairment and dementia prevalence: the Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS). Alzheimers Dement (Amst). 2016;2:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knopman DS, DeKosky ST, Cummings JL, Chui H, Corey-Bloom J, Relkin N, Small GW, et al. Practice parameter: diagnosis of dementia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56(9):1143–1153. [DOI] [PubMed] [Google Scholar]
- 35.Iddah MA, Macharia BN. Autoimmune thyroid disorders. ISRN Endocrinol. 2013;2013:509764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bensenor IM, Olmos RD, Lotufo PA. Hypothyroidism in the elderly: diagnosis and management. Clin Interv Aging. 2012;7:97–111. [DOI] [PMC free article] [PubMed] [Google Scholar]