Abstract
Background
The spontaneous breathing trial (SBT) is a well-established diagnostic test for predicting extubation failure in intubated intensive care unit (ICU) patients. However, the SBT has not been evaluated in a specific cohort of tracheostomized patients in whom weaning is prolonged and ultimately unsuccessful.
Objective
The aim of the trial was to investigate the relevance of SBT failure criteria in chronic respiratory failure subjects undergoing long-term invasive home mechanical ventilation following tracheostomy and weaning failure.
Methods
Measurement of all established failure criteria including pneumotachygraphical assessment of the rapid shallow breathing index (RSBI) took place during an SBT. The decision to continue spontaneous breathing was based on failure criteria as well as the subjective willingness of the patient.
Results
Fifteen subjects with a median age of 58 years (interquartile range [IQR] 44–74) were studied; 10 with COPD, 4 with neuromuscular diseases and 1 with both. Twelve subjects met the SBT failure criteria within 30 min, but one third of these subjects were still able to continue with spontaneous breathing. In contrast, 3 subjects could not be weaned despite the SBT being successful. An increased RSBI was the most frequently observed SBT failure criterion (57% of all SBT). However, the SBT varied substantially in individual subjects who were able to sustain spontaneous breathing, despite having reached the cut-off for SBT failure.
Conclusion
The SBT was of low predictive value regarding spontaneous breathing ability in chronic respiratory failure subjects with prolonged, unsuccessful weaning.
Keywords: Chronic respiratory failure, Invasive mechanical ventilation, Prolonged weaning, Rapid shallow breathing index, Home mechanical ventilation
Abstract
Hintergrund
Der Spontanatemversuch („spontaneous breathing trial“, SBT) ist ein etablierter Test, um ein Extubationsversagen bei intubierten Patienten auf der Intensivstation vorauszusagen. Bei tracheotomierten Patienten im prolongierten und schließlich erfolglosen Weaning wurde er bislang jedoch noch nicht systematisch untersucht.
Ziel der Arbeit
Ziel der Studie war es, die Relevanz der definierten SBT-Abbruchkriterien bei tracheotomierten Patienten mit chronischer respiratorischer Insuffizienz und außerklinischer invasiver Langzeitbeatmung nach Weaningversagen zu untersuchen.
Material und Methoden
Alle etablierten SBT-Abbruchkriterien inklusive pneumotachographischer Messungen des Rapid Shallow Breathing Index (RSBI) wurden während eines SBT systematisch gemessen. Die Entscheidung, den Spontanatemversuch fortzuführen, wurde anhand der Abbruchkriterien und des subjektiven Wunsches des Patienten getroffen.
Ergebnisse
In die Studie wurden 15 Patienten mit einem medianen Alter von 58 Jahren (Interquartilsabstand, IQR: 44–74) einbezogen (COPD: 10 Patienten, neuromuskulären Erkrankungen: 4 Patienten, beide Erkrankungen: 1 Patient). Bei 12 Patienten wurde innerhalb von 30 min ein Abbruchkriterium erreicht, dennoch konnte ein Drittel dieser Patienten die Spontanatmung fortführen. Jedoch konnten 3 dieser Patienten nicht von der Beatmung entwöhnt werden, obwohl der SBT erfolgreich war. Ein erhöhter RSBI war das am häufigsten erreichte Abbruchkriterium (in 57 % aller SBT). Bei den Patienten, welche die Spontanatmung trotz Erreichen eines SBT-Abbruchkriteriums fortsetzen konnten, variierte der weitere Verlauf des SBT erheblich.
Schlussfolgerung
Der SBT ist von geringem prädiktivem Wert, um die Fähigkeit zur Spontanatmung bei Patienten mit chronischer respiratorischer Insuffizienz und prolongiertem erfolglosem Weaning vorauszusagen.
Schlüsselwörter: Chronische respiratorische Insuffizienz, Invasive Beatmung, Prolongiertes Weaning, Rapid Shallow Breathing Index, Außerklinische Beatmung
Introduction
Weaning from invasive mechanical ventilation is an essential and universal element in the care of critically ill patients, particularly in those with weaning difficulties [3]. The time spent on the weaning process is estimated to represent 40–50% of the total duration of mechanical ventilation on the ICU [6, 8, 10, 18]. Accordingly, three different weaning categories were defined at an International Consensus Conference in 2005 and published in 2007: ‘simple’, ‘difficult’ and ‘prolonged’ [3]. Importantly, these categories are fundamentally based on the spontaneous breathing trial (SBT).
The SBT is a well-established tool in ICU medicine for predicting how successful the outcome of extubation will be [7, 8, 13]. According to the International Consensus Conference, SBT failure criteria are defined both by subjective indices (e.g. depressed mental status, diaphoresis, cyanosis) and objective measurements (e.g. low PaO2 values, high PaCO2 values, low pH values or a high rapid shallow breathing index [RSBI]) [3]. Here, the first 30 min are crucial to the success of the SBT [7, 30]. The reintubation rate is reportedly at only 13% in patients who pass the SBT [4, 11, 28], regardless of whether it lasts 30 or 120 min [9, 24]. However, recent data showed that there might be patients who succeed in the 30 min trial but fail the 120 min trial [20].
It is, however, important to point out that the SBT was originally established for intubated patients [4, 7, 10, 11, 15, 17, 22, 24, 25, 28, 30, 31] but has since been adopted for tracheostomized patients, even though there is no evidence to suggest that the classic criteria for the SBT are also valid for these patients. This is particularly important for patients with underlying severe chronic respiratory failure who are undergoing prolonged weaning, especially because the weaning process in this patient group takes up a significant proportion of the total mechanical ventilation time; if weaning eventually fails, it then becomes necessary to continue invasive mechanical ventilation in the home environment (home mechanical ventilation, HMV) via a tracheostoma [5, 14, 27]. The current trial therefore focused on these particular subjects and specifically tested whether the SBT is applicable to subjects who have undergone prolonged weaning.
Materials and methods
The study protocol was approved by the Ethics Committee at the Witten/Herdecke University, Witten, Germany (protocol number 98/2014). The study was prospectively performed at a specialized weaning unit for prolonged weaning at the Department of Pneumology, Cologne-Merheim Hospital, University Witten/Herdecke, Cologne, Germany. The study was carried out in accordance with the ethical standards laid down in the Declaration of Helsinki (last revised in October 2013). The study was registered at the German Clinical Trials Register (DRKS00006744; Universal Trial Number: U1111-1161-7111). Informed written consent was obtained from all subjects or legal guardians.
Subjects
Adult subjects (≥18 years) were consecutively enrolled if they were admitted to the specialized weaning unit for an elective check-up of the invasive HMV that they had been using for at least 2 months. Prior to the study, all subjects were established on invasive HMV following weaning failure. All subjects had to have been in a clinically stable disease state during the 2 weeks preceding recruitment, with no signs of respiratory infection (defined by at least two of the following criteria: purulent sputum, temperature >38.0 °C, pulmonary infiltrates on chest X‑ray, leukocyte count >10,000/L) and no changes in their pharmaceutical and non-pharmaceutical treatment regime. In addition, subjects had to fulfil the criteria for readiness to wean in accordance with the 2005 International Consensus Conference recommendations [3].
Data sources
The following data was collected from each patient: demographics; main diagnosis and co-morbidities; medication; mechanical ventilation details: ventilator type and settings; ventilator protocols established for the home environment (mechanical ventilation times and spontaneous breathing).
Study design
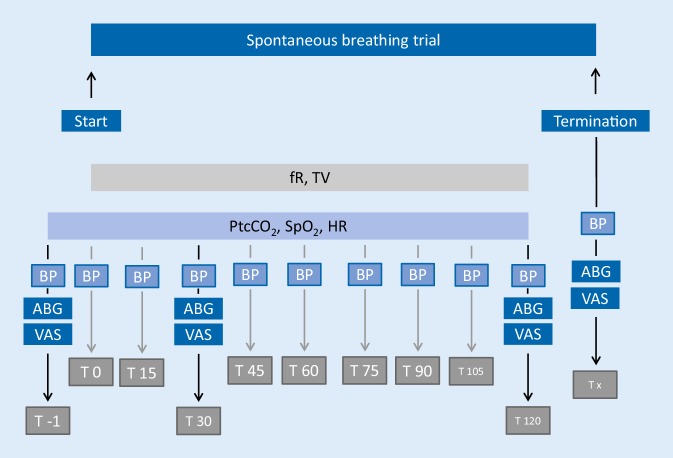
The study flowchart is presented in Fig. 1.
Fig. 1.
Study flowchart. ABG arterialized blood gas analysis, BP blood pressure (check for arrhythmias simultaneously), fR respiratory frequency, HR heart rate, PtcCO2 transcutaneous PCO2 monitoring, SpO2 peripheral oxygen saturation, T time (minutes), VT tidal volume, VAS visual analogue scale (dyspnoea, fear, pain)
Blood pressure was measured noninvasively (M1574A, Philips, Amsterdam, Netherlands; monitor: IntelliVue MP30; Philips Medical Systems, Boeblingen, Germany), while the degrees of dyspnoea, fear and pain were assessed via a 100 mm visual analogue scale (VAS) [23]. Blood gas analysis was performed on blood samples from the arterialized earlobe (ABL800 Flex, Radiometer Medical ApS, Brønshøj, Denmark) [21]. Transcutaneous PCO2 (PtcCO2) monitoring was performed throughout the entire study period (Sentec Digital Monitor System, SDMS; SenTec AG, Therwil, Switzerland, V07.03.0, V‑STATS® 4.00). For this purpose, the sensor was placed on the forehead at least 15 min prior to the SBT to allow for equilibrium measurements. Continuous heart rate (HR) and peripheral oxygen saturation (SpO2) measurements were made simultaneously using the same monitoring system.
For the purpose of the study, each patient underwent an SBT. Disconnecting the patient from the ventilator defined the start of the SBT (T0). To assess the breathing pattern during the SBT, pneumotachographical measurements were performed with a flow sensor (REF 281637; Hamilton Medical AG, Bonaduz, Switzerland) placed between the tracheal cannula and the heat and moisture exchanger (REF 1873000; Intersurgical GmbH, Sankt Augustin, Germany). Continuous spirometric measurements (AD Instruments, Therwil, Switzerland) were also performed. Data were assessed by PowerLab (AD Instruments, Sydney, Australia) and analysed using specialized software (labchart, AD Instruments, Sydney, Australia). The RSBI was calculated using the mean values for respiratory frequency (fR) and tidal volume (VT); here, a single value was exported from the software every 30 s and a mean value was calculated from groups of 10 single values at a time.
The SBT was scheduled for 120 min. SBT failure criteria were defined in accordance with the literature (Table 1 [3]).
Table 1.
Spontaneous breathing trial failure criteria [1]
| Subjective indices and clinical assessment | Agitation and anxiety |
| Depressed mental status | |
| Diaphoresis | |
| Cyanosis | |
| Evidence of increasing effort: increased accessory muscle activity, facial signs of distress, dyspnoea | |
| Objective measurements | PaO2 ≤50–60 mm Hg on FiO2 ≥0.5 or SaO2 <90% |
| PaCO2 >50 mm Hg or an increase in PaCO2 >8 mm Hg | |
| pH <7.32 or a decrease in pH ≥0.07 units | |
| fR/VT >105 breaths/min/l (=RSBI) | |
| fR >35 breaths/min or increased by ≥50% | |
| HR >140 beats/min or increased by ≥20% | |
| RR sys >180 mm Hg or increased by ≥20% or <90 mm Hg | |
| Cardiac arrhythmias |
Table modified according to [1]
PaO2 arterial partial pressure of oxygen, SaO2 arterial oxygen saturation, FiO2 inspired oxygen fraction, PaCO2 arterial partial pressure of carbon dioxide, fR respiratory frequency, VT tidal volume, RSBI rapid shallow breathing index, HR heart rate, RR sys systolic blood pressure
If a failure criterion was fulfilled, the SBT was terminated prior to its scheduled end and the reason for termination was documented. However, if a patient insisted on continuing with spontaneous breathing despite the fulfilment of a failure criterion, the SBT was continued under close observation. If the patient did not fulfil any failure criteria within 120 min, the SBT was rated as successful and spontaneous breathing was continued until it was no longer possible, at which point the reason for becoming reconnected to the ventilator was documented. If the SBT was terminated due to clinical criteria, the specific criterion that led to termination (fear, dyspnoea or pain) was documented.
Statistical analysis
All analyses were performed using SigmaPlot 12.0 (Systat Software GmbH, Erkrath, Germany). For continuous measurements (RSBI, VT, fR, PtcCO2, SpO2 and HR), a single parameter was exported from the software every 30 s. Normal data distribution was assessed using the Kolmogorov–Smirnov test. Statistical significance was calculated using the t-test for normally distributed data and the Mann–Whitney rank sum test for non-normally distributed data. Statistical significance was assumed at a p-value <0.05. All analyses are exploratory, without prior planning of sample size. No adjustment for multiple testing was performed. All p-values should be interpreted in a descriptive sense.
The Pearson correlation coefficient was used to compare the actual duration of the current SBT trial to the duration of spontaneous breathing in the outpatient setting, as well as to that recommended by the physician at the last in-patient check-up session. For further analysis, subjects with SBT failure were categorized according to the timepoint at which the trial failed: 1 = termination of the SBT after <30 min, 2 = termination of the SBT after ≥30 min.
Results
Fifteen subjects were consecutively enrolled from October 2014 to August 2015. The basic demographic data of the cohort were as follows: 60% (n = 9) were female, median age was 58 years (interquartile range [IQR] 44–74 years), median BMI was 26 kg/m2 (IQR 20–30), median duration of HMV was 17 months (IQR 6–27 months). The leading reasons for mechanical ventilation were as follows: COPD (n = 10), COPD and amyotrophic lateral sclerosis (n = 1), neuromuscular disorders (n = 4: amyotrophic lateral sclerosis, n = 1; Duchenne muscular dystrophy, n = 1; mitochondriopathies, n = 1; spinal muscular dystrophy, n = 1).
Nine subjects had cardiovascular comorbidities (e.g. arterial hypertension, atrial fibrillation, heart failure). Overall, 10 subjects were ventilated in an assisted pressure-controlled mode (aPCV), and 5 in a pressure-supported mode (PSV or ST).
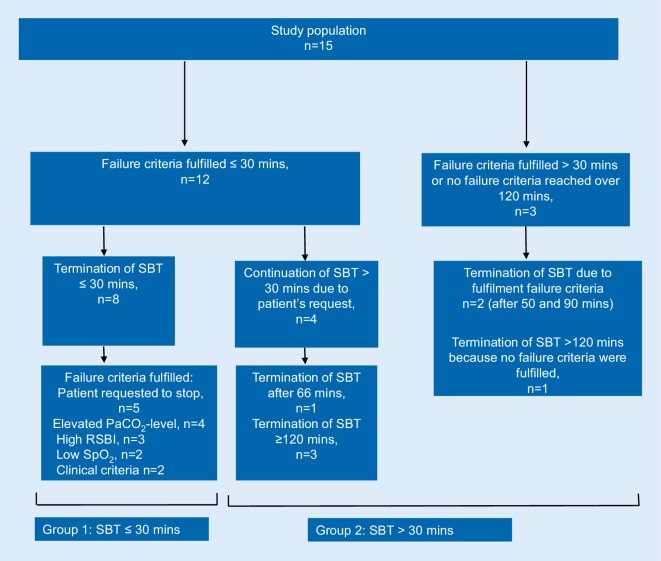
The results of the SBT are displayed in Fig. 2.
Fig. 2.
SBT results for the whole study population. mins minutes, PaCO2 arterialized partial pressure of carbon dioxide, RSBI rapid shallow breathing index, SBT spontaneous breathing trial, SpO2 peripheral oxygen saturation
Overall, twelve subjects fulfilled at least one failure criterion within the first 30 min of the SBT. Two subjects fulfilled at least one failure criterion between 30 and 120 min, and one patient did not fulfil any failure criteria within 120 min of the SBT (Fig. 2).
None of the subjects could eventually be weaned from invasive mechanical ventilation. This was also true for the subjects who had undergone a successful SBT after the first 30 min (n = 3). Table 2 shows the SBT results of the individual subjects.
Table 2.
Individual results of the spontaneous breathing trial (SBT)
| n = 15 | Time taken to fulfil the first failure criterion (min) | First failure criterion fulfilled | Duration of SBT (min) | Type of failure criteria fulfilled |
|---|---|---|---|---|
| 1 | 25 | Clinical criteria | 25 | PaCO2, clinical criteria |
| 2 | 30 | PaCO2 | 30 | PaCO2, pH |
| 3 | 25 | RSBI | 66 | RSBI |
| 4 | 8 | RSBI | 11 | PaCO2, RSBI, clinical criteria |
| 5 | 6 | Clinical criteria | 6 | Clinical criteria, PaCO2, SpO2 |
| 6 | 7 | RSBI | 13 | RSBI |
| 7 | 4 | RSBI | >120 | RSBI, HR |
| 8 | 0 | RSBI | >120 | PaCO2, RSBI, RR sys, clinical criteria |
| 9 | 90 | RR sys | >120 | RR sys |
| 10 | 24 | Clinical criteria | 24 | Clinical criteria |
| 11 | >120 | None | >120 | None |
| 12 | 0 | RSBI | 30 | PaCO2, RSBI, clinical criteria |
| 13 | 0 | RSBI | 21 | pH, SpO2, RSBI, fR |
| 14 | 50 | PaCO2 | 50 | PaCO2, clinical criteria |
| 15 | 0 | RSBI | >120 | RSBI, fR |
Clinical criteria = agitation and anxiety/depressed mental status/diaphoresis/cyanosis/evidence of increasing effort/increased accessory muscle activity/facial signs of distress/dyspnoea [3]
fR respiratory frequency >35 breaths/min or increased by ≥50%, HR heart rate >140 beats/min or increased by ≥20%, PaCO2 arterial partial pressure of oxygen >50 mm Hg, or an increase of >8 mm Hg, pH pH-value <7.32 or a decrease in pH of ≥0.07 pH units, RR sys systolic blood pressure >180 mm Hg or increased by ≥20% or <90 mm Hg, RSBI rapid shallow breathing index >105 breaths/min/l, SpO2 peripheral oxygen saturation <90%
Figure 3 shows the distribution of the first failure criterion that was fulfilled.
Fig. 3.
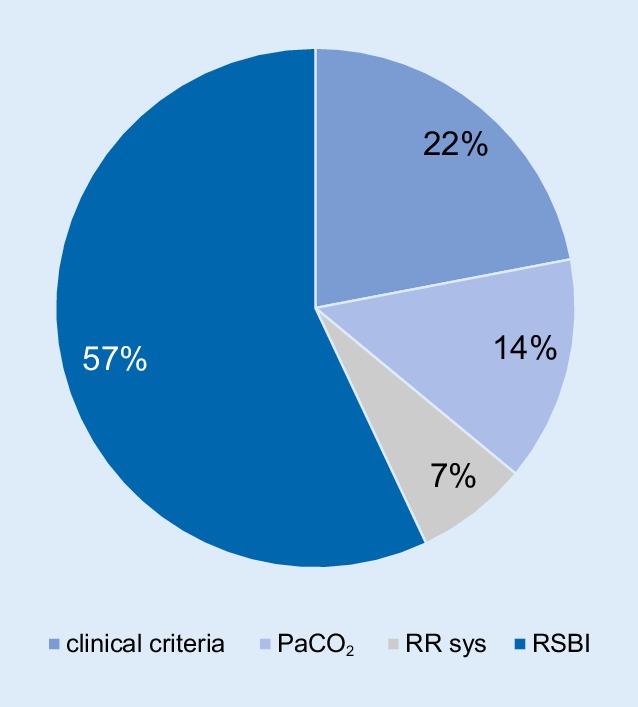
Distribution of the first failure criterion fulfilled (n = 14). PaCO2 arterial partial pressure of carbon dioxide, RR sys systolic blood ores sure, RSBI rapid shallow breathing index
Eight subjects terminated the SBT after ≤30 min (group 1), and seven subjects after >30 min (group 2), irrespective of the failure criteria being fulfilled or not.
Group 1 and 2 were comparable in terms of the following pre-SBT parameters: PaCO2 (p = 0.23), pH (p = 0.51), systolic blood pressure (p = 0.86), VAS score for dyspnoea (p = 0.17), fear (p = 0.61) and pain (p = 0.34). Furthermore, the RSBI (at the time of disconnection from the ventilator; T0) also did not differ between groups (p = 0.61). Overall, the RSBI showed broad intra-individual variation throughout the SBT assessment period, with a standard deviation of 17.6/18.3 breaths/min/l (median/mean; min–max: 1.7–64.9).
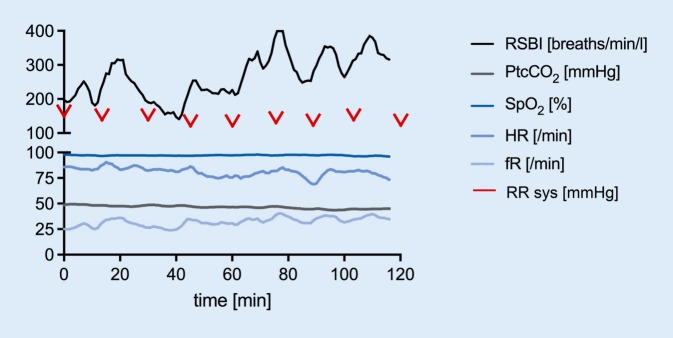
Figure 4 shows the individual recordings of a 32-year-old female patient with spinal muscular atrophy (Kugelberg–Welander) who was on spontaneous breathing for 16 h/day at home.
Fig. 4.
Individual recordings from a 32-year-old patient with spinal muscular atrophy. fR respiratory frequency, HR heart rate, PtcCO2 transcutaneous partial pressure of carbon dioxide, RR sys systolic blood pressure, RSBI rapid shallow breathing index, SpO2 peripheral oxygen saturation. VAS scores for pain, dyspnoea and fear were zero throughout the whole trial
Although RSBI measurements showed values greater than 105 breaths/min/l (=failure criteria) from the very start, the patient’s clinical status was stable throughout the whole trial and she insisted on continuing with spontaneous breathing. RSBI showed wide variability throughout the measurement period of 120 min. The second failure criterion that was fulfilled was breathing frequency >35 breaths/min after 17 min. VAS scores for pain, dyspnoea and fear were zero throughout the whole trial and are therefore not displayed in the figure.
The mean duration of the spontaneous breathing period recommended by the physician was 2.0 h/day (median; min–max: 0–19 h/day). This was highly correlated (r = 0.89, p < 0.001) with the actual duration of spontaneous breathing at home (median 3.0 h/d; min–max: 0–17 h/day), and moderately correlated with the duration of the experimental SBT in the current study (30 min median; min–max: 6–>120 min, r = 0.70, p = 0.004). Importantly, however, this did not correlate with the time taken until the first failure criterion was fulfilled in the current experimental set-up (8 min median; min–max: 0–>120 min, r = 0.12, p = 0.70).
Discussion
To the best of our knowledge this is the first trial to evaluate the diagnostic value of SBTs in subjects who were undergoing prolonged weaning from invasive HMV that was ultimately unsuccessful. The main result of the study was that the SBT did not provide useful information for predicting the ability to maintain spontaneous breathing in subjects with prolonged weaning. On one hand, none of the subjects who had a successful SBT were eventually able to be weaned from invasive mechanical ventilation. On the other, many of the subjects who fulfilled specific failure criteria for the SBT were still able to continue spontaneous breathing for many hours, even in cases of severe physiological disturbances. For example, even subjects with an extremely high RSBI during the first 30 min of spontaneous breathing were able to maintain spontaneous breathing for many hours. Finally, there was discordance between the subjective comfort during spontaneous breathing vs. the physiological parameters assessed during the SBT. Taken together, the SBT—which was originally transferred from an ICU setting to subjects with prolonged weaning—may therefore not be useful in this patient cohort.
Data for the prognostic value of the SBT stem from ICU medicine. Here, most patients are assigned to the ‘easy’ or ‘difficult’ weaning [4, 7, 9, 11, 12] rather than ‘prolonged’ weaning categories. In contrast to patients with easy or difficult weaning, patients undergoing prolonged weaning mostly suffer from chronic respiratory failure, and many also have significant comorbidities that affect respiratory mechanics [9]. This might explain why prolonged-weaning patients respond differently to the physiological parameters covered by the SBT. In addition, patients are likely to have adapted to a more ‘rapid shallow breathing’ pattern during the course of their disease and may therefore tolerate higher RSBIs for a longer period of time. Moreover, the RSBI in ICU medicine is usually measured via an orotracheal rather than a tracheal tube, which was exclusively the case in the current cohort.
As already emphasized by Yang and Tobin back in 1991, the RSBI is the best index for evaluating the SBT [30], although it has a variable specificity and sensitivity [16, 19, 26] and several trials observed a wide range of different predictive values for RSBI, depending on the different study population and classification of outcomes [1, 28, 29].
However, it has not been clarified how long the RSBI can remain above 105 breaths/min/l before the SBT has to be terminated. Interestingly, in this trial, high individual variations in RSBIs were documented amongst patients with prolonged weaning, which further supports the thesis that patients in prolonged weaning differ from intubated patients in the ICU. In particular, the individual variations in RSBIs observed amongst patients with prolonged weaning (see current data) would present a challenge when deciding whether to terminate the SBT based on these criteria. In the present trial, an elevated RSBI was the first failure criterion to be met within the first 30 min in 8 out of 15 subjects. However, the SBT had to be terminated within the first 30 min in just half of these subjects. Therefore, the RSBI does not seem to be an adequate proxy for SBT failure in prolonged-weaning patients. Furthermore, it was also shown that the duration of spontaneous breathing recommended upon clinical appraisal did not correlate at all with the duration of spontaneous breathing that occurred until the first failure criterion was fulfilled. It did, however, correlate with the duration of spontaneous breathing during the current experimental set-up, where maximal correlation was observed between the clinically recommended duration of spontaneous breathing and the actual time subjects spent on spontaneous breathing at home. It can hence be postulated from these data that clinical assessment is more useful in this case than following the rigid failure criteria for the SBT.
Even though the International Consensus Conference data published over 10 years ago described the SBT as a well-accepted predictor of successful weaning [3], a very recent epidemiological study covering 2729 subjects concluded that many of the subjects treated on the ICU cannot be categorized according to the results of the SBT, because this particular trial is not always performed in clinical practice [2]. A different categorization scheme for the degree of weaning failure severity was therefore proposed, which is independent from performing an SBT [2]. Indeed, the SBT is a technically complex procedure in patients with prolonged weaning, as demonstrated in the current study. Given the fact that the SBT is yet to be convincingly adapted for use in the specific cohort of prolonged weaning, in contrast to intubated patients receiving short-term invasive mechanical ventilation, the current study doubts that the SBT—as recommended by guidelines—is a worthwhile tool for predicting spontaneous breathing capabilities in prolonged, unsuccessful weaning. Clearly, further studies adding to the current pilot testing are necessary to validate this observation.
There are some limitations to this study that need to be addressed. Firstly, it is a strictly observational trial in which the statistical data serve as exploratory and descriptive findings only. Secondly, the sample size was rather small due to the special inclusion criteria.
Conclusion
In conclusion, the standard SBT established for intubated ICU patients is prone to both over- and underestimation of the ability to breathe spontaneously in patients with prolonged, unsuccessful weaning. Therefore, the SBT does not provide useful information for predicting subsequent spontaneous breathing patterns in the specific cohort of prolonged-weaning patients.
Acknowledgments
Acknowledgements
All participants are acknowledged for the effort they devoted to this study and Dr. Sandra Dieni for helpful comments on the manuscript.
Compliance with ethical guidelines
Conflict of interest
F.S. Magnet, E. Heilf, S.E. Huttmann, J. Callegari, S.B. Schwarz, J.H. Storre and W. Windisch report open research grants from Weinmann/Germany, Vivisol/Germany, Heinen und Löwenstein/Germany, VitalAire/Germany and Philips/Respironics/USA, during the conduct of the study. F.S. Magnet reports travel grants from Heinen und Löwenstein/Germany, grants for travel and advisory board from Philipps Respironics/Germany and speaker fees from SenTec/Switzerland, outside the submitted work. J.H. Storre reports personal fees for lectures from Heinen und Löwenstein/Germany, VitalAire/Germany and Vivisol GmbH; financial support for attending congresses from Vivisol GmbH; and personal fees for expertise/advisory board from Breas Medical during the conduct of the study. He reports personal honoraria for expertise/lectures and financial support for attending congresses from Boehringer Ingelheim Pharma; honoraria for expertise/lectures from SenTec AG, Keller Medical GmbH, Keller Medical GmbH, Linde Deutschland and Santis GmbH outside the submitted work. W. Windisch reports speaker fees from companies dealing with mechanical ventilation outside the submitted work.
The study protocol was approved by the Ethics Committee at the Witten/Herdecke University, Witten, Germany (protocol number 98/2014). The study was carried out in accordance with the ethical standards laid down in the Declaration of Helsinki (last revised in October 2013). Informed written consent was obtained from all subjects or legal guardians.
References
- 1.Banerjee A, Mehrotra G. Comparison of lung ultrasound-based weaning indices with rapid shallow breathing index: are they helpful? Indian J Crit Care Med. 2018;22:435–440. doi: 10.4103/ijccm.IJCCM_331_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beduneau G, Pham T, Schortgen F, et al. Epidemiology of weaning outcome according to a new definition. The WIND study. Am J Respir Crit Care Med. 2017;195:772–783. doi: 10.1164/rccm.201602-0320OC. [DOI] [PubMed] [Google Scholar]
- 3.Boles JM, Bion J, Connors A, et al. Weaning from mechanical ventilation. Eur Respir J. 2007;29:1033–1056. doi: 10.1183/09031936.00010206. [DOI] [PubMed] [Google Scholar]
- 4.Brochard L, Rauss A, Benito S, et al. Comparison of three methods of gradual withdrawal from ventilatory support during weaning from mechanical ventilation. Am J Respir Crit Care Med. 1994;150:896–903. doi: 10.1164/ajrccm.150.4.7921460. [DOI] [PubMed] [Google Scholar]
- 5.Bruells CS, Bickenbach J, Marx G. Weaning ward-different from the ICU? Med Klin Intensivmed Notfmed. 2018;113:94–100. doi: 10.1007/s00063-016-0192-6. [DOI] [PubMed] [Google Scholar]
- 6.Ely EW, Baker AM, Dunagan DP, et al. Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. N Engl J Med. 1996;335:1864–1869. doi: 10.1056/NEJM199612193352502. [DOI] [PubMed] [Google Scholar]
- 7.Esteban A, Alia I, Gordo F, et al. Extubation outcome after spontaneous breathing trials with T‑tube or pressure support ventilation. The Spanish Lung Failure Collaborative Group. Am J Respir Crit Care Med. 1997;156:459–465. doi: 10.1164/ajrccm.156.2.9610109. [DOI] [PubMed] [Google Scholar]
- 8.Esteban A, Alia I, Ibanez J, et al. Modes of mechanical ventilation and weaning. A national survey of Spanish hospitals. The Spanish Lung Failure Collaborative Group. Chest. 1994;106:1188–1193. doi: 10.1378/chest.106.4.1188. [DOI] [PubMed] [Google Scholar]
- 9.Esteban A, Alia I, Tobin MJ, et al. Effect of spontaneous breathing trial duration on outcome of attempts to discontinue mechanical ventilation. Spanish Lung Failure Collaborative Group. Am J Respir Crit Care Med. 1999;159:512–518. doi: 10.1164/ajrccm.159.2.9803106. [DOI] [PubMed] [Google Scholar]
- 10.Esteban A, Anzueto A, Frutos F, et al. Characteristics and outcomes in adult patients receiving mechanical ventilation: a 28-day international study. JAMA. 2002;287:345–355. doi: 10.1001/jama.287.3.345. [DOI] [PubMed] [Google Scholar]
- 11.Esteban A, Frutos F, Tobin MJ, et al. A comparison of four methods of weaning patients from mechanical ventilation. Spanish Lung Failure Collaborative Group. N Engl J Med. 1995;332:345–350. doi: 10.1056/NEJM199502093320601. [DOI] [PubMed] [Google Scholar]
- 12.Farias JA, Retta A, Alia I, et al. A comparison of two methods to perform a breathing trial before extubation in pediatric intensive care patients. Intensive Care Med. 2001;27:1649–1654. doi: 10.1007/s001340101035. [DOI] [PubMed] [Google Scholar]
- 13.Figueroa-Casas JB, Connery SM, Montoya R. Changes in breathing variables during a 30-minute spontaneous breathing trial. Respir Care. 2015;60:155–161. doi: 10.4187/respcare.03385. [DOI] [PubMed] [Google Scholar]
- 14.Geiseler J, Kelbel C. Weaning from mechanical ventilation. Weaning categories and weaning concepts. Med Klin Intensivmed Notfmed. 2016;111:208–214. doi: 10.1007/s00063-016-0147-y. [DOI] [PubMed] [Google Scholar]
- 15.Haberthur C, Mols G, Elsasser S, et al. Extubation after breathing trials with automatic tube compensation, T‑tube, or pressure support ventilation. Acta Anaesthesiol Scand. 2002;46:973–979. doi: 10.1034/j.1399-6576.2002.460808.x. [DOI] [PubMed] [Google Scholar]
- 16.Huang CT, Yu CJ. Conventional weaning parameters do not predict extubation outcome in intubated subjects requiring prolonged mechanical ventilation. Respir Care. 2013;58:1307–1314. doi: 10.4187/respcare.01773. [DOI] [PubMed] [Google Scholar]
- 17.Jones DP, Byrne P, Morgan C, et al. Positive end-expiratory pressure vs T‑piece. Extubation after mechanical ventilation. Chest. 1991;100:1655–1659. doi: 10.1378/chest.100.6.1655. [DOI] [PubMed] [Google Scholar]
- 18.Kollef MH, Shapiro SD, Silver P, et al. A randomized, controlled trial of protocol-directed versus physician-directed weaning from mechanical ventilation. Crit Care Med. 1997;25:567–574. doi: 10.1097/00003246-199704000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Lee KH, Hui KP, Chan TB, et al. Rapid shallow breathing (frequency-tidal volume ratio) did not predict extubation outcome. Chest. 1994;105:540–543. doi: 10.1378/chest.105.2.540. [DOI] [PubMed] [Google Scholar]
- 20.Liang G, Liu T, Zeng Y, et al. Characteristics of subjects who failed a 120-minute spontaneous breathing trial. Respir Care. 2018;63:388–394. doi: 10.4187/respcare.05820. [DOI] [PubMed] [Google Scholar]
- 21.Magnet FS, Majorski DS, Callegari J, et al. Capillary PO2 does not adequately reflect arterial PO2 in hypoxemic COPD patients. Int J Chron Obstruct Pulmon Dis. 2017;12:2647–2653. doi: 10.2147/COPD.S140843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matic I, Majeric-Kogler V. Comparison of pressure support and T‑tube weaning from mechanical ventilation: randomized prospective study. Croat Med J. 2004;45:162–166. [PubMed] [Google Scholar]
- 23.Mccormack HM, Horne DJ, Sheather S. Clinical applications of visual analogue scales: a critical review. Psychol Med. 1988;18:1007–1019. doi: 10.1017/S0033291700009934. [DOI] [PubMed] [Google Scholar]
- 24.Perren A, Domenighetti G, Mauri S, et al. Protocol-directed weaning from mechanical ventilation: clinical outcome in patients randomized for a 30-min or 120-min trial with pressure support ventilation. Intensive Care Med. 2002;28:1058–1063. doi: 10.1007/s00134-002-1353-z. [DOI] [PubMed] [Google Scholar]
- 25.Reissmann HK, Ranieri VM, Goldberg P, et al. Continuous positive airway pressure facilitates spontaneous breathing in weaning chronic obstructive pulmonary disease patients by improving breathing pattern and gas exchange. Intensive Care Med. 2000;26:1764–1772. doi: 10.1007/s001340000725. [DOI] [PubMed] [Google Scholar]
- 26.Savi A, Teixeira C, Silva JM, et al. Weaning predictors do not predict extubation failure in simple-to-wean patients. J Crit Care. 2012;27(221):e221–e228. doi: 10.1016/j.jcrc.2011.07.079. [DOI] [PubMed] [Google Scholar]
- 27.Schonhofer B, Geiseler J, Dellweg D, et al. S2k-Guideline “Prolonged Weaning”. Pneumologie. 2015;69:595–607. doi: 10.1055/s-0034-1392809. [DOI] [PubMed] [Google Scholar]
- 28.Vallverdu I, Calaf N, Subirana M, et al. Clinical characteristics, respiratory functional parameters, and outcome of a two-hour T‑piece trial in patients weaning from mechanical ventilation. Am J Respir Crit Care Med. 1998;158:1855–1862. doi: 10.1164/ajrccm.158.6.9712135. [DOI] [PubMed] [Google Scholar]
- 29.Yan S, Lichros I, Zakynthinos S, et al. Effect of diaphragmatic fatigue on control of respiratory muscles and ventilation during CO2 rebreathing. J. Appl. Physiol. 1993;75:1364–1370. doi: 10.1152/jappl.1993.75.3.1364. [DOI] [PubMed] [Google Scholar]
- 30.Yang KL, Tobin MJ. A prospective study of indexes predicting the outcome of trials of weaning from mechanical ventilation. N Engl J Med. 1991;324:1445–1450. doi: 10.1056/NEJM199105233242101. [DOI] [PubMed] [Google Scholar]
- 31.Zeggwagh AA, Abouqal R, Madani N, et al. Weaning from mechanical ventilation: a model for extubation. Intensive Care Med. 1999;25:1077–1083. doi: 10.1007/s001340051015. [DOI] [PubMed] [Google Scholar]