Abstract
Although penile carcinoma is a rare malignancy, there is still an unmet need to identify prognostic factors associated with poor survival. In this study, we utilized demographic and clinical information to identify the most informative variables associated with overall survival in patients with penile cancer. From a full model including all covariates found to be statistically significant in univariable analyses, we identified a parsimonious reduced model containing tumor site (penis glans: hazard ratio [HR] = 0.48; 95% CI: 0.28-0.85 and penis not otherwise specified: HR = 0.45; 95% CI: 0.25-0.84), undetermined tumor differentiation (HR = 0.48; 95% CI: 0.27-0.86), and TNM stage III/IV (HR = 2.83; 95% CI: 1.68-4.75). When all of the covariates from the full model were subjected to classification and regression tree analysis, we identified 6 novel risk groups. Of particular interest, we found marriage was associated with substantial improvement in survival among men with the same stage and disease site. Specifically, among single/widowed/divorced men with TNM stage 0-II and prepuce/penis corpus/overlapping lesions had worse survival (5-year survival = 18.2%) versus married men (5-year survival = 62.5%). Since marital status is linked to social support, these findings warrant a deeper investigation into the relationships between disease prognosis and social support in patients with penile carcinoma.
Keywords: penile cancer, penile cancer survival, prognostic factors, epidemiology, cancer survival
Introduction
Penile cancer is rate accounting for less than 1% of cancers in men in the United States. In 2019, in the United States, approximately 2,080 new cases of penile carcinoma are expected to be diagnosed and 410 deaths are estimated to occur.1 The overall 5-year relative survival rate for localized disease is 83% but drops to 48% with regional disease that has spread to the regional lymph nodes.2 Squamous cell carcinoma (SCC), which presents as a painless lump, ulcer, or irregular mass,3 is the most common subtype comprising over 90% of all invasive penile carcinomas in the United States.1 Although surgical resection with a partial or radical penectomy remains the gold standard therapy for advanced invasive penile carcinoma, the management of penile carcinoma has evolved in recent years resulting in a paradigm shift toward minimally invasive surgical treatment.4
Although penile cancer is rare and the overall prognosis is modestly optimistic, identifying prognostic factors is important to identify high-risk patients associated with poor outcomes. Previous studies have shown that presence and extent of regional inguinal lymph node metastases is an important prognostic factor for individuals with penile carcinoma.4-8 As a precursor to lymph node metastasis, the existence of lymphovascular invasion (LVI) has been shown to be associated with reduced survival among those without lymph node metastasis.9 Yet differing outcomes between studies warrant validation of the role of LVI as an independent prognostic indicator of reduced survival in patients with penile carcinoma.9 Studies have demonstrated clinicopathological variables, such as inguinal lymph node metastases, perineural invasion, tumor stage, and grade, to be predictors of poor survival10-12; however, there are socioeconomic and psychosocial factors that influence survival risk. Demographic and socioeconomic predictors, such as lower education attainment, African-American, and Hispanic ethnicity, have been shown to be associated with poor survival.13 Based on patient experiences including feelings of embarrassment and denial, there are psychosocial factors that may contribute to poor outcomes.4 In addition, men with penile cancer have expressed the importance of social support in coping with treatment and that social support helps to improve their mental well-being.14 There is a need for identifying men with penile cancer at high risk for poor survival, including assessment of sociodemographic factors with clinical factors. Thus, in this study, we utilized demographic and clinical data and a rigorous analytical approach to identify parsimonious models for identifying patients who are at risk of poor outcomes.
Patients and Methods
Study Population
This retrospective study included all 230 patients who were diagnosed with penile carcinoma at the H. Lee Moffitt Cancer Center & Research Institute from 1986 to 2013. There were no inclusion or exclusion criteria. Because penile cancer is a rare disease, this analysis included all available cases. The protocol for this study was approved by Advarra (IRB#Pro00014743; approval no. CR#00150095).
Cancer Registry Data
Patient data were obtained from electronic medical records and institutional databases including age at diagnosis, race/ethnicity, marital status, tobacco/alcohol use, and clinical covariates (tumor site, stage, histology, summary of first course of treatment, T stage, N stage, M stage, tumor size, surgical margins, and systemic surgery sequence). Pathologic staging was utilized and if these data were not available clinical staging was used. TNM staging was derived from the SEER Collaborative Staging which included site-specific factors SSF10 (involvement of corpus spongiosum or corpus cavernosum), SSF17 (extranodal extension of regional lymph nodes), and tumor size, extension, lymph nodes, and metastases. Moffitt’s Cancer Registry collects vital status through active (ie, chart review and contacting patients, relatives, and other medical providers) and passive methods (ie, mortality records). Summary of first course of treatment was defined as all treatment methods recorded in the treatment plan and administered to the patient before disease progression, recurrence, or death. Tobacco use was categorized as self-reported ever, never, or unknown. Alcohol use was categorized as self-reported ever or never drinker. Overall survival was the dependent variable and was defined as date of diagnosis to date of death or last follow-up and was right censored at 5 years.
Statistical Analysis
The log-rank test was used to test for differences in overall survival (censored at 12, 36, and 60 months) by demographic and clinical variables. We utilized a model building approach to identify the most informative demographic and clinical variables associated with overall survival. Overall survival was defined as date of cancer diagnosis to date of event or date of last follow-up. Among patients without an event, censoring occurred at either 5-years or date of last follow-up if it was less than 5 years.
Univariable Cox proportional hazards regression models were used to identify which demographic and clinical variables were significantly associated with overall survival. All covariates that were found to be statistically significant in the univariable analyses were then included into a single full multivariable model. To reduce the full model to the most informative covariates, 2 separate analyses were conducted: backward elimination analysis and classification and regression tree (CART) analysis. Using the covariates from the full model, a backward elimination approach was utilized using 0.05 as the pre-specified P value for removal from the model. Classification and regression tree analysis, adapted for survival time, was also employed to identify novel patient risk groups (nodes) based on the most informative covariates from the full models for model 1 based on TNM group stage and for model 2 based on T, N, and M stages. Classification and regression tree is a nonparametric tree-building technique that can reveal complex interactions between predictors and the outcome of interest (survival).15 Survival curves of the risk groups identified by CART were estimated using Kaplan-Meier method, and differences in survival time were tested for significance using the log-rank test. Receiver operating characteristics (ROC) curves were used to assess performance of TNM stage alone versus the CART models. P value <.05 was considered statistically significant. All statistical analyses were performed using Stata/MP 14.2 (StataCorp LP).
Results
Survival Time by Demographic and Clinical Characteristic
Overall survival rates, censored at 12, 36, and 60 months, were compared by demographic and clinical variables (Table 1). There were significant differences for the survival rates by marital status, tumor site, differentiation, stage of disease, T stage, N stage, M stage, tumor size, surgical margins, and systemic/surgery sequence. Specifically, single (61.8%) and widowed/divorced/unknown (45.4%) had lower 5-year survival rates compared to married men (68.7%; log-rank P value = .029). By site, there were significant differences by 5-year survival between those with prepuce/penis corpus/overlapping lesions (41.6%) compared to other penile tumor sites, penis glans (66.5%), and penis nos (71.2%; log-rank P value = .005). For tumor differentiation, significant differences were observed between poorly and undifferentiated (41.2%) compared to moderately and well-differentiated (56.4%), and undetermined (75.9%) at 5 years (log-rank P value < .001). For TNM staging, there were significant differences at 5 years (log-rank P value < .001) for TNM stage 0 (77.0%), 1 (70.4%), 2 (60.9%), 3 (41.9%), 4 (26.4%), and unknown (55.6%). For pathologic T stage (collapsed), pathologic N stage, and pathologic T stage there were also significant differences for 5-year survival (log-rank P value < .001) and significant differences for 5-year survival for tumor size <25 mm (71.9%) versus ≥25 mm (45.2%; log-rank P value < .001). For surgical margins, no primary surgery (47.5%), no residual tumor (68.8%), microscopic/macroscopic/residual tumor, not otherwise specified (NOS; 61.2%), and not evaluable/missing (76.8%) differed significantly for 5-year survival (log-rank P value = .015).
Table 1.
Overall Survival of Patients With Penile Cancer by Demographic and Clinical Characteristics.a
| Characteristics | N (%) | 12 months % | 36 months % | 60 months % |
|---|---|---|---|---|
| Year of diagnosis | ||||
| ≤2005 | 112 (48.7) | 86.5 | 68.3 | 59.9 |
| ≥2006 | 118 (51.3) | 92.6 | 75.9 | 66.0 |
| P value | .207 | .288 | .291 | |
| Age at diagnosis, years | ||||
| Mean (SD), 60 (13.7) | ||||
| ≤55 | 75 (32.6) | 94.2 | 70.8 | 67.1 |
| 56-69 | 92 (40.0) | 83.5 | 65.0 | 63.4 |
| ≥70 | 63 (27.4) | 91.8 | 81.2 | 55.6 |
| P value | .103 | .107 | .785 | |
| Race | ||||
| White | 211 (91.7) | 88.3 | 71.0 | 62.2 |
| Other | 19 (8.3) | 100.0 | 73.3 | 73.3 |
| P value | .144 | .495 | .370 | |
| Ethnicity | ||||
| Non-Hispanic | 199 (86.9) | 90.3 | 73.5 | 65.3 |
| Hispanic | 30 (13.1) | 81.9 | 57.6 | 46.1 |
| Unknown | 1.0 (0.4) | 0.0 | 0.0 | 0.0 |
| P value | .362 | .179 | .101 | |
| Marital status | ||||
| Married | 149 (64.8) | 90.8 | 76.8 | 68.7 |
| Single | 32 (13.9) | 93.1 | 67.9 | 61.8 |
| Widowed/divorced/missing | 49 (21.3) | 82.5 | 56.8 | 45.4 |
| P value | .308 | .061 | .029 | |
| Alcohol use | ||||
| Never | 121 (52.6) | 89.4 | 77.2 | 71.2 |
| Ever | 93 (40.4) | 88.2 | 65.9 | 56.4 |
| Missing | 16 (7.0) | 93.3 | 71.1 | 53.3 |
| P value | .788 | .292 | .168 | |
| Tobacco use | ||||
| Never/unknown | 74 (32.2) | 90.2 | 72.4 | 62.2 |
| Ever | 156 (67.8) | 88.7 | 70.9 | 63.1 |
| P value | .785 | .867 | .946 | |
| Tumor site | ||||
| Prepuce/penis corpus/overlapping lesion | 46 (20.0) | 81.7 | 53.2 | 41.6 |
| Penis glans | 94 (40.9) | 90.9 | 72.9 | 66.5 |
| Penis, NOS | 90 (39.1) | 91.4 | 80.1 | 71.2 |
| P value | .186 | .013 | .005 | |
| Histology | ||||
| Squamous cell carcinoma | 204 (88.7) | 89.4 | 70.7 | 63.1 |
| Nonsquamous cell carcinoma | 26 (11.3) | 87.9 | 75.4 | 61.7 |
| P value | .884 | .674 | .988 | |
| Tumor differentiation | ||||
| Poorly and undifferentiated | 38 (16.5) | 78.1 | 41.2 | 41.2 |
| Moderately and well-differentiated | 94 (40.9) | 85.4 | 62.5 | 56.4 |
| Undetermined | 98 (42.6) | 96.6 | 89.3 | 75.9 |
| P value | .005 | <.001 | <.001 | |
| TNM staging | ||||
| 0 | 68 (29.6) | 98.3 | 92.7 | 77.0 |
| 1 | 63 (27.4) | 91.6 | 72.6 | 70.4 |
| 2 | 39 (17.0) | 85.3 | 71.9 | 60.9 |
| 3 | 37 (16.1) | 83.0 | 41.9 | 41.9 |
| 4 | 13 (5.6) | 52.7 | 26.4 | 26.4 |
| Missing | 10 (4.3) | 100.0 | 77.8 | 55.6 |
| P value | <.001 | <.001 | <.001 | |
| TNM stage groupb | ||||
| I/II | 102 (67.1) | 89.3 | 72.5 | 66.9 |
| III/IV | 50 (32.9) | 74.9 | 38.2 | 38.2 |
| P value | .016 | <.001 | <.001 | |
| Pathologic T stage | ||||
| Overall | ||||
| 0/CIS | 62 (26.9) | 98.1 | 95.9 | 83.1 |
| 1 | 55 (23.9) | 88.7 | 62.9 | 60.8 |
| 1A | 9 (3.9) | 100.0 | 100.0 | 100.0 |
| 1B | 8 (3.5) | 85.7 | 85.7 | 0.0 |
| 2 | 34 (14.8) | 81.6 | 65.3 | 58.0 |
| 3 | 37 (16.1) | 78.9 | 45.7 | 45.7 |
| 3A | 1 (0.4) | 100.0 | 100.0 | 100.0 |
| 4 | 4 (1.7) | 50.0 | 50.0 | 50.0 |
| 4A | 1 (0.4) | 100.0 | 100.0 | 0.0 |
| Missing | 19 (8.3) | 100.0 | 71.3 | 44.4 |
| Collapsed | ||||
| 0/CIS | 62 (29.9) | 98.1 | 95.9 | 83.1 |
| 1 | 72 (31.3) | 89.5 | 66.5 | 64.3 |
| 2 | 34 (14.8) | 81.6 | 65.3 | 58.0 |
| 3 | 38 (16.5) | 79.5 | 47.6 | 47.6 |
| 4 | 5 (2.2) | 60.0 | 60.0 | 30.0 |
| Missing | 19 (8.3) | 100.0 | 71.3 | 44.4 |
| P value | .002 | <.001 | <.001 | |
| Pathologic N stage | ||||
| 0/CIS | 168 (73.0) | 91.5 | 78.9 | 70.7 |
| 1 | 9 (3.9) | 87.5 | 72.9 | 72.9 |
| 2 | 20 (8.7) | 80.0 | 33.6 | 0.0 |
| 3 | 10 (4.4) | 46.7 | 15.6 | 0.0 |
| Missing | 23 (10.0) | 100.0 | 72.7 | 54.2 |
| P value | <.001 | <.001 | <.001 | |
| Pathologic M stage | ||||
| 0 | 213 (92.6) | 88.8 | 72.2 | 64.8 |
| 1 | 2 (0.9) | 50.0 | 0.0 | 0.0 |
| Missing | 15 (6.5) | 100.0 | 71.4 | 48.9 |
| P value | .033 | <.001 | <.001 | |
| Tumor size, mm | ||||
| Mean (SD), 29.8 (22.3) | ||||
| <25 (median) | 43 (40.6) | 94.3 | 77.5 | 71.9 |
| ≥25 | 63 (59.4) | 83.4 | 47.9 | 45.2 |
| Missing | 124 (53.9) | 90.7 | 80.3 | 68.7 |
| P value | .131 | <.001 | <.001 | |
| Surgical margin | ||||
| No primary surgery | 70 (30.4) | 86.6 | 58.7 | 47.5 |
| No residual tumor | 114 (49.6) | 91.4 | 77.5 | 68.8 |
| Microscopic/macroscopic/residual tumor, NOS | 14 (6.1) | 85.7 | 61.2 | 61.2 |
| Not evaluable/missing | 32 (13.9) | 90.0 | 81.6 | 76.8 |
| P value | .676 | .031 | .015 | |
| Summary of first course treatment | ||||
| Surgery only | 172 (74.8) | 89.9 | 73.8 | 63.9 |
| Combination/other | 58 (25.2) | 87.2 | 64.0 | 61.1 |
| P value | .473 | .088 | .221 | |
| Systemic/surgery sequence | ||||
| No systemic and/or no surgery | 89 (38.7) | 93.6 | 74.9 | 66.3 |
| Surgery + adjuvant | 35 (15.2) | 96.9 | 81.7 | 75.8 |
| Surgery + neoadjuvant | 4 (1.7) | 100.0 | 0.0 | 0.0 |
| Surgery + neoadjuvant + adjuvant | 2 (0.9) | 50.0 | 50.0 | 0.0 |
| Missing | 100 (43.5) | 85.0 | 66.8 | 57.4 |
| P value | .045 | .345 | .316 |
Abbreviations: NOS, not otherwise specified; SD, standard deviation.
a Bold font indicates statistically significant P values
b TNM stages: Unknown values were excluded from the analysis.
Univariable Analyses
As presented in Table 2, statistically significant univariable hazard ratios (HRs) were observed for Hispanics (HR = 1.91, 95% CI = 1.04-3.50), single/widowed/divorced (HR = 1.71, 95% CI = 1.06-2.75), tumor site (penis glans: HR = 0.49, 95% CI = 0.28-0.87 and penis NOS: HR = 0.41, 95% CI = 0.22-0.74), for undetermined tumor differentiation (HR = 0.25, 95% CI = 0.13-0.49), TNM stage III/IV (HR = 4.70, 95% CI = 2.48-8.93), tumor size ≥25 mm (HR = 2.91, 95% CI = 1.32-6.39), surgical margins (HR = 0.49, 95% CI = 0.29-0.82), pathologic T stage (collapsed) for stage 1 (HR = 3.05, 95% CI = 1.30-7.18), 2 (HR = 3.87, 95% CI = 1.55-9.71), 3 (HR = 5.61, 95% CI = 2.30-13.67), 4 (HR = 9.17, 95% CI = 2.37-35.53), missing (HR = 4.48, 95% CI = 1.67-12.04), pathologic N stage for stage 2 (HR = 4.09, 95% CI = 2.11-7.91), 3 (HR = 9.93, 95% CI = 4.36-22.64), and pathologic M stage for those positive for metastasis (HR = 15.75, 95% CI = 3.65-67.96).
Table 2.
Univariable Analysis.a,b
| Characteristics | HR (95% CI) |
|---|---|
| Year of diagnosis | |
| ≤2005 | Ref. |
| ≥2006 | 0.77 (0.47-1.26) |
| Age at diagnosis | |
| ≤55 | Ref. |
| 56-69 | 1.22 (0.69-2.16) |
| ≥70 | 1.12 (0.60-2.08) |
| Race | |
| White | Ref. |
| Other | 0.59 (0.19-1.89) |
| Ethnicity | |
| Non-Hispanicc | Ref. |
| Hispanic | 1.91 (1.04-3.50) |
| Marital status | |
| Married | Ref. |
| Single/widowed/divorced/missing | 1.71 (1.06-2.75) |
| Alcohol use | |
| Never | Ref. |
| Ever | 1.60 (0.97-2.65) |
| Missing | 1.59 (0.65-3.87) |
| Tobacco use | |
| Never/unknown | Ref. |
| Ever | 0.98 (0.59-1.62) |
| Tumor site | |
| Prepuce/penis corpus/overlapping lesion | Ref. |
| Penis glans | 0.49 (0.28-0.87) |
| Penis, NOS | 0.41 (0.22-0.74) |
| Histology | |
| Squamous cell carcinoma | Ref. |
| Nonsquamous cell carcinoma | 0.99 (0.49–2.01) |
| Tumor differentiation | |
| Poorly and undifferentiated | Ref. |
| Moderately and well differentiated | 0.62 (0.34-1.13) |
| Undetermined | 0.25 (0.13-0.49) |
| TNM stage group | |
| 0/CIS/missing | Ref. |
| I/II | 1.62 (0.86-3.03) |
| III/IV | 4.70 (2.48-8.93) |
| Tumor size, mm (median) | |
| <25 | Ref. |
| ≥25 | 2.91 (1.32-6.39) |
| Missing | 1.18 (0.54-2.55) |
| Surgical margins | |
| No primary surgery | Ref. |
| No residual tumor | 0.49 (0.29-0.82) |
| Microscopic/macroscopic/residual, NOS | 0.85 (0.30-2.42) |
| Not evaluable/missing | 0.36 (0.15-0.86) |
| T stage (collapsed) | |
| 0/CIS | Ref. |
| 1 | 3.05 (1.30-7.18) |
| 2 | 3.87 (1.55-9.71) |
| 3 | 5.61 (2.30-13.67) |
| 4 | 9.17 (2.37-35.53) |
| Missing | 4.48 (1.67-12.04) |
| N stage | |
| 0 | Ref. |
| 1 | 1.07 (0.26-4.45) |
| 2 | 4.09 (2.11-7.91) |
| 3 | 9.93 (4.36-22.64) |
| Missing | 1.68 (0.83-3.36) |
| M stage | |
| 0 | Ref. |
| 1 | 15.75 (3.65-67.96) |
| Missing | 1.08 (0.61-1.91) |
| Summary of first course treatment | |
| Surgery only | Ref. |
| Combination/other | 1.39 (0.82-2.39) |
| Systemic/surgery sequence | |
| No systemic treatment and/or no surgery | Ref. |
| Systemic after surgery | 0.73 (0.29-1.83) |
| Systemic before surgery | 2.25 (0.30-17.0) |
| Systemic before and after surgery | 2.66 (0.36-19.9) |
| Missing | 1.46 (0.85-2.51) |
| Year of first contact | |
| ≤2005 | Ref. |
| ≥2006 | 0.71 (0.43-1.16) |
Abbreviations: CI, confidence interval; HR, hazards ratio; NOS, not otherwise specified; Ref., reference.
a N = 230.
b Bold font indicates a statistically significant HR.
c Non-Hispanic, includes one unknown.
Full and Reduced Models
The full multivariable models included all covariates found to be statistically significant from the univariable analyses (Table 3). However, model 1 considered TNM group stage (and excluded T stage, N stage, and M stage), while model 2 considered pathologic T stage, N stage, and M stage (and excluded TNM group stage). In the full model for model 1, the only statistically significant covariates were ever alcohol use (HR = 1.80; 95% CI: 1.06-3.05), tumor site (penis glans: HR = 0.53; 95% CI: 0.29-0.96, and penis NOS: HR = 0.42; 95% CI: 0.22-0.80), undetermined tumor differentiation (HR = 0.40; 95% CI: 0.18-0.91), and TNM stage III/IV (HR = 2.64; 95% CI: 1.11-6.30). The full model 1 was subjected to a backward elimination approach to yield a parsimonious model which contained tumor site (penis glans: HR = 0.48; 95% CI: 0.28-0.85 and penis NOS: HR = 0.45; 95% CI: 0.25-0.84), undetermined tumor differentiation (HR = 0.48; 95% CI: 0.27-0.86), and TNM stage III/IV (HR = 2.83; 95% CI: 1.68-4.75).
Table 3.
Univariable, Multivariable, Reduced Model Analyses.a
| Characteristics | Univariableb | Full multivariable model 1 | Reduced multivariable model 1 | Full multivariable model 2 | Reduced multivariable model 2 |
|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Ethnicity | |||||
| Non-Hispanicc | Ref. | Ref. | Ref. | Ref. | Ref. |
| Hispanic | 1.91 (1.04-3.50) | 1.81 (0.94-3.49) | – | 1.61 (0.80-3.26) | – |
| Marital status | |||||
| Married | Ref. | Ref. | Ref. | Ref. | Ref. |
| Single/widowed/divorced/missing | 1.71 (1.06-2.75) | 1.52 (0.91-2.50) | – | 1.51 (0.88-2.60) | 1.70 (1.05-2.76) |
| Alcohol use | |||||
| Never | Ref. | Ref. | Ref. | Ref. | Ref. |
| Ever | 1.60 (0.97-2.65) | 1.80 (1.06-3.05) | – | 2.04 (1.19-3.50) | 1.81 (1.08-3.03) |
| Missing | 1.59 (0.65-3.87) | 2.14 (0.83-5.53) | – | 2.56 (0.98-6.72) | 2.81 (1.11-7.12) |
| Tumor site | |||||
| Prepuce/penis corpus/overlapping lesion | Ref. | Ref. | Ref. | Ref. | Ref. |
| Penis glans | 0.49 (0.28-0.87) | 0.53 (0.29-0.96) | 0.48 (0.28-0.85) | 0.58 (0.30-1.12) | – |
| Penis NOS | 0.41 (0.22-0.74) | 0.42 (0.22-0.80) | 0.45 (0.25-0.84) | 0.35 (0.17-0.72) | 0.54 (0.31-0.94) |
| Tumor differentiation | |||||
| Poorly and undifferentiated | Ref. | Ref. | Ref. | Ref. | Ref. |
| Moderately and well differentiated | 0.62 (0.34-1.13) | 0.63 (0.33-1.22) | – | 0.64 (0.32-1.29) | – |
| Undetermined | 0.25 (0.13-0.49) | 0.40 (0.18-0.91) | 0.48 (0.27-0.86) | 0.46 (0.19-1.12) | 0.52 (0.28-0.96) |
| TNM stage group | |||||
| 0/CIS/missing | Ref. | Ref. | Ref. | ||
| I/ II | 1.62 (0.86-3.03) | 1.05 (0.48-2.31) | – | ||
| III/IV | 4.70 (2.48-8.93) | 2.64 (1.11-6.30) | 2.83 (1.68-4.75) | ||
| Tumor size (mm), median | |||||
| <25 | Ref. | Ref. | Ref. | Ref. | Ref. |
| ≥25 | 2.91 (1.32-6.39) | 1.76 (0.76-4.04) | – | 2.23 (0.94-5.31) | 1.99 (1.18-3.36) |
| Missing | 1.18 (0.54-2.55) | 1.42 (0.64-3.15) | – | 1.48 (0.63-3.50) | – |
| Surgical margins | |||||
| No primary surgery | Ref. | Ref. | Ref. | Ref. | Ref. |
| No residual tumor | 0.49 (0.29-0.82) | 1.01 (0.55-1.88) | – | 0.98 (0.49-1.93) | – |
| Microscopic/macroscopic/residual, NOS | 0.85 (0.30-2.42) | 1.38 (0.46-4.15) | – | 1.40 (0.44-4.43) | – |
| Not evaluable/missing | 0.36 (0.15-0.86) | 0.80 (0.30-2.17) | – | 0.86 (0.31-2.38) | – |
| T stage (collapsed) | |||||
| 0/CIS | Ref. | Ref. | Ref. | ||
| 1 | 3.05 (1.30-7.18) | 1.53 (0.54-4.34) | – | ||
| 2 | 3.87 (1.55-9.71) | 1.89 (0.57-6.29) | – | ||
| 3 | 5.61 (2.30-13.67) | 1.51 (0.45-5.07) | – | ||
| 4 | 9.17 (2.37-35.53) | 6.44 (1.46-28.38) | 6.28 (1.83-21.55) | ||
| Missing | 4.48 (1.67-12.04) | 1.33 (0.31-5.66) | – | ||
| N stage | |||||
| 0 | Ref. | Ref. | Ref. | ||
| 1 | 1.07 (0.26-4.45) | 0.71 (0.16-3.16) | – | ||
| 2 | 4.09 (2.11-7.91) | 2.93 (1.36-6.33) | 2.90 (1.48-5.68) | ||
| 3 | 9.93 (4.36-22.64) | 12.6 (4.01-39.56) | 13.17 (5.28-32.89) | ||
| Missing | 1.68 (0.83-3.36) | 1.68 (0.45-6.23) | – | ||
| M stage | |||||
| 0 | Ref. | Ref. | Ref. | ||
| 1 | 15.75 (3.65-67.96) | 0.77 (0.10-6.13) | – | ||
| Missing | 1.08 (0.61-1.91) | 0.97 (0.23-4.14) | – |
Abbreviations: CI, confidence interval; HR, hazard ratio; Ref., reference.
a Bold font indicates a statistically significant HR.
b For comparison to the full and reduced model, these are the significant HRs from the univariable analyses from Table 2.
c Non-Hispanic includes one unknown.
In the full model for model 2, statistically significant covariates were ever alcohol use (HR = 2.04, 95% CI = 1.19-3.50), tumor site (penis NOS: HR = 0.35, 95% CI = 0.17-0.72), pathologic T stage 4 (HR = 6.44, 95% CI = 1.46-28.38), and N stage (2: HR = 2.93, 95% CI = 1.36-6.33; 3: HR = 12.6, 95% CI = 4.01-39.56). Backward elimination resulted in a parsimonious model containing single/widowed/divorced/missing (HR = 1.70, 95% CI = 1.05-2.76), ever alcohol use (HR = 1.81, 95% CI = 1.08-3.03), penis NOS (HR = 0.54, 95% CI = 0.31-0.94), undetermined tumor differentiation (HR = 0.52, 95% CI = 0.28-0.96), ≥25 mm tumor size (HR = 1.99, 95% CI = 1.18-3.36), pathologic T stage 4 (HR = 6.28, 95% CI = 1.83-21.55), and pathologic N stage 2 (HR = 2.90, 95% CI = 1.48-5.68) and stage 3 (HR = 13.17, 95% CI = 5.28-32.89).
Classification and Regression Tree Analysis
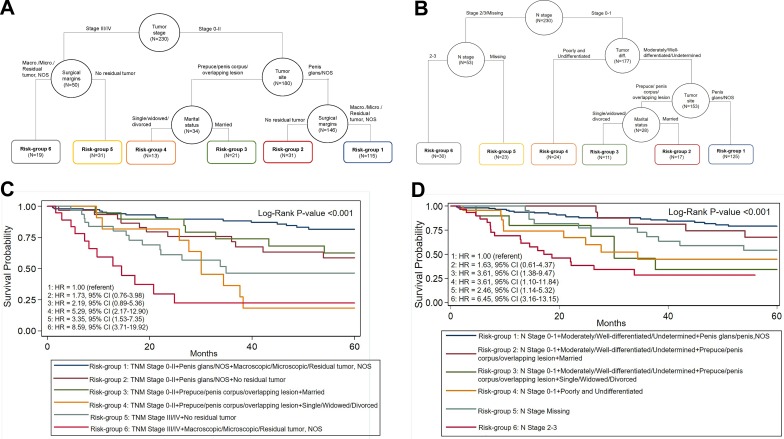
When all the covariates from the full model 1 were subjected to CART analysis, we identified 6 risk groups (Table 4 and Figure 1A-B) based on 4 covariates. Classification and regression tree model 1 included tumor stage, tumor site, surgical margins, and marital status. Compared to risk group 1 (stage 0-II + penis glans/NOS + macroscopic/microscopic/residual tumor, NOS), men in risk group 4 (stage 0-II, prepuce/penis corpus/overlapping lesion, single/widowed/divorced) exhibited a 6.5-fold increased risk of death (HR = 6.51; 95% CI = 2.86-14.81). Men in risk group 6 (stages III/IV + prepuce/penis corpus/overlapping lesion + macroscopic/microscopic/residual tumor, NOS) had an elevated risk of death (HR = 10.93; 95% CI = 5.11-23.36) compared to risk-group 1 (HR = 1.00).
Table 4.
Hazard Ratios for the Risk Groups Identified by CART Analysis.a
| Risk group | Covariates | 5-year survival rate (%) | Univariable, HR (95% CI) | Multivariable,b HR (95% CI) |
|---|---|---|---|---|
| Model 1 | ||||
| 1 | TNM stage 0-II + Penis glans/NOS + Macroscopic/microscopic/residual tumor, NOS | 81.2 | Ref. | Ref. |
| 2 | TNM stage 0-II + Prepuce/penis corpus/overlapping lesion + No residual tumor, NOS | 58.7 | 2.57 (1.19-5.54) | 1.73 (0.76-3.98) |
| 3 | TNM stage 0-II + Prepuce/penis corpus/overlapping lesion + married | 62.5 | 2.19 (0.90-5.31) | 2.19 (0.89-5.36) |
| 4 | TNM stage 0-II + Prepuce/penis corpus/overlapping lesion + Single/widowed/divorced | 18.2 | 6.51 (2.86-14.81) | 5.29 (2.17-12.90) |
| 5 | TNM stages III/IV + Prepuce/penis corpus/overlapping lesion + No residual tumor, NOS | 46.5 | 4.52 (2.20-9.31) | 3.35 (1.53-7.35) |
| 6 | TNM stages III/IV + Prepuce/penis corpus/overlapping lesion + Macroscopic/microscopic/residual tumor, NOS | 22.2 | 10.93 (5.11-23.36) | 8.59 (3.71-19.92) |
| Model 2 | ||||
| 1 | N stage 0-1 + Moderately/well-differentiated/undetermined + Penis glans/penis, NOS | 79.1 | Ref. | Ref. |
| 2 | N stage 0-1 + Moderately/well-differentiated/undetermined + Prepuce/penis corpus/overlapping lesion + Married | 67.7 | 1.49 (0.56-3.97) | 1.63 (0.61-4.37) |
| 3 | N stage 0-1 + Moderately/well-differentiated/undetermined + Prepuce/penis corpus/overlapping lesion + Single/widowed/divorced | 34.3 | 4.39 (1.76-10.94) | 3.61 (1.38-9.47) |
| 4 | N stage 0-1 + Poorly and undifferentiated | 45.0 | 3.79 (1.72-8.35) | 3.61 (1.10-11.84) |
| 5 | N stage missing | 54.2 | 2.46 (1.15-5.26) | 2.46 (1.14-5.32) |
| 6 | N stage 2-3 | – | 7.82 (4.11-14.85) | 6.45 (3.16-13.15) |
Abbreviations: CI, confidence interval; CART, classification and regression tree; HR, hazard ratio; NOS, not otherwise specified; Ref., reference.
a Bold font indicates a statistically significant HR.
b Adjusted for alcohol use, ethnicity, and tumor differentiation.
Figure 1.
A, Associations between tumor stage and overall survival were assessed by CART analysis. For model 1, while stage III/IV + surgical margins generated 2 terminal nodes (group 5 and group 6), stage 0 to II was segmented by tumor site forming terminal node for penis glans/NOS and was further segmented by macroscopic/microscopic/residual tumor, NOS (group 1) and no residual tumor (group 2), and prepuce/corpus/overlapping lesion as married (group 3), or single/divorced/widowed (group 4). B, N stage segmented to form terminal nodes for N stage 2 to 3 (group 5 and group 6), and N stage 0 to 1 which further segmented on tumor differentiation, resulting in terminal node for poorly and undifferentiated tumors (group 4). Tumor differentiation further segmented on tumor site with a terminal node for penis glans/NOS (group 1) and marital status segmented to 2 terminal nodes for married (group 2) and single/widowed/divorced (group 3). C, Overall differences in survival between CART groups for model 1 were significant (log rank P < .001). D, Overall differences in survival between CART groups for model 2 were significant (log rank P < .001). CART indicates classification and regression tree; NOS, not otherwise specified.
When all the covariates from the full model 2 were subjected to CART analysis, we identified 6 risk groups based on 4 covariates. The 4 covariates included in CART model 2 were N stage, tumor differentiation, site, and marital status. Risk group 3 (N stage 0-1 + moderately/well-differentiated/undetermined + prepuce/penis corpus/overlapping lesion + single/widowed/divorced) had an increased risk of dying (HR = 4.39; 95% CI = 1.76-10.94). Those in risk group 4 (N stage 0 -1 + poorly and undifferentiated tumors) had an increased risk of dying (HR = 3.79; 95% CI = 1.72-8.35) compared to risk group 1 (HR = 1.00). Risk group 6 (N stage 2-3) had the greatest increase in risk (HR = 7.82; 95% CI = 4.11-14.85).
When CART risk groups for both models (Table 4) were adjusted for alcohol use, ethnicity, and tumor differentiation, the point estimates were slightly somewhat attenuated, but the overall trend was the same. For risk groups identified in CART model 1 (Figure 1A), men with stage 0-II disease and were married (risk group 3) had better survival than single/divorced/widowed (risk group 4; 62.5% 5-year survival and HR = 2.19, 95% 0.89-5.36 for risk group 3 vs 18.0% 5-year survival and HR = 5.29, 95% CI: 2.17-12.90 for risk group 4). For risk groups identified in CART model 2 (Figure 1B), men with N stage 0-1 and married (risk group 2) had better survival than single/divorced/widowed (risk group 3; 67.7% 5-year survival and HR = 1.63, 95% CI: 0.61-4.37 vs 34.3% 5-year survival and HR = 3.61, 95% CI: 1.38-9.47).
Model Performance
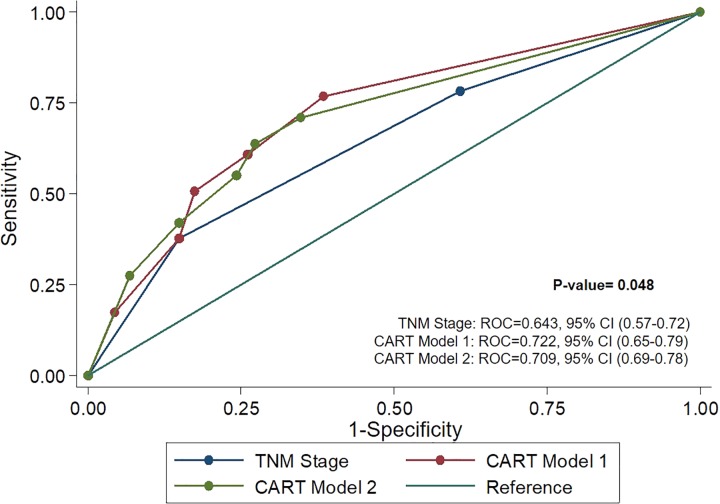
Receiver operating characteristics curves (Figure 2) were used to assess performance of TNM stage alone versus the models and revealed both CART model 1 (ROC = 0.722) and CART model 2 (ROC = 0.709) were better predictors than TNM stage alone (ROC = 0.643). Likewise, reduced model 1(AUC = 0.713) and 2 (AUC = 0.744) performed better than TNM stage alone (Table 5).
Figure 2.
Comparison of ROC curves show CART model 1 (ROC = 0.722) and CART model 2 (ROC = 0.709) to be more accurate predictors of prognosis than TNM stage (ROC = 0.643). CART indicates classification and regression tree; ROC, receiver operating characteristics.
Table 5.
Harrell’s C-index and ROC.
| Characteristics | C-index | ROC |
|---|---|---|
| TNM stage only | 0.674 | 0.643 |
| Model 1 (TNM stage included) | ||
| Full multivariable model | 0.741 | – |
| Reduced model | 0.713 | – |
| CART | 0.716 | 0.722 |
| Model 2 (TNM stage included) | ||
| Full multivariable model | 0.756 | – |
| Reduced model | 0.744 | – |
| CART | 0.707 | 0.709 |
Abbreviations: CART, classification and regression tree; ROC, receiver operating characteristics.
Discussion
This analysis of a cohort of patients diagnosed with penile cancer identified novel prognostic models containing the most informative covariates significantly associated with overall survival. The most informative covariates using a backward elimination approach were found to be tumor site, tumor differentiation, tumor size, TNM stage, pathologic N stage, and marital status. However, after using a decision tree approach (CART), novel patient risk groups were identified for 2 separate CART models and both showed a beneficial impact on survival among married men. Specifically, among men with TNM stage 0-II and prepuce/penis corpus/overlapping lesions, single/widowed/divorced men had worse survival (5-year survival = 18.2%) versus married men (5-year survival = 62.5%). Moreover, late-stage men with prepuce/penis corpus/overlapping lesion and no residual tumor (5-year survival = 46.5%) and macroscopic/microscopic/residual tumor, NOS (5-year survival = 22.2%) had better survival than single/widowed/divorced men with early stage and prepuce/penis corpus/overlapping lesions. In addition to clinicopathologic covariates, these data may suggest that social support network has a beneficial impact on outcomes of patients diagnosed with early-stage disease.
From the full model which contained all significant univariable covariates (Table 3), we utilized feature reduction analyses which yielded 2 reduced models and 2 CART models. These 4 models yielded 7 covariates: tumor site, staging variables (TNM stage, N stage, and T stage), tumor differentiation, marital status, alcohol use, surgical margins, and tumor size. Staging variables and tumor site were found in all 4 models which are well-established cancer prognostic factors. Consistent with previous reports,4-8,16 our analysis found that diagnosis of advanced disease stage significantly associated with decreased patient survival for penile cancer. In support of this, a report by Pandey et al indicated positive inguinal and pelvic nodal metastasis as predictors for shorter survival.6 These results are in concordance with our finding that men with TNM stage III/IV have worse outcomes compared to those with TNM stage 0/CIS/I/II. Our analyses also demonstrated that men with tumors located at the penis glans had a reduced risk of mortality compared to those with prepuce/penis corpus/overlapping lesions. About 70% of penile SCCs originate in the mucosal epithelium of the glans17 and typically tumors at the glans are diagnosed at earlier stages, while corpus is diagnosed at later stages.18 Men diagnosed with poorly differentiated tumors are associated with having advanced stage and nodal metastasis. There are several studies that have found that those with poorly differentiated tumors are at increased risk of poor prognosis.19 Tumor size, which is correlated with tumor stage, is also associated with survival outcomes. A prior study found that 5-year survival rate was 56% to 78% for men with tumor thickness of ≤5 mm and decreases to 48% to 64% for tumors that are ≥5 mm.20 We also found that alcohol use was also significantly associated with survival. Alcohol consumption is a potent modulator of immune function,21 associated with an increased risk of penile cancer,22 and classified as a “Group 1 Carcinogen” by the International Agency for Research of Cancer.23 It is believed that alcohol consumption may impair host defense against viral infections.24 An analysis of alcohol consumption in men revealed that alcohol consumption is associated with an elevated risk for human papillomavirus (HPV) infection.24 Considering that HPV is detected in 48% of penile cancers,25 we can speculate that HPV infections may have played a biological role in the increased risk of mortality observed in our cohort of patients with penile cancer who were alcohol users.
Our novel decision tree approach (i.e., CART analysis) demonstrated that a subset of unmarried early-stage patients had worse outcomes than late-stage patients (Figure 1C-D). Due to the potentially mutilating surgery that patients may face, patients with penile cancer may experience an increase in psychological distress that requires psychosocial care.26 The emotional stress they experience can result from a multitude of factors, including the diagnosis itself, treatment, and fear of recurrence.26 A study about marital status among men with penile cancer demonstrated marital status to be an independent prognostic factor for early stage but not for late-stage disease.27 Additionally, other studies have also revealed significant associations between marital status and penile cancer. A population-based registry study by Torbrand et al28 showed that married men with penile cancer had lower risk of late-stage tumors compared to divorced and never married men. However, the authors did not find an association between marital status and cause-specific mortality. Other studies have revealed an increased risk of mortality for nonmarried men with localized advanced penile cancer29 and an increased risk of invasive penile cancer for nonmarried men.29,30 Similar to the results found in our analyses, Rippentrop et al showed that married men with localized or regional penile carcinoma had significantly longer survival time compared to never married patients. However, among patients with distant disease, there were no significant differences by marital status.31 Our study provides further evidence of the importance of marital status on outcomes in patients with penile cancer.
We acknowledge that there are limitations with these analyses. The higher number of non-Hispanic whites from a single comprehensive cancer center may limit the generalizability of our findings. The incidence of penile carcinoma is higher among Hispanics and African American men compared to non-Hispanic whites32 and despite having a small cohort of Hispanics within our study, we observed Hispanics to have an increased risk of dying. The status of other prognostic factors such as HPV type and molecular markers (p16 expression) in our study cohort is not known. As basaloid carcinomas are more often diagnosed over other subtypes among those infected with HPV, a future stratified analysis by HPV status may be possible with a larger cohort of patients with penile cancer. An investigation of whether patients diagnosed with early-stage penile cancer are likely to depend more on psychosocial support and how it effects outcomes is important to understanding the influences of these potential risk factors on prognosis.33,34
In summary, this study identified novel prognostic models for penile cancer that were largely concordant in terms of the covariates that were identified based on the 2 analytical approaches (backward elimination and decision tree analysis). Importantly, our decision tree analyses showed that men diagnosed with early-stage penile cancer and that were not currently married had poorer survival compared to men that were currently married. The coexistence of poor clinicopathologic variables in combination with lack of social support may negatively impact prognosis. It can be concluded from this study that the assessment of clinicopathologic risk factors, including socioeconomic factors, may help to identify those at high risk and that would benefit from psychosocial support.
Acknowledgments
Appreciation is expressed to the Collaborative Data Services at the H. Lee Moffitt Cancer Center and Research Institute for their contributions in data abstraction, curation, and management.
Authors’ Note: The protocol for this study (IRB#Pro00014743) was approved by Advarra (CR# 00150095), and waiver of patient consent was obtained. M.E.R. and H.B. contributed equally.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Phillipe E. Spiess serves as Vice-chair of the NCCN Bladder and Penile Cancer Panel.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by a Cancer Center Support Grant (CCSG grant P30-CA76292) at the H. Lee Moffitt Cancer Center and Research Institute, a National Cancer Institute-Designated Comprehensive Cancer Center. Dr. Reyes was supported by Moffitt’s postdoctoral training program in molecular epidemiology (5T32CA147832-09).
ORCID iD: Monica E. Reyes, PhD  https://orcid.org/0000-0003-1433-0126
https://orcid.org/0000-0003-1433-0126
Matthew B. Schabath, PhD  https://orcid.org/0000-0003-3241-3216
https://orcid.org/0000-0003-3241-3216
References
- 1. Hernandez BY, Jill BS, Robert RG, et al. Burden of invasive squamous cell carcinoma of the penis in the United States, 1998-2003. Cancer. 2008;113(10 suppl):2883–2891. doi:10.1002/cncr.23743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. American Cancer Society. Survival Rates for Penile Cancer. 2019. https://www.cancer.org/cancer/penile-cancer/detection-diagnosis-staging/survival-rates. (Updated January 9, 2020. Accessed February 2, 2020).
- 3. Teichman JM, Sea J, Thompson IM, Elston DM. Noninfectious penile lesions. Am Fam Physician. 2010;81(2):167–174. [PubMed] [Google Scholar]
- 4. Spiess PE, Horenblas S, Lance CP, et al. Current concepts in penile cancer. J Natl Compr Canc Netw. 2013;11(5);617–624. [DOI] [PubMed] [Google Scholar]
- 5. Lopes A, Hidalgo GS, Kowalski LP, Torloni H, Rossi BM, Fonseca FP. Prognostic factors in carcinoma of the penis: multivariate analysis of 145 patients treated with amputation and lymphadenectomy. J Urol. 1996;156(5):1637–1642. doi:10.1016/s0022-5347(01)65471-5 [DOI] [PubMed] [Google Scholar]
- 6. Pandey D, Mahajan V, Kannan RR. Prognostic factors in node-positive carcinoma of the penis. J Surg Oncol. 2006;93(2):133–138. doi:10.1002/jso.20414 [DOI] [PubMed] [Google Scholar]
- 7. Wiechno P, Kalinowski T, Itrych B, Bożena SK, Demkow T, Karwański M. Prognostic factors in patients undergoing lymphadenectomy for squamous cell carcinoma of the penis. Urol Int. 2014;92(2):194–201. doi:10.1159/000353095 [DOI] [PubMed] [Google Scholar]
- 8. Novara G, Galfano A, Marco VD, Artibani W, Ficarra V. Prognostic factors in squamous cell carcinoma of the penis. Nat Clin Pract Urol. 2007;4(3):140–146. doi:10.1038/ncpuro0751 [DOI] [PubMed] [Google Scholar]
- 9. Li K, Jian S, Xuedong W, et al. Prognostic value of lymphovascular invasion in patients with squamous cell carcinoma of the penis following surgery. BMC Cancer. 2019;19(1):476 doi:10.1186/s12885-019-5714.-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Albersen M, Parnham A, Muneer A. Re: Russel et al: minimally invasive inguinal lymphadenectomy in the management of penile carcinoma (Urology 2017;106:113-118). Urology. 2018;113:254 doi:10.1016/j.urology.2017.10.046 [DOI] [PubMed] [Google Scholar]
- 11. Hu J, Cui Y, Liu P, et al. Predictors of inguinal lymph node metastasis in penile cancer patients: a meta-analysis of retrospective studies. Cancer Manag Res. 2019;11:6425–6441. doi:10.2147/CMAR.S206579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhou X, Feng Q, Ruhua Z, et al. The role of perineural invasion in penile cancer: a meta-analysis and systematic review. Biosci Rep. 2018;38(5):BSR20180333 doi:10.1042/BSR20180333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Attalla K, David JP, Kyle B, et al. Demographic and socioeconomic predictors of treatment delays, pathologic stage, and survival among patients with penile cancer: a report from the National Cancer Database. Urol Oncol. 2018;36(1):14.e17–e14.e24. doi:10.1016/j.urolonc.2017.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Witty K, Peter B, Julie E, Kate B, Alan W, Ian E. The impact of surgical treatment for penile cancer—patients’ perspectives. Eur J Oncol Nurs. 2013;17(5):661–667. doi:10.1016/j.ejon.2013.06.004 [DOI] [PubMed] [Google Scholar]
- 15. Lemon SC, Roy J, Clark MA, Friedmann PD, Rakowski W. Classification and regression tree analysis in public health: methodological review and comparison with logistic regression. Ann Behav Med. 2003;26(3):172–181. doi:10.1207/S15324796ABM2603_02 [DOI] [PubMed] [Google Scholar]
- 16. Ries LAG, Young JL, Keel GE, et al. SEER Survival Monograph: Cancer Survival Among Adults: U.S. SEER Program, 1988-2001, Patient and Tumor Characteristics. 2007. https://seer.cancer.gov/archive/publications/survival/. (Accessed February 2, 2020).
- 17. Portillo SC, Sanchez DF, Cubilla AL. Pathology of invasive and intraepithelial penile neoplasia. Eur Urol Focus. 2019;5(5):713–717. doi:10.1016/j.euf.2019.06.013 [DOI] [PubMed] [Google Scholar]
- 18. Engelsgjerd JS, LaGrange CA. Cancer, Penile. Statpearls; 2020. [PubMed] [Google Scholar]
- 19. Jayaratna IS, Mitra AP, Schwartz RL, Dorff TB, Schuckman AK. Clinicopathologic characteristics and outcomes of penile cancer treated at tertiary care centers in the Western United States. Clin Genitourin Cancer. 2014;12(2):138–142, doi:10.1016/j.clgc.2013.09.006 [DOI] [PubMed] [Google Scholar]
- 20. Krishna S, Krishna S, Nicola S, et al. Role of MRI in staging of penile cancer. J Magn Reson Imaging. 2020. doi:10.1002/jmri.27060 [DOI] [PubMed] [Google Scholar]
- 21. Szabo G. Alcohol’s contribution to compromised immunity. Alcohol Health Res World. 1997;21(1):30–41. [PMC free article] [PubMed] [Google Scholar]
- 22. Roswall N, Weiderpass E. Alcohol as a risk factor for cancer: existing evidence in a global perspective. J Prev Med Public Health. 2015;48(1):1–9. doi:10.3961/jpmph.14.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Testino G. The burden of cancer attributable to alcohol consumption. Maedica (Buchar). 2011;6(4):313–320. [PMC free article] [PubMed] [Google Scholar]
- 24. Schabath MB, Zachary JT, Kathleen ME, et al. Alcohol consumption and prevalence of human papillomavirus (HPV) infection among US men in the HPV in Men (HIM) study. Sex Transm Infect. 2015;91(1):61–67. doi:10.1136/sextrans-2013-051422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stratton KL, Culkin DJ. A contemporary review of HPV and penile cancer. Oncology (Williston Park). 2016;30(3):245–249. [PubMed] [Google Scholar]
- 26. Drager DL, Protzel C, Hakenberg W. Identifying psychosocial distress and stressors using distress-screening instruments in patients with localized and advanced penile cancer. Clin Genitourin Cancer. 2017;15(5):605–609. doi:10.1016/j.clgc.2017.04.010 [DOI] [PubMed] [Google Scholar]
- 27. Mao W, Zhang Z, Huang X, Fan J, Geng J. Marital status and survival in patients with penile cancer. J Cancer. 2019;10(12):2661–2669. doi:10.7150/jca.32037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Torbrand C, Annette W, Linda D, et al. Socioeconomic factors and penile cancer risk and mortality; a population-based study. BJU Int. 2017;119(2):254–260. doi:10.1111/bju.13534 [DOI] [PubMed] [Google Scholar]
- 29. Thuret R, Maxine S, Lars B, et al. A population-based analysis of the effect of marital status on overall and cancer-specific mortality in patients with squamous cell carcinoma of the penis. Cancer Causes Control. 2013;24(1):71–79. doi:10.1007/s10552-012-0091-y [DOI] [PubMed] [Google Scholar]
- 30. Ulff Moller CJ, Simonsen J, Frisch M. Marriage, cohabitation and incidence trends of invasive penile squamous cell carcinoma in Denmark 1978-2010. Int J Cancer 2013;133(5):1173–1179. doi:10.1002/ijc.28107 [DOI] [PubMed] [Google Scholar]
- 31. Rippentrop JM, Joslyn SA, Konety BR. Squamous cell carcinoma of the penis: evaluation of data from the surveillance, epidemiology, and end results program. Cancer 2004;101(6):1357–1363. doi:10.1002/cncr.20519 [DOI] [PubMed] [Google Scholar]
- 32. Sharma P, Ashouri K, Zargar Shoshtari K, Luchey AM, Spiess PE. Racial and economic disparities in the treatment of penile squamous cell carcinoma: results from the national cancer database. Urol Oncol. 2016;34(3):122 e129–e115 doi:10.1016/j.urolonc.2015.10.001 [DOI] [PubMed] [Google Scholar]
- 33. Gregoire L, Cubilla AL, Reuter VE, Haas GP, Lancaster WD. Preferential association of human papillomavirus with high-grade histologic variants of penile-invasive squamous cell carcinoma. J Natl Cancer Inst. 1995;87(22):1705–1709. doi:10.1093/jnci/87.22.1705 [DOI] [PubMed] [Google Scholar]
- 34. Rubin MA, Kleter B, Zhou M, et al. Detection and typing of human papillomavirus DNA in penile carcinoma: evidence for multiple independent pathways of penile carcinogenesis. Am J Pathol. 2001;159(4):1211–1218. doi:10.1016/S0002-9440(10)62506-0 [DOI] [PMC free article] [PubMed] [Google Scholar]