Abstract
Background
New patient-centric integrated care models are enabled by the capability to exchange the patient’s data amongst stakeholders, who each specialise in different aspects of the patient’s care. This requires a robust, trusted and flexible mechanism for patients to offer consent to share their data. Furthermore, new IT technologies make it easier to give patients more control over their data, including the right to revoke consent. These characteristics challenge the traditional paper-based, single-organisation-led consent process. The Dovetail digital consent application uses a mobile application and blockchain based infrastructure to offer this capability, as part of a pilot allowing patients to have their data shared amongst digital tools, empowering patients to manage their condition within an integrated care setting.
Objective
To evaluate patient perceptions towards existing consent processes, and the Dovetail blockchain based digital consent application as a means to manage data sharing in the context of diabetes care.
Method
Patients with diabetes at a General Practitioner practice were recruited. Data were collected using focus groups and questionnaires. Thematic analysis of the focus group transcripts and descriptive statistics of the questionnaires was performed.
Results
There was a lack of understanding of existing consent processes in place, and many patients did not have any recollection of having previously given consent. The digital consent application received favourable feedback, with patients recognising the value of the capability offered by the application. Patients overwhelmingly favoured the digital consent application over existing practice.
Conclusions
Digital consent was received favourably, with patients recognising that it addresses the main limitations of the current process. Feedback on potential improvements was received. Future work includes confirmation of results in a broader demographic sample and across multiple conditions.
Keywords: Blockchain, digital consent, dynamic consent, eHealth, EHRs
Introduction
Background
Integrated care is often synonymous with coordinated and seamless care, usually describing a multi-disciplinary service structured around the patient, and integrated at multiple levels such as organisation, functional, service and clinical.1 A number of healthcare services are offering new digital technologies to patients (e.g. mobile applications and integration of health systems), which contribute to the transformation of service towards achieving the goals of integrated care.2 Examples of such services provide a number of advantages, such as on-demand personalised advice and education; and continuous, remote (and thus low-resource) monitoring of health conditions. Such services typically combine data from multiple sources, which can be used to inform patients, support self-management, and show to healthcare practitioners a more complete picture.3
Management of health data is a rapidly growing challenge worldwide. A global increase in smart healthcare, telecare and telehealth, means that there is a shift toward digital health data on a significant scale, which will eventually result in growing amounts of shared data. Patients are far more mobile in a geographical sense than ever before, and may access healthcare in varied locations for numerous reasons.4 The UK’s National Health Service (NHS) has been criticised in recent years for continuing the use of ‘outdated’ technology, such as fax machines to transfer data, giving rise to more calls for an update in the technology employed within the system.5 NHS England’s Five Year Forward View pledged in 2014 to implement ‘fully interoperable electronic health records so that patients’ records are largely paperless. Patients will have full access to these records, and be able to write into them. They will retain the right to opt out of their record being shared electronically’ and presented a vision of a largely paperless NHS.6 To achieve this, action is needed now, for sophisticated e-health solutions to be developed, which can not only integrate with current systems but also evolve alongside future implementations.
Digital transformation is necessary to achieve services exhibiting these qualities, including easier ways to share data, and offering better consent models.7,8 The UK, responding to a report by the National Data Guardian,9 has introduced a national data opt-out policy,10 which is implemented by compliance to the NHS Digital DCB 3058.11 Managing and revoking consent are also parts of the EU’s General Data Protection Regulation, adopting good practice recommendation into law.12,13 Patients need to be able to give consent to data sharing or transfer of information between healthcare organisations (e.g. General Practitioner (GP) practices, pharmacies and specialist hospital care) and clearly understand what data are involved, as well as be able to revoke consent when they wish to do so. Given the complexity of integrated care service, informed consent can be difficult to maintain amongst all participating stakeholders, something that is exacerbated by IT systems.5 The traditional, paper-based approach to consent may hinder provision of integrated services to patients.
Digital approaches to consent have increasingly found their way into practice, offering advantages such as accessibility to patient, enrichment with educational material, and electronic signature of the consent.14–19 Transferring consent to health data sharing amongst multiple systems is a considerable challenge,4 which does not easily allow the patient to maintain clear knowledge of who has access to their health data, how to amend these permissions when required, or how to update their data across all of the different systems. There are a number of barriers to efficient data sharing within modern-day health IT systems and how this impacts collaborative clinical decision making, such as security and privacy concerns, lack of trust relationships between healthcare entities, scalability concerns and lack of interoperable data standards enforcement.20
The Dovetail Consent App is a mobile application for patients to digitally consent to sharing their data between services, using blockchain. This paper presents an evaluation of patients’ attitudes towards the deployed digital consent application as part of a pilot for a phase 2 Small Business Research Initiative and NHS England project. In the pilot, the Dovetail application allowed patients with type 2 diabetes to offer digital consent to having structured data relevant to their condition, transferred from their GP to a diabetes specific IT system.
Blockchain is a cryptographic, distributed peer-to-peer ledger system which operates via a number of nodes, all jointly responsible for the maintenance of a database. The ledger is a virtual container of the data of the transactions (e.g. financial transactions in finance or consent requests in healthcare) that have taken place within the system. The information that constitutes the ledger is usually stored in multiple computers, making it distributed. The data are contained within ‘blocks’, each forming part of a chain. The blocks are linked to each other using cryptography, thus ensuring the integrity of the chain. Each time an update is required to a block within the chain, nodes in the network must collectively accept or decline the change, and once this is agreed the network updates accordingly.4 Blockchains can operate either a public or a private system. In public systems (such as bitcoin), anyone can join and a large amount of computational power is required across the network. In a private system, all participants are known and require permission to join.21 Following the introduction and proof of concept of blockchain technology in recent years, it has become apparent that there is an opportunity to apply the same technology to healthcare.20,22,23 Blockchain is appreciated as a useful technology in healthcare.24 In particular, blockchain’s smart contract (i.e. a transaction between two parties, satisfying the requirements of both, guaranteed by the IT infrastructure) can be used as immutable authentication for digital consent.25–27 Using such an approach, a patient would be able to consent to a transaction (e.g. sharing of their data between two healthcare systems), with the details of the transaction recorded in the chain. As the transaction would be stored using cryptography, any change would invalidate the chain. As the chain is distributed to multiple computers, the system would be able to retrieve the last valid chain (also containing the patient’s approved transaction). Subsequent transactions would be stacked in the chain without amending the previous ones, offering traceability.
This paper presents a cross-sectional exploratory study, evaluating the perception of patients towards the use of dynamic digital consent technology. The technology was implemented by the Dovetail digital consent mobile application, deployed as part of a broader pilot; offering a suite of self-management digital tools to patients with diabetes.
The Dovetail digital consent application
The Dovetail digital consent application is a mobile application, for iOS and Android operating systems, which enables users (patients) to consent to the sharing of their data and subsequently triggers the transfer of these data. The mobile application connects to the Dovetail distributed ledger network where the consent transaction is recorded. The Dovetail network, through trusted integration, will notify local practice systems that a patient has been informed of, and consented to, a data exchange. A data exchange will take place directly between the local GP practice and the systems of the diabetes digital tools that participate in the pilot. As a result, patients will be able to see their data on diabetes specific applications, follow the progress of their care plan, add measurements, and request expert advice. The Dovetail infrastructure does not access any medical data, and only provides a description, and the provider and recipient of the data, as well as the ID of the patient, which will be stored as a transaction in the chain.
The Dovetail consent application and Dovetail network manage consent according to the dynamic consent model.28 Consent is stored with reference to the data being transferred and can be toggled independently. The Dovetail network stores consent in private channels between the sender, receiver, and Dovetail (representing the patient). Consent to share additional data can be added at later stages, and existing instances of consent can be withdrawn or augmented by the user (patient).
The Dovetail consent application provides a user interface for patients to consent to their data being transferred (Figure 1). Patients must first sign up to the application, which includes confirming their identity by using the camera on their phone to photograph their face, and a photographic identification document. These two images are compared by the Dovetail application to verify the identity of the patient. An identified patient can use the application to give consent by selecting a sender and a receiver of the data. After the consent is created, the patient can view and manage all their consents and the history of data transfers associated with those consents.
Figure 1.
Screenshots of the Dovetail digital consent application. (a) Description of purpose for sharing the data, (b) overview of active consents, (c) revocation of data sharing consent.
GP: General Practitioner
Materials and methods
This is a cross-sectional exploratory mixed-methods study. The goal of the study was to identify the patients’ attitude towards the capability offered by the Dovetail consent application. The study was conducted using a mixed methods approach, in order to yield both qualitative and quantitative data. Semi-structured focus groups were carried out to gather qualitative feedback on the application and digital consent technology. The focus groups covered previous experiences and perception with giving/taking consent for health data to be shared; attitudes towards and experience with technology, in general as well as for healthcare; perceived strengths and limitations of the app capability in comparison with current methods; and open discussion about any related issues. Discussions were semi-structured, which allowed participants to direct the conversation to a certain extent. This allows rich, organic feedback to develop by allowing the conversations to flow naturally (with some guidance from the researcher to stay on-topic). This methodology means that some topics of conversation were not present in all focus groups, as they resulted from discussions among participants or further questioning on a specific point, rather than from the listed questions. The focus group discussions were audio recorded, transcribed and analysed according to the themes of the discussion.
All participants at the end of the focus groups completed questionnaires. This provided the opportunity to explain the context, scope and terminology as well as the capability of the digital consent application, offering confidence that participants have understood the basic technological and capability tenets. Questionnaire data were captured using a five step Likert scale with 1 showing strong disagreement and 5 strong agreement (or strongly against and strongly in favour respectively, depending on the phrasing of the question). All questions were optional to participants, to avoid potential noise in the data through forced questions, and to offer a comfortable experience. Quantitative analysis of the data was performed using descriptive statistics. Where not all participants answered a question, frequencies are expressed using the valid percentage. The number of participants who answered each question (n) out of the 23 patients and 13 staff is included in the results. The questionnaires focused on three main areas: 1) identification of patients’ perceptions towards existing consent approaches, 2) identification of patients’ perceptions towards the digital consent capability offered through the Dovetail application, and 3) identification of patients’ perception towards the overall effect of the digital consent capability on the care they were receiving during the pilot.
One technology expert, one clinician and one integrated care expert reviewed the questionnaires. A Cronbach alpha test was performed to measure internal consistency, in order to assess the reliability of the Likert-based questionnaire.
The setting of the study was a GP practice in England. The Dovetail digital consent application was used to receive consent from the patients to have their data shared between the practice and other diabetes self-management mobile applications.
Participants were invited to take part in the study based on two criteria: being patients of the GP practice in which the demonstrator was offered, and having a diagnosis of either diabetes or pre-diabetes. Staff of the GP practice with involvement in diabetes care and/or patient record management were also invited. The staff perspective allowed us to understand whether certain attitudes were unique to patients, or could also be corroborated by staff. Before the questionnaire and focus group, the participants watched a video tutorial of how the app works, as well as screenshots of the application with further explanation. Following the video, questions were answered. The focus group preceded the questionnaire to ensure that all participants had understood the purpose and operation of the application. The study focused on patient perception of the deployed application, and evaluation of the technical implementation and its limitations was out of scope. Use of blockchain as the underlying technology was mentioned during the video and explained in layman terms. The explanation did not go into technical details, but was limited to highlighting: a) use of cryptography in line with best practice in the finance industry to guarantee integrity and immutability, b) storage of information in the ledger containing the transaction information and not any medical data.
The University of Warwick Biomedical & Scientific Research Ethics Committee reviewed and approved the study protocol with number REGO-2018-2277. All the participants were given a participant leaflet with details of the study and signed a consent form, according to the approved protocol. Questionnaires were anonymous and the participants were given a time window following the focus groups, during which they could retract their questionnaire data using an unidentifiable unique code.
Results
The sample consisted of a total of 36 participants, of which 23 were patients and 13 were staff in the practice.
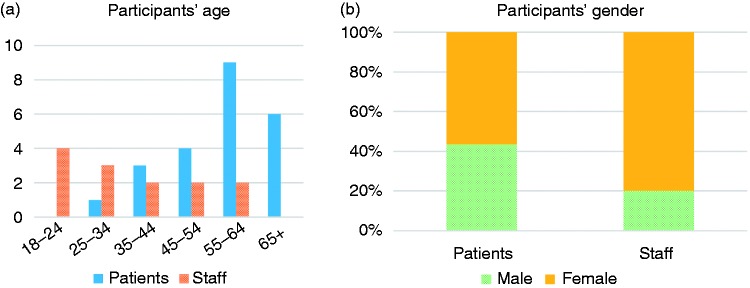
Figure 2 shows the demographics of the sample. The staff sample covered all roles in the practice including two GPs, two nurses, one healthcare assistant and eight clerical staff.
Figure 2.
(a) Age of participants (n = 36), (b) gender of participants (n = 33).
Figure 3 illustrates the participants’ perceived skill level in terms of using a smartphone device (this was an open-ended question, which was further investigated during the focus groups). Although smartphone skills level could be considered as a potential barrier to participants’ understanding the concepts of the study, technology literacy was not a recruitment criterion.
Figure 3.
Smartphone skill level (n = 35).
The evaluation focused on three themes: a) awareness and understanding of existing consent practice, b) evaluation of the dynamic patient-managed digital consent Dovetail application, and c) evaluation of the technology against current practice. Albeit the majority of patients were over 55 years of age, all of them declared they were at least somewhat confident using a smartphone. An internal reliability Cronbach’s alpha test was performed for the patient questions using the Likert scale (α = .856), indicating good consistency.
Awareness and understanding of existing consent practice
The patients were generally not familiar with consenting procedures, and most could not recall being asked for their consent (Table 1). Some had experience of operations and referrals, but still could not recall the specific process of giving their consent for their health data to be shared. FG3F1: ‘I don’t remember ever being given something to sign’.
Table 1.
Attitudes of patients towards awareness on consent.
| Question | f(1) | f(2) | f(3) | f(4) | f(5) |
|---|---|---|---|---|---|
| I have a good idea of who currently has access to my health data (n = 23) | 4 (17.4%) | 3 (13%) | 8 (34.8%) | 3 (13%) | 5 (21.7%) |
| If I wanted to change my preferences of who has access to my health data, I would know how to do this (n = 23) | 8 (34.8%) | 1 (4.3%) | 8 (34.8%) | 2 (8.7%) | 4 (17.4%) |
| If I wanted to find out who has access to my health data, I would know how to do this (n = 23) | 8 (34.8%) | 2 (8.7%) | 6 (26.1%) | 4 (17.4%) | 3 (13%) |
| If I wanted to change my preferences of who has access to my health data, I would find this easy to do this (n = 23) | 8 (34.8%) | 1 (4.3%) | 6 (26.1%) | 4 (17.4%) | 4 (17.4%) |
| If I wanted to find out who has access to my health data, I would find this easy to do this (n = 23) | 9 (39.1%) | 2 (8.7%) | 4 (17.4%) | 4 (17.4%) | 4 (17.4%) |
| I trust the current process of consenting to share my health data (n = 23) | 3 (39.1%) | 1 (4.3%) | 5 (21.7%) | 5 (21.7%) | 9 (39.1%) |
| I feel in control of who has access to my health data (n = 23) | 5 (21.7%) | 2 (8.7%) | 7 (30.4%) | 2 (8.7%) | 7 (30.4%) |
Two participants had recollections of complex consenting processes due to the management of long term conditions, and could identify some of the inefficiencies of the current processes (in large part due to having no shared care pathway with their particular specialists). FG1M3: ‘I think when I did it, it would be over five years ago’; FG1M1: ‘we were given something to sign … it was on the iPad’. It was apparent that giving consent to sharing data was not a significant event for most of the patients (evidenced by the use of language such as ‘I think …’, and the inability to recall specific details of the process), despite the discussion suggesting that some patients do hold strong opinions on the subject of data sharing.
Healthcare professionals who were more involved in this process were able to identify the point when patients had given consent. Health data sharing between systems or organisations was reported to sometimes be difficult and even be a ‘nightmare’:
SFG3C8: ‘… a nightmare trying to get the shared care sometimes, if we can’t get hold of the patient or the GP practice, or the service they want to share it, and it does delay the referral. Because we can’t actually action it without getting that consent.’
This may affect the quality of care. SFG2N: ’ … I came across this yesterday with a diabetic patient who’d been for a foot check and has not shared care, so we cannot see that diabetic patient’s foot review on the self-management app’. Visibility between organisations seems to be an issue that may result in additional overheads.
SFG3C8: ‘If we weren’t aware …we find out, we’d still continue to follow the process that we are, and asking them and chasing it up. Because we wouldn’t know that they’ve already consented, if it doesn’t link through … to let us know somehow’.
It was also apparent that the staff had varied experience of consenting for health data sharing, due to differences in their role responsibilities. For example, nurses were less aware of consent practices mentioning: SFG2N3: ‘I have no idea’, SFGN1: ‘Does reception; I think it’s something that reception asks’, and SFG2N3: ‘occasionally a screen pops up … and you click a button if they consent to you looking at their data … it’s a little bit grey…’. Whereas clerical staff appeared more certain of the process: SFG3C8: ‘you can ask them verbal consent over the telephone … we don’t have to ask them every time’, which also is an indication that the patients may lose touch of what they have consented to after some time.
The attitudes of the patients towards data sharing (in the context of receiving care) were generally relaxed, with the proviso that the data sharing is to be with only relevant entities within the NHS. Patients were unaware who currently has access to their data, how to find out, or how to withdraw their consent for their data to be shared, which, however, did not prevent patients from making assumptions about how their data might be shared.
The issue of relevance was very important to patients, and even those with a relaxed view on data sharing were very clear that their data must only be: FG1M1: ‘…shared relevantly, to the right department or right people, not everyone’.
Evaluation of the dynamic patient-managed digital consent capability offered by the Dovetail application
A number of benefits to using the app were identified, in comparison with paper-based consenting processes. The primary benefits were that paper can be lost either through human error or environmental issues such as flooding or fire, and that paper is more difficult to control and track (Table 2). Electronic consent was viewed as a potential solution to these problems and therefore the app was viewed favourably in this respect; for example: FG1M1: ‘the electronic is better because there’s no paper involved and you can track it down, and that’s how the systems – all over everywhere, is a paperless system’, FG1F2: ‘paper gets lost doesn’t it?’; FG6M2: ‘It wouldn’t go missing’; however, some participants still favoured paper processes, as this was what they were used to: FG1F1: ‘I like my paper … because it’s on hand, you can read it, you can pick it up’.
Table 2.
Patients’ perceptions towards the Dovetail app.
| Question | f(1) | f(2) | f(3) | f(4) | f(5) |
|---|---|---|---|---|---|
| How easy do you think this app would be to use? (n = 20) | 1 (5%) | 1 (5%) | 5 (25%) | 5 (25%) | 8 (40%) |
| How suitable do you think this app is for managing consent online? (n = 20) | 1 (5%) | 0 (0%) | 8 (40%) | 5 (25%) | 6 (30%) |
| How likely would you be to try this app if it were available to you? (n = 20) | 1 (5%) | 1 (5%) | 1 (5%) | 4 (20%) | 13 (65%) |
| How well do you think this app provides solutions to the drawbacks of paper based consent? (n = 20) | 1 (5%) | 0 (0%) | 4 (20%) | 6 (30%) | 9 (45%) |
| How empowered would you feel using this app to manage your own healthcare? (n = 21) | 2 (9.5%) | 1 (4.8%) | 2 (9.5%) | 6 (28.6%) | 10 (47.6%) |
| How do you think this app would affect the number of visits you would make to the GP? (1 = greatly increased, 2 = increased, 3 = no change, 4 = reduced, 5 = greatly reduced) (n = 20) | 1 (4.3%) | 0 (0%) | 10 (50%) | 2 (10%) | 7 (35%) |
Another benefit identified was that the application allows the patient to take more control, which previously had been something the participants did not feel they had. Being able to access the app at any time (FG1M2: ‘… you can do it from home’), from anywhere, was viewed as a positive and something that would allow them to be more involved with their health data. Furthermore, being able to view and amend/update consents easily was regarded as a positive and as something which is not currently easy to do (FG6M1: ‘It’s good, because you can see what information is being shared, and if there’s anything on there that you’re not happy about sharing, you can withdraw your consent to share it’). The ability for patients to be empowered through taking control of their own data, will support wider patient self-management and health empowerment goals.
Being able to see a visual confirmation that data have been processed, being able to view current and past consents and having control over data sharing at any given time was seen as a benefit, for example:
FG6M1: ‘If you are the one who’s gone on the app to give consent, then at least you know it’s been processed. There’s nothing worse than turning up at the hospital appointment and they’ve no idea what you’re talking about.’
It was generally agreed that the app could improve feelings of control over one’s data.
FG3F1: ‘… you’ve got the option of stopping a consent if you want to, so you’re still in control of what happens so I think that’s very good … You always feel like you’re not allowed to look, whereas with the app if the information is there that you’re feeding to the app, then you know what it is anyway, so you know, you’ve got more knowledge.’
Evaluation of the technology against current practice
Figure 4 shows the Likert frequencies of patient responses to questions asked that compared their experiences with the existing service, against the capability of the digital consent application by Dovetail. From front to back the questions asked of patients, focused on: Q30) receiving better advice from healthcare professionals (n = 23); Q31) controlling their medical conditions (n = 21); Q32) improving the relationship and interactions with healthcare professionals (n = 23); Q33) provision of better access to specialists; Q34) keeping track of who has access to the their data (n = 22); Q35) removing data sharing when no longer needing a specific service (n = 23); and Q36) updating their health data across a number of services (n = 24). Patients’ attitudes overwhelmingly shifted towards strong agreement, with the capability improving a number of aspects. It is noteworthy that there was a significant shift from strong disagreement towards the other extreme of the Likert scale.
Figure 4.
Perception of patients towards service improvement using digital consent (frequency of responses in valid percentages).
A favourable opinion towards the application when compared with existing service also prevailed in the staff responses, although with more moderate shift in attitude (Figure 5). From front to back the questions asked focused on: S25) reduction of time spent on administration (n = 13); S28) keeping track of who has access to the practice’s patients’ health data (n = 13); S33) supporting patients to manage their own health data (n = 13); S35) supporting transparency regarding health data sharing (n = 13); and S38) adhering to data protection standards and requirements (n = 13).
Figure 5.
Attitude of staff towards current practice and digital consent (frequency of responses in valid percentages).
Discussion and conclusions
Overall, the capability of digital consent offered by the Dovetail application was received favourably. Patients saw a number of advantages of the application, particularly when compared with the existing process of paper-based consent. The digital consent approach was found to be easy to understand and use, and also gave the perception of more control on the patients’ data. It is noteworthy that patients acknowledged that the application offers a transparent and traceable process, which can be reviewed and amended at any time, also highlighted in other uses of digital consent.19 There were benefits seen to a paperless system, including time saving and increased control, as well as reduced risk of data loss (though there were slight concerns about hacking and electronic data). When discussing current processes and consent to share health data, it was evident that patients were not clear about how things work or who currently has access to their health data. Patients felt that they were not in control of their own data, and were unaware of their options to be more involved in their own data management. This demonstrates a gap at the functionality level, which is met by the Dovetail application.
In general, the patients were relaxed about sharing their data, under the assumption that they would not be shared outside the NHS, appreciating the value of sharing data towards getting a better service. Although not explicitly identified, a digital consent application would further allow them to share the data with non-NHS entities, as this would be declared in the application. This is something that may not be apparent with the current paper based approach, as being handled by the GP practice. There is sometimes the mistaken assumption that the sharing consent only refers to sharing the data within an organisation, and not across organisations. Staff agreed with the perception of the patients, although they were more moderate towards favouring digital consent. One major issue highlighted was a potential added workload. This is probably due to the fact that the pilot, in which the application was used, took place in addition to their existing responsibilities. Hence the pilot overall, may have actually contributed to more workload. A different protocol would need to conclusively investigate this, by comparing the transformed, with the traditional service. Patients also expressed that they would generally be willing to submit anonymous data to researchers via the application. The application has also managed to offer clarity to an issue that both patients and staff were not confident about. A technology assured consent approach would transcend organisation changes, and would offer the patients long-term continuous control over consent, which in other cases they may forget about.
Participants in this study were recruited from one local GP practice, therefore any generalisation should be considered carefully. The pilot itself also experienced technical difficulties due to network outages at the pilot site, during the data collection period of the study. Although the evaluation protocol was designed based on the application mock-ups and instruction videos, the technical difficulties may have introduced biases in the responses of patients who anticipated having used the application by that time. Furthermore, the study evaluated the perception of the patients towards the overall capability of the Dovetail application, based on their experience during the pilot, and not the merits of the technical advantages of blockchain. Although the strengths of the technology were presented, isolating the effect of blockchain on the patients’ perception needs a more targeted approach.
Finally, when compared with current practice, the patients overwhelmingly responded favourably to the digital consent capability, confirming the technology benefit. Future work will incorporate evaluation with broader demographic geographical diversity, as well as larger patient sample. Additionally, evaluation of the value to clinical researchers, as well as integrated care delivery experts, has been identified as future work.
In conclusion, the patients received both the potential of the technology and the specific application positively. It was considered that it will allow patients to manage their consent to having their data shared more effectively. Staff identified that the digital consent approach could help with delays in processing consent, as well integrating services and organisations such as GP practices and self-management applications. Further research would need to confirm the results in a wider geographical area, and strongly establish confidence in the technology.
Contributorship
All authors contributed significantly to the study and write-up of the article. Author #1 (GD): principal investigator of the study. Contributed to the design, execution of the study and write-up of the article. Author #2 (JE): contributed to the design, execution of the study and write-up of the article. Author #3 (WN): contributed to the theoretical background, and technical parts of the article. Author #4 (AE): lead of the SBRI project on development of the digital consent service. Contributed to the logistics and the theoretical background of the study. Author #5 (TR): contributed to the design of the study, discussion and conclusions sections and reviewed the article. Author #6 (TNA): contributed to the design of the study, discussion and conclusions sections and reviewed the article.
Conflict of interest
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The authors’ organisations were part of the same publically funded research and development project, led by Dovetail digital, without any commercial interests between them. Dovetail digital are developing the application into a commercial service.
Ethical approval
The University of Warwick Biomedical & Scientific Research Ethics Committee reviewed and approved the study protocol with number REGO-2018-2277.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Small Business Research Initiative (SBRI) in healthcare, an Academic Health Science Network (AHSN) led programme for NHS England (grant number A-1296).
Guarantor
GD.
Peer review
Dr Gary Leeming, University of Manchester, has reviewed the manuscript.
ORCID iDs
George Despotou https://orcid.org/0000-0003-3437-6412
Theodoros N Arvanitis https://orcid.org/0000-0001-5473-135X
References
- 1.World Health Organization, Regional Office for Europe. Integrated care models: An overview. Working document, http://www.euro.who.int/__data/assets/pdf_file/0005/322475/Integrated-care-models-overview.pdf (2016, accessed 1 May 2019.
- 2.Carewell project. Guidelines for implementing integrated care in policy and practice; the journey to deploying scalable integrated healthcare services, http://carewell-project.eu/fileadmin/carewell/deliverables/d8.6_v2.0_carewell_guidelines_for_deployment_printable_version.pdf (2017, accessed 1 May 2019)
- 3.Erturkmen GBL, Yuksel M, Sarigul B A, et al. collaborative platform for management of chronic diseases via guideline-driven individualized care plans. Comput Struct Biotechnol J 2019; 17: 869–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akarca D, Saleh K, Xiu P. Upgrading our digital health infrastructures with blockchain-based records. Cambridge Med J 2018; 1–4. [Google Scholar]
- 5.O’Connor Y, Rowan W, Lynch L, et al. Privacy by design: Informed consent and internet of things for smart health. Procedia Comput Sci 2017; 113: 653–658. [Google Scholar]
- 6.Maruthappu M, Sood HS, Keogh B. The NHS Five Year Forward View: Transforming care. Br J Gen Pract 2014; 64: 635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The King’s Fund. A digital NHS? An introduction to the digital agenda and plans for implementation, https://www.kingsfund.org.uk/sites/default/files/field/field_publication_file/A_digital_NHS_Kings_Fund_Sep_2016.pdf (2016, accessed 3 October 2019)
- 8.UK Department of Health and Social Care. The future of healthcare: Our vision for digital, data and technology in health and care. Policy Paper, https://www.gov.uk/government/publications/the-future-of-healthcare-our-vision-for-digital-data-and-technology-in-health-and-care/the-future-of-healthcare-our-vision-for-digital-data-and-technology-in-health-and-care (2018, accessed 3 October 2019).
- 9.UK National Data Guardian. Review of data security, consent and opt-outs, https://www.gov.uk/government/publications/review-of-data-security-consent-and-opt-outs (2017, accessed 5 February 2020.
- 10.NHS Digital. National data opt-out operational policy guidance document, https://digital.nhs.uk/services/national-data-opt-out/operational-policy-guidance-document (2019, accessed 5 February 2020.
- 11.NHS Digital, Data Coordination Board. DCB3058 compliance with national data opt-outs standard, https://digital.nhs.uk/data-and-information/information-standards/information-standards-and-data-collections-including-extractions/publications-and-notifications/standards-and-collections/dcb3058-compliance-with-national-data-opt-outs (2018, accessed 12 February 2020).
- 12.Official Journal of the European Union. Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the protection of natural persons with regard to the processing of personal data and on the free movement of such data, and repealing Directive 95/46/EC (General Data Protection Regulation), https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32016R0679&from=EN (2016, accessed 1 May 2019.
- 13.Lea N. How will the general data protection regulation affect healthcare? Acta Médica Portuguesa 2018; 31: 363. [DOI] [PubMed] [Google Scholar]
- 14.Blake K, Hollbrock JT, Antal H, et al. Use of mobile devices and the internet for multimedia informed consent delivery and data entry in pediatric asthma trial: Study design and rationale. Contemp Clin Trials 2015; 14: 05–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilber M, Bonnell A, Farrell J, et al. Click yes to consent: Acceptability of incorporating informed consent into an internet-based testing program for sexually transmitted and blood-borne infections. Int J Med Inform 2017; 105:38–48. [DOI] [PubMed] [Google Scholar]
- 16.Welch BM, Marshall E, Qanungo S, et al. Teleconsent: A novel approach to obtain informed consent for research. Contemp Clin Trials Commun 2016; 3: 4–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.US Food and Drug Administration. Use of electronic informed consent questions and answers; guidance for institutional review boards, investigators, and sponsors, https://www.fda.gov/media/116850/download (2016, accessed 3 October 2019).
- 18.Budin-Ljosne I, Teare HJA, Kaye J, et al. Dynamic consent: A potential solution to some of the challenges of modern biomedical research. BMC Med Ethics 2017; 18: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teare HJA, Morrison M, Whitley EA, et al. Towards ‘Engagement 2.0’: Insights from a study of dynamic consent with biobank participants. Digit Health 2015; 1: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang P, White J, Schmidt D, et al. FHIRChain: Applying blockchain to securely and scalably share clinical data. Comput Struct Biotechnol J 2018; 16: 267–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pirtle C, Ehrenfeld J. Blockchain for healthcare: The next generation of medical records? J Med Sys 2018; 42: 172. [DOI] [PubMed] [Google Scholar]
- 22.Roehrs A, da Costa C, da Rosa Righi R. OmniPHR: A distributed architecture model to integrate personal health records. J Biomed Inform 2019; 71: 70–81. [DOI] [PubMed] [Google Scholar]
- 23.Casino F, Dasaklis TK, Ptsakis C. A systematic review of Blockchain-based applications: Current status, classification and open issues. Telemat Inform 2019; 36: 55–81. [Google Scholar]
- 24.Gordon W, Catalini C. Blockchain technology for healthcare: Facilitating the transition to patient-driven interoperability . Comput Struct Biotechnol J 2018; 16: 224–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drosatos G, Kaldoudi E. Blockchain applications in the biomedical domain: A scoping review. Comput Struct Biotechnol J 2019; 17: 29–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGhin T, Choo KR, Liu CZ, et al. Blockchain in healthcare applications: Research challenges and opportunities. J Network Comput Appl 2019; 135: 62–75. [Google Scholar]
- 27.Vazirani AA, O’Donoghue O, Brindley D, et al. Implementing blockchains for efficient health care: Systematic review. J Med Internet Res 2019; 21: e12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaye J, Whitley EA, Lund D, et al. Dynamic consent: A patient interface for twenty-first century research networks. Eur J Hum Genet 2015; 23: 141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]